Abstract
Previous studies have shown that the promoter from the barley (Hordeum vulgare) phosphate transporter gene, HvPht1;1, activates high levels of expression in rice (Oryza sativa) roots and that the expression level was induced by up to 4-fold in response to phosphorus (P) deprivation. To identify promoter regions controlling gene regulation specificities, successive promoter truncations were made and attached to reporter genes. Promoters of between 856 and 1,400 nucleotides activated gene expression in a number of cell types but with maximal expression in trichoblast (root hair) cells. For shorter promoters the trichoblast specificity was lost, but in other tissues the distribution pattern was unchanged. The low P induction response was unaffected by promoter length. Domain exchange experiments subsequently identified that the region between −856 and −547 nucleotides (relative to the translational start) is required for epidermal cell expression. A second region located between 0 and −195 nucleotides controls root-tip expression. The HvPht1;1 promoter contains one PHO-like motif and three motifs similar to the dicot P1BS element. Analysis of promoters from which the PHO-like element was eliminated (by truncation) showed no change in the gene induction response to P deficiency. In contrast, mutation of the P1BS elements eliminated any induction of gene expression in response to low P. An internal HvPht1;1 promoter fragment, incorporating a single P1BS element, had an increased response to P deprivation in comparison with the unmodified promoter (containing three elements). Together these findings further our understanding of the regulation of the HvPht1;1 gene and provide direct evidence for a functional role of the P1BS element in the expression of P-regulated genes.
Phosphorus (P) is an essential macronutrient required for a range of key biochemical processes associated with plant growth and function and is taken up by plant roots from soil solution as inorganic phosphate (Pi). However, most soils throughout the world are deficient in readily available forms of P, and in many natural ecosystems, poor availability of P may limit plant growth. Plants have therefore evolved a range of strategies to increase the availability of soil P. These include both morphological and biochemical changes at the soil-root interface. For example, increased root growth and branching, proliferation of root hairs, and release of root exudates can increase plant access to Pi from otherwise poorly available sources (Raghothama, 1999; Vance et al., 2003). Plant roots also possess membrane-bound transporters specifically associated with the uptake of Pi from soil solution. Expression of these transporters is induced in response to P deficiency and enables Pi to be effectively taken up against the large concentration gradient that occurs between the soil solution and internal plant tissues (Smith et al., 2000).
Pi transporters belonging to two major gene families (Pht1 and Pht2) have now been identified for a range of plant species (for review, see Rausch and Bucher, 2002) and have been most extensively studied in Arabidopsis (Arabidopsis thaliana; Muchhal et al., 1996; Mitsukawa et al., 1997; Smith et al., 1997, 2000; Okumura et al., 1998; Karthikeyan et al., 2002; Mudge et al., 2002). The members of the gene family that are expressed in roots are typically up-regulated under P deficiency, but the molecular basis of the regulation has not been investigated. Relatively little work has been reported on the Pi transporter family of genes in cereals, although at least eight Pht1 genes have been identified in barley (Hordeum vulgare; Rae et al., 2003).
To provide a better understanding of the regulation of the Pi-uptake mechanism, the promoter regions from two of the barley Pht1 genes (HvPht1;1 and HvPht1;2) were investigated using reporter genes (Schünmann et al., 2004). When transformed into rice (Oryza sativa), the promoters activated highest levels of gene expression in the trichoblast (root hair) cells of the nodal root. Expression was also evident in the stele of the nodal root, throughout secondary roots, and at a low level in some leaf tissues. The expression level was induced by up to 4-fold in response to P deprivation. Since root hairs are recognized as being the major site for Pi uptake from soil (Gahoonia and Nielsen, 1998), such observations are consistent with a role for the Pi transporters encoded by these genes in the uptake of Pi from soil solution.
The promoter regions of the Pht1 genes from barley were also analyzed for the presence of regulatory elements for which there is evidence of a functional role in plants (Schünmann et al., 2004). Several motifs were identified, including a motif similar to the putative P-responsive P1BS element (GnATATnC) identified by Rubio et al. (2001). The P1BS-like motif was present in all HvPht1 promoters for which sequence was available (Pht1;1, Pht1;2, Pht1;4, Pht1;5, Pht1;6, and Pht1;7; Schünmann et al., 2004). In Arabidopsis a MYB transcription factor, PHR1, has been identified which, when mutated, resulted in impaired P-regulated expression for a range of genes. The PHR1 protein binds to the P1BS element, implying that the element is associated with the P-starvation response (Rubio et al., 2001). The presence of P1BS-like elements in the HvPht1 promoters suggests that the motif may serve a similar role in monocot promoters. However, P1BS-like elements are also present in multiple copies within the ubiquitin promoter, a promoter which does not respond to P deficiency (Schünmann et al., 2004), and few cis elements have been shown to be functional in both monocots and dicots. Furthermore, even in Arabidopsis, the element was identified in the promoter regions of 15% to 20% of all genes and was not preferentially in promoters of P-regulated genes (Hammond et al., 2003). There is therefore, to date, no direct functional evidence that P1BS-like elements are associated with P-regulated expression of the Pi transporter genes, and the presence of the motif in itself is insufficient evidence to conclude a role in P regulation.
A second element identified in the Arabidopsis AtPT2 (also known as ARAth;Pht1;4) promoter with similarity to the PHO element of yeast (Saccharomyces cerevisiae) was described by Mukatira et al. (2001). The observation that nuclear proteins bound specifically to this region under high P nutrition but not low P nutrition suggested that the element was involved in the negative regulation of Pi starvation-induced genes. In addition, Hammond et al. (2003) showed from microarray screening that the PHO-like element (G(G/T/A)(C/T/A)GTGG) was found preferentially in promoter regions of P-responsive genes (30% of the genes identified, compared with 7% occurrence for unselected genes). The PHO-like element is also present in four of the HvPht1 promoters (Pht1;1, Pht1;2, Pht1;4, and Pht1;6; P. Schünmann, unpublished data) and is also a candidate motif for controlling the expression of P-regulated genes.
In this report the specificity of expression and P regulation of the HvPht1;1 gene promoter was investigated through a series of promoter modifications. From the results presented here, promoter fragments required for expression in both the root epidermis and in the apical region of the root were identified. Further, in the HvPht1;1 promoter, P responsiveness was shown to be associated specifically with the P1BS-like motif, confirming the importance of the motif in regulating the level of gene expression in vivo in response to P deprivation.
RESULTS
Construction of a Promoter Deletion Series for HvPht1;1
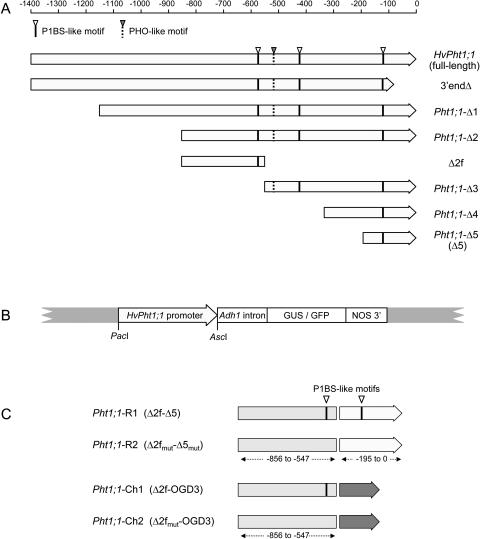
The HvPht1;1 promoter, designated here as full-length HvPht1;1, is derived from the 1,400 nucleotides immediately upstream from the translation start of the HvPht1;1 gene (GenBank accession number AF543197) and has been described previously (Schünmann et al., 2004). A promoter deletion series was prepared as outlined in Figure 1A and used to generate reporter gene constructs (Fig. 1B). The constructs included an intron derived from the maize (Zea mays) Adh1 gene as this had previously been shown to increase expression levels by more than 15-fold without altering promoter specificity (Schünmann et al., 2004). In addition to the five deletion constructs (Pht1;1-Δ1 to Pht1;1-Δ5), a negative control (3′endΔ) was designed that lacked 85 nucleotides of 3′ sequence including the putative transcription start and 5′ untranslated region of the HvPht1;1 gene. The HvPht1;1 promoter constructs were analyzed using a rice system since its high transformation efficiency enabled numerous promoter variants to be assessed in detail as stably transformed lines, and many studies support the hypothesis that cloned promoters commonly retain their native expression profile when transformed into related species (see Komari et al., 1998). Preliminary experiments showed that the full-length HvPht1;1 promoter attached to green fluorescent protein (GFP) conferred expression in similar tissues in both barley and rice (data not shown).
Figure 1.
A, The HvPht1;1 full-length promoter and truncated derivatives. Approximate promoter lengths (in nucleotides) measured from the ATG start codon are indicated by the scale bar. The position of the P1BS-like elements and a PHO-like motif are indicated. The Δ2f fragment used to generate additional promoters (Fig. 1C) is also shown. B, Structure of the HvPht1;1 promoter::reporter gene expression cassette, described in detail in Schünmann et al. (2004): The PacI and AscI sites flanking the promoter enable substitutions to be made directly within the binary vector. C, Structure of the rearranged (R1 and R2) and chimeric (Ch1 and Ch2) HvPht1;1 promoters derived from the Δ2f, Δ5, and OGD3 promoter fragments. The relative positions of functional P1BS-like elements (Δ2f and Δ5 regions) and the corresponding fragments in which specific mutations were introduced (Δ2fmut and Δ5mut) are shown.
Truncation of the HvPht1;1 Promoter Does Not Affect Promoter Strength or P Responsiveness
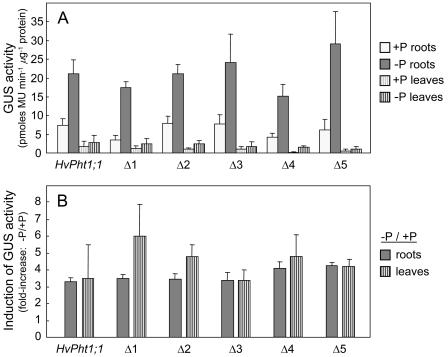
Following transformation of the β-glucuronidase (GUS)-containing constructs into rice, 15 independent lines were regenerated for each construct, and the 8 highest expressing lines were selected for further study. Plants were initially grown in hydroponic culture with a high Pi supply (0.1 mm). After 4 weeks, one set of primary transformant (T0) clones (derived from separating tillers of the original T0 plants) was transferred to Pi-free nutrient solution. GUS activity was measured in roots and leaves 14 and 21 d following the minus P treatment, at which time internal leaf Pi levels were 0.084 ± 0.007 and 0.057 ± 0.003 μg Pi mg−1 leaf fresh weight, respectively (mean ± se). This compared with 0.342 ± 0.024 μg Pi mg−1 leaf fresh weight for control plants that were maintained with Pi. Consistent with previous findings (Schünmann et al., 2004), GUS activities increased steadily over the 21 d of P deprivation for all promoter constructs. In a parallel experiment under similar experimental conditions, reporter gene expression levels driven by the ubiquitin control promoter remained stable during the 3 weeks of P deprivation (Schünmann et al., 2004). Following truncation of the Pht1;1 promoter, no differences were observed in either promoter strength (Fig. 2A) or in the low P induction of GUS expression (Fig. 2B) for the deletion series compared with the full-length Pht1;1 promoter. All promoter deletions also had similar GUS levels in leaves (approximately 10% of the root expression level) to the full-length promoter, with the exception of the Pht1;1-Δ5 promoter, which showed a reduction in relative leaf level (Fig. 2A). No GUS activity was observed for the 3′endΔ promoter fragment in any tissue examined (data not shown).
Figure 2.
GUS activities and response to P deprivation for root and leaf tissues from rice plants transformed with the HvPht1;1::GUS construct and promoter deletion derivatives. A, GUS activities (mean ± se) for the three highest expressing transgenic lines selected from a total of 15 independent lines for each construct. B, Induction response (fold-increase) of reporter gene expression following P deprivation. Values shown are mean inductions (ratio of −P to +P) for eight independent transgenic lines (±se). All measurements were taken following 21 d of treatment.
The low-P-induction response was not affected for any promoters of the deletion series despite changes to the number of P1BS-like elements (reduced from three to one) and the loss of the PHO-like element (absent from promoters Pht1;1-Δ4 and Pht1;1-Δ5). This suggests that the observed gene induction is independent of the PHO-like element and that either the P1BS element is not functional in cereals or that the number of P1BS elements per se is not important.
Identification of Promoter Regions Conferring Trichoblast-Specific Gene Expression
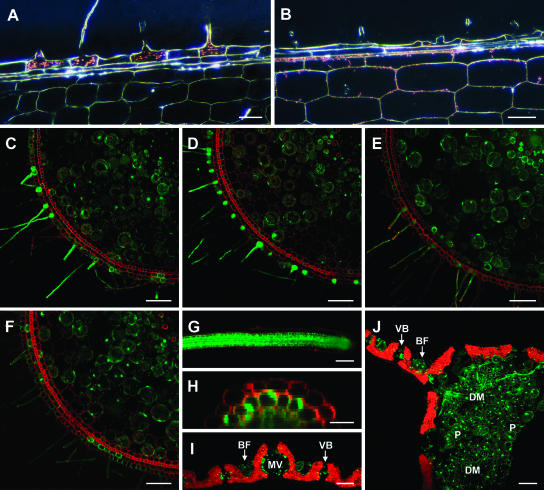
To determine whether the spatial distribution of gene expression was affected by the promoter truncations, P-deficient nodal roots were stained for GUS activity. While expression patterns for the Pht1;1-Δ1 and Pht1;1-Δ2 promoters were indistinguishable from the full-length HvPht1;1 promoter (Fig. 3A), the shorter promoters (Pht1;1-Δ3–Δ5) showed greatly reduced or no expression in the trichoblast cells but apparent expression in the hyperdermis and cortex (Fig. 3B; Supplemental Fig. 1, available at www.plantphysiol.org).
Figure 3.
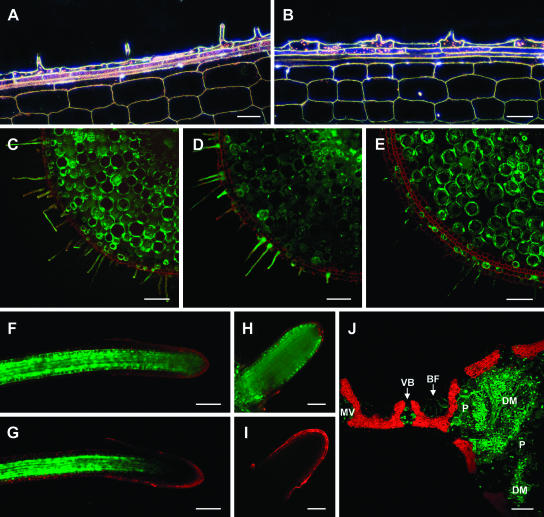
Tissue specificity of the HvPht1;1 promoter constructs analyzed by dark-field microscopy (GUS-containing constructs) or confocal microscopy (GFP-containing constructs). At least three independent transgenic lines were examined for each genotype, and typical results are presented. Shown are A and B, Longitudinal sections of P-deficient nodal roots transformed with the promoter::GUS constructs, taken at the site of initial root-hair emergence, stained for GUS activity (bio-refringence of GUS crystals appearing red) for plants containing Pht1;1-Δ2 (A) and Pht1;1-Δ5 (B) promoter::GUS constructs. C to F, Transverse sections of P-deficient nodal roots from plants transformed with full-length HvPht1;1 (C) and truncated Pht1;1-Δ2 (D), Pht1;1-Δ3 (E), and Pht1;1-Δ5 (F) promoter::GFP constructs. The Pht1;1-Δ5 promoter had a similar distribution of activity to Pht1;1-Δ4, and the Pht1;1-Δ2 promoter had a similar distribution of activity to Pht1;1-Δ1 (not shown). Roots were stained with propidium iodide (red fluorescence) to show cell walls. G, Secondary root tip of a plant transformed with the Pht1;1-Δ5 promoter::GFP construct. H, Transverse sections through a secondary root of a plant transformed with the Pht1;1-Δ5 promoter::GFP construct showing the reduced expression in the outer epidermal layer. I and J, Transverse section through leaf blade (I) and leaf midrib (J) for the Pht1;1-Δ5 promoter::GFP construct (chlorophyll autofluorescence shown in red). Leaf cell types showing GFP fluorescence include bulliform cells (BF), vascular bundles (VB), midvein (MV), parenchyma cells (P), and the star-shaped cells of the leaf diaphragm (DM). Scale bars are indicated in μm. Bars = 20 μm (A, B, and H) and 80 μm (C–G and I and J).
The loss of trichoblast specificity in the truncated Pht1;1 promoters was further investigated in rice plants transformed with GFP-containing constructs (Pht1;1 full-length promoter plus deletion derivatives), assessed by confocal microscopy. Similar to the full-length promoter (Fig. 3C), plants transformed with the Pht1;1-Δ1 and Δ2 promoters showed high levels of expression in trichoblast cells of the nodal root and lower levels in the root cortex (Fig. 3D). In contrast, the shorter promoters expressed GFP with markedly reduced (Pht1;1-Δ3; Fig. 3E) or minimal (Pht1;1-Δ4 and Δ5; Fig. 3F; Supplemental Fig. 2) expression in the epidermal cells. Expression in other tissues was not affected by promoter length; for example, in secondary roots, GFP expression was expressed throughout the root but frequently at a lower level in the outer epidermal layer compared with other cell types (Fig. 3, G and H), and in leaves expression was specific to a few cell types including the auricle and ligule (not shown) in the leaf diaphragm and parenchyma cells of the midrib and in the bulliform cells and vascular bundles of the leaf blade (Fig. 3, I and J). Consistent with earlier observations (Schünmann et al., 2004), comparison with constructs lacking the inclusion of the Adh1 intron at the 3′ end of the promoter showed that the presence of the intron did not affect spatial expression in any tissues examined while significantly increasing the level of expression for all constructs (data not shown).
Promoter Modifications Designed to Identify Functional Domains
Reducing the length of the promoter from 856 to 546 nucleotides (relative to the translational start) resulted in an altered expression profile with reduced expression in the trichoblast cells (Fig. 3). To establish whether the −547 to −856 region contained promoter elements that controlled cell specificity, the fragment (named Δ2f; Fig. 1A) was isolated and joined to the Pht1;1-Δ5 promoter to make a rearranged promoter designated as Pht1;1-R1 (Δ2f-Δ5). A chimeric promoter, Pht1;1-Ch1 (Δ2f-OGD3), was also constructed by joining the Δ2f fragment to an unrelated minimal promoter (OGD3) derived from an endosperm-specific gene and was used to identify promoter activity controlled by the Δ2f promoter fragment without influence from other regions of the HvPht1;1 promoter.
Two further promoters were prepared whereby mutations to the P1BS-like motifs (GnATATnC) were introduced. P1BS elements have been implicated in the P-regulated expression of genes in Arabidopsis (Rubio et al., 2001), and previous analysis of upstream regions from the barley Pht1 genes identified P1BS-like domains in all Pht1 promoters for which sequence was available (Schünmann et al., 2004). The Δ2f and Δ5 fragments each contain a single copy of the motif. In each case, the P1BS sequence was mutated to GnAGAG (to make fragments Δ2fmut and Δ5mut, respectively) using PCR primers outlined in Table I. The derived promoters were designated Pht1;1-R2 (Δ2fmut-Δ5mut) and Pht1;1-Ch2 (Δ2fmut-OGD3). Promoters Pht1;1-R2 and Pht1;1-Ch2 therefore differ from Pht1;1-R1 and Pht1;1-Ch1 (respectively) only by two base substitutions at each P1BS motif (Fig. 1C).
Table I.
Primers used to generate the HvPht1;1 promoter deletion series and modified promoters
| Promoter/Fragment | Primer ID | Primer Sequence |
|---|---|---|
| HvPht1;1 (full-length) | Pht1;1 5′ (forward) | ctcactagaGCGGCCGCTTAATTAAGAGCTCCGACTACCCCCG |
| Pht1;1-Δ1 | −1151 5′ (forward) | ctcactagaTTAATTAACAAAATGCAATCAACCGAC |
| Pht1;1-Δ2 | −856 5′ (forward) | ctcactagaTTAATTAACTCGTAAACTGATAGCACG |
| Pht1;1-Δ3 | −546 5′ (forward) | ctcactagaTTAATTAAGTTGCTAAGTTTGACATAGC |
| Pht1;1-Δ4 | −333 5′ (forward) | ctcactagaTTAATTAAGCTCAATCCTTGCGATAAC |
| Pht1;1-Δ5 | −195 5′ (forward) | ctcactagaTTAATTAATGTACGTACGTGCAATGC |
| HvPht1;1, plus Δ1-Δ5 | Pht1;1 3′ (reverse) | gatccagtgGGCGCGCCGGTCGCCGGCGATCTC |
| 3′end Δ | −1151 5′ (forward | (as above) |
| −86Δ 3′ (reverse) | gatccagtgGGCGCGCCGTAGTACGTATATATAGGCA | |
| Δ2f | −856 5′ (forward) | (as above) |
| LinkΔ5-Δ2 (reverse) | tacggcattgcaCGTACGTACATGTAAACGAGAGAAAATAATGAG | |
| Δ2fmut | −856 5′ (forward) | (as above) |
| LinkΔ5-Δ2mut (reverse) | tacggcattgcaCGTACGTACATGTAAACGAGAGAAAATAATGAGAAGATCCGACTCTGCCTGAG | |
| Δ5 | LinkΔ2-Δ5 (forward) | cgtttacatgtaCGTACGTGCAATGC |
| Pht1;1 3′ (reverse) | (as above) | |
| Δ5mut | LinkΔ2-Δ5mut (forward) | cgtttacatgtaCGTACGTGCAATGCCGTACATGGAGATTTTAATATTTACAAGTAGCGAGGAAATGTCCCTTTGGCAGAGCCGCCGAAC |
| Pht1;1 3′ (reverse) | (as above) | |
| OGD3 | LinkΔ2-OGD3 (forward) | cgtttacatgtaCGTACGCATCATGTTTATCCTGGAC |
| OGD3 3′ (reverse) | gatccagtgGGCGCGCCTGTTTATTGCTCTTGCTTGC |
The mutated P1BS-like motifs (GnAGAGnC) contained within the linkΔ5-Δ2mut (reverse) primer and the linkΔ2-Δ5mut (forward) primer are shown in bold. Incorporated endonuclease restriction site sequences are underlined. 5Δ Spacer sequences are indicated in lowercase.
The P1BS-Like Motif Is a Functional Phosphate Responsive Element in Rice
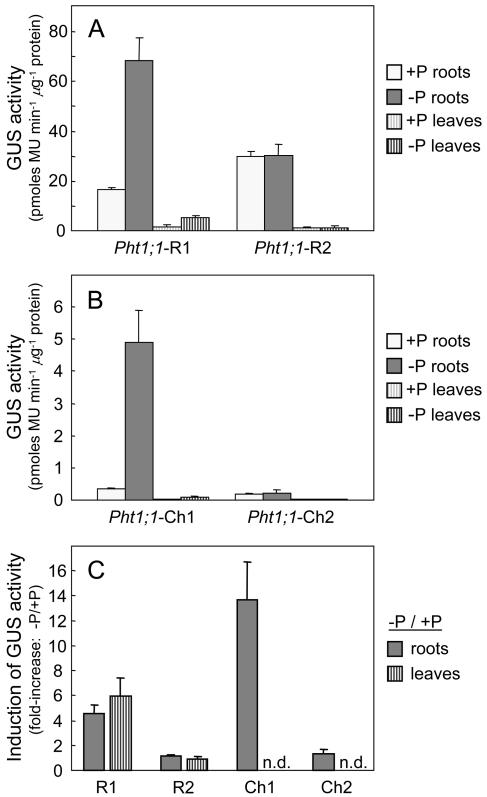
Following transformation of the GUS-containing constructs into rice, 20 independent lines were regenerated for each of the 4 modified Pht1;1 constructs, and the 8-highest expressing T0 lines of each construct were selected and grown for further study as described previously. Analysis of GUS activities (Fig. 4A) for the Pht1;1-R1 promoter (Δ2f-Δ5) showed that gene activity was approximately 2-fold higher than for the full-length Pht1;1 promoter (i.e. in comparison with Fig. 2A), but both the level of gene induction following P deficiency and the relative distribution of GUS activities between roots and leaves were similar. For the Pht1;1-R2 promoter (in which the two P1BS elements present in the promoter region were mutated), the low P induction of gene expression was completely abolished for both root and leaf tissues (Fig. 4, A and C).
Figure 4.
GUS activities and response to P deprivation for root and leaf tissues for rice plants transformed with the Pht1;1-R1, R2, Ch1, and Ch2 promoter::GUS constructs. A and B, GUS activities (mean ± se) for the three highest expressing transgenic lines (to maximize any differences between the promoters) selected from a total of 20 independent lines for each construct. C, Induction response (fold-increase) of reporter gene expression following P deprivation. Values shown are mean inductions (ratio of −P to +P) for eight independent transgenic lines (± se). All measurements were taken following 21 d of treatment, and n.d. denotes activities that were below the limits of detection.
The chimeric promoter Pht1;1-Ch1 (Δ2f-OGD3) activated low levels of expression, less than 10% of that observed for promoter Pht1;1-R1 (Fig. 4, A and B); however, the level of gene induction following P deficiency was increased by approximately 3-fold over Pht1;1-R1 to 14-fold overall (Fig. 4C). For the Pht1;1-Ch2 promoter, mutation of the P1BS element present on the Δ2f fragment again completely abolished the low P-induction response in root tissues, resulting in GUS activities that were both low and independent of P nutrition. Levels of expression for the Pht1;1-Ch1 and Ch2 promoters in leaf tissue were too low to enable a measure of the induction response to be determined.
The Δ2f Promoter Fragment Confers Epidermal Expression
In nodal roots, constructs driven by the Pht1;1-R1 (Δ2f-Δ5) and Pht1;1-R2 (Δ2fmut-Δ5mut) promoters resulted in activities prevalent throughout the root including the epidermal and hyperdermal cell layers (Fig. 5, A and C; Supplemental Figs. 3 and 4). For the Pht1;1-Ch1 (Δ2f-OGD3) promoter, maximal expression was found in the epidermal cell layer, predominantly in trichoblast cells (Fig. 5, B and D; Supplemental Figs. 3 and 4). The distinctiveness of the expression profile for the Pht1;1-Ch1 construct is particularly evident when compared with that of the constitutive promoter ubiquitin (Schünmann et al., 2004; also shown in Fig. 5E). No differences in the spatial expression profile were observed with the ubiquitin promoter that could be attributed to P nutrition. For plants transformed with the Pht1;1-Ch2 promoter (Δ2fmut-OGD3), absolute levels of reporter gene expression were too low for the expression profile to be determined by microscopy.
Figure 5.
Tissue specificity of promoter constructs Pht1;1-R1 and Pht1;1-Ch1 analyzed by dark-field microscopy (GUS-containing constructs) or confocal microscopy (GFP-containing constructs). The Pht1;1-R2 promoter showed a similar distribution of activity to Pht1;1-R1 (not shown). At least three independent transgenic lines were examined for each genotype, and typical results are presented. Shown are A and B, Longitudinal sections of P-deficient nodal roots taken at the site of initial root-hair emergence. Root segments were stained for GUS activity (bio-refringence of GUS crystals appearing red) for plants containing Pht1;1-R1 (A) and Pht1;1-Ch1 (B) promoter::GUS constructs. C to E, Transverse sections of P-deficient nodal roots from plants transformed with Pht1;1-R1 (C) and Pht1;1-Ch1 (D) promoter::GFP constructs. E, Transverse sections through P-deficient nodal roots from plants transformed with the constitutive control promoter, ubiquitin (described in Schünmann et al., 2004). Roots were stained with propidium iodide (red fluorescence) to show cell walls. F and G, Secondary root tips for plants transformed with Pht1;1-R1 (F) and Pht1;1-Ch1 (G) promoter::GFP constructs. The confocal software was standardized for equivalent signal intensity in the mature region of the root to negate the 10-fold difference in level of expression between the Pht1;1-R1 and Pht1;1-Ch1 constructs. H and I, Expression in emerging secondary roots for plants containing Pht1;1-R1 (H) and Pht1;1-Ch1 (I) promoter::GFP constructs. Imaging levels are shown standardized for more mature regions of the secondary roots. J, Transverse section through a leaf blade and midrib for the Pht1;1-Ch1 promoter::GFP construct (chlorophyll autofluorescence shown in red). Leaf cell types showing GFP fluorescence include bulliform cells (BF), vascular bundles (VB), midvein (MV), parenchyma cells (P), and the star-shaped cells of the leaf diaphragm (DM). Bars = 20 μm (A and B), 40 μm (H and I), and 80 μm (C–G and J).
The Δ5 Promoter Fragment Is Required for Root-Tip Expression
Within secondary roots, gene expression controlled by the Pht1;1-R1 (Δ2f-Δ5) promoter and mutated Pht1;1-R2 promoter was similar to the Pht1;1-Δ5 (isolated Δ5) promoter (Fig. 5F). In contrast, the Pht1;1-Ch1 promoter (Δ2f-OGD3), which lacked the Δ5 region, showed markedly reduced expression near the root tip (Fig. 5G; Supplemental Fig. 5). Since absolute levels of GFP expression differed markedly between the different transgenic lines, images shown in Figure 5, F and H, were taken with imaging levels standardized to the GFP intensity approximately 600 μm from the root tip. Relative levels of GFP expression at the root tips were quantified using the TCS SP2 confocal software. The intensity of GFP measured 50 to 100 μm from the tip for the Pht1;1-Ch1 promoter was 15.8% ± 1.4% of that measured approximately 600 μm from the tip (average for 5 different root tips). This compared with 45.8% ± 4.6% for the Pht1;1-R1 promoter and indicates a 3-fold reduction in GFP expression at the root tip of the Pht1;1-Ch1 promoter. In young secondary roots, the effect was even more marked, with no GFP detectable in emerging root tips from any Pht1;1-Ch1 plants (Fig. 5, H and I). These data suggest that the Δ5 promoter domain contains regulatory elements required for gene expression in the root tip. In contrast to the observations for root tissues, the specificities of leaf expression did not appear to be altered by any of the promoter modifications (e.g. as shown in Fig. 5J for Pht1;1-Ch1 as compared to Fig. 3J).
DISCUSSION
Following deletion analyses, the smallest Pht1;1-derived promoter (truncated to 195 nucleotides; Pht1;1-Δ5) was similar to the full-length promoter for absolute gene expression levels in roots and leaves. The low P induction of gene expression was also similar for all of the truncated promoters. This indicates that the promoter's activity is predominantly controlled by the 195 nucleotides located immediately upstream of the translation start site of the gene. However, while the full-length promoter expressed high levels in the trichoblast cells of the epidermis, promoters truncated to less than 546 nucleotides showed reduced expression within the epidermis, suggesting that a promoter element controlling trichoblast specificity was located between −547 and −856 nucleotides (relative to the translational start). To test this hypothesis, the region (Δ2f) was isolated and attached to the Δ5 fragment and to an unrelated minimal promoter (OGD3; Fig. 1). Promoter Pht1;1-Δ5 showed expression throughout the root but not in epidermal cells. In contrast, promoter Pht1;1-R1 (Δ2f-Δ5) activated expression in most cell types including those of the epidermal layer, while chimeric promoter Pht1;1-Ch1 (Δ2f-OGD3) characteristically showed a more extreme profile with highest levels of expression in the trichoblast cells. It is unlikely that the expression profile shown by the Pht1;1-Ch1 promoter was influenced by the OGD3 minimal promoter since the minimal promoter had previously been used in transient assays and did not activate expression in any tissue (endosperm, embryo, root, or shoot), while a longer promoter (additional 60 nucleotides at the 5′ end taken from the native promoter) conferred expression only in the seed (data not shown). It is therefore likely that the Δ2f region contains elements for epidermal cell expression. Root-tip expression controlled by the Δ2f fragment in chimeric promoters Pht1;1-Ch1 and Pht1;1-Ch2 also differed from that of the full-length and truncated promoters, with expression being notably absent in tips of emerging secondary roots. This indicates that although the cellular localization of root expression for the HvPht1;1 promoter is controlled by multiple cis elements located throughout the promoter region, specificity for root-tip expression resides within the Δ5 fragment.
In leaf tissues, the HvPht1;1 promoters result in a distinct cellular expression profile in rice (Figs. 3, I and J, and 5J), similar to results observed following transformation of the HvPht1;1::GFP constructs into barley, and markedly different from that of a constitutive promoter (Schünmann et al., 2003, 2004). In contrast to the observations for roots, promoter modifications resulted in no apparent effects on the leaf expression profile. It therefore seems likely that leaf expression is controlled by elements that are present in both the Δ2f and Δ5 fragments, suggesting a degree of redundancy for motifs within the promoter.
Promoter analysis has previously identified P1BS-like elements as being present in all six Pht1 genes for which promoter regions are available (Schünmann et al., 2004). Although the element is a binding site for the PHR1 transcription factor (Rubio et al., 2001), no direct evidence for a functional role of this motif in the P-regulated response of gene expression has previously been demonstrated. The native Pht1;1 promoter contains three P1BS-like motifs. Following truncation of the promoter, this was reduced to two copies for the Pht1;1-Δ3 promoter and only a single copy for the Pht1;1-Δ4 and Pht1;1-Δ5 promoters. Despite the reduction in number, no effect on the low P-induction response was observed. However, mutation of the motif (i.e. both elements within the Pht1;1-R2 promoter and the single element within the Pht1;1-Ch2 promoters) resulted in a complete loss of gene induction following P deficiency (Fig. 4). Together these findings illustrate that the element is a key regulator of gene expression in response to P availability, that a single copy of the element is sufficient to confer a P response, and that multiple elements do not confer a greater induction response.
Consistent with the observation that the number of elements does not correlate with the level of the gene induction response, the Δ2f fragment, which contains a single P1BS motif, resulted in a 14-fold increase in gene induction when attached to the OGD3 minimal promoter, 3-fold higher than that for the full-length promoter which contains three copies of the motif. In addition, although our results illustrate that the presence of the P1BS element is essential for P-regulated expression, the presence of four P1BS elements in the ubiquitin promoter does not result in P-regulated expression, implying that the response is also dependent upon other sequences within the promoter. To identify other regions of commonality, promoters from a range of P-responsive and nonresponsive monocot genes were aligned using the promoter sequence flanking the P1BS elements (50 bases both upstream and downstream). However, no other significant regions of homology between the various P-responsive promoters were identified (data not shown).
To conclude, the findings reported here have identified specific regions in the Pht1;1 promoter required for expression in both epidermal cells (primarily trichoblasts) and apical tissues of the root (summarized in Supplemental Fig. 6). In addition, the functionality of the P1BS element in the P regulation of gene expression in cereals was clearly demonstrated and, to our knowledge, provides the first direct evidence for the role of this element in the low P response for any plant species.
MATERIALS AND METHODS
Preparation of the HvPht1;1 Promoter Deletion Series
The barley (Hordeum vulgare) Pht1 genes were named according to the Commission on Plant Gene Nomenclature (http://mbclserver.rutgers.edu/CPGN/Guide.html) as HORvu;Pht1;1 through to HORvu;Pht1;8; however, for simplification, they are referred to here as HvPht1;1 to HvPht1;8, respectively.
The HvPht1;1 gene promoter has been described previously (Schünmann et al., 2004). A series of deletions (Pht1;1-Δ1 to Δ5, and 3′endΔ; Fig. 1A) were made using the primers shown in Table I. The fragments were amplified by PCR, digested with PacI and AscI, and cloned by promoter exchange into binary vectors containing either the Pht1;1 promoter::GUS or Pht1;1 promoter::GFP expression cassettes (Fig. 1; Schünmann et al., 2004). PCR products were sequenced to confirm base identity. As shown in Figure 1, an intron derived from the maize (Zea mays) Adh1 gene was incorporated at the 3′ end of the promoter as this had previously been shown to increase expression levels by more than 15-fold without altering promoter specificity (Schünmann et al., 2004). Expression constructs were transferred to Agrobacterium tumefaciens strain AGL1 by triparental mating (Lazo et al., 1991). Rice (Oryza sativa cv Taipei 309) was transformed as described by Upadhyaya et al. (2000).
Generation of Modified Promoters and P1BS Element Mutation
A series of promoter modifications were generated in which the fragment between −856 and −547 relative to the translational start (Δ2f) was joined to the Pht1;1-Δ5 promoter fragment (Δ5) or to the OGD3 minimal promoter (Fig. 1C). The fragments were amplified by PCR using the primers shown in Table I. The Δ2f and Δ5 promoter regions each possess a single P1BS-like motif (GnATATnC; Fig. 1). Where applicable, the motif was mutated to GnAGAGnC by use of specific PCR primers (Table I). The rearranged Pht1;1-R1 promoter was generated by joining the Δ2f and Δ5 regions (using link primers as outlined in Table I) and cloning into PacI/AscI digested GUS and GFP binary vectors (Fig. 1B). A comparable promoter containing mutated P1BS elements, Pht1;1-R2 (Δ2fmut and Δ5mut fragments), was also generated. Chimeric promoters, Pht1;1-Ch1 and Pht1;1-Ch2, were similarly generated by joining the Δ2f region, either with or without a mutated P1BS element (Δ2f and Δ2fmut, respectively) to the OGD3 promoter (Fig. 1C). OGD3 is a synthetic minimal promoter generated by PCR from the 3′ end of the promoter region of an endosperm-specific globulin gene from oats (AsGlo1; Shotwell et al., 1990) to which a synthetic 5′ untranslated leader (Patel et al., 2000) was attached to the 3′ end. The derived OGD3 sequence is as follows: 5′-CATCATGTTTATCCTGGACTACTTTTTATGGCTATAAAATCAAACTTACAATTAGGAAACTAGCACCAATCCACCTTCTACAATCTCggatccGTCCTAAAGCAAGCAAGAGCAATAAACA (total length 121 bp, incorporated BamHI site shown in lowercase, TATA box shown in bold). All PCR products were sequenced to confirm base identity. Unless otherwise indicated, all gene constructs referred to include the Adh1 intron incorporated at the 3′ end of the promoter. Modified gene constructs were transformed into rice as described previously.
Plant Growth Conditions
Plants were grown hydroponically in 10-L containers, 12 plants/container, with 29°C/22°C (day/night) temperatures and a 16-/8-h photoperiod (glasshouse conditions with controlled heating and supplemental lighting). The nutrient solution was changed twice weekly and contained 0.5 mm KNO3, 0.5 mm Ca(NO3)2, 0.25 mm NH4NO3, 0.15 mm MgSO4, 0.1 mm KH2PO4, 10 μm FeEDTA, 12 μm Na2EDTA, 44 μm H3BO3, 8 μm MnCl2, 1.4 μm ZnCl2, and 0.8 μm CuCl2. Duplicate sets of T0 clonal plants were prepared by separating tillers derived from single primary transformants, enabling plants deprived of P to be compared with P-fed controls of the same T0 line. After approximately 4 weeks growth, the clonal plant sets were transferred to 2× strength nutrient solution with or without the inclusion of 0.1 mm KH2PO4. Plants were grown for a further 3 weeks. In a previous paper, the ubiquitin promoter was studied in detail in parallel to the HvPht1;1 promoter. Under these experimental conditions, reporter gene expression levels driven by the constitutive control promoter, ubiquitin, were previously shown to remain stable during the 3 weeks of P deprivation (Schünmann et al., 2004).
Measurement of Pi in Leaf Tissue
Pi content of plants was determined from leaf tissue taken 5 cm from the tip of the first mature leaf using a modification of the method of Irving and McLaughlin (1990) as described by Schünmann et al. (2004).
Protein Extraction and GUS Assays
GUS activities were assayed in 0- to 8-cm sections of the nodal root and in leaf samples taken 5 cm from the tip. Plant tissues were ground with fine sand in 50 mm sodium phosphate buffer (pH 7.0, 10 mm EDTA, 10 mm β-mercaptoethanol, 0.1% [v/v] Triton X-100). GUS activities were measured using a modification of the method of Breyne et al. (1993) as described previously (Schünmann et al., 2004).
Histochemical Localization of GUS Expression
Histochemical analysis of GUS activity was performed essentially as described by Stomp (1992). Excised root tissues were incubated at 25°C for 12 h in 100 mm sodium phosphate buffer (pH 7.2, 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, 0.1% [v/v] Triton X-100) containing 0.1 to 1 mm 5-bromo-4-chloro-3-indoyl glucuronide. After fixation, roots were sectioned and visualized under dark-field microscopy.
Confocal Microscopy
For plants transformed with GFP-containing constructs, confocal microscopy images of cross sections of nodal roots, intact secondary roots, and cross sections of leaf tissue were captured and processed using an upright Leica (Wetzlar, Germany) DMRXE microscope and Leica TCS SP2 confocal software.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Supplementary Material
Acknowledgments
We thank John Graham, Celia Miller, Rosemary White, Carl Davies, and Sandie McIntosh for excellent technical assistance and are extremely grateful to Donna Glassop for transforming the HvPht1;1::GFP construct into barley and providing us with regenerated transgenic lines.
This work was supported by Graingene: a joint venture between Australian Wheat Board Limited, Commonwealth Scientific and Industrial Research Organization, Grains Research and Development Corporation, and Syngenta Seeds.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045823.
References
- Breyne P, De Loose M, Dedonder A, Van Montagu M, Depicker A (1993) Quantitative kinetic analysis of β-glucuronidase activities using a computer-directed microtiter plate reader. Plant Mol Biol Rep 11: 21–31 [Google Scholar]
- Gahoonia TS, Nielsen NE (1998) Direct evidence on participation of root hairs in phosphorus (32P) uptake from soil. Plant Soil 198: 147–152 [Google Scholar]
- Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, May ST, Rahn C, Swarup R, Woolaway KE, White PJ (2003) Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol 132: 578–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving GCJ, McLaughlin MJ (1990) A rapid and simple field test for phosphorus in Olsen and Bray no. 1 extracts of soil. Commun Soil Sci Plant Anal 21: 2245–2255 [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komari T, Hiei Y, Ishida Y, Kumashiro T, Kubo T (1998) Advances in cereal gene transfer. Curr Opin Plant Biol 1: 161–165 [DOI] [PubMed] [Google Scholar]
- Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology (N Y) 9: 963–967 [DOI] [PubMed] [Google Scholar]
- Mitsukawa N, Okumura S, Shirano Y, Sato S, Kato T, Harashima S, Shibata D (1997) Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc Natl Acad Sci USA 94: 7098–7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal US, Pardo JM, Raghothama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 96: 5868–5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353 [DOI] [PubMed] [Google Scholar]
- Mukatira UT, Liu C, Varadarajan DK, Raghothama KG (2001) Negative regulation of phosphate starvation-induced genes. Plant Physiol 127: 1854–1862 [PMC free article] [PubMed] [Google Scholar]
- Okumura S, Mitsukawa N, Shirano Y, Shibata D (1998) Phosphate transporter gene family of Arabidopsis thaliana. DNA Res 5: 261–269 [DOI] [PubMed] [Google Scholar]
- Patel M, Johnson JS, Brettell RIS, Jacobsen J, Xue G-P (2000) Transgenic barley expressing a fungal xylanase gene in the endosperm of the developing grains. Mol Breed 6: 113–124 [Google Scholar]
- Rae AL, Cybinski DH, Jarmey JM, Smith FW (2003) Characterization of two phosphate transporters from barley; evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol Biol 53: 27–36 [DOI] [PubMed] [Google Scholar]
- Raghothama K (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216: 23–37 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signalling both in vascular plants and in unicellular algae. Genes Dev 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünmann PHD, Richardson AE, Smith FW, Delhaize E (2004) Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.). J Exp Bot 55: 855–865 [DOI] [PubMed] [Google Scholar]
- Schünmann PHD, Surin B, Waterhouse PM (2003) A suite of novel promoters and terminators for plant biotechnology II. The pPLEX series for use in monocots. Funct Plant Biol 30: 453–460 [DOI] [PubMed] [Google Scholar]
- Shotwell MA, Boyer SK, Chesnut RS, Larkins BA (1990) Analysis of seed storage protein genes of oats. J Biol Chem 265: 9652–9658 [PubMed] [Google Scholar]
- Smith FW, Ealing PM, Dong B, Delhaize E (1997) The cloning of two Arabidopsis genes belonging to a phosphate transporter family. Plant J 11: 83–92 [DOI] [PubMed] [Google Scholar]
- Smith FW, Rae AL, Hawkesford MJ (2000) Molecular mechanisms of phosphate and sulphate transport in plants. Biochim Biophys Acta 1465: 236–245 [DOI] [PubMed] [Google Scholar]
- Stomp A-M (1992) Histochemical localization of β-glucuronidase. In SR Gallagher, ed, GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression. Academic Press, London, pp 103–113
- Upadhyaya NM, Surin B, Ramm K, Gaudron J, Schünmann PHD, Taylor W, Waterhouse PM, Wang MB (2000) Agrobacterium-mediated transformation of Australian rice cultivars Jarrah and Amaroo using modified promoters and selectable markers. Aust J Plant Physiol 27: 201–210 [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a non-renewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.