Abstract
The intestine exerts a considerable influence over urinary oxalate in two ways, through the absorption of dietary oxalate and by serving as an adaptive extra-renal pathway for elimination of this waste metabolite. Knowledge of the mechanisms responsible for oxalate absorption and secretion by the intestine therefore have significant implications for understanding the etiology of hyperoxaluria, as well as offering potential targets for future treatment strategies for calcium oxalate kidney stone disease. In this review, we present the recent developments and advances in this area over the past 10 years, and put to the test some of the new ideas that have emerged during this time, using human and mouse models. A key focus for our discussion are the membrane-bound anion exchangers, belonging to the SLC26 gene family, some of which have been shown to participate in transcellular oxalate absorption and secretion. This has offered the opportunity to not only examine the roles of these specific transporters, revealing their importance to oxalate homeostasis, but to also probe the relative contributions made by the active transcellular and passive paracellular components of oxalate transport across the intestine. We also discuss some of the various physiological stimuli and signaling pathways which have been suggested to participate in the adaptation and regulation of intestinal oxalate transport. Finally, we offer an update on research into Oxalobacter formigenes, alongside recent investigations of other oxalate-degrading gut bacteria, in both laboratory animals and humans.
Keywords: Caco-2, Chloride/bicarbonate exchange, DRA, PAT1, Ussing chamber
Introduction
The vast majority of oxalate in the body is excreted via the kidneys where 90–95% of circulating oxalate is eliminated in the urine [1–3], with the remaining fraction secreted into the intestine. The renal contribution to keeping blood oxalate levels low is therefore obvious, but the value of the intestinal tract to oxalate homeostasis can easily be overlooked. The importance of the intestine stems from its role in dietary oxalate absorption, together with its ability to serve as an extra-renal pathway for excretion. The intestine also houses the gut microbiome. Some of these commensal bacteria are capable of degrading oxalate, thus limiting its absorption and, in the case of Oxalobacter formigenes, avidly promoting intestinal oxalate secretion. The influence of the intestine is most evident, and indeed clinically relevant, in cases of disease. Gastrointestinal (GI) disorders (e.g., inflammatory bowel disease), and/or surgery (e.g., gastric bypass), can lead to excessive oxalate absorption and secondary enteric hyperoxaluria [4]. In chronic renal failure, where urinary oxalate clearance is limited, compensatory oxalate secretion by the large intestine is triggered to increase fecal elimination [3, 5, 6]. A final example are the large amounts of oxalate generated by metabolic overproduction in the liver (the primary hyperoxalurias), where this excess has been successfully targeted via the gut, at least in animal models, through colonization with Oxalobacter [7–9].
As a valuable extra-renal pathway for eliminating oxalate, knowing how the intestine transports this anion is essential. Illuminating the mechanisms responsible for absorption and secretion has garnered considerable interest, not only for understanding oxalate homeostasis but also for the development of future therapeutic approaches to tackling hyperoxaluria and kidney stone disease. Realizing this potential demands a fundamental understanding of oxalate transport and how it is regulated. Over the past 35 years, four major discoveries have come to shape our present knowledge. The first came in 1980 with the report of an active component to intestinal oxalate transport [10]. The second was subsequent studies revealing the remarkable adaptive capacity of the intestine, where it could be induced to either actively absorb or secrete oxalate on a net basis in response to various local and systemic stimuli [5, 11–13]. The third came with the isolation and identification of Oxalobacter [14, 15], but more specifically, its unique ability to induce active oxalate secretion by the intestine [7–9]. The final key development has been identification of the SLC26 (SoLute Carrier) gene family of anion exchangers and the pivotal roles some of these individual transporters play in oxalate transport by the intestine [16–19]. For more expansive background information on these and other facets of intestinal oxalate transport readers are directed to prior authoritative reviews [20, 21]. The intention of this present review is to provide an update of recent developments and advances that have taken place in the field over the past 10 years.
The pathways and mechanisms for oxalate transport across the intestine
Overview
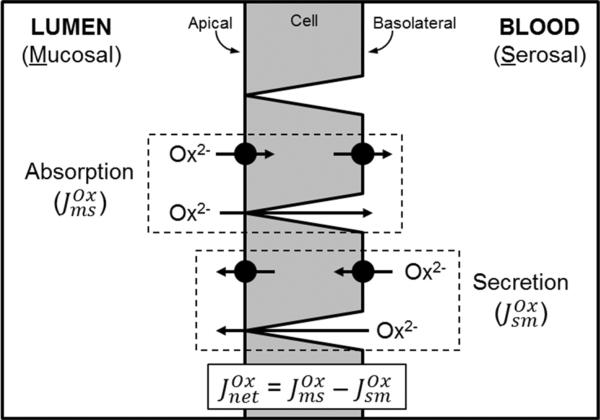
The transport of oxalate by the intestine can be categorized based on the pathway it takes across the epithelium and the underlying mechanism involved. Broadly speaking, these are ‘paracellular and passive’ and ‘transcellular and active’. The former involves oxalate moving between the epithelial cells in response to the prevailing transepithelial electrical and concentration gradients acting upon the oxalate anion, and also the properties of the tight junctions. For the transcellular pathway, oxalate moves through the cells and this must be facilitated by membrane-bound transport proteins located within the apical and basolateral membranes (Fig. 1). The absorption and secretion of oxalate occur simultaneously across the intestinal epithelium. The absorptive oxalate flux from the lumen (mucosal) to the blood (serosal), denoted , involves paracellular and transcellular pathways, while in the opposite direction, oxalate secretion ( ) is presently understood to be largely transcellular, although the paracellular route may be significant in cases of hyperoxalemia. In sum, the relative contribution of each of these unidirectional fluxes determines the overall net direction of oxalate movement across a particular segment of the intestine (Fig. 1).
Fig. 1.
Pathways for oxalate (Ox2−) transport across the intestinal epithelium. An illustration of the unidirectional absorptive ( ) and secretory ( ) oxalate fluxes, which are composed of both transcellular and paracellular pathways. Active, transcellular oxalate movements are facilitated by transport proteins, such as the SLC26 anion exchangers, expressed in the apical and basolateral membranes. The paracellular component involves oxalate moving passively through the tight junctions and between cells in response to the prevailing electrical and concentration gradients across the epithelium, and relative ionic permeability of this pathway. These processes of absorption and secretion occur simultaneously across the epithelium; and in the Ussing chamber they can be directly measured and used to calculate the overall net direction of oxalate movement ( ) across the epithelium. See text for further details
The active transcellular movement of oxalate has been characterized as performed by a variety of anion exchange proteins based on their sensitivity to disulfonic stilbenes (i.e., DIDS and SITS) and the associated interactions of oxalate with the prominent anions, chloride (Cl−), bicarbonate (HCO3−) and sulfate (SO42−). In the intestine, these particular anion exchangers are responsible for Cl−/HCO3− exchange, mediating Cl− absorption and HCO3− secretion, as well as SO42− absorption, and have been identified as belonging to the multi-functional SLC26 gene family where they also have an affinity for, and transport, oxalate. Of its 11 members, at least 4 SLC26 transporters are prominently expressed in the intestine and pertinent to the following discussions, they are: Slc26a1 (SAT1: Sulfate Anion Transporter 1), Slc26a2 (DTDST: DiasTrophic Dysplasia Sulfate Transporter), Slc26a3 (DRA: DownRegulated in Adenoma) and Slc26a6 (PAT1: Putative Anion Transporter 1).
The presence of simultaneous, bi-directional oxalate transport, plus the low concentrations of oxalate typically found within the body, demands a technique sensitive enough to detect and parse these opposing fluxes across the intestinal epithelium. Over the years, this has been most conveniently studied with the in vitro Ussing chamber with a range of animal models (various strains of rabbits, rats and mice) and different segments of the small and large intestine, utilizing 14C-oxalate as a tracer. A variety of other experimental approaches have also been employed with these same models such as gut sacs and membrane vesicles. Other studies have included immortalized, epithelia-like human cell lines (i.e., Caco-2 and T84), together with other cultured cells (e.g., HEK, Sf9 and MDCK), where transporters of interest such as the Slc26s have been either (over-) expressed or knocked-down; the Xenopus oocyte expression system has also been commonly used in this regard. Furthermore, the experimental conditions and how oxalate transport has been measured in all of these different systems vary too, from transepithelial fluxes and calculations of permeability, to cellular uptakes and efflux. Such diversity has produced a wealth of valuable information contributing enormously to advancing this area, but at the same time it has generated complexity and lack of consensus. As such, the data presented in the published literature necessitates careful interpretation. We recommend the reader bear this in mind when drawing their own conclusions from the following discussions.
Intestinal oxalate absorption
The favorable transepithelial electrochemical gradient that exists in vivo (i.e., typical lumen-negative potential difference and low micro-molar blood oxalate) makes the paracellular route a large contributor to absorption in this setting—depending on the amount of soluble, unbound oxalate available within the lumen and the corresponding permeability of this pathway—which will be segment specific. In general, the small intestine tends to be ‘leakier’ to ions and small molecules (such as oxalate) than the large intestine [22]. Under the controlled, symmetrical conditions imposed by the Ussing chamber, investigations confirmed that the absorptive flux of oxalate across the intestine, from the lumen to the blood, does indeed vary with paracellular permeability [10], but also revealed evidence of an active transcellular component that in some segments (notably, the large intestine) can result in net absorption [5, 11–13]. The relative contribution of transcellular and paracellular pathways to oxalate absorption has not been formally distinguished in vitro or in vivo [20], but a recent investigation addressing this concluded that absorption is, in fact, predominantly passive and paracellular in vitro [23]. However, this appeared just prior to another report identifying a significant contribution of DRA (Slc26a3) to transcellular oxalate absorption in the small and large intestine [17].
The discussion of Freel et al. [17] presented a number of reasons that might account for the disparities between these two studies. Among them was omission of critical electro-physiological data by Knauf et al. [23], specifically TER (TransEpithelial Resistance; Ω cm2), or its reciprocal, conductance (GT; mS/cm2). GT is a measure of the composite ionic permeability of the epithelium and the sum of transcellular conductance (GC) and the paracellular or ‘shunt’ conductance (GS). In terms of its permeability to ions, the intestine is classified as ‘leaky’ since GS constitutes 53–95% of overall GT [22]. This means that GT is a close approximation of ionic permeability through the paracellular pathway—and thus an important indicator of epithelial integrity—so it was perplexing that this was neglected by the authors. Instead, Knauf et al. [23] utilized equivalence between oxalate permeability (POx)—calculated based on the unidirectional absorptive oxalate flux, —and the permeability of mannitol (PMan), a non-metabolized solute confined to the paracellular pathway [24], as evidence of paracellular oxalate absorption.
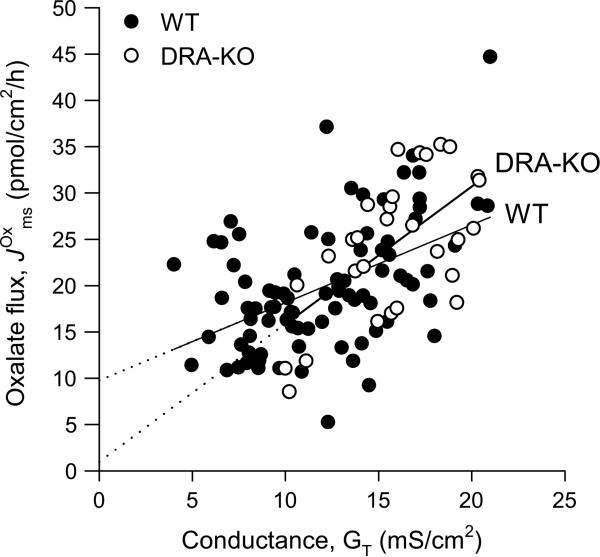
If the assertion made by Knauf et al. [23] is indeed correct and is overwhelmingly paracellular for all regions of the intestine, then the relationship between and GT should be direct and linear, and the intercept of this line should pass through zero. To test this, we examined the relationship between and GT across the distal colon of wild-type (WT) mice. There was indeed a direct, linear relationship, although the predicted flux at the intercept was 9.75 ± 2.13 pmol/cm2/h and significantly different from zero (T = 4.58, P ≤ 0.001) (Fig. 2). This indicates that oxalate absorption by the distal colon is not entirely paracellular with this intercept representing 49% of the mean in Fig. 2 (19.92 ± 0.75 pmol/cm2/h; n 85). Remarkably, this value (9.75 pmol/cm2/h) is very similar to the proposed contribution of DRA to in this same segment (11.4 pmol/cm2/h or 41% of ) [17]. When we undertook the same analysis for DRA-knockout (KO) mice, the projected intercept was just 0.97 ± 5.91 pmol/cm2/h, and not significantly different from zero (T = 0.16, P 0.870), predicting that in the absence of DRA might be paracellular. These results concur with Freel et al. [17] and earlier studies, that there is indeed an active (transcellular) component to oxalate absorption in the large intestine and this is represented by DRA. Furthermore, the comparison between WT and DRA-KO mice in Fig. 2 leads us to tentatively suggest that DRA is the sole contributor to transcellular oxalate absorption, at least in the mouse distal colon. Thus, with 41% of attributed to DRA [17], the remaining 59% of this flux would therefore be paracellular and passive.
Fig. 2.
The relationship between transepithelial conductance, GT and the unidirectional absorptive oxalate flux, , across the distal colon from WT (filled circle) and DRA-KO (open circle) mice. Individual data points were collated from published studies [17, 45] together with the results from previously unpublished studies in our laboratory. and GT were measured on isolated segments of the distal colon mounted in the Ussing chamber under symmetrical, short-circuited conditions with standard bicarbonate buffer. Further methodological details are available in the supplementary material. The accompanying linear regression equation and analysis summary for WT mice is: = 9.75 + (0.84 × GT) (R2 = 0.233) and F1,83 = 25.15, P < 0.001. The regression equation and analysis summary for DRA-KO mice is: = 0.97 + (1.49 × GT) (R2 = 0.378) and F1,27 = 16.40, P < 0.001
The above analysis and the recent report by Freel et al. [17] concentrated on the lower intestine (distal ileum, cecum and distal colon), whereas the principal focus of Knauf et al. [23] has been the duodenum. It is conceivable that DRA does not participate in oxalate absorption in the proximal small intestine, even though it is prominently expressed there and responsible for 50–60% of baseline duodenal HCO3− secretion [25–28]. In the Ussing chamber, the duodenum, unlike more distal segments, deteriorates rapidly with observable damage to the villi after 2 h followed by their near-complete destruction at 4 h [29]. Since Knauf et al. [23] instituted a 2.5 h “equilibration period” prior to beginning their measurements, and with DRA functionally active in the villus [25, 30, 31], it is possible that they missed the contribution of DRA because the villus epithelium had largely deteriorated. This compromise to epithelial integrity would also amplify the amount of oxalate moving passively across the tissue, further diminishing their ability to detect any active, transcellular component. While 41–57% of appears to be transcellular and DRA mediated in the lower half of the mouse intestine [17], whether there is a similar contribution from DRA in the upper small intestine remains undetermined.
Intestinal oxalate secretion
Of the possible apical transporters involved in transcellular oxalate secretion, only PAT1 (Slc26a6) has been definitively identified as a participant. In the mouse small intestine, PAT1 has a prominent role in oxalate secretion by the distal ileum [16] and duodenum [18]. Previously, oxalate secretion by the rabbit ileum and colon resembled cAMP-stimulated Cl− secretion [12, 13], involving a conductive channel in the apical membrane [32]. The centerpiece of cAMP-stimulated anion secretion by various epithelia is the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) channel [33], but when human CFTR was expressed in Xenopus oocytes it turned out not to transport oxalate [34]. Furthermore, unlike the rabbit, oxalate secretion by the ileum of the contemporary mouse model is not initiated by cAMP [16]. Despite this, a role for CFTR cannot be discounted; after all, cystic fibrosis patients have an elevated risk for kidney stones associated with hyperoxaluria [35] and higher than normal rates of intestinal oxalate absorption [36]. Indeed, the CFTR-KO mouse also displays hyperoxaluria, with hyperoxalemia [37]. This phenotype was recently linked to reduced expression of PAT1 in the villus duodenum and defective oxalate secretion, predicated on the ability of CFTR to facilitate Cl−/oxalate exchange by PAT1 when the two are co-expressed in oocytes [37]. This conclusion was based on a two-third reduction in (shown as POx) in the CFTR-KO mouse duodenum, but such an intriguing finding is unfortunately incomplete without the corresponding absorptive flux ( ), to determine how overall net oxalate transport was affected (Fig. 1). Using PMan, Knauf et al. [37] subtracted this from POx to show that transepithelial oxalate secretion was all but eliminated in the CFTR-KO duodenum. However, this hinges on the critical assumption that PMan is equivalent to [23] which, to the best of our understanding, has not been directly demonstrated [17], and is further complicated by a protracted 2.5 h equilibration period [37], discussed earlier. The absence of CFTR is particularly relevant in relation to DRA and its potential contribution to in the proximal small intestine. Like PAT1, DRA can also directly interact with and bind to CFTR [38–40]; plus, CFTR is required for DRA-mediated HCO3− secretion by the mouse duodenum [26, 41, 42]. If DRA does contribute to unidirectional oxalate absorption in the duodenum, then one cannot assume is exclusively paracellular and overlook the interaction between DRA and CFTR.
The corresponding transporter at the basolateral membrane supplying oxalate for apical secretion is unknown, but one candidate is SAT1 (Slc26a1). Primarily, an SO42− transporter, SAT1, is the only member of the SLC26 family localized to the basolateral membrane in the intestine [19]. Recent development and study of the SAT1-KO mouse implied there was diminished intestinal oxalate secretion in the absence of SAT1, contributing to higher rates of oxalate absorption, causing hyperoxaluria, hyperoxalemia and nephrolithiasis in these animals [19]. With SAT1 present throughout the small intestine [19, 43, 44], this opened up the exciting possibility that it might be functioning in series with apical PAT1 to mediate transcellular oxalate secretion [19], a prospect explored by Ko et al. [44]. However, they were forced to concede that SAT1 was “dispensable for active oxalate secretion”, at least in the duodenum. This appeared to be confirmed after finding that the secretory oxalate flux ( ), expressed as POx, was unchanged in the SAT1-KO duodenum [44]. Although proposed to be involved in oxalate secretion [19], Ko et al. [44] do not present (or any electrophysiology) and, similar to Knauf et al. [23, 37], allowed 2.5 h before commencing their experiments. As discussed earlier, such an extended period of time for the duodenum in the Ussing chamber may compromise villus integrity. Nevertheless, the hypothesis that ‘the basolateral step for oxalate secretion is mediated via exchange with SO42− and HCO3− on SAT1′ [44] is intriguing. In the presence of SO42−, we found oxalate transport by the distal ileum to be fully independent of extracellular HCO3− and carbonic anhydrase (CA) [45]. For the mouse, this appears to rule out a SO42−/HCO3−(oxalate) exchange mechanism, at least in this segment of the small intestine. Furthermore, SAT1 mRNA is present in human Caco-2 cells [46], but the absence of SO42− does not attenuate transepithelial oxalate fluxes [47], ruling against a prominent role for SAT1.
Even less is known about the mechanism and identities of the transporters responsible for oxalate secretion by the large intestine. In the mouse distal colon, is dependent on extracellular HCO3− and requires CA activity [45]. This might be suggestive of HCO3−/oxalate exchange at either the apical or basolateral membrane, although Whittamore et al. [45] did not probe this possibility any further with anion exchange inhibitors such as DIDS or SITS. It is uncertain how CA is involved in oxalate secretion, but the mechanism does not appear to be related to a non-catalytic role for this enzyme [45]. We considered whether the extracellular, membrane-bound isoform CA-IV might be supplying HCO3− for exchange with intracellular oxalate at the apical membrane. However, was unaffected when CA-IV was targeted with a specific inhibitor, possibly implicating one of the cytosolic isoforms such as CAI or CAII [45].
The SLC26 anion exchangers and intestinal oxalate transport
While the contributions of some SLC26 anion exchangers to transcellular oxalate transport have begun to emerge, such as PAT1 and DRA, there remain many unanswered questions concerning these and, indeed, other members of this gene family expressed along the gut. There are no selective inhibitors available for the anion exchangers to help distinguish their respective roles, hence the development of KO mice and targeted knock-down (KD) of the SLC26 transporters in cultured cell models have been instrumental. The following discussion will elaborate on the intestinal SLC26 anion exchangers, considering each one in turn. For a broader perspective on the SLC26 gene family and their associated physiological roles, we direct the interested reader to a number of excellent reviews [48–51].
SAT1 (Slc26a1)
A number of studies, the majority using Xenopus oocytes, characterized human, rat and mouse SAT1 as a DIDS-sensitive SO42− exchanger displaying an affinity for Cl−, HCO3−, and oxalate [43, 52–56]. The report on the SAT1-KO mouse makes a compelling case for SAT1 as an important oxalate transporter, whereby oxalate uptakes into basolateral membrane vesicles made from the distal ileum, cecum and proximal colon were reduced 40–60% [19]. Furthermore, the luminal contents of the SAT1-KO cecum contained higher concentrations of SO42− and were correspondingly lower in oxalate, consistent with the absence of a SO42−/oxalate exchanger and impaired oxalate secretion [19]. However, as discussed above, the characteristics of oxalate fluxes by the mouse distal ileum are not compatible with a SO42−/oxalate exchange mechanism. SAT1 is also functionally expressed at the sinusoidal membrane of hepatocytes transporting oxalate into the blood [19, 57] and at the basolateral membrane of cells in the renal proximal tubule moving oxalate from the blood into the tubular lumen [19, 52]. This expression is sex dependent, with greater functional expression in males than females [58, 59]. This appears to be under the influence of female sex hormones [58] and has been linked to the higher incidence of calcium oxalate kidney stones in men [58, 59]. However, this particular hypothesis may be undermined somewhat if the male-dominant expression of SAT1 in the liver and kidney extends to the intestine, given the evidence for its contribution to oxalate secretion in male mice [19]. With respect to the intestine, we have not encountered any differences in transepithelial oxalate fluxes between male and female mice in our collective experiments on either the distal ileum, cecum or distal colon (Whittamore, J. M. and Hatch, M., unpublished observations). Despite the disruption to oxalate homeostasis and other pathologies exhibited by SAT1-KO mice [19], this gene has not yet been associated with any human disease. However, a recent pilot study examined a small cohort of recurrent, idiopathic calcium oxalate stone formers revealing a number of sequence variants in the SAT1 gene that may be of significance [60].
DTDST (Slc26a2)
DTDST is an essential SO42− transporter expressed prominently in chondrocytes [53, 61, 62], and mutations of this gene have been associated with a number of inherited chondrodysplasias [62–66]. There is also abundant DTDST mRNA in the small and large intestine of mice, rats and humans [46, 53, 61, 63, 67, 68]. In human colon, DTDST is present in the upper third of the crypts, as determined by in situ hybridization and immunohistochemistry, where it is directed toward the apical membrane [61, 67]. A number of studies characterizing DTDST in oocytes have revealed its ability to transport oxalate alongside Cl−, OH− and possibly HCO3− [53, 69, 70]. These findings have therefore led to speculation on a possible role in oxalate transport, particularly the large intestine, where it might contribute to secretion by operating in either oxalate/Cl− exchange or oxalate/SO42− exchange modes [69, 70]. The DIDS sensitivity of DTDST appears to preclude a role in oxalate absorption, since just 50 μM was sufficient to completely inhibit mouse DTDST [70] while across the distal ileum from WT and PAT1-KO mice were unaffected by 200 μM DIDS [16].
The rate of oxalate efflux from oocytes expressing either mouse [70] or human [69] DTDST increased with extracellular pH. Raising the pH from 7.5 to 8.2 enhanced oxalate/OH− exchange by mouse DTDST >2.5-fold [70], although recent work found by the mouse distal ileum and distal colon to be unresponsive between pH 6.9 and 7.9 [45]. Furthermore, oxalate transport by mouse, but not human DTDST [69], also displayed characteristics of inverse regulation by extracellular Cl− [70]. Stool Cl− concentrations within the rodent ileum and colon are low, <20 mM [71], which would likely compel oxalate absorption, given the enhanced electrochemical gradient for Cl−/oxalate exchange, as well as reduced competition between luminal Cl− and oxalate for uptake across the apical membrane. Indeed, reduced Cl− concentrations are well known to affect intestinal oxalate transport [12, 13, 16, 47]. A caveat to extrapolating from oocyte studies is the reliance on the observed functional characteristics being retained when the transporter is studied as part of the native epithelium in vitro, and then subsequently in vivo. The lethality of certain DTDST mutations [65] precludes development of a KO mouse, but a ‘knock-in’ mouse possessing a non-lethal, loss-of-function mutation has been produced [66]. In humans, this particular mutation (A386V) exhibits a near 50% reduction in oxalate uptake when expressed in oocytes [69]; however, no evidence of nephrocalcinosis was seen in these transgenic mice [69].
DRA (Slc26a3)
DRA was initially considered a transporter of SO42− and oxalate, similar to SAT-1 [72], but mutations in this gene were subsequently associated with congenital chloride diarrhea [73], a rare, inherited disease characterized by defective Cl−/HCO3− exchange in the ileum and large intestine [74–76]. Until recently, the contribution of DRA to intestinal oxalate handling was unknown and development of the DRA-KO mouse [77] afforded the opportunity to examine this. Using this KO model, DRA was found to contribute significantly to transcellular oxalate absorption, accounting for 42, 57 and 41% of across the distal ileum, cecum and distal colon, respectively [17]. These reductions in oxalate absorption produced overwhelming net oxalate secretion by each of these segments, associated with a 66% decline in urinary oxalate excretion and a 60% lower plasma oxalate concentration compared to their WT counterparts [17]. Considering the limited distribution of DRA within the body, which does not extend to either the liver or kidney [68, 72, 78–82]; this further emphasizes the degree to which changes to intestinal oxalate handling can impact overall oxalate homeostasis. We do note, however, that one study has localized DRA to the apical pole of a subset of cells in the connecting tubule and cortical collecting duct of the human nephron [83].
How this new role for DRA in oxalate absorption by the mouse intestine translates to humans is unclear. Only one study [83] has reported oxalate in patients with congenital chloride diarrhea, revealing a median urinary Ox:Cr excretion ratio well within the normal range [17]. Studies of recombinant human DRA in oocytes have indicated that it is not a convincing transporter of oxalate [84]. Although we must emphasize that oxalate transport by mouse DRA under similar experimental conditions has not been described, thus it is unclear if there are species differences in oxalate affinity, as reported for PAT1 [85]. If DRA directly mediates oxalate absorption, as indicated by the DRA-KO mouse model [17], then the transport mode will likely be Cl−(oxalate)/HCO3− exchange, with oxalate directly competing with Cl−. Consistent with this proposal, oxalate competitively inhibited 36Cl− uptake by rat DRA expressed in HEK cells [82]. Indeed, Cl− strongly affects oxalate transport, this is evident in the significantly elevated across the mouse distal ileum when luminal Cl− was removed [16], or in Caco-2 cell monolayers where mucosal Cl− was varied between 0 and 120 mM with 50% inhibition of achieved at 20mM [47]. This competition between oxalate and Cl− might explain why oxalate uptakes by human DRA expressed in oocytes were in fact so low where the buffers contained >100 mM Cl− [72, 84], leading to its characterization as an unconvincing oxalate transporter.
Previously, fluxes across the PAT1-KO mouse ileum revealed a very large, disproportionate increase in that was unaffected by 200 μM DIDS [16]. In light of the contribution of DRA to [17] and its relative insensitivity to DIDS [82, 84, 86–88], this likely represented an increase in DRA-mediated oxalate absorption. This could not be explained by a transcriptional upregulation of DRA [16], but may be evidence of a direct functional coupling between DRA and PAT1 [38, 89–91]. This is not surprising given that they are both apical Cl−/HCO3− exchangers with an overlapping distribution along the crypt–villus axis of the small intestine [25, 28, 30, 31, 80, 92]. In the absence of DRA, there was no corresponding increase in (PAT1-mediated) by the distal ileum [17], suggesting PAT1 does not compensate in terms of oxalate secretion in the DRA-KO ileum. This would be consistent with the lack of evidence for an upregulation of PAT1 mRNA in the small intestine of DRA-KO mice [16, 25, 27, 93, 94]. One exception is the original report on the DRA-KO mouse by Schweinfest et al. [77], who state, but do not show, there was a threefold upregulation of PAT1 in the duodenum and jejunum, and it is unclear whether they were referring to mRNA or protein.
PAT1 (Slc26a6)
There has been considerable interest in PAT1 given its demonstrated importance to intestinal oxalate secretion and consequent influence on urinary oxalate excretion [16, 18]. PAT1 is commonly thought of as being almost exclusive to the small intestine, but PAT1 mRNA is also present in the mouse and human large intestine, typically at much lower levels [38, 92, 95, 96]. In the mouse, PAT1 protein expression did not correlate with oxalate fluxes across the large intestine [8]. To help distinguish the role of PAT1 in a human model, Freel et al. [47] examined its contribution to oxalate transport across Caco-2 cell monolayers. Utilizing an siRNA approach, PAT1 mRNA was knocked-down >75% which translated to a corresponding >60% reduction in PAT1 protein expression. This, in turn, reduced oxalate fluxes by half and, since there would have been residual PAT1, this can be considered a minimal contribution [47]. PAT1 was also shown to be a major Cl−/HCO3− exchanger in this system, and its ability to mediate oxalate influx or efflux across the apical membrane was dependent on the size and direction of the prevailing Cl− and HCO3− gradients [47]. Adopting another RNA interference technique, shRNA, with human T84 cell monolayers, Hassan et al. [97] reduced PAT1 protein expression by around 64% leading to a >57% reduction in oxalate uptake across the apical membrane, similarly showing a prominent role for PAT1 in oxalate transport in this model.
When expressed in Xenopus oocytes, Cl−/oxalate exchange by human PAT1 demonstrated a far lower affinity for extracellular Cl− than mouse PAT1 (62 mM compared to 8 mM) and mediated electroneutral (i.e., 2Cl−/Ox2−) exchange unlike mouse PAT1 which was electrogenic (i.e., Cl−/Ox2−) [85]. These differing properties imply that the mouse may be capable of secreting oxalate into the intestine via PAT1 at low luminal Cl− concentrations; such efficiency may explain why mice are considered “refractory” to kidney stone formation relative to humans [85]. Consistent with this, Clark et al. [85] cited that the postprandial Cl− concentration within the human lower small intestine averaged 60 mM [98], similar to the corresponding Cl− affinity of PAT1 (62 mM). However, this intriguing proposition is dependent on intraluminal Cl− concentrations within the small intestine of mice being close to humans. For rats fed a standard rodent diet, the ileal contents contained ~14 mM Cl− [71], fitting the lower Cl− affinity of mouse PAT1 (8 mM); hence it might not necessarily be more efficient in vivo than its human counterpart.
PAT1 was not among the differentially expressed genes in the jejunum of an idiopathic hyperoxaluric rat model [99], and no human disease has yet been associated with PAT1. One common variant of PAT1 in the human population (V185M) produced a slight, but significant 25% decline in Cl−/oxalate exchange activity in oocytes [85]. This same variant (V206M) also reduced oxalate uptake by 30% and did not cause any changes to the abundance of PAT1 in the oocyte membrane [100]. Heterozygosity for the V206M variant did not correlate significantly with plasma or urine oxalate in patients with primary hyperoxaluria or matched controls [100]. Furthermore, in a population with primary hyperparathyroidism, the homozygous V206M genotype did not associate with urinary oxalate or kidney stone prevalence [101].
Adaptations and regulation of intestinal oxalate transport
With the identification of the Slc26 anion exchangers, in particular the respective roles of PAT1 and DRA in oxalate secretion and absorption, there has been growing interest in how these transporters are regulated. The rate, mechanism and locations of electrolyte absorption and secretion along the intestine are tightly regulated and coordinated by a broad, complex multitude of local and systemic inputs integrated through the endocrine, immune and autonomic nervous systems. At the cellular level, various hormones, cytokines and neurotransmitters can frequently exert their effects on transporter expression and activity by triggering signaling pathways often involving one or more distinct intracellular second messengers (cAMP, Ca2+ and cGMP) and protein kinases [102, 103]. Being able to distinguish the pathways involved in regulating oxalate transport is necessary to understand: (1) the contribution of the intestine to systemic oxalate homeostasis, (2) the pathophysiology of oxalate-associated disorders, particularly those linked to GI dysfunction, (3) how the oxalate-degrading gut bacterium Oxalobacter interacts with the epithelium to modify oxalate transport and (4) to assist identifying potential targets and pathways that might serve the development of future therapeutics.
Protein Kinase C (PKC) has received a great deal of attention recently, having been identified as a key negative regulator of PAT1-mediated oxalate transport, across species (human or mouse PAT1) and in different experimental systems (Xenopus oocytes, T84 cells, Caco-2 cells, native epithelium) [97, 104, 105]. Indeed, this role for PKC is not limited to mouse and human PAT1, but includes human DRA and DTDST, and the transport activity of all these exchangers can be inhibited by PKC activation in oocytes [69, 106]. There are at least 11 isoforms of PKC divided into three sub-families (conventional, novel and atypical) depending on their various requirements for activating cofactors—Ca2+, diacylglycerol and phosphatidylserine [107–109]. In the gastrointestinal tract, various PKC iso-forms are involved in the development and function of the epithelial barrier, immune responses, gut motility and carcinogenesis, as well as electrolyte transport [110].
Protein Kinase C as a regulator of oxalate transport
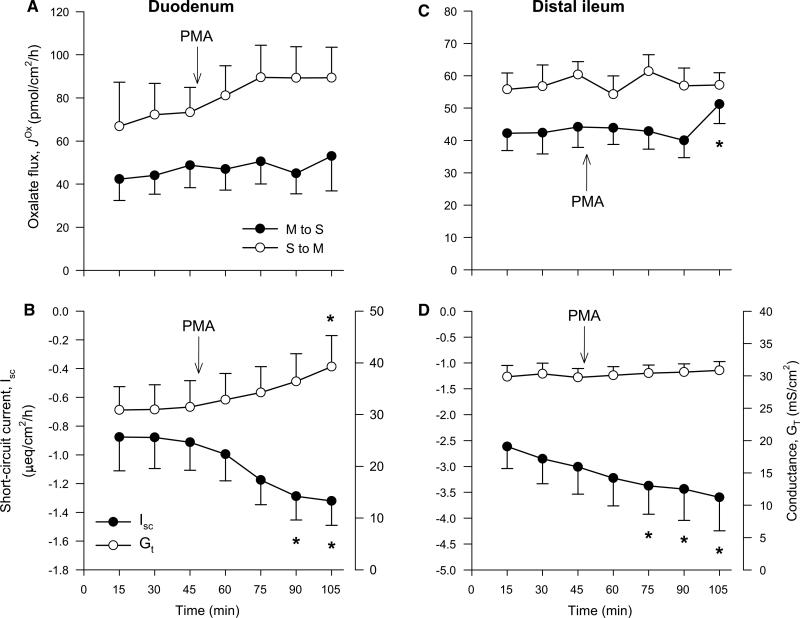
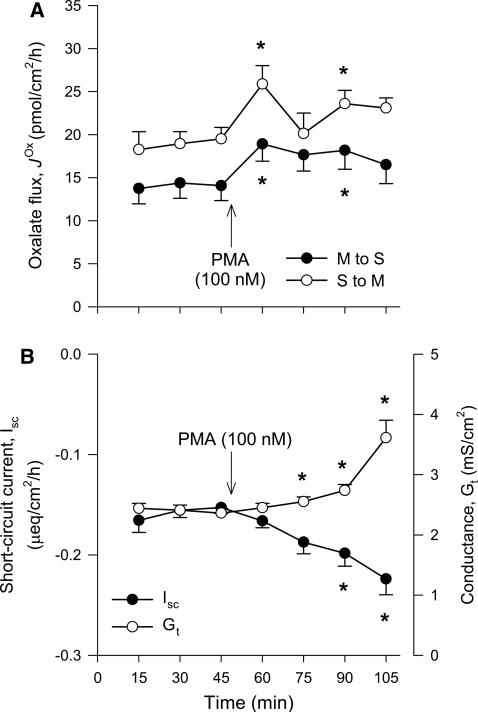
The phorbol esters such as PMA (Phorbol 12-Myristate 13-Acetate) can mimic diacylglycerol and are widely used PKC activators [107, 111]. PMA was found to inhibit oxalate uptake by mouse PAT1 in oocytes by reducing the abundance of this exchanger at the cell membrane [104]. When PMA was subsequently applied to the isolated mouse duodenum it acutely inhibited > 50%, suggesting that constitutively expressed PAT1 is regulated by a PKC-dependent pathway too [104]. In addition, PAT1 also mediates oxalate secretion by the mouse ileum [16], while DRA is involved in [17], and both of these transporters can be negatively regulated by PMA in oocytes [104, 106]. However, we found no effect of PMA on either of these unidirectional oxalate fluxes across the mouse distal ileum (Fig. 3c) and were also unable to reproduce the same inhibitory effect on by the duodenum (Fig. 3a), as previously reported [104]. Despite similarities in our respective experimental approaches, there are a number of disparities that may explain our inability to reproduce this result: (1) Hassan et al. [104] used a BALB/c mouse strain; hence, strain-related differences in responses cannot be ruled out [112]. (2) Only was presented by Hassan et al. [104]. Without the corresponding absorptive flux ( ), it is unclear how the net flux changes (see Fig. 1), since we found no statistically significant changes to net oxalate transport (Fig. 3a, c). (3) Hassan et al. [104] did not list calcium in their buffers for these experiments, potentially confounding their results, because the absence of extracellular calcium is well established as compromising epithelial integrity with consequences for oxalate fluxes [10]. (4) The inhibition of by the mouse duodenum was ascribed to the actions of isoform PKC-δ based on the ability of rottlerin to block the effect of PMA [104]. Not only has rottlerin been discredited as a specific PKC-δ inhibitor [113–116], but Hassan et al. [104] did not determine whether PKC-δ was expressed in their mouse model. Of the seven PKC isoforms detected in the (Swiss) mouse duodenum, PKC-δ was not among them [117]. Furthermore, this same study showed that 10 μM PMA (a concentration 50–100 times greater than used in Fig. 3) had no effect on basal duodenal HCO3− secretion in vitro [117], even though 20–30% of this HCO3− secretion has been attributed to PAT1 [26, 27, 81, 118].
Fig. 3.
The Protein Kinase C (PKC) activator phorbol-12-myristate-13-acetate (PMA) does not inhibit oxalate transport by the mouse small intestine in vitro. The unidirectional oxalate fluxes measured across isolated short-circuited segments of the duodenum (a) and distal ileum (c) following the addition of PMA (100 or 200 nM) to the mucosal and serosal baths under symmetrical buffer conditions. The accompanying panels show the associated measures of short-circuit current, Isc (filled circle) and transepithelial conductance, GT (open circle) for duodenum (b) and distal ileum (d). Each data point represents the mean ± SE from n = 6 (duodenum) and n = 8 (distal ileum) GT-matched tissue pairs. An asterisk indicates a statistically significant difference from the preceding control period (0–45 min) determined by repeated measures one-way ANOVA followed by multiple, pairwise post hoc comparisons. Further details are available in the supplementary material. There were no differences between any of the transport characteristics in the presence of either 100 or 200 nM PMA; hence, these data sets were combined to produce this figure
Cholinergic regulation
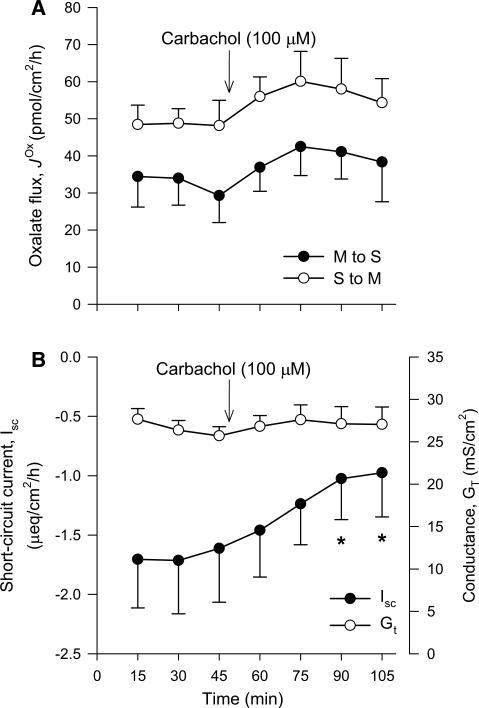
Using the human T84 cell line, cholinergic regulation has been shown to be one of the specific physiological stimuli triggering PKC-δ activation, which acutely inhibits oxalate uptake in association with reduced expression of PAT1 [97], most likely through membrane endocytosis. Pre-incubation of T84 monolayers with the acetylcholine analog carbachol inhibited Cl−-driven oxalate uptake between 25 and 35%, a response that was attenuated by rottlerin and coincident with translocation of PKC-δ from the cell cytosol to the membrane [97]. By association, PKC-δ was concluded as responsible, but crucially Hassan et al. [97] do not show whether PKC-δ activation and the changes to PAT1 expression could actually be blocked by rotterlin after treatment with carbachol. This is important because, in T84 cells, carbachol has been reported to neither activate PKC-δ [119], nor are the effects of carbachol on membrane endocytosis blocked by rottlerin [120]. Nevertheless, Hassan et al. [97] projected that the direct inhibitory effects of cholinergic stimulation on T84 cells would translate to the native intestinal epithelium, generating excitement about the prospect of antagonizing this pathway to enhance enteric oxalate secretion [121]. However, this overlooked previous work on the autonomic control of oxalate transport where adrenergic stimulation with epinephrine reversed net secretion to absorption by the rabbit proximal colon [12]. This is consistent with the characteristic role of the enteric nervous system in regulating electrolyte transport by the mammalian intestine, whereby adrenergic agonists promote absorption and/or reduce secretion [122–125], while cholinergic stimulation conversely elicits anion secretion [126]. The actions of cholinergic agents (e.g., acetylcholine, carbachol and bethanechol) are thus more likely to promote, rather than inhibit, oxalate secretion. Unfortunately, we found that neither was the case when we tested the effects of carbachol on oxalate fluxes across the mouse distal ileum (Fig. 4a).
Fig. 4.
Cholinergic stimulation does not inhibit oxalate transport by the mouse distal ileum in vitro. The unidirectional oxalate fluxes measured across isolated short-circuited segments of the distal ileum (a) following the addition of carbachol (100 μM) to the serosal bath under symmetrical buffer conditions. The accompanying measures of short-circuit current, Isc (filled circle), and transepithelial conductance, GT (open circle), are shown in b. Each data point represents the mean ± SE from n = 6 GT-matched tissue pairs. An asterisk indicates a statistically significant difference from the preceding control period (0–45 min) determined by repeated measures one-way ANOVA followed by multiple, pairwise post hoc comparisons. Further details are available in the supplementary material
Purinergic regulation
In addition to being an intracellular energy source used to drive active epithelial transport, ATP and other purine nucleotides can also function as extracellular messengers regulating intestinal absorption and secretion either directly, following local cellular release, or indirectly, by acting as neurotransmitters [127, 128]. A purinergic signaling pathway has also been proposed to negatively regulate PAT1-mediated oxalate transport by Caco-2 cell monolayers via PKC-δ [105]. Luminal application of either ATP or UTP inhibited oxalate uptake by 25–30%, coincident with a 30–35% reduction in PAT1 expression at the cell membrane [105]. These responses were attenuated by the broad-spectrum PKC inhibitor Gö-6983 and targeting PKC-δ expression with siRNA [105]. However, none of the other eight isoforms (i.e., PKC-ι, PKC-βI, PKC-βII, PKC-γ, PKC-η, PKC-ζ) known to be present in Caco-2 cells [129–131] were examined, including PKC-α—associated with downregulation of the apical Na+/H+ exchanger NHE3 (SLC9A3) [132], and PKC-ε—suggested to inhibit a DIDS-sensitive Cl−/OH− exchanger [133], in this same model. Since PAT1 mediates a large fraction of DIDS-sensitive oxalate transport in Caco-2 cells [47], we reasoned that using PMA to activate PKC should demonstrably inhibit oxalate fluxes, regardless of the isoform involved (excepting the atypical isoform PKC-ζ which is not activated by diacylglycerol [108, 110]). On the contrary, Fig. 5 shows that PKC activation does not inhibit oxalate fluxes by Caco-2 monolayers, but to our surprise elicited stimulation. Within 15 min of PMA addition, there was a sharp increase in oxalate transport which appears to peak at 60 min (Fig. 5a). A remarkably similar pattern of PKC translocation is exhibited in response to PMA in Caco-2 cells over the same time frame [134], where a rapid rise in membrane-associated PKC activity peaked at 1 min before subsequently declining, but remained elevated above baseline levels for at least 15 min (see Fig. 6a in [134]). For the remainder of the second experimental period (75–105 min), both and are, on average, 17.4 ± 2.0 and 22.3 ± 1.0 pmol/cm2/h, and significantly higher by 24 and 18%, respectively, compared to these corresponding fluxes in the preceding control period (P < 0.05, Paired t test) (Fig. 5a). Accompanying these changes in oxalate fluxes is a reduction in short-circuit current (Isc), indicative of net electrogenic anion secretion, and increasing paracellular permeability (GT) which rises dramatically between 90 and 105 min (Fig. 5b). This latter observation agrees well with the reduction in TER and threefold increase in mannitol permeation previously seen by Caco-2 monolayers following exposure to 50 nM PMA [134], but contrasts with another study finding no effect of 60 nM PMA on TER [135]. The potential reasons for these disparities were discussed by Turner et al. [135] and they are clarified no further by our data, but a number of PKC isoforms (α, βI, and η), which are associated with the regulation of Caco-2 cell permeability [110, 131], will have been activated by PMA. Whether the increases to oxalate transport in Fig. 5a represent movement through the paracellular pathway cannot be determined. However, we note that the stimulation of oxalate fluxes between 60 and 105 min does not synchronize with GT as one might expect if this was entirely due to changes in paracellular oxalate movements.
Fig. 5.
The Protein Kinase C (PKC) activator phorbol-12-myristate-13-acetate (PMA) stimulates oxalate transport by human Caco-2 cell monolayers. a displays the unidirectional oxalate fluxes measured across cultured Caco-2 cell monolayers at 19–24 days post-seeding under symmetrical, short-circuited conditions following the addition of PMA (100 nM) to the mucosal and serosal baths. The accompanying measures of short-circuit current, Isc (filled circle), and transepithelial conductance, GT (open circle), are shown in b. Each data point represents the mean ± SE from n = 9 GT-matched pairs of monolayers. An asterisk indicates a statistically significant difference from the preceding control period (0–45 min) determined by repeated measures one-way ANOVA followed by multiple, pairwise post hoc comparisons. Further details are available in the supplementary material
Fig. 6.
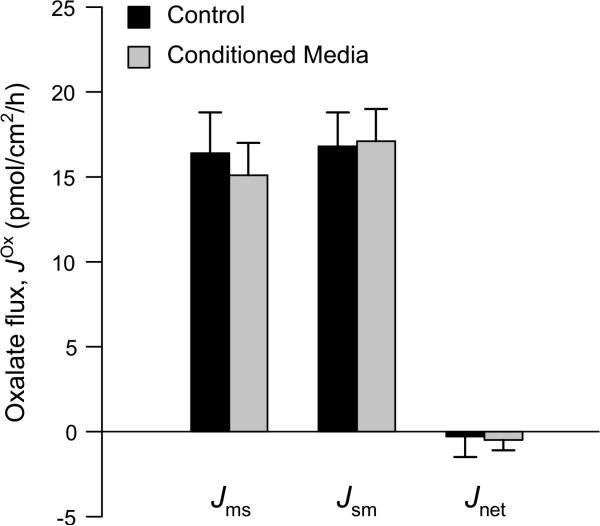
Exposing human Caco-2 cell monolayers to ‘Conditioned Media’ (CM) from a culture of Oxalobacter formigenes does not significantly enhance oxalate transport. The unidirectional oxalate fluxes measured across cultured Caco-2 cell monolayers at either 11 or 28 days post-seeding under symmetrical, short-circuited conditions following prior mucosal (luminal) exposure to ‘conditioned media’ from an O/N culture of Oxalobacter strain HC-1 (1:50 dilution) for 18–24 h are shown alongside concurrent controls exposed to sterile media only. There were also no significant differences to the electrophysiological parameters. Short-circuit current (Isc) was −0.09 ± 0.02 μeq/cm2/h (control) compared to −0.09 ± 0.02 μeq/cm2/h (conditioned media), and transepithelial conductance (GT) was 6.2 ± 0.4 mS/cm2 (control) compared to 6.4 ± 0.3 mS/cm2 (conditioned media). The data represent the mean SE from a total of n = 16 and n = 20 GT-matched pairs of monolayers for the control ± and conditioned media treatments, respectively. Further details are available in the supplementary material. There were no statistically significant differences between any of the transport characteristics of monolayers at 11 days compared to 28 days post-seeding; hence, monolayers from these two time points were combined to produce this figure
Reciprocal regulation of oxalate and citrate transport
An intriguing interaction has been revealed between oxalate transport by PAT1 and citrate transport by the sodium dicarboxylate cotransporter, NaDC1 (SLC13A2) [136]. Urinary citrate is an important chelator of calcium, consequently reducing the potential for calcium and oxalate to interact. Citrate thus exerts a protective effect by limiting calcium oxalate crystal formation and growth; hence hypocitraturia, like hyperoxaluria, is a major risk factor for kidney stones [137, 138]. Based on this relationship, Ohana et al. [136] hypothesized that oxalate and citrate levels are coordinated by their respective transporters, namely PAT1 and NaDC1, which are localized to the apical membrane in both the proximal tubule and small intestine [92, 139, 140]. This was supported by the observations that PAT1-KO mice, in addition to being hyperoxaluric, also display hypocitraturia [136, 141]. The reduction in urinary citrate in these animals corresponded to increased Na+-dependent succinate uptake by kidney cortical slices (70%) and isolated sheets of jejunum (35%), considered representative of increased NaDC1 activity, implying that the absence of PAT1 enhanced renal citrate recovery and intestinal citrate absorption [136]. Suspecting there might be a functional interaction between NaDC1 and PAT1, Ohana et al. [136] went on to show that co-expression of mouse PAT1 with either opossum or human NaDC1 in oocytes enhanced PAT1-mediated oxalate transport, while Na+-dependent succinate transport by NaDC1 was concomitantly reduced [136]. This reciprocity was determined to be the result of a direct physical and functional interaction between these transporters and suggested to serve as a mechanism for sensing and regulating urinary oxalate and citrate levels [136], which may be relevant to the changes in renal citrate and oxalate handling seen during a metabolic acidosis [142]. It was notable that DRA, but not DTDST, also inhibited succinate uptake by NaDC1 in a similar manner to PAT1 in oocytes [136]. The significance that such reciprocal regulation between PAT1 (or indeed DRA) and NaDC-1 might have for oxalate transport by the small intestine was not considered.
Adaptations to acid–base status
Even though the mammalian intestine does not directly participate in maintaining acid–base balance, it makes a major, clinically relevant contribution [143, 144] and also responds to acid–base abnormalities, most prominently through modifications to sodium chloride (NaCl) cotransport [145, 146]. These consist of acute, reversible responses by the apical Na+/H+ and Cl−/HCO3− exchangers to variations in systemic pH, HCO3− and CO2, in conjunction with CA, in a distinct, segment-specific manner [145–148]. Since DRA and PAT1 are the main Cl−/HCO3− exchangers involved in intestinal oxalate transport [16–18], this raised the question of whether oxalate also displays characteristics of regulation by acid–base variables, particularly since PAT1-mediated HCO3− secretion appears sensitive to short-term (<1 h) systemic acid–base disturbances [41]. We recently explored how oxalate transport by the mouse distal ileum and distal colon responded to acute alterations in buffer pH, HCO3− and CO2 in vitro, and the involvement of CA [45]. Contrary to expectation, net oxalate secretion by the distal ileum, which involves PAT1 [16], was completely insensitive to any changes in pH, HCO3− or CO2, and unaffected by CA inhibition. This latter observation is noteworthy given that PKC disrupts the physical interaction between CAII and PAT1 in HEK cells [149]. Clearly, no such arrangement is needed to support oxalate secretion in the mouse ileum. In contrast, by the distal colon required CA activity, was dependent on the presence of extracellular HCO3−/CO2 and could be acutely stimulated by increasing PCO2, but was impervious to pH [45], suggesting colonic oxalate transport may indeed be responsive to systemic acid–base disorders. This was confirmed by a report of net oxalate absorption being completely reversed to net secretion by the rat distal colon following induction of a chronic metabolic acidosis [142]. The initiation of colonic oxalate secretion was associated with a dramatic 75% reduction in urinary oxalate excretion [142]. Importantly, we do not discount the impact of an acidosis on renal oxalate handling, especially since clearance ratios indicated enhanced net re-absorption by the kidneys, yet plasma oxalate levels remained stable [142]. Unless hepatic oxalate production was diminished by this protracted acidosis, as suggested by Bushinsky et al. [150], these circumstances necessitate an alternate route for elimination, coinciding with the initiation of colonic oxalate secretion [142]. These adaptations during a metabolic acidosis offer additional evidence for a dynamic gut–kidney axis in the management of oxalate excretion alongside chronic renal failure [3, 5, 6], oxalate loading [151] and colonization with Oxalobacter [7–9]. Even though the signaling pathways and transporters involved remain unidentified, these new findings offer a glimpse at the presence of a previously unrecognized regulatory mechanism. How this might translate to humans is presently unclear. An acute acid load reportedly increased urinary oxalate and the risk for calcium oxalate crystallization [152], while the impacts of drugs which antagonize CA activity (potentially generating an acidosis) are limited and inconclusive. For example, a significant rise in urinary oxalate was apparent for patients taking acetazolamide, but this did not exceed the normal range [153], while studies of other CA-inhibiting drugs revealed no effects on urinary oxalate excretion [154–156].
Hyperoxaluria following gastric bypass
With the increasing prevalence of obesity and the modern bariatric surgical procedures implemented in an effort to reduce body weight, urinary tract stone disease is in the top ten primary diagnoses for post-operative conditions [157]. A number of clinical reports have suggested the hyperoxaluria associated with Roux-en-Y gastric bypass (RYGB) could be explained by enteric hyperoxaluria caused by steatorrhea [158]. The mechanistic basis of RYGB-induced hyperoxaluria was directly addressed using an RYGB/SHAM obese rat model [159]. The results show that oxalate bioavailability (for intestinal absorption) is largely dependent on the fecal fat content and there is a threshold level for fecal fat which, when reached, significantly alters colonic permeability to oxalate. Clearly, there is an effect of steatorrhea on both the paracellular and transcellular movement of oxalate in the distal colon and each pathway may involve different signaling mechanisms which remain unexplained at present. It was concluded that passive, paracellular oxalate absorption and more importantly oxalate bioavailability are promoted by steatorrhea following RYGB. A novel finding was that the Roux limb is not hyper-absorbing oxalate, which was previously unknown. In fact, both unidirectional and net oxalate fluxes are abolished in the Roux limb compared to robust oxalate fluxes across that segment of intestine when left intact. This study underscores the need for examining other intestinal segments, surgically reconfigured or not, for their contributions to enteric hyperoxaluria following bariatric procedures.
Gut microbiota and oxalate homeostasis
A number of intestinal bacteria degrade oxalate and these are divided into two groups: (1) the “generalist oxalotrophs”, including some strains of Bifidobacterium and Lactobacillus, which can utilize oxalate in addition to other carbon sources and (2) the “specialist oxalotrophs”, such as Oxalobacter formigenes, a commensal anaerobe that relies on oxalate as its sole carbon source [15, 160]. This section primarily focuses on Oxalobacter in laboratory rodents since it has been the most intensely studied with respect to mammalian oxalate homeostasis and intestinal transport. For background information, we recommend the section on the role of oxalate-degrading bacteria from a previous review [21] for the following discussion, in addition to other recent reviews bearing relevant sections on this topic [161–164].
Oxalate-degrading bacteria in humans
Human studies [165–168] and earlier work [21, 163] have focused primarily on Oxalobacter, in addition to some commercial probiotic preparations of various formulations of Lactobacillus and Bifidobacterium strains (Oxadrop, VSL#3, and including a symbiotic preparation Agri-King Synbiotic [167]). However, these studies have shown inconsistent results. Potential reasons for the conflicting and sometimes transient probiotic effects may include a lack of standardization of the various formulae and poor control of dietary oxalate and calcium. Indeed, in vitro analysis of one commercial probiotic showed it contained no viable oxalate-degrading bacteria [169]. More rigorously designed clinical studies including patient groups with various etiologies of hyperoxaluria are warranted to reconcile the beneficial probiotic effects in reducing hyper-oxaluria and hyperoxalemia evident in laboratory animals with the inconsistent probiotic effects observed in human studies. In addition, the importance of determining how colonization with beneficial oxalate degraders is established and maintained, as well as the intraluminal factors affecting colonization (apart from the obvious antibiotic treatments), is essential for a therapeutic approach utilizing probiotics to be realized. Finally, it cannot be overstated that methodologies for the collection and measurements of oxalate in blood and urine are in need of rigorous attention and quality control across all studies, animal and human.
Oxalobacter formigenes in laboratory animals
Initial studies in rats using a variety of experimental approaches showed that Oxalobacter can derive oxalate from systemic sources by inducing active intestinal oxalate secretion [7], followed by intraluminal enzymatic oxalate degradation. The latter is important to provide a concentration gradient for the movement of oxalate into the gut lumen, including through the passive paracellular pathway (Fig. 1), which may be especially enhanced in cases of hyperoxalemia. The beneficial consequences of these combined actions of Oxalobacter will result in an efficient enteric secretion leading to a reduced renal oxalate burden [7–9]. These actions of Oxalobacter were also recently confirmed in an obese rat model following bariatric surgery resulting in a >70% reduction in urinary oxalate excretion over an 8-week period following a combined manipulation in dietary fat and colonization with the wild rat strain, OxWR [158]. The beneficial probiotic effect is perhaps best highlighted in a KO mouse model of primary hyperoxaluria, type 1, having a deficiency of the liver enzyme Alanine–Glyoxylate-aminoTransferase (AGT). Artificially colonizing AGT-KO mice resulted in a normalization of both blood and urinary oxalate levels in otherwise hyperoxalemic/hyperoxaluric animals. The basic mechanism of action of Oxalobacter (both OxWR and the human strain, HC-1) was confirmed to be alterations in the net directional movements of oxalate resulting in enteric oxalate elimination [9]. Although attempts were made to identify the transport proteins involved, this proved difficult due to the lack of specific primary antibodies to the SLC26s. Currently, these questions are being addressed using single KO mouse models of SAT1, DRA and PAT1.
More in-depth studies with mice have revealed Oxalobacter can colonize various segments of both the large and small intestine for varying time periods and are more persistent in the colon compared to more proximal segments [9]. It is notable that urinary oxalate excretion remains persistently low over a 75-day colonization period in WT mice, presumably due to development of a highly robust colonization and possibly adaptations in the efficiency of oxalate transport [8]. In addition, modulation of oxalate secretion by Oxalobacter was also evident in the distal ileum and cecum, but curiously absent in the proximal colon, of both rats and mice [7, 9]. The refractory nature of this latter segment has not been explained and warrants further study given so little is known about the bacterium–host interaction. About a decade ago, we proposed that the mechanism of action for Oxalobacter was possibly due to elaboration of a bacterial secretagogue, since delivery of an encapsulated and freeze-dried, cell-free Oxalobacter lysate had the same effect on colonic oxalate transport as found in animals colonized with Oxalobacter [7]. However, reproducing this effect in vitro has not been successful in our hands despite testing multiple preparations including whole bacterial cells, cell membranes and heat-treated lysates [7]. A confounding issue is the oxalate-degrading activity of Oxalobacter enzymes and degradation of the 14C-oxalate tracer used to measure intestinal fluxes together with the highly oxygenated buffers used in Ussing chamber experiments [7]. A number of intriguing recent reports have shown that exposure to Oxalobacter conditioned media (CM) at a 1:50 dilution for 6–24 h stimulates oxalate influx by Caco-2 cells and a >4-fold increase in DRA mRNA expression, with no changes to DTDST or PAT1 [170, 171]. We repeated this experiment measuring unidirectional oxalate fluxes across Caco-2 monolayers in Ussing chambers using a 1:50 dilution (and also a 1:25 dilution; see Supplementary Table 1) of CM from the cultured human strain of Oxalobacter, HC-1. Exposing the mucosal compartment of these monolayers to CM from Oxalobacter for 18–24 h had no effects on oxalate transport (Fig. 6). In addition to these contrasting results, it is difficult to reconcile why Oxalobacter CM targets DRA [170, 171], and not PAT1, since the latter is the major oxalate transporter in Caco-2 cells [47], and given the respective contributions of PAT1 and DRA to oxalate secretion and absorption in mice [16–18]. It is also notable that Hassan et al. reported no effect of CM from Lactobacillus acidophilus [170, 171], whereas several studies have previously shown that Lactobacillus significantly upregulates the functional expression of DRA in both Caco-2 and mouse models [172–174].
Bifidobacterium and oxalate homeostasis
Recently, we studied the efficacy of the probiotic Bifidobacteria sp. and their contribution to oxalate homeostasis in WT and AGT-KO mice. Groups of mice were artificially colonized with one of two Bifidobacteria strains, an oxalate-degrader B. animalis and a non-degrader B. adolescentis [175]. As anticipated, urinary oxalate was reduced in both WT (↓44%) and AGT-KO (↓33%) mice receiving B. animalis. However, the question was whether B. animalis achieved this by altering intestinal oxalate transport, which is the major characteristic of Oxalobacter. Neither of the two Bifidobacteria strains had significant effects on net oxalate transport in the three segments examined (distal ileum, cecum and distal colon). Thus, the reductions in urinary oxalate in animals colonized with B. animalis occurred solely by intraluminal oxalate degradation. It is notable here that Kumar et al. [176] looked at three other strains of Bifidobacteria and reported an increased expression of DRA in both mice and Caco-2 cells. This work focused on Cl− uptake by Caco-2 monolayers using a surrogate tracer (125I) to show an approximate twofold induction of DRA function, although we saw no clear changes to the DRA-mediated absorptive oxalate flux, [175]. The current work in our laboratory is addressing the same question of Lactobacillus sp., since the ability to induce net intestinal oxalate secretion by interacting with the host enterocyte appears to be a trait unique to Oxalobacter. A prior study by Murphy et al. [177] has already shown that Lactobacillus animalis significantly reduces urinary oxalate excretion in rats, but the effects on intestinal transport of oxalate were not the focus of this work.
Supplementary Material
Acknowledgements
The authors wish to thank Tara Braun, Kristina Fernandez, Heran Getachew, Shreya Mishra, Candi Morris, Susie Robertson and Tisha Van Pelt who provided valuable technical assistance and animal husbandry over the period spanning the collection of data presented here. We are also grateful to Dr. Robert W. Freel, who performed some of the experiments reported and for many useful discussions on epithelial oxalate transport and the transporters involved. This work, and the data presented herein, has been supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK-056245, DK-081624, DK-088892 to M. Hatch, and U54 DK-083908 (sub-award to J. M. Whittamore), from the National Institutes of Health, together with funding from the Oxalosis and Hyperoxaluria Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00240-016-0952-z) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures involving animals were performed under protocols approved by the University of Florida Institutional Animal Care and Use Committee (IACUC), in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”.
References
- 1.Osswald H, Hautmann R. Renal elimination kinetics and plasma half-life of oxalate in man. Urol Int. 1979;34(6):440–450. doi: 10.1159/000280294. [DOI] [PubMed] [Google Scholar]
- 2.Prenen JAC, Boer P, Mees EJD, Endeman HJ, Spoor SM, Oei HY. Renal clearance of C-14-labeled oxalate—comparison of constant-infusion with single-injection techniques. Clin Sci. 1982;63(1):47–51. doi: 10.1042/cs0630047. [DOI] [PubMed] [Google Scholar]
- 3.Costello JF, Smith M, Stolarski C, Sadovnic MJ. Extra-renal clearance of oxalate increases with progression of renal-failure in the rat. J Am Soc Nephrol. 1992;3(5):1098–1104. doi: 10.1681/ASN.V351098. [DOI] [PubMed] [Google Scholar]
- 4.Worcester EM. Stones from bowel disease. Endocrinol Metab Clin North Am. 2002;31(4):979–999. doi: 10.1016/s0889-8529(02)00035-x. doi:10.1016/s0889-8529(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 5.Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic-renal-failure. J Am Soc Nephrol. 1994;5(6):1339–1343. doi: 10.1681/ASN.V561339. [DOI] [PubMed] [Google Scholar]
- 6.Hatch M, Freel RW. Angiotensin II involvement in adaptive enteric oxalate excretion in rats with chronic renal failure induced by hyperoxaluria. Urol Res. 2003;31(6):426–432. doi: 10.1007/s00240-003-0367-5. doi:10.1007/s00240-003-0367-5. [DOI] [PubMed] [Google Scholar]
- 7.Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69(4):691–698. doi: 10.1038/sj.ki.5000162. doi:10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 8.Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G461–G469. doi: 10.1152/ajpgi.00434.2010. doi:10.1152/ajpgi.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatch M, Freel RW. A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis. 2013;41(5):379–384. doi: 10.1007/s00240-013-0601-8. doi:10.1007/s00240-013-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freel RW, Hatch M, Earnest DL, Goldner AM. Oxalate transport across the isolated rat colon—a reexamination. Biochim Biophys Acta. 1980;600(3):838–843. doi: 10.1016/0005-2736(80)90486-1. doi:10.1016/0005-2736(80)90486-1. [DOI] [PubMed] [Google Scholar]
- 11.Hatch M, Freel RW, Goldner AM, Earnest DL. Oxalate and chloride absorption by the rabbit colon: sensitivity to metabolic and anion transport inhibitors. Gut. 1984;25(3):232–237. doi: 10.1136/gut.25.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatch M, Freel RW, Vaziri ND. Characteristics of the transport of oxalate and other ions across rabbit proximal colon. Pflug Archiv Eur J Physiol. 1993;423(3–4):206–212. doi: 10.1007/BF00374396. doi:10.1007/bf00374396. [DOI] [PubMed] [Google Scholar]
- 13.Hatch M, Freel RW, Vaziri ND. Mechanisms of oxalate absorption and secretion across the rabbit distal colon. Pflug Archiv Eur J Physiol. 1994;426(1–2):101–109. doi: 10.1007/BF00374677. doi:10.1007/bf00374677. [DOI] [PubMed] [Google Scholar]
- 14.Dawson KA, Allison MJ, Hartman PA. Isolation and some characteristics of anaerobic oxalate-degrading bacteria from the rumen. Appl Environ Microbiol. 1980;40(4):833–839. doi: 10.1128/aem.40.4.833-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol. 1985;141(1):1–7. doi: 10.1007/BF00446731. [DOI] [PubMed] [Google Scholar]
- 16.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G719–G728. doi: 10.1152/ajpgi.00481.2005. doi:10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 17.Freel RW, Whittamore JM, Hatch M. Transcellular oxalate and Cl− absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol. 2013;305(7):G520–G527. doi: 10.1152/ajpgi.00167.2013. doi:10.1152/ajpgi.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang ZR, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38(4):474–478. doi: 10.1038/ng1762. doi:10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 19.Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Investig. 2010;120(3):706–712. doi: 10.1172/JCI31474. doi:10.1172/jci31474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res. 2005;33(1):1–16. doi: 10.1007/s00240-004-0445-3. doi:10.1007/s00240-004-0445-3. [DOI] [PubMed] [Google Scholar]
- 21.Hatch M, Freel RW. The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol. 2008;28(2):143–151. doi: 10.1016/j.semnephrol.2008.01.007. doi:10.1016/j.semnephrol.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell DW. Barrier function of epithelia. Am J Physiol Gastrointest Liver Physiol. 1981;241(4):G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 23.Knauf F, Ko N, Jiang ZR, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol. 2011;22(12):2247–2255. doi: 10.1681/ASN.2011040433. doi:10.1681/asn.2011040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappenheimer JR. Paracellular intestinal-absorption of glucose, creatinine, and mannitol in normal animals—relation to body size. Am J Physiol Gastrointest Liver Physiol. 1990;259(2):G290–G299. doi: 10.1152/ajpgi.1990.259.2.G290. [DOI] [PubMed] [Google Scholar]
- 25.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology. 2009;136(3):893–901. doi: 10.1053/j.gastro.2008.11.016. doi:10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AK, Riederer B, Chen MM, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U. The switch of intestinal Slc26 exchangers from anion absorptive to HCO3− secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol. 2010;298(5):C1057–C1065. doi: 10.1152/ajpcell.00454.2009. doi:10.1152/ajpcell.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh AK, Liu YJ, Riederer B, Engelhardt R, Thakur BK, Soleimani M, Seidler U. Molecular transport machinery involved in orchestrating luminal acid-induced duodenal bicarbonate secretion in vivo. J Physiol Lond. 2013;591(21):5377–5391. doi: 10.1113/jphysiol.2013.254854. doi:10.1113/jphysiol.2013.254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XM, Li TL, Riederer B, Lenzen H, Ludolph L, Yeruva S, Tuo BG, Soleimani M, Seidler U. Loss of Slc26a9 anion transporter alters intestinal electrolyte and HCO3− transport and reduces survival in CFTR-deficient mice. Pflug Archiv Eur J Physiol. 2015;467(6):1261–1275. doi: 10.1007/s00424-014-1543-x. doi:10.1007/s00424-014-1543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki E, Natori Y, Ohgishi Y, Hayashi H, Suzuki Y. Segmental difference of mucosal damage along the length of a mouse small intestine in an Ussing chamber. J Nutr Sci Vita-minol. 2005;51(6):406–412. doi: 10.3177/jnsv.51.406. [DOI] [PubMed] [Google Scholar]
- 30.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol. 2007;292(4):G1079–G1088. doi: 10.1152/ajpgi.00354.2006. doi:10.1152/ajpgi.00354.2006. [DOI] [PubMed] [Google Scholar]
- 31.Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL. Functional activity of Pat-1 (Slc26a6) Cl−/HCO3− exchange in the lower villus epithelium of murine duodenum. Acta Physiol. 2011;201(1):21–31. doi: 10.1111/j.1748-1716.2010.02210.x. doi:10.1111/j.1748-1716.2010.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freel RW, Hatch M, Vaziri ND. Conductive pathways for chloride and oxalate in rabbit ileal brush-border membrane vesicles. Am J Physiol Cell Physiol. 1998;275(3):C748–C757. doi: 10.1152/ajpcell.1998.275.3.C748. [DOI] [PubMed] [Google Scholar]
- 33.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. doi:10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 34.Freel RW, Hatch M. Enteric oxalate secretion is not directly mediated by the human CFTR chloride channel. Urol Res. 2008;36(3–4):127–131. doi: 10.1007/s00240-008-0142-8. doi:10.1007/s00240-008-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibney EM, Goldfarb DS. The association of nephrolithiasis with cystic fibrosis. Am J Kidney Dis. 2003;42(1):1–11. doi: 10.1016/s0272-6386(03)00403-7. doi:10.1016/s0272-6386(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 36.Hoppe B, von Unruh GE, Blank G, Rietschel E, Sidhu H, Laube N, Hesse A. Absorptive hyperoxaluria leads to an increased risk for urolithiasis or nephrocalcinosis in cystic fibrosis. Am J Kidney Dis. 2005;46(3):440–445. doi: 10.1053/j.ajkd.2005.06.003. doi:10.1053/j.ajkd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Knauf F, Thomson RB, Heneghan JF, Jiang Z, Adebamiro A, Thomson CL, Barone C, Asplin JR, Egan ME, Alper SL, Aronson PS. Loss of cystic fibrosis transmembrane regulator impairs intestinal oxalate secretion. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016030279. doi:10.1681/asn.2016030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko SBH, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 2002;21(21):5662–5672. doi: 10.1093/emboj/cdf580. doi:10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko SBH, Zeng WZ, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6(4):343–350. doi: 10.1038/ncb1115. doi:10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossmann H, Jacob P, Baisch S, Hassoun R, Meier J, Natour D, Yahya K, Yun C, Biber J, Lackner KJ, Fiehn W, Gregor M, Seidler U, Lamprecht G. The CFTR associated protein CAP70 interacts with the apical Cl−/HCO3− exchanger DRA in rabbit small intestinal mucosa. Biochemistry. 2005;44(11):4477–4487. doi: 10.1021/bi048828b. doi:10.1021/bi048828b. [DOI] [PubMed] [Google Scholar]
- 41.Singh AK, Sjoblom M, Zheng W, Krabbenhoft A, Riederer B, Rausch B, Manns MP, Soleimani M, Seidler U. CFTR and its key role in in vivo resting and luminal acid-induced duodenal HCO3− secretion. Acta Physiol. 2008;193(4):357–365. doi: 10.1111/j.1748-1716.2008.01854.x. doi:10.1111/j.1748-1716.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 42.Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Investig. 2009;119(3):540–550. doi: 10.1172/JCI35541. doi:10.1172/jci35541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regeer RR, Lee A, Markovich D. Characterization of the human sulfate anion transporter (hsat-1) protein and gene (SAT1; SLC26A1). DNA Cell Biol. 2003;22(2):107–117. doi: 10.1089/104454903321515913. doi:10.1089/104454903321515913. [DOI] [PubMed] [Google Scholar]
- 44.Ko N, Knauf F, Jiang Z, Markovich D, Aronson PS. Sat1 is dispensable for active oxalate secretion in mouse duodenum. Am J Physiol Cell Physiol. 2012;303(1):C52–C57. doi: 10.1152/ajpcell.00385.2011. doi:10.1152/ajpcell.00385.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittamore JM, Frost SC, Hatch M. Effects of acid-base variables and the role of carbonic anhydrase on oxalate secretion by the mouse intestine in vitro. Physiol Rep. 2015;3(2) doi: 10.14814/phy2.12282. doi:10.14814/phy2.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morozumi M, Green M, Freel RW, Hatch M. The effect of oxalate loading or acidified media on the expression of mRNA encoding candidate oxalate transporters in Caco-2 monolayers.. 10th international symposium on urolithiasis; Hong Kong. 2004.2004. pp. 178–180. [Google Scholar]
- 47.Freel RW, Morozumi M, Hatch M. Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G918–G929. doi: 10.1152/ajpgi.00251.2009. doi:10.1152/ajpgi.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflug Archiv Eur J Physiol. 2004;447(5):710–721. doi: 10.1007/s00424-003-1090-3. doi:10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 49.Dorwart MR, Shcheynikov N, Yang DK, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology. 2008;23(2):104–114. doi: 10.1152/physiol.00037.2007. doi:10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- 50.Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol Asp Med. 2013;34(2–3):494–515. doi: 10.1016/j.mam.2012.07.009. doi:10.1016/j.mam.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shcheynikov N, Ohana E, Muallem S. Properties and function of the solute carrier 26 family of anion transporters. In: Hamilton KL, Devor DC, editors. Ion channels and transporters of epithelia in health and disease. physiology in health and disease. Springer; New York: 2016. pp. 465–490. [Google Scholar]
- 52.Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber J, Murer H. Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol Renal Physiol. 1998;275(1):F79–F87. doi: 10.1152/ajprenal.1998.275.1.F79. [DOI] [PubMed] [Google Scholar]
- 53.Satoh H, Susaki M, Shukunami C, Iyama K, Negoro T, Hiraki Y. Functional analysis of diastrophic dysplasia sulfate transporter—its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J Biol Chem. 1998;273(20):12307–12315. doi: 10.1074/jbc.273.20.12307. doi:10.1074/jbc.273.20.12307. [DOI] [PubMed] [Google Scholar]
- 54.Xie QH, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283(4):F826–F838. doi: 10.1152/ajprenal.00079.2002. doi:10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- 55.Lee A, Beck L, Markovich D. The mouse sulfate anion transporter gene Sat1 (Slc26a1): cloning, tissue distribution, gene structure, functional characterization, and transcriptional regulation by thyroid hormone. DNA Cell Biol. 2003;22(1):19–31. doi: 10.1089/104454903321112460. doi:10.1089/104454903321112460. [DOI] [PubMed] [Google Scholar]
- 56.Krick W, Schnedler N, Burckhardt G, Burckhardt BC. Ability of sat-1 to transport sulfate, bicarbonate, or oxalate under physiological conditions. Am J Physiol Renal Physiol. 2009;297(1):F145–F154. doi: 10.1152/ajprenal.90401.2008. doi:10.1152/ajprenal.90401.2008. [DOI] [PubMed] [Google Scholar]
- 57.Quondamatteo F, Krick W, Hagos Y, Kruger MH, Neubauer-Saile K, Herken R, Ramadori G, Burckhardt G, Burckhardt BC. Localization of the sulfate/anion exchanger in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G1075–G1081. doi: 10.1152/ajpgi.00492.2005. doi:10.1152/ajpgi.00492.2005. [DOI] [PubMed] [Google Scholar]
- 58.Brzica H, Breljak D, Krick W, Lovric M, Burckhardt G, Burckhardt BC, Sabolic I. The liver and kidney expression of sulfate anion transporter sat-1 in rats exhibits male-dominant gender differences. Pflug Archiv Eur J Physiol. 2009;457(6):1381–1392. doi: 10.1007/s00424-008-0611-5. doi:10.1007/s00424-008-0611-5. [DOI] [PubMed] [Google Scholar]
- 59.Breljak D, Brzica H, Vrhovac I, Micek V, Karaica D, Ljubojevic M, Sekovanic A, Jurasovic J, Rasic D, Peraica M, Lovric M, Schnedler N, Henjakovic M, Wegner W, Burckhardt G, Burckhardt BC, Sabolic I. In female rats, ethylene glycol treatment elevates protein expression of hepatic and renal oxalate transporter sat-1 (Slc26a1) without inducing hyperoxaluria. Croat Med J. 2015;56(5):447–459. doi: 10.3325/cmj.2015.56.447. doi:10.3325/cmj.2015.56.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dawson PA, Sim P, Mudge DW, Cowley D. Human SLC26A1 gene variants: a pilot study. Sci World J. 2013 doi: 10.1155/2013/541710. doi:10.1155/2013/541710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haila S, Hastbacka J, Bohling T, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U. SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J Histochem Cytochem. 2001;49(8):973–982. doi: 10.1177/002215540104900805. [DOI] [PubMed] [Google Scholar]
- 62.Park M, Ohana E, Choi SY, Lee M-S, Park JH, Muallem S. Multiple Roles of the SO 24−/Cl−/OH− exchanger protein Slc26a2 in chondrocyte functions. J Biol Chem. 2014;289(4):1993–2001. doi: 10.1074/jbc.M113.503466. doi:10.1074/jbc.M113.503466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hastbacka J, Delachapelle A, Mahtani MM, Clines G, Reevedaly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander ES. The diastrophic dysplasia gene encodes a novel sulfate transporter—positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78(6):1073–1087. doi: 10.1016/0092-8674(94)90281-x. doi:10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 64.Karniski LP. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene: correlation between sulfate transport activity and chondrodysplasia phenotype. Hum Mol Genet. 2001;10(14):1485–1490. doi: 10.1093/hmg/10.14.1485. doi:10.1093/hmg/10.14.1485. [DOI] [PubMed] [Google Scholar]
- 65.Rossi A, Superti-Furga A. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene (SLC26A2): 22 Novel mutations, mutation review, associated skeletal phenotypes, and diagnostic relevance. Hum Mutat. 2001;17(3):159–171. doi: 10.1002/humu.1. doi:10.1002/humu.1. [DOI] [PubMed] [Google Scholar]
- 66.Forlino A, Piazza R, Torre SD, Tatangelo L, Bonafe L, Gualeni B, Romano A, Pecora F, Superti-Furga A, Cetta G, Rossi A. A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum Mol Genet. 2005;14(6):859–871. doi: 10.1093/hmg/ddi079. doi:10.1093/hmg/ddi079. [DOI] [PubMed] [Google Scholar]
- 67.Haila S, Saarialho-Kere U, Karjalainen-Lindsberg ML, Lohi H, Airola K, Holmberg C, Hastbacka J, Kere J, Hoglund P. The congenital chloride diarrhea gene is expressed in seminal vesicle, sweat gland, inflammatory colon epithelium, and in some dysplastic colon cells. Histochem Cell Biol. 2000;113(4):279–286. doi: 10.1007/s004180000131. [DOI] [PubMed] [Google Scholar]
- 68.Dawson PA, Rakoczy J, Simmons DG. Placental, renal, and ileal sulfate transporter gene expression in mouse gestation. Biol Reprod. 2012;87(2) doi: 10.1095/biolreprod.111.098749. doi:10.1095/biolreprod.111.098749. [DOI] [PubMed] [Google Scholar]
- 69.Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL. Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol. 2010;298(6):C1363–C1375. doi: 10.1152/ajpcell.00004.2010. doi:10.1152/ajpcell.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohana E, Shcheynikov N, Park M, Muallem S. Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SO42−/OH−/Cl− exchanger regulated by extra-cellular Cl−. J Biol Chem. 2012;287(7):5122–5132. doi: 10.1074/jbc.M111.297192. doi:10.1074/jbc.M111.297192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Talbot C, Lytle C. Segregation of Na+/H+ exchanger-3 and Cl−/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G358–G367. doi: 10.1152/ajpgi.00151.2010. doi:10.1152/ajpgi.00151.2010. [DOI] [PubMed] [Google Scholar]
- 72.Silberg DG, Wang W, Moseley RH, Traber PG. The down-regulated in adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem. 1995;270(20):11897–11902. doi: 10.1074/jbc.270.20.11897. [DOI] [PubMed] [Google Scholar]
- 73.Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14(3):316–319. doi: 10.1038/ng1196-316. doi:10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 74.Turnberg LA. Abnormalities in intestinal electrolyte transport in congenital chloridorrhoea. Gut. 1971;12(7):544–551. doi: 10.1136/gut.12.7.544. doi:10.1136/gut.12.7.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bieberdorf FA, Fordtran JS, Gorden P. Pathogenesis of congenital alkalosis with diarrhea—implications for physiology of normal ileal electrolyte absorption and secretion. J Clin Investig. 1972;51(8):1958–1968. doi: 10.1172/JCI107002. doi:10.1172/jci107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmberg C, Perheentupa J, Launiala K. Colonic electrolyte transport in health and in congenital chloride diarrhea. J Clin Investig. 1975;56(2):302–310. doi: 10.1172/JCI108094. doi:10.1172/jci108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang ZH, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281(49):37962–37971. doi: 10.1074/jbc.M607527200. doi:10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 78.Schweinfest CW, Henderson KW, Suster S, Kondoh N, Papas TS. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc Natl Acad Sci USA. 1993;90(9):4166–4170. doi: 10.1073/pnas.90.9.4166. doi:10.1073/pnas.90.9.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byeon MK, Westerman MA, Maroulakou IG, Henderson KW, Suster S, Zhang XK, Papas TS, Vesely J, Willingham MC, Green JE, Schweinfest CW. The down-regulated in adenoma (DRA) gene encodes an intestine-specific membrane glycoprotein. Oncogene. 1996;12(2):387–396. [PubMed] [Google Scholar]
- 80.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology. 2002;122(3):709–724. doi: 10.1053/gast.2002.31875. doi:10.1053/gast.2002.31875. [DOI] [PubMed] [Google Scholar]
- 81.Wang ZH, Wang T, Petrovic S, Tuo BG, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol. 2005;288(4):C957–C965. doi: 10.1152/ajpcell.00505.2004. doi:10.1152/ ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 82.Barmeyer C, Ye JHQ, Sidani S, Geibel J, Binder HJ, Rajendran VM. Characteristics of rat downregulated in adenoma (rDRA) expressed in HEK 293 cells. Pflug Archiv Eur J Physiol. 2007;454(3):441–450. doi: 10.1007/s00424-007-0213-7. doi:10.1007/s00424-007-0213-7. [DOI] [PubMed] [Google Scholar]
- 83.Wedenoja S, Ormala T, Berg UB, Halling SFE, Jalanko H, Karikoski R, Kere J, Holmberg C, Hoglund P. The impact of sodium chloride and volume depletion in the chronic kidney disease of congenital chloride diarrhea. Kidney Int. 2008;74(8):1085–1093. doi: 10.1038/ki.2008.401. doi:10.1038/ki.2008.401. [DOI] [PubMed] [Google Scholar]
- 84.Chernova MN, Jiang LW, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol Lond. 2003;549(1):3–19. doi: 10.1113/jphysiol.2003.039818. doi:10.1113/jphysiol.2003.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark JS, Vandorpe DH, Chernova MN, Heneghan JF, Stewart AK, Alper SL. Species differences in Cl− affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl− exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis. J Physiol Lond. 2008;586(5):1291–1306. doi: 10.1113/jphysiol.2007.143222. doi:10.1113/jphysiol.2007.143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamprecht G, Baisch S, Schoenleber E, Gregor M. Transport properties of the human intestinal anion exchanger DRA (down-regulated in adenoma) in transfected HEK293 cells. Pflug Archiv Eur J Physiol. 2005;449(5):479–490. doi: 10.1007/s00424-004-1342-x. doi:10.1007/s00424-004-1342-x. [DOI] [PubMed] [Google Scholar]
- 87.Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol. 2011;301(2):C289–C303. doi: 10.1152/ajpcell.00089.2011. doi:10.1152/ajpcell.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whittamore JM, Freel RW, Hatch M. Sulfate secretion and chloride absorption are mediated by the anion exchanger DRA (Slc26a3) in the mouse cecum. Am J Physiol Gastrointest Liver Physiol. 2013;305(2):G172–G184. doi: 10.1152/ajpgi.00084.2013. doi:10.1152/ajpgi.00084.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shcheynikov N, Wang Y, Park M, Ko SBH, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127(5):511–524. doi: 10.1085/jgp.200509392. doi:10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohana E, Shcheynikov N, Yang DK, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol. 2011;137(2):239–251. doi: 10.1085/jgp.201010531. doi:10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song Y, Yamamoto A, Steward MC, Ko SBH, Stewart AK, Soleimani M, Liu BC, Kondo T, Jin CX, Ishiguro H. Deletion of Slc26a6 alters the stoichiometry of apical Cl−/HCO3− exchange in mouse pancreatic duct. Am J Physiol Cell Physiol. 2012;303(8):C815–C824. doi: 10.1152/ajpcell.00151.2012. doi:10.1152/ajpcell.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang ZH, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282(3):G573–G579. doi: 10.1152/ajpgi.00338.2001. doi:10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 93.Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl−/HCO3− exchanger couples with Na+/H+ exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology. 2008;135(5):1645–1653. doi: 10.1053/j.gastro.2008.07.083. doi:10.1053/j.gastro.2008.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xia WL, Yu Q, Riederer B, Singh AK, Engelhardt R, Yeruva S, Song PH, Tian DA, Soleimani M, Seidler U. The distinct roles of anion transporters Slc26a3 (DRA) and Slc26a6 (PAT-1) in fluid and electrolyte absorption in the murine small intestine. Pflug Archiv Eur J Physiol. 2014;466(8):1541–1556. doi: 10.1007/s00424-013-1381-2. doi:10.1007/s00424-013-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol. 2003;284(3):C769–C779. doi: 10.1152/ajpcell.00270.2002. doi:10.1152/ajpcell.00270.2002. [DOI] [PubMed] [Google Scholar]
- 96.Anderle P, Sengstag T, Mutch DM, Rumbo M, Praz V, Mansourian R, Delorenzi M, Williamson G, Roberts MA. Changes in the transcriptional profile of transporters in the intestine along the anterior–posterior and crypt-villus axes. BMC Genom. 2005;6 doi: 10.1186/1471-2164-6-69. doi:10.1186/1471-2164-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hassan HA, Cheng M, Aronson PS. Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells. Am J Physiol Cell Physiol. 2012;302(1):C46–C58. doi: 10.1152/ajpcell.00075.2011. doi:10.1152/ajpcell.00075.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966;11(7):503–521. doi: 10.1007/BF02233563. doi:10.1007/bf02233563. [DOI] [PubMed] [Google Scholar]
- 99.Li H, Ye ZQ, He W, Xia D, Aliya AY, Shen JH, Chen ZQ. Screening of differentially expressed genes in the jejunum of rats with idiopathic hyperoxaluria. Chin Med J. 2012;125(2):312–315. doi:10.3760/cma.j.issn.0366-6999.2012.02.027. [PubMed] [Google Scholar]
- 100.Monico CG, Weinstein A, Jiang ZR, Rohlinger AL, Cogal AG, Bjornson BB, Olson JB, Bergstralh EJ, Milliner DS, Aronson PS. Phenotypic and functional analysis of human SLC26A6 variants in patients with familial hyperoxaluria and calcium oxalate nephrolithiasis. Am J Kidney Dis. 2008;52(6):1096–1103. doi: 10.1053/j.ajkd.2008.07.041. doi:10.1053/j.ajkd.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Corbetta S, Eller-Vainicher C, Frigerio M, Vataperta R, Costa E, Vicentini L, Baccarelli A, Beck-Peccoz P, Spada A. Analysis of the 206M polymorphic variant of the SLC26A6 gene encoding a Cl− oxalate transporter in patients with primary hyperparathyroidism. Eur J Endocrinol. 2009;160(2):283–288. doi: 10.1530/EJE-08-0623. doi:10.1530/eje-08-0623. [DOI] [PubMed] [Google Scholar]
- 102.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Investig. 2003;111(7):931–943. doi: 10.1172/JCI18326. doi:10.1172/jci200318326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol. 2011;73:261–281. doi: 10.1146/annurev-physiol-012110-142244. doi:10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS. Regulation of anion exchanger Slc26a6 by protein kinase C. Am J Physiol Cell Physiol. 2007;292(4):C1485–C1492. doi: 10.1152/ajpcell.00447.2006. doi:10.1152/ajpcell.00447.2006. [DOI] [PubMed] [Google Scholar]
- 105.Amin R, Sharma S, Ratakonda S, Hassan HA. Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-delta activation. Am J Physiol Cell Physiol. 2013;305(1):C78–C89. doi: 10.1152/ajpcell.00339.2012. doi:10.1152/ajpcell.00339.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reimold FR, Heneghan JF, Stewart AK, Zelikovic I, Vandorpe DH, Shmukler BE, Alper SL. Pendrin function and regulation in Xenopus oocytes. Cell Physiol Biochem. 2011;28(3):435–450. doi: 10.1159/000335106. doi:10.1159/000335106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Newton AC. Protein-kinase-C - Structure, function, and regulation. J Biol Chem. 1995;270(48):28495–28498. doi: 10.1074/jbc.270.48.28495. doi:10.1074/jbc.270.43.25526. [DOI] [PubMed] [Google Scholar]
- 108.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng L, Webster SV, Newton PM. The biology of protein kinase C. Calcium Signal. 2012;740:639–661. doi: 10.1007/978-94-007-2888-2_28. doi:10.1007/978-94-007-2888-2_28. [DOI] [PubMed] [Google Scholar]
- 110.Di Mari JF, Mifflin RC, Powell DW. The role of protein kinase C in gastrointestinal function and disease. Gastroenterology. 2005;128(7):2131–2146. doi: 10.1053/j.gastro.2004.09.078. doi:10.1053/j.gastro.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 111.Nishizuka Y. The role of Protein Kinase-C in cell-surface signal transduction and tumor promotion. Nature. 1984;308(5961):693–698. doi: 10.1038/308693a0. doi:10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 112.Flores CA, Cid LP, Sepulveda FV. Strain-dependent differences in electrogenic secretion of electrolytes across mouse colon epithelium. Exp Physiol. 2010;95(6):686–698. doi: 10.1113/expphysiol.2009.051102. doi:10.1113/expphysiol.2009.051102. [DOI] [PubMed] [Google Scholar]
- 113.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. doi:10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. doi:10.1042/bj20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKC delta. Trends Pharmacol Sci. 2007;28(9):453–458. doi: 10.1016/j.tips.2007.07.003. doi:10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 116.Wu-Zhang AX, Newton AC. Protein kinase C pharmacology: refining the toolbox. Biochem J. 2013;452:195–209. doi: 10.1042/BJ20130220. doi:10.1042/bj20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tuo BG, Chow JYC, Barrett KE, Isenberg JI. Protein kinase C potentiates cAMP-stimulated mouse duodenal mucosal bicarbonate secretion in vitro. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G814–G821. doi: 10.1152/ajpgi.00251.2003. doi:10.1152/ajpgi.00251.2003. [DOI] [PubMed] [Google Scholar]
- 118.Tuo BG, Riederer B, Wang ZH, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger SLC26A6 in prostaglandin E2- but not forskolin-stimulated duodenal HCO3− secretion. Gastroenterology. 2006;130(2):349–358. doi: 10.1053/j.gastro.2005.10.017. doi:10.1053/j.gastro.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 119.Song JC, Hanson CM, Tsai V, Farokhzad OC, Lotz M, Matthews JB. Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am J Physiol Cell Physiol. 2001;281(2):C649–C661. doi: 10.1152/ajpcell.2001.281.2.C649. [DOI] [PubMed] [Google Scholar]
- 120.Del Castillo IC, Fedor-Chaiken M, Song JC, Starlinger V, Yoo J, Matlin KS, Matthews JB. Dynamic regulation of Na+−K+−2Cl− cotransporter surface expression by PKC-ε in Cl− secretory epithelia. Am J Physiol Cell Physiol. 2005;289(5):C1332–C1342. doi: 10.1152/ajpcell.00580.2004. doi:10.1152/ajpcell.00580.2004. [DOI] [PubMed] [Google Scholar]
- 121.Heneghan JF, Alper SL. This, too, shall pass—like a kidney stone: a possible path to prophylaxis of nephrolithiasis? Focus on “Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells”. Am J Physiol Cell Physiol. 2012;302(1):C18–C20. doi: 10.1152/ajpcell.00389.2011. doi:10.1152/ajpcell.00389.2011. [DOI] [PubMed] [Google Scholar]
- 122.Field M, McColl I. Ion-transport in rabbit ileal mucosa 3. Effects of catecholamines. Am J Physiol. 1973;225(4):852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- 123.Hubel KA. Intestinal ion-transport—effect of norepinephrine, pilocarpine, and atropine. Am J Physiol. 1976;231(1):252–257. doi: 10.1152/ajplegacy.1976.231.1.252. [DOI] [PubMed] [Google Scholar]
- 124.Chang EB, Field M, Miller RJ. Alpha-2-adrenergic receptor regulation of ion-transport in rabbit ileum. Am J Physiol Gastrointest Liver Physiol. 1982;242(3):G237–G242. doi: 10.1152/ajpgi.1982.242.3.G237. [DOI] [PubMed] [Google Scholar]
- 125.Sellin JH, Desoignie R. Regulation of Na-Cl absorption in rabbit proximal colon in vitro. Am J Physiol Gastroint Liver Physiol. 1987;252(1):G45–G51. doi: 10.1152/ajpgi.1987.252.1.G45. [DOI] [PubMed] [Google Scholar]
- 126.Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol. 2006;149(5):463–479. doi: 10.1038/sj.bjp.0706889. doi:10.1038/sj.bjp.0706889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Novak I. Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol. 2011;202(3):501–522. doi: 10.1111/j.1748-1716.2010.02225.x. doi:10.1111/j.1748-1716.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 128.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2014;10(1):3–50. doi: 10.1007/s11302-013-9397-9. doi:10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abraham C, Scaglione-Sewell B, Skarosi SF, Qin WY, Bissonnette M, Brasitus TA. Protein kinase C alpha modulates growth and differentiation in Caco-2 cells. Gastroenterology. 1998;114(3):503–509. doi: 10.1016/s0016-5085(98)70533-5. doi:10.1016/s0016-5085(98)70533-5. [DOI] [PubMed] [Google Scholar]
- 130.Wald FA, Oriolo AS, Mashukova A, Fregien NL, Langshaw AH, Salas PJI. Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J Cell Sci. 2008;121(5):644–654. doi: 10.1242/jcs.016246. doi:10.1242/jcs.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC-η regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA. 2009;106(1):61–66. doi: 10.1073/pnas.0802741106. doi:10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alrefai WA, Scaglione-Sewell B, Tyagi S, Wartman L, Brasitus TA, Ramaswamy K, Dudeja PK. Differential regulation of the expression of Na+/H+ exchanger isoform NHE3 by PKC-alpha in Caco-2 cells. Am J Physiol Cell Physiol. 2001;281(5):C1551–C1558. doi: 10.1152/ajpcell.2001.281.5.C1551. [DOI] [PubMed] [Google Scholar]
- 133.Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Inhibition of apical Cl−/OH− exchange activity in Caco-2 cells by phorbol esters is mediated by PKC-ε. Am J Physiol Cell Physiol. 2002;283(5):C1492–C1500. doi: 10.1152/ajpcell.00473.2001. doi:10.1152/ajpcell.00473.2001. [DOI] [PubMed] [Google Scholar]
- 134.Stenson WF, Easom RA, Riehl TE, Turk J. Regulation of paracellular permeability in Caco-2 cell monolayers by protein-kinase-C. Am J Physiol Gastrointest Liver Physiol. 1993;265(5):G955–G962. doi: 10.1152/ajpgi.1993.265.5.G955. [DOI] [PubMed] [Google Scholar]
- 135.Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB, Madara JL. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol Cell Physiol. 1999;277(3):C554–C562. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- 136.Ohana E, Shcheynikov N, Moe OW, Muallem S. SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J Am Soc Nephrol. 2013;24(10):1617–1626. doi: 10.1681/ASN.2013010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moe OW, Preisig PA. Dual role of citrate in mammalian urine. Curr Opin Nephrol Hypertens. 2006;15(4):419–424. doi: 10.1097/01.mnh.0000232882.35469.72. doi:10.1097/01.mnh.0000232882.35469.72. [DOI] [PubMed] [Google Scholar]
- 138.Sakhaee K, Maalouf NM, Sinnott B. Kidney Stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012;97(6):1847–1860. doi: 10.1210/jc.2011-3492. doi:10.1210/jc.2011-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA. 2001;98(16):9425–9430. doi: 10.1073/pnas.141241098. doi:10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pajor AM. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflug Archiv Eur J Physiol. 2014;466(1):119–130. doi: 10.1007/s00424-013-1369-y. doi:10.1007/s00424-013-1369-y. [DOI] [PubMed] [Google Scholar]
- 141.Garcia-Perez I, Villasenor A, Wijeyesekera A, Posma JM, Jiang ZR, Stamler J, Aronson P, Unwin R, Barbas C, Elliott P, Nicholson J, Holmes E. Urinary metabolic phenotyping the slc26a6 (Chloride-oxalate exchanger) null mouse model. J Proteome Res. 2012;11(9):4425–4435. doi: 10.1021/pr2012544. doi:10.1021/pr2012544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Whittamore JM, Hatch M. Chronic metabolic acidosis reduces urinary oxalate excretion and promotes intestinal oxalate secretion in the rat. Urolithiasis. 2015;43(6):489–499. doi: 10.1007/s00240-015-0801-5. doi:10.1007/s00240-015-0801-5. [DOI] [PubMed] [Google Scholar]
- 143.Charney AN, Goldfarb DS, Dagher PC. Metabolic disorders associated with gastrointestinal disease. In: Arieff AI, DeFronzo RA, editors. Fluid, electrolyte, and acid-base disorders. 2nd edn. Churchill Livingstone; New York: 1995. pp. 813–836. [Google Scholar]
- 144.Gennari FJ, Weise WJ. Acid-base disturbances in gastrointestinal disease. Clin J Am Soc Nephrol. 2008;3(6):1861–1868. doi: 10.2215/CJN.02450508. doi:10.2215/cjn.02450508. [DOI] [PubMed] [Google Scholar]
- 145.Charney AN, Feldman GM. Systemic acid-base-disorders and intestinal electrolyte transport. Am J Physiol Gastroin-test Liver Physiol. 1984;247(1):G1–G12. doi: 10.1152/ajpgi.1984.247.1.G1. [DOI] [PubMed] [Google Scholar]
- 146.Charney AN, Dagher PC. Acid-base effects on colonic electrolyte transport revisited. Gastroenterology. 1996;111(5):1358–1368. doi: 10.1053/gast.1996.v111.agast961111358. doi:10.1053/gast.1996.v111.agast961111358. [DOI] [PubMed] [Google Scholar]
- 147.Charney AN, Egnor RW, Alexander-Chacko J, Cassai N, Sidhu GS. Acid-base effects on intestinal Na+ absorption and vesicular trafficking. Am J Physiol Cell Physiol. 2002;283(3):C971–C979. doi: 10.1152/ajpcell.00079.2002. doi:10.1152/ajpcell.00079.2002. [DOI] [PubMed] [Google Scholar]
- 148.Charney AN, Egnor RW, Henner D, Rashid H, Cassai N, Sidhu GS. Acid-base effects on intestinal Cl− absorption and vesicular trafficking. Am J Physiol Cell Physiol. 2004;286(5):C1062–C1070. doi: 10.1152/ajpcell.00454.2003. doi:10.1152/ajpcell.00454.2003. [DOI] [PubMed] [Google Scholar]
- 149.Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J. 2005;24(14):2499–2511. doi: 10.1038/sj.emboj.7600736. doi:10.1038/sj.emboj.7600736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bushinsky DA, Grynpas MD, Asplin JR. Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2001;59(4):1415–1423. doi: 10.1046/j.1523-1755.2001.0590041415.x. doi:10.1046/j.1523-1755.2001.0590041415.x. [DOI] [PubMed] [Google Scholar]
- 151.Hatch M, Freel RW. Renal and intestinal handling of oxalate following oxalate loading in rats. Am J Nephrol. 2003;23(1):18–26. doi: 10.1159/000066300. doi:10.1159/000066300. [DOI] [PubMed] [Google Scholar]
- 152.Osther PJ, Bollerslev J, Norgard JR, Engel K, Kildeberg P. Effects of acute acid loading on the risk of calcium-phosphate and calcium-oxalate crystallization in urine. Scanning Microsc. 1994;8(1):63–69. [Google Scholar]
- 153.Ahlstrand C, Tiselius HG. Urine composition and stone formation during treatment with acetazolamide. Scand J Urol Nephrol. 1987;21(3):225–228. doi: 10.3109/00365598709180326. [DOI] [PubMed] [Google Scholar]
- 154.Higashihara E, Nutahara K, Takeuchi T, Shoji N, Araie M, Aso Y. Calcium-metabolism in acidotic patients induced by carbonic-anhydrase inhibitors—responses to citrate. J Urol. 1991;145(5):942–948. doi: 10.1016/s0022-5347(17)38496-3. [DOI] [PubMed] [Google Scholar]
- 155.Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K. Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis. 2006;48(4):555–563. doi: 10.1053/j.ajkd.2006.07.003. doi:10.1053/j.ajkd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 156.Kaplon DM, Penniston KL, Nakada SY. Patients with and without prior urolithiasis have hypocitraturia and incident kidney stones while on topiramate. Urology. 2011;77(2) doi: 10.1016/j.urology.2010.06.048. doi:10.1016/j.urology.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 157.Encinosa WE, Bernard DM, Chen CC, Steiner CA. Healthcare utilization and outcomes after bariatric surgery. Med Care. 2006;44(8):706–712. doi: 10.1097/01.mlr.0000220833.89050.ed. doi:10.1097/01.mlr.0000220833.89050.ed. [DOI] [PubMed] [Google Scholar]
- 158.Canales BK, Hatch M. Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis. 2014;10(4):734–742. doi: 10.1016/j.soard.2014.03.026. doi:10.1016/j.soard.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hatch M, Canales BK. The mechanistic basis of hyperoxaluria following gastric bypass in obese rats. Urolithiasis. 2015 doi: 10.1007/s00240-015-0836-7. doi:10.1007/s00240-015-0836-7. [DOI] [PubMed] [Google Scholar]
- 160.Dawson KA, Allison MJ, Hartman PA. Characteristics of anaerobic oxalate-degrading enrichment cultures from the rumen. Appl Environ Microbiol. 1980;40(4):840–846. doi: 10.1128/aem.40.4.840-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Knight J, Deora R, Assimos DG, Holmes RP. The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis. 2013;41(3):187–196. doi: 10.1007/s00240-013-0566-7. doi:10.1007/s00240-013-0566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Asplin JR. The management of patients with enteric hyperoxaluria. Urolithiasis. 2016;44(1):33–43. doi: 10.1007/s00240-015-0846-5. doi:10.1007/s00240-015-0846-5. [DOI] [PubMed] [Google Scholar]
- 163.Liebman M, Al-Wahsh IA. Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr. 2011;2(3):254–260. doi: 10.3945/an.111.000414. doi:10.3945/an.111.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abratt VR, Reid SJ. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv Appl Microbiol. 2010;72:63–87. doi: 10.1016/S0065-2164(10)72003-7. [DOI] [PubMed] [Google Scholar]
- 165.Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschenes G, Unwin R, Milliner D. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant. 2011;26(11):3609–3615. doi: 10.1093/ndt/gfr107. doi:10.1093/ndt/gfr107. [DOI] [PubMed] [Google Scholar]
- 166.Jiang J, Knight J, Easter LH, Neiberg R, Holmes RP, Assimos DG. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol. 2011;186(1):135–139. doi: 10.1016/j.juro.2011.03.006. doi:10.1016/j.juro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lieske JC, Tremaine WJ, De Simone C, O'Connor HM, Li X, Bergstralh EJ, Goldfarb DS. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 2010;78(11):1178–1185. doi: 10.1038/ki.2010.310. doi:10.1038/ki.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Siener R, Bade DJ, Hesse A, Hoppe B. Dietary hyper-oxaluria is not reduced by treatment with lactic acid bacteria. J Transl Med. 2013;11:306. doi: 10.1186/1479-5876-11-306. doi:10.1186/1479-5876-11-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ellis ML, Shaw KJ, Jackson SB, Daniel SL, Knight J. Analysis of commercial kidney stone probiotic supplements. Urology. 2015;85(3):517–521. doi: 10.1016/j.urology.2014.11.013. doi:10.1016/j.urology.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hassan H, Arvans D, Cheng M, Musch MW, Chang EB. Oxalobacter formigenes conditioned medium stimulates oxalate transport by human intestinal cells. J Am Soc Nephrol. 2011;22:383A. (Abstract) [Google Scholar]
- 171.Arvans D, Musch M, Chang E, Hassan H, Cheng M. Oxalobacter formigenes conditioned medium stimulates oxalate transport by human intestinal cells. J Investig Med. 2012;60(4):738–738. (Abstract) [Google Scholar]
- 172.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G395–G401. doi: 10.1152/ajpgi.00465.2009. doi:10.1152/ajpgi.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr. 2008;138(7):1355–1359. doi: 10.1093/jn/138.7.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus attenuates down-regulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol. 2014;307(6):G623–G631. doi: 10.1152/ajpgi.00104.2014. doi:10.1152/ajpgi.00104.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Klimesova K, Whittamore JM, Hatch M. Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis. 2015;43(2):107–117. doi: 10.1007/s00240-014-0728-2. doi:10.1007/s00240-014-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, Alrefai WA, Gill RK, Dudeja PK. Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol. 2014;307(12):C1084–C1092. doi: 10.1152/ajpcell.00194.2014. doi:10.1152/ajpcell.00194.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Murphy C, Murphy S, O'Brien F, O'Donoghue M, Boileau T, Sunvold G, Reinhart G, Kiely B, Shanahan F, O'Mahony L. Metabolic activity of probiotics-oxalate degradation. Vet Microbiol. 2009;136(1–2):100–107. doi: 10.1016/j.vetmic.2008.10.005. doi:10.1016/j.vetmic.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 178.Sheldon RJ, Malarchik ME, Fox DA, Burks TF, Porreca F. Pharmacological characterization of neural mechanisms regulating mucosal ion-transport in mouse jejunum. J Pharmacol Exp Ther. 1989;249(2):572–582. [PubMed] [Google Scholar]
- 179.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3− secretion. J Physiol Lond. 1997;505(2):411–423. doi: 10.1111/j.1469-7793.1997.411bb.x. doi:10.1111/j.1469-7793.1997.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.