Abstract
Background
Several observational studies have suggested that outdoor air pollution may induce or aggravate asthma. However, epidemiological results are inconclusive due to the presence of numerous moderators which influence this association. The goal of this study was to assess the relationship between outdoor air pollutants and moderate or severe asthma exacerbations in children and adults through a systematic review and multilevel meta-analysis.
Material and methods
We searched studies published in English on PubMed, Scopus, and Google Scholar between January 2000 and October 2016. Studies following a case-crossover design with records of emergency departments and/or hospital admissions as a surrogate of moderate or severe asthma exacerbations were selected. A multilevel meta-analysis was employed, taking into account the potential clustering effects within studies examining more than one lag. Odds ratios (ORs) and 95% confidence intervals were estimated. A subgroup analysis in children aged 0 to 18 years and a sensitivity analysis based on the quality of the included studies as defined in the Newcastle-Ottawa Scale were performed. Publication bias was evaluated through visual inspection of funnel plots and by a complementary search of grey literature. (Prospero Registration number CRD42015032323).
Results
Database searches retrieved 208 records, and finally 22 studies were selected for quantitative analysis. All pollutants except SO2 and PM10 showed a significant association with asthma exacerbations (NO2: 1.024; 95% CI: 1.005,1.043, SO2: 1.039; 95% CI: 0.988,1.094), PM10: 1.024; 95% CI: 0.995,1.053, PM2.5: 1.028; 95% CI: 1.009,1.047, CO: 1.045; 95% CI: 1.005,1.086, O3: 1.032; 95% CI: 1.005,1.060. In children, the association was significant for NO2, SO2 and PM2.5.
Conclusion
This meta-analysis provides evidence of the association between selected air pollutants and asthma exacerbations for different lags.
Introduction
Asthma can be defined as a chronic inflammatory disorder of the airways associated with bronchial hyper-responsiveness, reversible airflow limitation and recurrent symptoms of wheezing, chest tightness, and cough [1]. Worldwide, asthma accounts for nearly 1% of all disability adjusted life years (DALYs) lost [2]. According to recent estimates, as many as 623 million people are currently living with some level of asthma-related symptoms [3], while 250,000 deaths can be attributed to this disease each year [4]. Economic losses due to asthma are estimated to be the highest among patients with chronic diseases due to significant healthcare utilization. Hospitalization and medications are the most important associated direct costs, while work and school absenteeism account for the greatest percentage of indirect costs [5]. On the other hand, asthma exacerbations are common in asthmatic patients [6], and the main goal of asthma treatment is the prevention of exacerbations and fixed airflow limitation [7].
Asthma exacerbations can be classified as mild, moderate, and severe, with the latter two generally requiring an emergency department visit and likely hospitalization [8]. In children, several risk factors for asthma exacerbations have been identified, including poor asthma control, individual susceptibility, viral infections, allergen exposure, environmental tobacco smoke (ETS) exposure, and outdoor air pollution [9].
Several studies have confirmed that air pollution from ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2), and particulate matter (PM) may induce or aggravate asthma [10]. A large number of observational studies have been conducted to assess the effect of air pollutants in asthma prevalence, incidence, and exacerbations. According to a number of published meta-analysis [11,12,13,14], these pollutants in the atmosphere are associated with higher incidence, prevalence, hospitalizations, or worsening of symptoms of asthma. Regarding the short-term effects of air pollutants in terms of exacerbations or worsening of symptoms, three studies have found an association with PM, NO2, SO2, carbon monoxide (CO) and O3 [15,16,17]. In one of these studies analyzing data from Asia, the association was not significant in people aged 15–64 years [17].
The present study provides complementary results to these previous meta-analyses, with two main methodological differences. First, our study has estimated a pooled association measure considering all lag times between air pollution increase and asthma exacerbations for each individual study, using a multilevel analysis. In contrast, previous meta-analyses either used only one lag for study, selecting them through predetermined rules of choice, or made subgroup analysis of specific lags. Second, our meta-analysis selected only studies following case-crossover designs, while previous meta-analyses included all time-series designs. The pros and cons of these choices are examined in the discussion section. Thus, the goal of this study was to assess the association between the increase in concentration of outdoor air pollutants and moderate or severe asthma exacerbations in children and adults, through a systematic review, multilevel meta-analysis, and meta-regression of case-crossover studies, pooling all the lag times and taking into account potential moderators.
Materials and methods
We prepared this article according to the PRISMA guidelines for systematic reviews and meta-analysis [18] (S1 Table). The protocol for this study was registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/) under registration number CRD42015032323 before the formal screening of search results (S1 File). A pilot of the study was previously carried out to adjust the search strategy.
Search strategy and sources
We searched studies published in English in PubMed, Scopus, and Google Scholar between January 2000 and October 2016. The searched studies should explore the relationship between outdoor air pollution and acute exacerbations of asthma in children and adults through a case-crossover observational design. Moderate and severe exacerbations were represented as visits to emergency departments or hospitalizations by this cause. A combination of the following terms combined using Boolean connectors was used: “asthma”, “wheeze”, “pollut*”, “contamin*”, “hospitaliz*”, “admission*”, “emergenc*”, “attack*”, “case crossover”. A detailed description of the search strategy in PubMed, Scopus, and Google Scholar is shown in the supplementary material (S2 Table). In addition, we have manually searched in reference lists from other systematic reviews in order to find additional studies.
Study selection
Two reviewers (PO and JR) independently screened all records retrieved from database searches in three stages. First they searched the assessment of titles, second the assessment of abstracts, and finally they screened the assessment of full-text articles. Any disagreement was resolved by consensus with the help of a third reviewer (BB). The inclusion criteria for the retrieved articles were: (1) studies following a case-crossover design; (2) assessment of outdoor air pollution represented by NO2, SO2, PM10, PM2.5, CO and O3; (3) studies working with records of emergency departments or hospital admissions as a surrogate of moderate or severe asthma exacerbations; (4) studies reporting odds ratio (OR) and 95% confidence intervals (95%CI) as the measure of association; and (5) articles published as a full-text document.
Data extraction
Data were extracted by means of a data extraction form developed in Microsoft Excel®. These data included: (1) first author and year of publication; (2) participant’s age and gender; (3) OR and 95% confidence intervals (95%CI); (4) the lags, understood as the time distance between the increase of the selected pollutant and the date of the asthma exacerbation. Other variables considered were latitude and elevation of the study sites. These variables were used in the meta-regression analysis. In the case of multicity studies, a separate OR was extracted for each city. When this information was not available, the data was considered as missing.
Risk of bias and quality assessment
Two reviewers (PO and JR) independently evaluated the methodological quality of each selected study, in order to determine the risk of bias. The Newcastle–Ottawa scale (NOS) for case-control studies was used as a measure of the quality of individual studies [19]. In this scale, there are three dimensions: selection of the study group, comparability of the groups, and exposure ascertainment. A total of seven questions are raised, with a minimum of zero and a maximum of nine stars. Study quality is then graded as poor (1–3 stars), intermediate (4–6 stars), or high (7–9 stars). An explanation of each question adapted for this study is presented in the supporting material (S3 Table). The potential risk of publication bias was evaluated by visual examination of funnel plots asymmetry [20]. In addition, a grey literature search was performed in order to identify studies from conference proceedings and other sources that may have had null results and were not published as articles in journals. The sources for the grey literature search were Google Scholar, Scopus, Biomed Central (http://www.biomedcentral.com) and NLM Gateway (https://gateway.nlm.nih.gov/gw/Cmd).
Sensitivity analysis
We performed a sensitivity analysis based on the quality of the included studies as defined in the NOS. Studies classified with 6 or more stars (intermediate-high quality) were selected for the sensitivity analysis.
Statistical analysis
The ORs and 95%CIs derived from single-pollutant models were retrieved and weighted based on the inverse of the variance method, assuming that observations with lower variances should be given more weight in the analysis. Where necessary, the coefficient estimates were recalculated to reflect a 10-μg/m3 increase in PM10 and PM2.5, a 10 ppb increase in NO2, SO2, and O3, and a 1 ppm increase in CO, assuming a linear relationship within the considered range. The majority of studies contributed with more than one lag between the increases in pollutant concentration up to the date of the asthma exacerbation. Accordingly, we used two or more effect sizes from each study, one for each lag. Thus, a multilevel meta-analytic model was employed, taking into account the potential clustering effects within studies examining more than one lag at a time. Other meta-analyses considered only one lag per study using a preset rule. On the contrary, the multilevel meta-analysis allows for the hierarchical structure of the data, in which lags are nested within studies. The multilevel model was then extended using a multilevel meta-regression model to test the modifying effect of the lag and other moderators, i.e. the latitude and elevation. The heterogeneity was measured with the QE-test for residual heterogeneity, which tests whether the variability in the observed effect sizes that is not accounted for by the moderators is larger than one would expect based on sampling variability [21]. A subgroup analysis in children aged 0 to 18 years was also performed, to evaluate the possible association in this age group. All analyses were performed using the “metafor” package (version 1.9–4) [21] in the statistical software R, version 3.2.2 (https://www.r-project.org/) [22]. The latitude and elevation of the cities were obtained through packages “ggmap” [23] and “weatherr” [24] from the same software.
Results
Characteristics and quality of studies
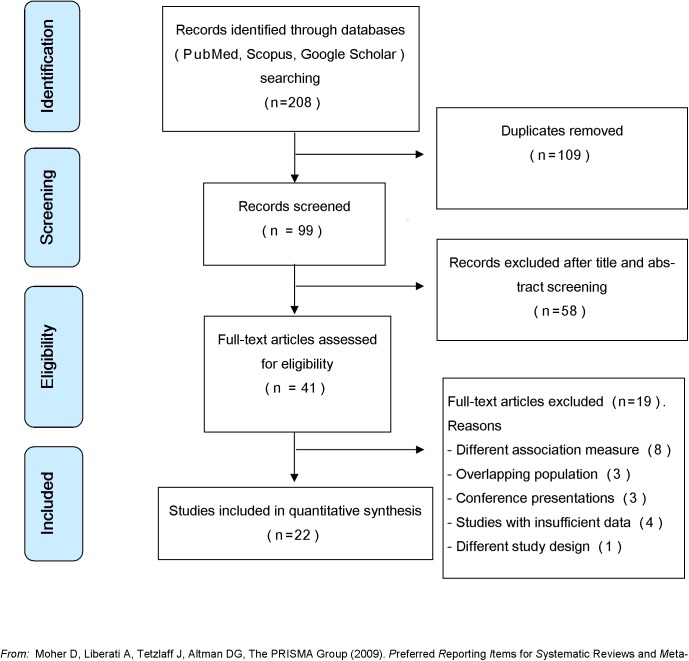
Database searches retrieved 208 records. After removing duplicates, title and abstract screen on 99 records were completed by reviewers, and 41 articles were selected for full-text eligibility assessment. Nineteen studies were excluded due to different reasons (Fig 1). Finally, 22 studies were selected for quantitative analysis (Fig 1), representing 267,413 visits to emergency departments, emergency calls, or hospitalizations due to asthma exacerbations in all age groups.
Fig 1. PRISMA flowchart of the study identification and selection process.
Of these studies, 12 analyzed only children and one analyzed only adults. The outcomes were hospitalizations (seven), emergency department visits (10), both (four) or emergency telephone calls (one). Studies were carried out in 12 countries. 10 were classified as high-income countries, and two were classified as upper-middle-income countries according to the World Bank classification [25]. The elevation of the cities where the studies were carried out were between eight and 1,134 meters over the sea level, and absolute latitudes were between 25° and 54°, while tropical climate zones were not represented. A general description of each included study can be seen in Table 1.
Table 1. Characteristics of the included studies.
| First author, year | Country | Country classification | N | Ages (years) | Pollutants | NOS scale | Ref. |
|---|---|---|---|---|---|---|---|
| Alman, 2016 | USA | High-income | 1,136 | 0–≥ 65 | PM2.5 | 5 | [26] |
| Canova, 2012 | UK | High-income | 234 | 18–≥ 75 | PM10 | 7 | [27] |
| Chen, 2013 | Taiwan | High-income | 1,912 | 5–15 | PM2.5, O3 | 6 | [28] |
| Ding, 2016 | China | Upper-middle-income | 2,507 | 0–18 | NO2, SO2, PM10, PM2.5, CO, O3 | 5 | [29] |
| Glad, 2012 | USA | High-income | 6,979 | 0–≥ 75 | PM2.5 | 6 | [30] |
| Gleason, 2014 | USA | High-income | 21,854 | 3–17 | PM2.5, O3 | 6 | [31] |
| Grineski, 2011 | USA | High-income | 3,504 | 1–≥ 65 | NO2, PM2.5 | 6 | [32] |
| Iskandar, 2012 | Denmark | High-income | 8,226 | 0–18 | NO2, PM10, PM2.5 | 5 | [33] |
| Laurent, 2008 | France | High-income | 4,677 | 0–≥ 65 | NO2, SO2, PM10, O3 | 5 | [34] |
| Lavigne, 2012 | Canada | High-income | 3,728 | 2–≥ 60 | NO2, SO2, PM2.5, CO, O3 | 6 | [35] |
| Lewin, 2013 | Canada | High-income | 429 | 0–4 | SO2, PM2.5 | 5 | [36] |
| Li, 2011 | USA | High-income | 7,063 | 2–18 | NO2, SO2, PM2.5, CO | 6 | [37] |
| Lin, 2003 | Canada | High-income | 7,319 | 6–12 | NO2, SO2, CO, O3 | 5 | [38] |
| Pereira, 2010 | Australia | High-income | 603 | 0–19 | NO2, CO | 5 | [39] |
| Sacks, 2014 | USA | High-income | 121,621 | 0–≥ 65 | O3 | 6 | [40] |
| Santus, 2012 | Italy | High-income | 3,579 | 0–≥ 75 | NO2, SO2, PM10, PM2.5, CO, O3 | 5 | [41] |
| Smargiassi, 2009 | Canada | High-income | 1,842 | 2–4 | SO2 | 6 | [42] |
| Sunyer, 2002 | Spain | High-income | 4,635 | 14–≥ 80 | NO2, SO2, PM10, CO, O3 | 8 | [43] |
| Tecer, 2008 | Turkey | Upper-middle-income | 2,779 | 0–14 | PM10, PM2.5 | 5 | [44] |
| Ueda 2010 | Japan | High-income | 3,427 | 0 – 12 | NO2, SO2, PM10 | 5 | [45] |
| Villeneuve 2007 | Canada | High-income | 57,912 | 2–≥ 75 | NO2, SO2, PM10, PM2.5, O3 | 6 | [46] |
| Yamazaki 2015 | Japan | High-income | 1,447 | 0–14 | NO2, PM10, PM2.5, O3 | 5 | [47] |
NO2: nitrogen dioxide, SO2: sulfur dioxide, O3: ozone, CO: carbon monoxide, PM10: particulate matter < 10 μm, PM2.5: particulate matter < 2.5 μm, N: number of emergency department visits, hospitalizations or participants, NOS scale: Newcastle-Ottawa scale.
The lags considered included single-day lags (0 to 6 days) and cumulative lags (1 to 6 days moving average) before the date of the event. The majority of studies assessed the effect of more than one pollutant (16 studies), while eight studies considered two or multiple pollutant models in addition to single pollutant models. All studies employed generalized linear model (GLM) techniques to estimate the regressions, but two studies also used a generalized additive model (GAM). The other variables considered for adjusting the regressions were temperature, relative humidity, dew point, barometric pressure, wind speed, global radiation, cloudiness, and one study took into account the influenza and soybean asthma outbreaks, while four studies failed to consider the adjustment for other variables.
According to the NOS, 20 studies were classified as intermediate quality, and the remaining two studies were classified as high quality. The two main problems related to studies’ quality were the adequate case definition and the ascertainment of exposure, because in most articles there was no independent validation and the residential address of cases was not verified.
Data preprocessing, heterogeneity, and publication bias
In quantitative analysis, the 22 studies contributed with 345 effect sizes corresponding to NO2 (68), SO2 (60), PM10 (44), PM2.5 (68), CO (50) and O3 (55) were included.
Based on the results of the meta-regression, the lag significantly affected the relationship between the increases in pollutant concentrations and severe asthma exacerbations for the NO2 and the O3 (Table 2). For SO2, PM10, PM2.5 and CO, the lag had no influence on the associations. Other moderators that influenced these associations were the elevation over the sea level for the PM10 and the latitude for the PM10, PM2.5 and CO (Table 2). The QE for each pollutant showed a significant heterogeneity for the SO2, PM10, PM2.5, and O3, which could possibly indicate that other moderators not considered in the models are influencing the association between these pollutants and the occurrence of asthma exacerbations (Table 2).
Table 2. Multilevel meta-regression analysis.
| Pollutant | QE (P-value) | Moderator | P-value |
|---|---|---|---|
| NO2 | 0.33 | Lag | <0.01 |
| Latitude | 0.80 | ||
| Elevation | 0.14 | ||
| SO2 | 0.02 | Lag | 0.36 |
| Latitude | 0.51 | ||
| Elevation | 0.16 | ||
| PM10 | <0.01 | Lag | 0.4 |
| Latitude | <0.01 | ||
| Elevation | <0.01 | ||
| PM2.5 | <0.01 | Lag | 0.76 |
| Latitude | 0.01 | ||
| Elevation | 0.81 | ||
| CO | 0.85 | Lag | 0.39 |
| Latitude | 0.01 | ||
| Elevation | 0.55 | ||
| O3 | 0.03 | Lag | <0.01 |
| Latitude | 0.22 | ||
| Elevation | 0.54 |
NO2: nitrogen dioxide; SO2: sulfur dioxide; O3: ozone; CO: carbon monoxide; PM10: particulate matter < 10 μm; PM2.5: particulate matter < 2.5 μm; QE: test for residual heterogeneity.
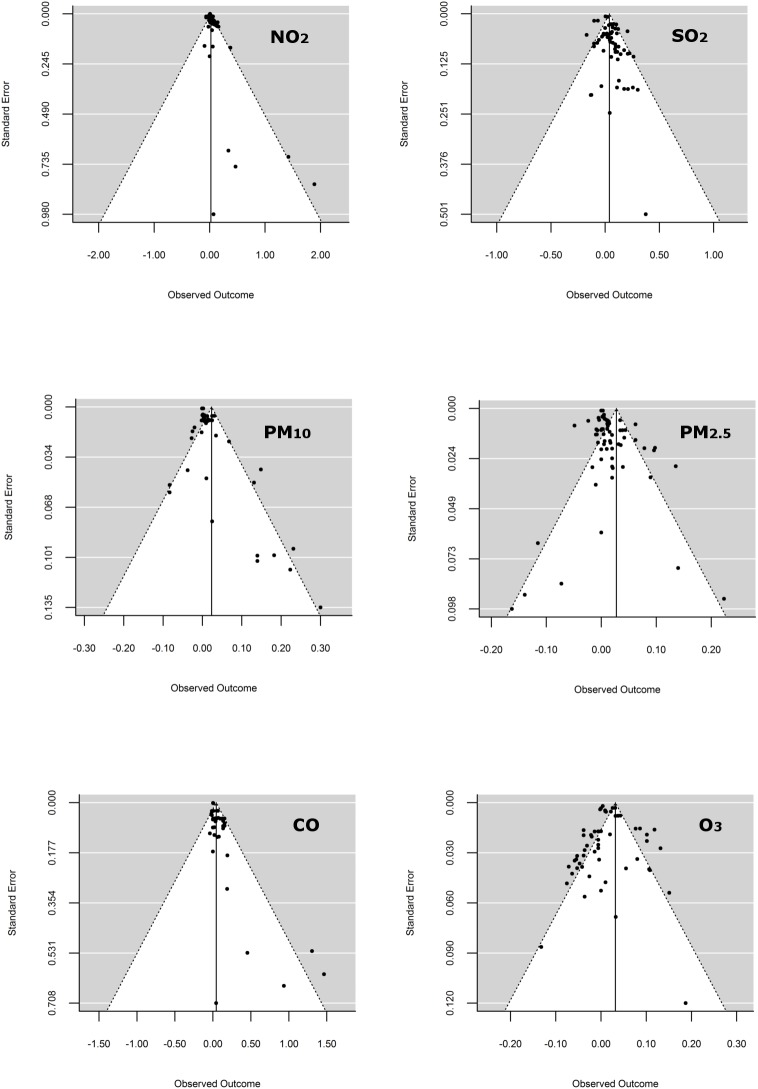
An asymmetry in data points can be observed for NO2, PM10, and CO, where the absence of negative outcomes produced by less precise studies suggested potential publication biases (Fig 2).
Fig 2. Funnel plot to explore publication bias for each pollutant.
The figure shows the observed outcomes (Log odds ratios) on the horizontal axis against their corresponding standard errors.
The plots show the observed outcomes (Log odds ratios) on the horizontal axis against their corresponding standard errors for nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), carbon monoxide (CO), particulate matter < 10 μm (PM10) and particulate matter < 2.5 μm (PM2.5). The search of grey literature identified seven additional studies not included in our analysis; five were conference abstracts [48–52] and two were articles published in local languages (Italian and Korean) [53,54]. In none of these studies the association of pollutants with asthma exacerbations was negative, null or non-significant.
Association between pollutants and asthma exacerbations
The only pollutants that did not show a significant association with asthma exacerbations within the considered lags were SO2 (OR: 1.039; 95% CI: 0.988,1.094) and PM10 (OR: 1.024; 95% CI: 0.995,1.053), while this association was significant for NO2 (OR: 1.024; 95% CI: 1.005,1.043), PM2.5 (OR: 1.028; 95% CI: 1.009,1.047), CO (OR: 1.045; 95% CI: 1.005,1.086), and O3 (OR: 1.032; 95% CI: 1.005,1.060). Figures in S2 File show forest plots with adjusted ORs and their 95%CI for the associations between pollutants and asthma exacerbations, as reported in each original article. In the subgroup analysis of children aged 0 to 18 years, the association was significant for NO2 (OR: 1.040; 95% CI: 1.001,1.081), SO2 (OR: 1.047; 95% CI: 1.009,1.086), and PM2.5 (OR: 1.022; 95% CI: 1.000,1.045). A subgroup analysis of adults was not performed as only one study was focused exclusively on this age group, while few studies reported results of adults separately from those of children.
Sensitivity analysis
For the sensitivity analysis, 11 studies with a NOS scale ≥ 6 (intermediate-high quality) were selected, representing 113 effect sizes. According to these analyses, the association was significant for NO2 (OR: 1.009; 95% CI: 1.004,1.013), PM2.5 (OR: 1.023; 95% CI: 1.006,1.041) and O3 (OR: 1.020; 95% CI: 1.013,1.027). Heterogeneity could be controlled after the sensitivity analysis for all pollutants (S4 Table). Publication bias in this subanalysis was not evaluated due to the more reduced sample size.
Discussion
This meta-analysis showed a significant association between the main pollutants, with the exception of SO2 and PM10, and moderate or severe exacerbations of asthma. Moreover, this approach allowed the simultaneous analysis of all lags considered in different studies, obtaining one polled measure of association. In the subgroup analysis of children, the exacerbations were associated with NO2, SO2 and PM2.5. In the sensitivity analysis, where only studies with a NOS scale ≥ 6 were selected, the association was significant for NO2, PM2.5 and O3. The most important outdoor air pollutants are PM, O3, SO2, NO2, CO and Lead (Pb) [55]. The main anthropogenic sources of PM are traffic and transportation, electricity generation and other combustion processes [56]. With respect to gases, the main sources of SO2 in the developed world are primary emissions during energy production or industrial processes [57], while NO2 and CO are principally emitted from fossil fuel combustion in urban environments [58]. Ozone is a secondary pollutant formed by photochemical reactions between sunlight and pollutant precursors, such as nitrogen oxides and volatile organic compounds [59]. Increased pollution exposures have been associated with increased numbers of hospital admissions and emergency-room visits, mainly due to exacerbations of chronic obstructive pulmonary disease (COPD) and asthma [60]. Air pollution may be related to asthma exacerbations through oxidative stress, airway remodeling and inflammation, and sensitization to aeroallergens [61,62]. Further, pulmonary inflammation can indirectly influence the worsening of asthma symptoms by affecting host defenses [63] and enhancing infections with rhinovirus (RV), influenza, and respiratory syncytial virus (RSV), which in turn are considered the main cause of asthma exacerbations [64]. Specifically, O3 exposure causes airway inflammation, airway hyper-responsiveness, and decrements in lung function, while SO2 mainly leads to bronchoconstriction [64] and NO2 probably triggers bronchial inflammation as a precursor of O3 [64]. On the other hand, exposure to PM might cause oxidative stress, airway hyper-responsiveness, and airway remodeling, either alone or in combination with allergic sensitization [65]. In the atmosphere, different PM sizes can be found. The coarse fraction (PM10–2.5) can penetrate into the upper airways, but the fine fraction (PM2.5-1) can be deposited in the lung, especially in the alveoli, although it could pass to the systemic circulation [63]. It should be noted that besides the size of PM, the chemical composition is very important to understand the health effects of particulate matter. There are also differences in the individual susceptibility to air pollutants. Children are more affected than adults and boys more affected than girls, while a diet high in fruits and vegetables and of antioxidant vitamin supplements could be a protective factor, and obesity might increase susceptibility to the adverse effects of air pollution [62].
In this article, the heterogeneity of the included studies was significant for SO2, PM10, PM2.5 and O3, and could not be completely controlled by the modifying effect of the moderators that were considered here, including the lag, the elevation and the latitude of the cities that were under analysis. It is possible that other effect modifiers, which were not available in the included articles, could have influenced these results. However, the heterogeneity was resolved for all pollutants in the sensitivity analysis.
The funnel plots showed a degree of asymmetry for the NO2, the PM10 and the CO. This asymmetry may suggest that small studies showing no statistically significant effects remain unpublished, and then the true effect could be overestimated [66]. Another possible explanation could be that small studies may have weaker methodological quality than larger studies, leading to an overestimation of the true effects [67]. Regarding the first possibility, our complementary search of grey literature allowed us to identify seven additional studies with the same design and objectives. However, in these studies the association between air pollutants and asthma exacerbations was positive and significant, meaning that the reason to be unpublished in journals was not related to null results. As to the second possible explanation, for NO2 and CO all the effect sizes that presented the highest standard errors came from a single study [39] that showed a low value (5 stars) of the NOS scale. Nonetheless, for PM10 the effect sizes that showed the maximum values of standard errors came from one study that was classified as high-quality [27] according to the NOS scale. It is worth noting that this study was the one that included only adults. Taking these results into consideration, it is possible that the asymmetry of funnel plots showed by the NO2 and the CO can be due to a weak methodological quality of one study which contributes with 5 observations, and not to publication bias. On the other hand, for PM10 the funnel plot asymmetry does not seem to be related to methodological quality, and thus publication bias cannot be ruled out.
In general, the relatively small risks and ORs detected through this meta-analysis may lead one to assume that the potential effect of outdoor air pollutants at a population level is negligible, and thus the impact of public health measures could be dismissed. However, nearly 300 million people suffer from asthma globally [4]. A large amount of people are susceptible to moderate and severe exacerbations related to outdoor air pollution, and a very large number of people are exposed to outdoor pollutants, for example by living near polluted roads. Considering these two facts, a combination of small relative risks and high prevalence of exposure can contribute to a moderate population attributable fraction. Thus, a public health intervention aimed at mitigating the effects of air pollutants and targeted to the entire population might have significant benefits for the society.
This study had several limitations. The first was the use of non-randomized observational studies that failed to control bias due to the confounding effects of several factors. The second limitation was that the majority of studies which were included used secondary data sources for the asthma hospitalizations and emergency visits. These two points do not allow the control of confounding factors, for example by controlling the change in medications or the exposure to secondhand smoke in the evaluated cases. A third limitation was that air pollutant concentrations were estimated in a different way in different studies, such as using alternative models or an unequal number of monitoring sites. Besides, the lags considered were often different, and these points would have caused the high heterogeneity between studies. The limited number of moderators could be considered the fourth limitation, as there are probably several other factors that modify the association between air pollutants and asthma exacerbations. One of these factors is the country income: almost all studies were conducted in high-income countries, and accordingly the effect of these pollutants in the asthma exacerbations could not be extrapolated to developing countries. Finally, this article only considered studies following a case-crossover design. This might be seen as a limitation in the sense that a number of studies have likely been excluded from the analysis, particularly time-series studies. The equivalence of results obtained from time-series Poisson regression and from case-crossover studies using conditional logistic regression has been proposed by Lu et al. [68]. However, there exist at least three differences between these methods. First, case-crossover designs appear to be less efficient than Poisson time-series designs, i.e. showing lower statistical power [69–71]. Second, case-crossover designs are less vulnerable to bias [69–71] and, unlike time-series analyses, individual data can be included to estimate effect modifications and to control for confounders by design [72]. Third, time-series regression based on generalized additive models involves several arbitrary decisions, e.g. the type of smoother or the number of degrees of freedom [69]. Carracedo-Martínez et al. [72] performed a thorough review of the literature comparing these methodologies. Taking these points into consideration, both study designs are potentially exposed to different biases, are subject to other assumptions, and display a dissimilar efficiency. Accordingly, we consider that our choice of a unique study design could be considered as a major strength, because it enables the obtention of more compatible and precise association values. However, our results should be interpreted as complementary and not antagonistic to other broader studies that included both case-crossover and time-series designs.
Conclusions
In conclusion, this meta-analysis provides evidence of the association between major air pollutants and moderate or severe asthma exacerbations. Moreover, this study proposes a methodological approach to obtain a single association value pooling different lags and studies, through the use of a multilevel meta-analytic model. Other similar studies should be carried out to confirm or discard the present findings, to update these results, and to achieve more accurate association values. This article also highlights the importance of confounders in the association of air pollutants and asthma exacerbations. The implications of these results for public health interventions and individual prevention are also suggested. Finally, it was pointed out that almost all observational studies included in this meta-analysis come from developed countries, and therefore extrapolating these results to developing countries requires caution. In this sense, the development of similar studies should be promoted to evaluate the associations between air pollutants and asthma under different income scenarios.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors would like to thank the anonymous reviewers and the academic editor for their insightful suggestions and support provided during the editorial process.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study did not receive any specific funding, but was supported by the research budget of the Universidad Tecnológica Nacional, Facultad Regional San Nicolas. PO is supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and NQ by the Comisión de Investigaciones Científicas (CIC).
References
- 1.Mathew J, Aronow WS, Chandy D. Therapeutic options for severe asthma. Arch Med Sci. 2012; 8:589–97. 10.5114/aoms.2012.30280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R; Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–78. 10.1111/j.1398-9995.2004.00526.x [DOI] [PubMed] [Google Scholar]
- 3.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012; 12:204 10.1186/1471-2458-12-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Globe G, Martin M, Schatz M, Wiklund I, Lin J, von Maltzahn R, et al. Symptoms and markers of symptom severity in asthma—content validity of the asthma symptom diary. Health Qual Life Outcomes. 2015; 13:21 10.1186/s12955-015-0217-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009; 9:24 10.1186/1471-2466-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Byrne PM. Therapeutic strategies to reduce asthma exacerbations. J Allergy Clin Immunol. 2011; 128:257–63. 10.1016/j.jaci.2011.03.035 [DOI] [PubMed] [Google Scholar]
- 7.Bostantzoglou C, Delimpoura V, Samitas K, Zervas E, Kanniess F, Gaga M. Clinical asthma phenotypes in the real world: opportunities and challenges. Breathe (Sheff). 2015; 11:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollart SM, Compton RM, Elward KS. Management of acute asthma exacerbations. Am Fam Physician. 2011; 84:40–7. [PubMed] [Google Scholar]
- 9.Forno E, Celedón JC. Predicting asthma exacerbations in children. Curr Opin Pulm Med. 2012; 18:63–9. 10.1097/MCP.0b013e32834db288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spann K, Snape N, Baturcam E, Fantino E. The Impact of Early-Life Exposure to Air-borne Environmental Insults on the Function of the Airway Epithelium in Asthma. Ann Glob Health. 2016; 82(1):28–40. 10.1016/j.aogh.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 11.Romeo E, De Sario M, Forastiere F, Compagnucci P, Stafoggia M, Bergamaschi A, et al. PM 10 exposure and asthma exacerbations in pediatric age: a meta-analysis of panel and time-series studies. Epidemiol Prev. 2006; 30:245–54. [PubMed] [Google Scholar]
- 12.Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res. 2012; 117:36–45. 10.1016/j.envres.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Barone-Adesi F, Dent JE, Dajnak D, Beevers S, Anderson HR, Kelly FJ, et al. Long-Term Exposure to Primary Traffic Pollutants and Lung Function in Children: Cross-Sectional Study and Meta-Analysis. PLoS One. 2015; 10:e0142565 10.1371/journal.pone.0142565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015; 70:245–56. 10.1111/all.12561 [DOI] [PubMed] [Google Scholar]
- 15.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010; 118:449–57. 10.1289/ehp.0900844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng XY, Ding H, Jiang LN, Chen SW, Zheng JP, Qiu M, et al. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLoS One. 2015; 10:e0138146 10.1371/journal.pone.0138146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Li G, Tian L, Guo Q, Pan X. Short-term exposure to air pollution and morbidity of COPD and asthma in East Asian area: A systematic review and meta-analysis. Environ Res. 2016; 148:15–23. 10.1016/j.envres.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 18.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011; 39:91–2. 10.1016/j.jcms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O’ Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale for assessing quality if nonrandomized studies in meta-analyses. 2009. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 20.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343:d4002 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 21.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010; 36: 1–48. [Google Scholar]
- 22.Crawley MJ. The R book. 2nd ed. Chichester: Wiley; 2013. [Google Scholar]
- 23.Kahle D, Wickham H. Package ‘ggmap’. 2015. Available: https://cran.r-project.org/web/packages/ggmap/ggmap.pdf.
- 24.Yip S. Package ‘weatherr’. 2016. Available: https://cran.r-project.org/web/packages/weatherr/weatherr.pdf.
- 25.The World Bank. Country and Lending Groups. 2016. Available: http://data.worldbank.org/about/country-and-lending-groups.
- 26.Alman BL, Pfister G, Hao H, Stowell J, Hu X, Liu Y, et al. The association of wildfire smoke with respiratory and cardiovascular emergency department visits in Colorado in 2012: a case crossover study. Environ Health. 2016; 15(1):64 10.1186/s12940-016-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canova C, Dunster C, Kelly FJ, Minelli C, Shah PL, Caneja C, et al. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: the modifying effect of individual characteristics. Epidemiology. 2012; 23:607–15. 10.1097/EDE.0b013e3182572563 [DOI] [PubMed] [Google Scholar]
- 28.Chen BY, Chen CH, Chen PC, Wang GS, Guo YL. Air pollution, allergic co-morbidity, and emergency department visit for pediatric asthma in Taiwan. Aerosol Air Qual Res. 2013; 13:1847–52. [Google Scholar]
- 29.Ding L, Zhu D, Peng D, Zhao Y. Air pollution and asthma attacks in children: A case-crossover analysis in the city of Chongqing, China. Environ Pollut. 2016. pii: S0269-7491(16)31449-X. [DOI] [PubMed] [Google Scholar]
- 30.Glad JA, Brink LL, Talbott EO, Lee PC, Xu X, Saul M, et al. The relationship of ambient ozone and PM(2.5) levels and asthma emergency department visits: possible influence of gender and ethnicity. Arch Environ Occup Health. 2012; 67:103–8. 10.1080/19338244.2011.598888 [DOI] [PubMed] [Google Scholar]
- 31.Gleason JA, Bielory L, Fagliano JA. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: a case-crossover study. Environ Res. 2014; 132:421–9. 10.1016/j.envres.2014.03.035 [DOI] [PubMed] [Google Scholar]
- 32.Grineski SE, Staniswalis JG, Bulathsinhala P, Peng Y, Gill TE. Hospital admissions for asthma and acute bronchitis in El Paso, Texas: do age, sex, and insurance status modify the effects of dust and low wind events? Environ Res. 2011; 111:1148–55. 10.1016/j.envres.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iskandar A, Andersen ZJ, Bønnelykke K, Ellermann T, Andersen KK, Bisgaard H. Coarse and fine particles but not ultrafine particles in urban air trigger hospital admission for asthma in children. Thorax. 2012; 67:252–7. 10.1136/thoraxjnl-2011-200324 [DOI] [PubMed] [Google Scholar]
- 34.Laurent O, Pedrono G, Segala C, Filleul L, Havard S, Deguen S, et al. Air pollution, asthma attacks, and socioeconomic deprivation: a small-area case-crossover study. Am J Epidemiol. 2008; 168:58–65. 10.1093/aje/kwn087 [DOI] [PubMed] [Google Scholar]
- 35.Lavigne E, Villeneuve PJ, Cakmak S. Air pollution and emergency department visits for asthma in Windsor, Canada. Can J Public Health. 2012; 103:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewin A, Buteau S, Brand A, Kosatsky T, Smargiassi A. Short-term risk of hospitalization for asthma or bronchiolitis in children living near an aluminum smelter. J Expo Sci Environ Epidemiol. 2013; 23:474–80. 10.1038/jes.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Batterman S, Wasilevich E, Wahl R, Wirth J, Su FC, et al. Association of daily asthma emergency department visits and hospital admissions with ambient air pollutants among the pediatric Medicaid population in Detroit: time-series and time-stratified case-crossover analyses with threshold effects. Environ Res. 2011; 111:1137–47. 10.1016/j.envres.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 38.Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. Effect of short-term exposure to gaseous pollution on asthma hospitalisation in children: a bi-directional case-crossover analysis. J Epidemiol Community Health. 2003; 57:50–5. 10.1136/jech.57.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira G, Cook A, De Vos AJ, Holman CD. A case-crossover analysis of traffic-related air pollution and emergency department presentations for asthma in Perth, Western Australia. Med J Aust. 2010; 193:511–4. [DOI] [PubMed] [Google Scholar]
- 40.Sacks JD, Rappold AG, Davis JA Jr, Richardson DB, Waller AE, Luben TJ. Influence of urbanicity and county characteristics on the association between ozone and asthma emergency department visits in North Carolina. Environ Health Perspect. 2014; 122:506–12. 10.1289/ehp.1306940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santus P, Russo A, Madonini E, Allegra L, Blasi F, Centanni S, et al. How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Respir Res. 2012; 13:95 10.1186/1465-9921-13-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smargiassi A, Kosatsky T, Hicks J, Plante C, Armstrong B, Villeneuve PJ, et al. Risk of asthmatic episodes in children exposed to sulfur dioxide stack emissions from a refinery point source in Montreal, Canada. Environ Health Perspect. 2009; 117:653–9. 10.1289/ehp.0800010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunyer J, Basagaña X, Belmonte J, Antó JM. Effect of nitrogen dioxide and ozone on the risk of dying in patients with severe asthma. Thorax. 2002; 57:687–93. 10.1136/thorax.57.8.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tecer LH, Alagha O, Karaca F, Tuncel G, Eldes N. Particulate matter (PM(2.5), PM(10-2.5), and PM(10)) and children's hospital admissions for asthma and respiratory diseases: a bidirectional case-crossover study. J Toxicol Environ Health A. 2008; 71:512–20. 10.1080/15287390801907459 [DOI] [PubMed] [Google Scholar]
- 45.Ueda K, Nitta H, Odajima H. The effects of weather, air pollutants, and Asian dust on hospitalization for asthma in Fukuoka. Environ Health Prev Med. 2010; 15:350–7. 10.1007/s12199-010-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villeneuve PJ, Chen L, Rowe BH, Coates F. Outdoor air pollution and emergency department visits for asthma among children and adults: a case-crossover study in northern Alberta, Canada. Environ Health. 2007; 6:40 10.1186/1476-069X-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki S, Shima M, Yoda Y, Oka K, Kurosaka F, Shimizu S, et al. Exposure to air pollution and meteorological factors associated with children's primary care visits at night due to asthma attack: case-crossover design for 3-year pooled patients. BMJ Open. 2015; 5:e005736 10.1136/bmjopen-2014-005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Im HJ, Lee SY, Yun KJ, Ju YS, Kang DH, Cho SH. A case-crossover study between air pollution and hospital emergency room visits by asthma attack. Korean Journal of Occupational and Environmental Medicine. 2000; 12(2):249–57. [Google Scholar]
- 49.Jalaludin B, Khalaj B, Sheppeard V, Morgan G. Effects of ambient air pollution on asthma visits by children to emergency department, Sydney, Australia. Epidemiology. 2005; 16(5):S57–8. [Google Scholar]
- 50.Strickland M, Darrow L, Klein M, Sarnat J, Flanders WD, Sarnat S, et al. Acute associations between ambient air pollution and pediatric asthma emergency department visits in Atlanta, 1993–2004. Epidemiology. 2009; 20(6):S163. [Google Scholar]
- 51.Iskandar A, Andersen ZJ, Bønnelykke K, Andersen K, Bisgaard H. Air pollution and asthma hospitalizations in children. European Respiratory Journal. 2011; 38(Suppl 55):p1131. [Google Scholar]
- 52.Tecer LH. The effects of air pollution and local meteorology on children’s respiratory health: a case-crossover study in Balikesir. 11th International Multidisciplinary Scientific GeoConference SGEM2011. 2011; 2:1263-70.
- 53.Lee JT. Associations between Air Pollution and Asthma-related Hospital Admissions in Children in Seoul, Korea: A Case-crossover Study. Korean Journal of Preventive Medicine. 2003; 36(1):47–53. [Google Scholar]
- 54.Colais P, Serinelli M, Faustini A, Stafoggia M, Randi G, Tessari R, et al. Air pollution and urgent hospital admissions in nine Italian cities. Results of the EpiAir Project. Epidemiologia e prevenzione. 2008; 33(6 Suppl 1):77–94. [PubMed] [Google Scholar]
- 55.Jiang XQ, Mei XD, Feng D. Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis. 2016; 8:E31–40. 10.3978/j.issn.2072-1439.2015.11.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falcon-Rodriguez CI, Osornio-Vargas AR, Sada-Ovalle I, Segura-Medina P. Aeroparticles, Composition, and Lung Diseases. Front Immunol. 2016; 7:3 10.3389/fimmu.2016.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014; 383:1581–92. 10.1016/S0140-6736(14)60617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masiol M, Agostinelli C, Formenton G, Tarabotti E, Pavoni B. Thirteen years of air pollution hourly monitoring in a large city: potential sources, trends, cycles and effects of car-free days. Sci Total Environ. 2014; 494-495:84–96. 10.1016/j.scitotenv.2014.06.122 [DOI] [PubMed] [Google Scholar]
- 59.Arbex MA, Santos Ude P, Martins LC, Saldiva PH, Pereira LA, Braga AL. Air pollution and the respiratory system. J Bras Pneumol. 2012; 38:643–55. [DOI] [PubMed] [Google Scholar]
- 60.Simoni M, Baldacci S, Maio S, Cerrai S, Sarno G, Viegi G. Adverse effects of outdoor pollution in the elderly. J Thorac Dis. 2015; 7:34–45. 10.3978/j.issn.2072-1439.2014.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auerbach A, Hernandez ML. The effect of environmental oxidative stress on airway inflammation. Curr Opin Allergy Clin Immunol. 2012; 12:133–9. 10.1097/ACI.0b013e32835113d6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esposito S, Tenconi R, Lelii M, Preti V, Nazzari E, Consolo S, et al. Possible molecular mechanisms linking air pollution and asthma in children. BMC Pulm Med. 2014; 14:31 10.1186/1471-2466-14-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brugha R, Grigg J. Urban air pollution and respiratory infections. Paediatr Respir Rev. 2014; 15:194–9. 10.1016/j.prrv.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 64.Botturi K, Langelot M, Lair D, Pipet A, Pain M, Chesne J, et al. Preventing asthma exacerbations: what are the targets? Pharmacol Ther. 2011; 131:114–29. 10.1016/j.pharmthera.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 65.Stanek LW, Brown JS, Stanek J, Gift J, Costa DL. Air pollution toxicology—a brief review of the role of the science in shaping the current understanding of air pollution health risks. Toxicol Sci. 2011; 120 Suppl 1:S8–27. [DOI] [PubMed] [Google Scholar]
- 66.Rothstein H, Sutton AJ, Borenstein M. Publication bias in meta-analysis: Prevention, assessment and adjustments. New York: Wiley; 2005. [Google Scholar]
- 67.Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003; 7(1):1–76. [PubMed] [Google Scholar]
- 68.Lu Y, Zeger SL. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics. 2007; 8(2):337–44. 10.1093/biostatistics/kxl013 [DOI] [PubMed] [Google Scholar]
- 69.Bateson TF, Schwartz J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology. 1999; 10(5):539–44. [PubMed] [Google Scholar]
- 70.Fung KY, Krewski D, Chen Y, Burnett R, Cakmak S. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. Int J Epidemiol. 2003; 32(6):1064–70. [DOI] [PubMed] [Google Scholar]
- 71.Figueiras A, Carracedo-Martínez E, Saez M, Taracido M. Analysis of case-crossover designs using longitudinal approaches: a simulation study. Epidemiology. 2005; 16(2):239–46. [DOI] [PubMed] [Google Scholar]
- 72.Carracedo-Martínez E, Taracido M, Tobias A, Saez M, Figueiras A. Case-crossover analysis of air pollution health effects: a systematic review of methodology and application. Environ Health Perspect. 2010; 118(8):1173–82. 10.1289/ehp.0901485 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.