Abstract
Background
Autologous chondrocyte implantation (ACI) is a durable treatment for patients with chondral defects. This study presents the comprehensive evaluation of patients with patella defects treated with ACI at medium- to long-term follow-up.
Methods
Thirty consecutive patients with isolated chondral lesions of the patella were enrolled prospectively. Primary outcome measures were validated patient reported outcome measures and objective magnetic resonance imaging.
Results
Nineteen of 30 patients underwent tibial tubercle osteotomy (TTO) to correct lateral maltracking in combination with soft tissue balancing. The defect sizes were large, averaging 4.7 ± 2.1 cm2 (range 2.2-30.0 cm2). Pidoriano/Fulkerson classification revealed that 3 defects were type II (lateral), 9 were type III (medial), and 18 were type IV (central/panpatella). Age at the time of surgery was 32 ± 10 years. At follow-up of 2 to 14 years, knee function was rated good to excellent in 25 (83%) patients, fair in 4 (13%) patients, and poor in 1 (3%) patient. Three patients failed treatment after a mean of 75 months (6.25 years). All 3 failures were Workers Compensation (WC) cases. They were older than the non-WC patients, 42 ± 6 years compared with the non-WC 28 ± 9 years (P = 0.0019). Significant increases in all clinical and health utility outcome scores were seen. Magnetic resonance imaging demonstrated that the fill grade, surface and integrity of the repair tissue correlated with clinical scores.
Conclusion
ACI to isolated patella defects results in significant functional improvement at a minimum of 24 months, with the results remaining durable at latest follow-up of 15 years.
Level of evidence
Level 4.
Keywords: articular cartilage, tissue, magnetic resonance imaging, diagnostics, chondromalacia, diagnosis, knee, joint involved, cartilage repair, repair
Introduction
Anterior knee pain is a complex problem that most commonly affects young active females.1 Patellofemoral chondral defects are thought to be the primary pain generator.2 In 63% of 31,516 knee arthroscopies, Curl et al.3 detected chondral lesions with an average of 2.7 lesions per knee. Combined patellar and medial femoral condyle lesions were the most common locations. Isolated patellar defects were reported in more than 20% of patients younger than 40 years with grade III Outerbridge chondral lesions. Arøen et al.4 reported an incidence of 11% of ICRS (International Cartilage Repair Society) grade 3 or 4 chondral lesions in 993 consecutive arthroscopies, among which 23% were patellar defects.
Cartilage lesions over 0.9 cm2 are biomechanically unstable5 and may degenerate at an unpredictable rate that ultimately leads to osteoarthritic changes.6,7 Cartilage restoration procedures using a variety of techniques, do less well in patellar defects than condylar defects.8-13 Microfracture has been shown to result in short-term improvement in pain and function with subsequent decline after 18 months, with the unfavorable results occurring for the patella or trochlea.14 Osteochondral allografts have also yielded only 60% good to excellent results in the patella.15 Bentley et al.16 showed poor results for patella osteochondral autografts and subsequently recommended that it be abandoned for patella lesions. More recently, studies have suggested acceptable outcomes with either fresh osteochondral allografting or autologous chondrocyte implantation (ACI).17-19
Our study aims to report on clinical outcome, survivorship of patellar ACI and the correlation between postoperative magnetic resonance imaging and clinical outcome.
Methods and Materials
Between 1995 and 2009, more than 500 patients were treated with ACI (Carticel; Genzyme BioSurgery, Cambridge, MA) at our institution. Institutional review board approval was obtained in March 1995 for prospective evaluation of all patients. Patients provided informed written consent at the time they entered into the database and were followed up with annual validated patient reported outcomes, which included the Short Form–36 (SF-36),20 Western Ontario McMaster Universities Osteoarthritis Index (WOMAC),21 Modified Cincinnati22 activity score ( Table 1 ), Knee Society Score (KSS),23 and a patient satisfaction survey. Thirty consecutive patients had lesions of the patella treated with ACI and formed the cohort of this study.
Table 1.
Modified Cincinnati Score.
| □1 □2 | □3 □4 | □5 □6 | □7 □8 | □9 □10 |
| Poor | Fair | Good | Very Good | Excellent |
|
| ||||
| Poor (1-2) | I have significant limitations that affect activities of daily living. | |||
| Fair (3-4) | I have moderate limitations that affect activities of daily living. No sports possible. | |||
| Good (5-6) | I have some limitations with sports but I can participate; I compensate. | |||
| Very Good (7-8) | I have only a few limitations with sports. | |||
| Excellent (9-10) | I am able to do whatever I wish (any sport) with no problem. | |||
Indications
Autologous chondrocyte implantation was considered as a treatment option if there was an isolated patellar cartilage defect Outerbridge grade 3 or 424 that was larger than 2 cm2.
All patients were primarily treated with conservative treatment for at least 6 months including physical therapy and anti-inflammatory medications. Surgery was indicated if there was disabling and persistent anterior knee pain.
Exclusion criteria included patients with multifocal defects in the trochlea or condyles, concomitant cruciate ligament repair, osteotomy or evidence of degenerative disease in the patellofemoral joint on plain radiographs.
Surgical Technique
A standard first-generation ACI technique was used until 2007, after which a second-generation technique was employed.25 Briefly, once the patellar defect was identified, it was radically debrided back to stable and healthy cartilage margins. A periosteal membrane harvested from the tibia or femur and was then microsutured flush to the surface of the defect26 to restore the articular topography. This involved oversizing the template medial to lateral and starting at the apex median ridge to restore the tent shape of the patella surface and not have the membrane bottom out on the subchondral bone. The membrane was then sealed with Tisseel fibrin glue (Tisseel, Baxter Biosurgery, Deerfield, IL) circumferentially to ensure water tightness. Autologous cultured chondrocytes (Carticel, Genzyme Biosurgery, Cambridge, MA) were implanted beneath the membrane to fill the defect. After 2007, a type I/III collagen membrane (Biogide, Geistlich Pharma AG, Wolhusen, Switzerland) was used instead of a periosteal patch.
Concurrent Procedures
Tibial Tubercle Osteotomy
Tibial tubercle osteotomy (TTO) was performed as a concurrent procedure in patients with lateral maltracking, patellar instability or an abnormal tibial tuberosity–trochlear grove (TT-TG) distance (>15 mm). A standard anteromedialization technique was used to realign the patellofemoral joint.27 None of the patients underwent additional distal femoral or high tibial osteotomy.
Soft Tissue Balancing
A lateral subvastus release to the lateral intermuscular septum (in 28 cases) was performed either in combination with an anatomic closure of the medial arthrotomy or a VMO (vastus medialis obliqus) advancement (23 cases) as necessary as to obtain patella stability without overconstraining the patellofemoral joint.
Trocheoplasty
Trocheoplasty was performed in 5 patients who demonstrated patellar instability due to hypoplastic trochlea using the surgical technique described by Peterson et al.28 and Minas.29 The technique involves removing the prominent proximal most trochlear bone and cartilage centrally toward the distal sulcus and advancing the synovium to the articular cartilage. The distal one-third of the patella engages this new sulcus and tracks more favorably. It is performed after distal tubercle osteotomy and proximal soft tissue balancing are deemed inadequate in the presence of dysplasia as the final step in order to accomplish stability of the patella.
Functional Outcome Evaluation
Clinical outcome was evaluated pre-operatively and at a minimum of 2 years and yearly afterward, after index surgery by an independent observer. The average time from index surgery to latest evaluation was 88 months (7.3 years; range, 24-175 months or 14.6 years). The patient-reported outcome measures were collected by mail or at the clinic visits. Patients completed the SF-36,20 KSS,23 WOMAC,21 the modified Cincinnati rating scale (a score from 0 to 10) activity-based score,22,30 and a patient satisfaction survey. Treatment failure was defined as the need for revision surgery due to structural failure of the ACI graft diagnosed on magnetic resonance imaging (MRI) or arthroscopy.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed with a Philips Medical Systems Achieva 1.5-tesla unit, using a SENSE- knee coil (Phased-Array coil with 8 elements) in 24 patients after a mean of 31 months (range, 2-89 months). A specific knee MRI protocol31 was used for the assessment of the ACI graft.
All images were assessed by 2 musculoskeletal radiologists first independently and then reviewed in consensus. Initial filling of the defect with cartilage repair tissue was measured in percentages with use of coronal and sagittal images and was graded as good (67% to 100%), moderate (34% to 66%), or poor (0% to 33%).
For detailed analysis of the cartilage repair filling we used the MRI score proposed by Marlovits et al.32,33: the magnetic resonance observation of cartilage repair tissue (MOCART) score. These elements recorded included the degree of defect repair and filling of the defect, integration to border zone, surface of the repair tissue, structure of the repair tissue, signal intensity of the repair tissue, subchondral lamina, subchondral bone, adhesions, and effusion.
Rehabilitation
There are 3 stages to the postoperative rehabilitation protocol. The first stage ranging from week 1 to 6 has the patient’s knee locked for ambulation, with a continuous passive motion for 6 to 8 hours starting at 0° to 40° with incrememental increase by 5° a day. If an additional TTO was performed, weightbearing was restricted to touch down weightbearing.
The second stage (week 7-12) consisted of active range of motion exercises with a gradual increased to full range of motion and full weightbearing. Stationary bike with low resistance and increasing time was implemented, then resistance as comfort allows occurred at this stage. Treadmill walking progressing to an elliptical trainer toward 12 to 13 weeks is encouraged for functional activities of daily living preparation. The third stage (week 12+) focuses on advancing to functional activities. Patients were discouraged from open chain exercises starting until 18 months. At 18 to 24 months, patients are allowed to return to cutting sports when clinical examination demonstrated no swelling, crepitus, good muscle tone and strength, and MRI scan demonstrated no bone marrow edema and a good graft fill.
Statistical Analysis
For testing relationships between variables, nonparametric Spearman correlation analysis using outcome scores and the 9 MOCART scores in addition to the 3 general MRI score (good, moderate, poor) were used. We determined differences in functional scores (WOMAC, KSS, SF-36, and modified Cincinnati) between 2 time points (preoperatively vs. 24-month follow-up and preoperatively vs. at latest follow- up) using 2-sample Student’s t test or Wilcoxon rank test and 1-way analysis of variance for the analysis of the subgroups. All reported P values are 2-tailed with a level of 0.05 indicating statistical significance (Graphpad Prism 5, La Jolla, CA) The Kaplan-Meier method was used to analyze time to an end point, which was the implant of a prosthesis or cartilage repair revision surgery.
Results
Thirty consecutive patients were followed for a mean of 88 ± 45 months (7.3 years). Three patients were subsequently lost to follow-up, and only their last scores were included. Twelve patients were male and 18 were female, with an average age of 32 ± 10 years (range, 15-49 years) at the index surgery. Previous surgery was undertaken prior to ACI in 4 patients and included 1 TTO, 1 ORIF (open reduction internal fixation) of the patella with concomitant TTO, and 1 arthroscopic lateral release. The mean body mass index was 27 kg/m2 (range, 18.9-43.6 kg/m2). The mean defect size averaged at 4.7 ± 2.1 cm2. According to Pidoriano/Fulkerson classification,34 3 defects were type II (lateral), 9 type III (medial), and 18 type IV (central/panpatella). The etiology of the defect was traumatic in 24 (80%) patients; 2 were sustained in road traffic accidents, 12 chondral defects were sports-related, and 10 had a history of a prior fall and subluxation or dislocation of the patella. In the nontraumatic group, 8 (20%) patients’ etiology was due to chronic maltracking and resulted in early degenerative changes. Five patients underwent trocheoplasty for restoration of a normal trochlear groove to improve maltracking. Soft tissue balancing was achieved with lateral release was performed in 28 patients, and 23 also required VMO advancement ( Table 2 ).
Table 2.
Demographics and Treatment of the Patient Cohort.
| Patient | Age (Years) at ACI | BMI (kg/m2) | Trauma | Patella Location Type | TTO | Anterialization | Medialization | Additional Procedures Performed With ACI or Osteotomy | Length (cm) | Width (cm) | Procedure for Failed ACI Graft | Workers Compensation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 23.2 | Yes | 4 | No | n/a | n/a | None | 1 | 3.4 | No | |
| 2 | 46 | 29 | Yes | 4 | No | n/a | n/a | None | 2.5 | 1 | PF replacement | Yes |
| 3 | 49 | 26.7 | Yes | 2 | Yes | 10 | 10 | None | 1.8 | 1 | PF replacement | Yes |
| 4 | 21 | 26 | Yes | 3 | Yes | 14 | 8 | None | 1.6 | 2.5 | No | |
| 5 | 36 | 30.4 | Yes | 4 | Yes | 15 | 10 | Trocheoplasty | 2 | 3 | Yes | |
| 6 | 43 | 24.3 | Yes | 3 | Yes | 15 | 10 | None | 2 | 4 | Yes | |
| 7 | 15 | 31.7 | Yes | 3 | Yes | 0 | 15 | Trochleoplasty | 1.5 | 2 | No | |
| 8 | 15 | 24 | Yes | 3 | Yes | 10 | 15 | None | 1.5 | 3 | No | |
| 9 | 35 | 24.6 | Yes | 4 | No | 0 | 0 | None | 1.5 | 3.5 | No | |
| 10 | 46 | 31.7 | No | 4 | No | 0 | 0 | None | 2 | 3.5 | Bicompartmental replacement | Yes |
| 11 | 36 | 31.5 | No | 2 | Yes | 0 | 10 | Trochleoplasty | 2.2 | 1 | No | |
| 12 | 39 | 29 | Yes | 2 | Yes | 0 | 15 | Trocheoplasty | 1.7 | 3 | No | |
| 13 | 31 | 35.6 | Yes | 3 | No | 0 | 0 | None | 1.5 | 2.5 | Yes | |
| 14 | 39 | 25.1 | Yes | 4 | No | 0 | 0 | None | 1 | 2.5 | No | |
| 15 | 35 | 30.1 | Yes | 4 | **Yes (previous) | n/a | n/a | Removal of TTO hardware | 2 | 3 | No | |
| 16 | 19 | 23.1 | Yes | 4 | Yes | 0 | 10 | None | 3 | 3 | No | |
| 17 | 22 | 43.1 | Yes | 4 | Yes | 10 | 5 | None | 2 | 3 | No | |
| 18 | 47 | 22 | Yes | 4 | Yes | 10 | 10 | None | 1.8 | 2 | Yes | |
| 19 | 20 | 22 | Yes | 3 | Yes | 15 | 10 | None | 2 | 15 | No | |
| 20 | 27 | 27.2 | No | 4 | No | 0 | 0 | None | 1.3 | 2 | No | |
| 21 | 18 | 23 | Yes | 4 | Yes | 10 | 10 | None | 2.5 | 4 | No | |
| 22 | 24 | 25.5 | No | 4 | Yes | 15 | 10 | None | 1 | 2.5 | No | |
| 23 | 44 | 23.6 | Yes | 4 | **Yes (previous) | n/a | n/a | Removal of TTO hardware | 2.1 | 2.3 | No | |
| 24 | 18 | 22.1 | Yes | 3 | Yes | 10 | 15 | None | 2.5 | 1 | No | |
| 25 | 39 | 22 | Yes | 3 | No | 0 | 0 | None | 1.5 | 2 | No | |
| 26 | 41 | 21 | Yes | 4 | Yes | 10 | 5 | None | 2 | 3 | Yes | |
| 27 | 33 | 43.6 | No | 3 | Yes | 10 | 15 | None | 2 | 1.5 | No | |
| 28 | 25 | 18.9 | Yes | 4 | No | 0 | 0 | Trochleoplasty | 2 | 2.5 | No | |
| 29 | 29 | 22 | No | 4 | Yes | 10 | 15 | None | 3.5 | 1.5 | No | |
| 30 | 40 | 30.5 | No | 4 | Yes | 10 | 15 | Notchplasty | 2 | 2 | No |
ACI, autologous chondrocyte implantation; BMI, body mass index; n/a, not applicable; PF, Patellofemoral; TTO, tibial tubercle osteotomy.
patient underwent previous TTO.
At a mean follow-up of 88 ± 45 months (7.3 years) after ACI, 25 (83%) patients reported good or excellent subjective results, 4 (13%) rated their knee function as fair, and only 1 (3%) had a poor knee function. All functional scores improved significantly at 24 months in all 30 patients. The Knee Society pain and function score increased from 55.7 ± 12.8 to 73 ± 14.7 (P < 0.001) and from 63.9 ± 12.9 to 81.8 ± 12.9 (P < 0.001), respectively. The WOMAC scale improved from 52.2 ± 16.9 to 27.9 ± 23.6 (P < 0.001) ( Tables 3 and 4 ; Fig. 1 ).
Table 3.
Comparison of Clinical Outcome Scores of Patients Undergoing Autologous Chondrocyte Implantation to Isolated Patella Defects.
| Score |
P | ||
|---|---|---|---|
| Preoperative | Latest Follow-up (>2 Years) (n = 27) | ||
| Modified Cincinnati | 3.1 ± 1.1 | 5.7 ± 1.5 | <0.01 |
| KSS–Function | 55.7 ± 12.8 | 73.0 ± 14.7 | <0.01 |
| KSS–Pain | 63.9 ± 12.9 | 81.8 ± 12.9 | <0.01 |
| WOMAC | 52.2 ± 16.9 | 27.9 ± 23.6 | <0.01 |
| SF-36–PCS | 40.0 ± 8.2 | 47.0 ± 10.0 | 0.01 |
| SF-36–MCS | 47.0 ± 8.4 | 53.0 ± 8.8 | 0.02 |
KSS, Knee Society Score; MCS, mental component summary; PCS, physical component summary; SF-36, Short Form–36; WOMAC, Western Ontario McMaster Universities Osteoarthritis Index.
Table 4.
Clinical Outcome Scores of Patients Undergoing Autologous Chondrocyte Implantation to Isolated Patella Defects with More Than 6-Year Follow-up.
| Score |
P | ||
|---|---|---|---|
| Preoperative | n = 17 | ||
| Modified Cincinnati | 3.3 ± 1.6 | 5.5.5 ± 1.5 | <0.01 |
| KSS–Function | 50.0 ± 20.6 | 70.0 ± 17.0 | <0.01 |
| KSS–Pain | 59.4 ± 14.1 | 78.8 | <0.01 |
| WOMAC | 57.6 ± 19.2 | 30.8 ± 27.9 | <0.01 |
| SF-36–PCS | 40.2 ± 9.1 | 46.6 ± 9.5 | 0.03 |
| SF-36–MCS | 46.2 ± 9.8 | 52.1 ± 9.7 | 0.03 |
KSS, Knee Society Score; MCS, mental component summary; PCS, physical component summary; SF-36, Short Form–36; WOMAC, Western Ontario McMaster Universities Osteoarthritis Index.
Figure 1.
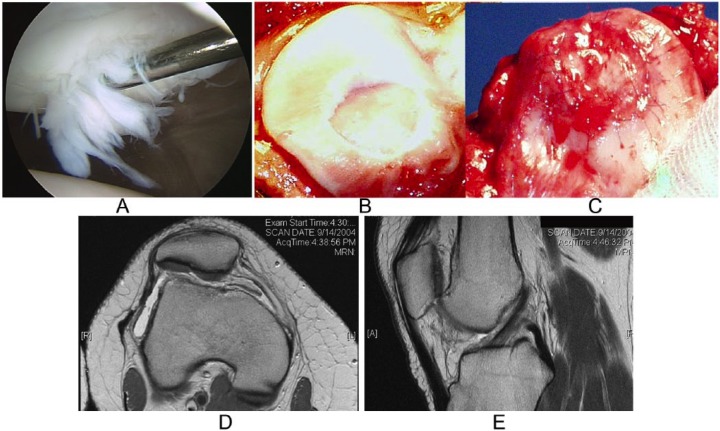
Patient who underwent patellar autologous chondrocyte implantation (ACI), tibial tubercle osteotomy (TTO), and trocheoplasty (patient 7; Table 2 ). (A) Preoperative arthroscopy image demonstrating chondral injury to the patella. (B, C) Debridement of the chondral defect and the autologous chondrocyte implantation to the patella. (D) Magnetic resonance image showing good cartilage repair tissue in the patella at 4 years postoperatively. (E) Sagittal magnetic resonance image of the patella with good repair tissue.
Twenty-four (80%) patients described their knee condition as better compared with their preoperative status, 5 as similar, and 1 described it as much worse. Twenty (66.6%) patients would definitely choose ACI surgery again, 8 (26.6%) declared that they would probably opt to have the procedure, and 2 were uncertain about the choice.
Survivorship
There were 3 failures out of 30 patients treated. All 3 were Workers Compensation (WC) patients, of the 8 WC treated, 3 failed. WC patients tended to be older, average age at 42 ± 6 years compared with the non-WC patients who were on average 28 ± 9 years old (P = 0.0019). This trend for WC patients to do less favorably was also documented in an earlier publication and was not an age related phenomenon.35 Two patients underwent subsequent patellofemoral joint replacement and the third patient was treated with a bicompartmental arthroplasty.
Based on estimates using the Kaplan-Meier methodology up to 15 years ( Fig. 2 ), 75% patients treated with ACI for isolated chondral defects on the patella are able to retain their native knee joint. Additional TTO was not found to increase the survivorship.
Figure 2.
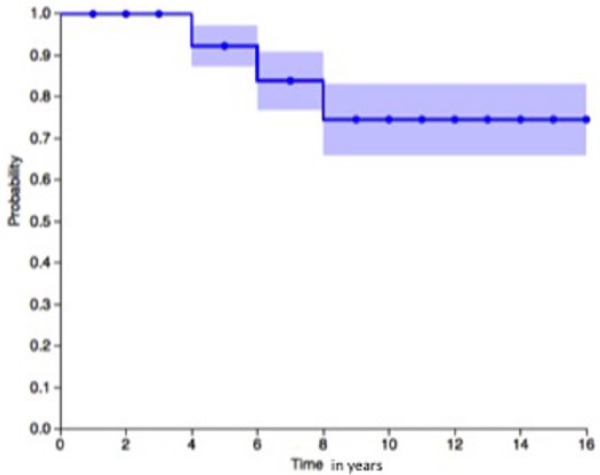
Kaplan-Meier curve for autologous chondrocyte implantation in isolated cartilage defects.
Reoperation Rate
The reoperation rate was in 18 (60%) out of 30 patients and was most commonly performed for graft hypertrophy (n = 7), chondroplasty (n = 5), arthrofibrosis (n = 4), and removal of hardware (n = 2), or in combination.
Magnetic Resonance Imaging
Magnetic resonance imaging was assessed in 24 patients at minimum 24 months postoperatively, and demonstrated complete fill of the defect in 18 (75%) patients. Five patients demonstrated moderate fill and 1 showed poor fill of the graft site. The symptomatic patient with poor fill was subsequently revised. Two patients with exposed subchondral bone still had up to 66% fill of the graft.
Four patients had graft hypertrophy beyond the level of the surrounding native host cartilage. Three (18.8%) out of the 16 patients who underwent ACI with periosteum developed graft hypertrophy in comparison with 1 (12.5%) out of the 8 patients who received a Biogide membrane. A complete list of the MRI findings is shown in Table 5 .
Table 5.
Magnetic Resonance Imaging Findings after Autologous Chondrocyte Implantation at a Mean of 31 Months’ Follow-up by Number and Percentage.
| Variables | Findings | No. (%) of Knees |
|---|---|---|
| 1. Degree of defect repair and filling of the defect: | ||
| Complete | 15 (62.5) | |
| Hypertrophy | 4 (16.7) | |
| Incomplete >50% of the adjacent cartilage | 2 (8.3) | |
| Subchondral bone exposed | 3 (12.5) | |
| 2. Integration to border zone: | ||
| Complete | 17 (70.8) | |
| Fissure <50% of the length of the repair tissue | 6 (25.0) | |
| No integration | 1 (4.2) | |
| 3. Surface of repair tissue: | ||
| Surface intact without any irregularities | 14 (58.3) | |
| Slight surface irregularities | 10 (41.7) | |
| 4. Structure of repair tissue: | ||
| Homogenous | 14 (58.3) | |
| Inhomogeneous | 10 (41.7) | |
| 5. Signal intensity of the repair tissue (Dual T2 FSE): | ||
| Isointense | 21 (87.5) | |
| Moderately hyperintense | 2 (8.3) | |
| Markedly hyperintense | 1 (4.2) | |
| 6. Subchondral lamina: | ||
| Intact | 24 (100) | |
| Nonintact | 0 (0.0) | |
| 7. Subchondral bone: | ||
| Intact | 14 (58.3) | |
| Edema | 10 (41.7) | |
| Subchondral sclerosis | 0 (0.0) | |
| 8. Adhesions: | ||
| Adhesions | 2 (8.3) | |
| No adhesions | 22 (91.7) | |
| 9. Effusion: | ||
| No | 21 (87.5) | |
| Yes | 3 (12.5) | |
The fill grade on MRI according to the aforementioned criteria showed a moderate positive correlation with postoperative KSS (rs = 0.53, P = 0.01) and mental health (rs = 0.43, P = 0.03), which indicated that a lower fill grade was associated with lower scores. All other markers did not demonstrate any significant correlation (data not shown).
Discussion
Several different cartilage restoration techniques have been used for patellofemoral cartilage lesions; microfracture, abrasion, chondroplasty, osteochondral autograft, allograft, and autologous ACI. Microfracture,36 although it is inexpensive, with a short learning curve, is not cost-effective as the results are not durable in the patella. Microfracture produces fibrocartilage containing type I collagen, which has less resistance to shear and compressive forces in comparison with type II collagen found in hyaline cartilage.37,38 Microfracture disrupts the subchondral bone and may produce intralesional osteophytes. Revision cartilage repair surgery with ACI after microfracture results in a failure rate 3 to 6 times worse than a primary ACI.19,39-41
Kreuz et al.14 reported on transient improvement of patellofemoral defects treated with microfracture, but deterioration after 18 to 36 months following index surgery. Long-term MRI at a mean of 48 months confirmed the deterioration of cartilage repair tissue after microfracture treatment.42
Bentley et al.16 compared ACI with osteochondral autografts and reported at 1 year postoperatively 85% good-to-excellent results in the ACI group. For patellar lesions, mosaicplasty to the patella failed in all cases (0/5 cases) and the recommendation was to abandon mosaicplasty in the patella. Second-look arthroscopy of the patients treated either with ACI or mosaicplasty demonstrated at 1 year postoperatively ICRS grades of 1 or 2 in 82% and 34%, respectively. Hangody and Fules43 found that the use of autologous osteochondroplasty may provide increased outcomes with up to 79% good to excellent results in the patellofemoral joint. Recently, Gracitelli et al.18 reported on 27 patients with fresh osteocondral allografts for isolated patellar defects with a failure rate of 28.6%. Despite their higher failure rate than reported for ACI in the patellofemoral joint of 8%,17 the authors found a significant increase in all clinical outcome scores and patient satisfaction. Nho et al.44 assessed the clinical outcome and the integration of autologous osteochondral transplantation in 22 patients with full thickness patellar cartilage lesions. They reported that all of their grafts appeared to have good cartilage fill, defined as 67% to 100% fill and 71% flush plug appearance.
Gobbi et al.45 have reported encouraging results with a new method of patellar cartilage restoration. It is a cell-based cartilage repair using scaffold-associated bone marrow aspirate concentrate however longer clinical follow-up is necessary to compare the durability of this treatment to ACI.
Biant et al.19 reported on a series of 104 patients treated with ACI at minimum 10 years follow-up. Thirty-seven of these were patella defects in patients with normal patella tracking. The results of ACI in the patella subgroup were comparable to the condylar group. Patients requiring TTO were excluded.
Originally, patellar defects treated with ACI without corrective osteotomy in one of the first patient series showed only 62% good to excellent results.46 Patients with chronic patellar malalignment may benefit from concurrent alignment correction. We believe that the corrective osteotomy has a significant impact on achieving a >85% good to excellent result, as previous demonstrated in another study.25 This may be the result of reduced pressure and shear in the patellofemoral joint which allows the ACI technique to restore articular cartilage and reduce pain.47 A few short-term to medium-term outcome studies have investigated isolated patellar chondral defects treated either with ACI25,48-50 or a modification of the ACI technique51-53 with clinical outcome scores that are comparable to our findings. However, to our knowledge, this study is the first mid-to-long-term outcome study of isolated patellar defects with a comprehensive evaluation using objective imaging. In this subset of patients the addition of a TTO may be beneficial to the survival of the graft.
Among the limitations that are inherent to a prospective cohort, this study lacked a control group. Furthermore, the inability to retrieve magnetic resonance images from all patients may have biased our radiological findings. The 2 scoring systems used have their own limitations when analyzed together. This is particularly evident in 2 patients where there was evidence of exposed subchondral bone on the MOCART Score; however, the graft still demonstrated a fill grade up to 67%.
In conclusion, this long-term outcome study with MRI data confirms the durability of autologous chondrocyte implantation tissue for the treatment of isolated patella defects after a mean of 7.3 years follow-up. Patients were satisfied with their results up to fifteen years postoperatively. Anteromedialization may provide additional benefit for maintaining graft integrity and correcting maltracking, implying its important role in the restoration of normal patellar tracking when addressing patella defects by ACI. ACI for patella defect management of Fulkerson type III (medial facet) and type IV (panpatellar) defects is the most effective and durable treatment option available and has optimal results when performed as the primary surgery.
Footnotes
Acknowledgments and Funding: We would like to thank the musculoskeletal radiology fellow for assistance with the radiological assessment of the images.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval for this study was obtained from INSTITUTIONAL REVIEW BOARD (APPROVAL NUMBER: 2007-P-00470/4; BWH).
Informed Consent: Written informed consent was obtained from all patients at the time they entered into the database.
Trial Registration: Not applicable.
References
- 1. Grelsamer RP, Stein DA. Patellofemoral arthritis. J Bone Joint Surg Am. 2006;88(8):1849-60. [DOI] [PubMed] [Google Scholar]
- 2. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-74, v. [DOI] [PubMed] [Google Scholar]
- 3. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 4. Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-5. [DOI] [PubMed] [Google Scholar]
- 5. Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med. 2004;32(6):1451-8. [DOI] [PubMed] [Google Scholar]
- 6. Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52(7):2033-9. [DOI] [PubMed] [Google Scholar]
- 7. Davies-Tuck ML, Wluka AE, Wang Y, Hanna F, Bell RJ, Davis SR, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):337-42. [DOI] [PubMed] [Google Scholar]
- 8. Dahm DL, Al-Rayashi W, Dajani K, Shah JP, Levy BA, Stuart MJ. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop (Belle Mead NJ). 2010;39(10):487-91. [PubMed] [Google Scholar]
- 9. Moen TC, Laskin W, Puri L. The lateral compartment in knees with isolated medial and patellofemoral osteoarthritis: a histologic analysis of articular cartilage. J Arthroplasty. 2011;26(5):783-7. [DOI] [PubMed] [Google Scholar]
- 10. van Jonbergen HP, Poolman RW, van Kampen A. Isolated patellofemoral osteoarthritis. Acta Orthop. 2010;81(2):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. Does isolated patellofemoral osteoarthritis matter? Osteoarthritis Cartilage. 2009;17(9):1151-5. [DOI] [PubMed] [Google Scholar]
- 12. Becker R, Ropke M, Krull A, Musahl V, Nebelung W. Surgical treatment of isolated patellofemoral osteoarthritis. Clin Orthop Relat Res. 2008;466(2):443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donell ST, Glasgow MM. Isolated patellofemoral osteoarthritis. Knee. 2007;14(3):169-76. [DOI] [PubMed] [Google Scholar]
- 14. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. [DOI] [PubMed] [Google Scholar]
- 15. Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts: results in the patellofemoral joint. Clin Orthop Relat Res. 2005;(437):176-85. [PubMed] [Google Scholar]
- 16. Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-30. [DOI] [PubMed] [Google Scholar]
- 17. Gomoll AH, Gillogly SD, Cole BJ, Farr J, Arnold R, Hussey K, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-81. [DOI] [PubMed] [Google Scholar]
- 18. Gracitelli GC, Meric G, Pulido PA, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43(4):879-84. [DOI] [PubMed] [Google Scholar]
- 19. Biant LC, Bentley G, Vijayan S, Skinner JA, Carrington RW. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am J Sports Med. 2014;42(9):2178-83. [DOI] [PubMed] [Google Scholar]
- 20. Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-83. [PubMed] [Google Scholar]
- 21. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-40. [PubMed] [Google Scholar]
- 22. Agel J, LaPrade RF. Assessment of differences between the modified Cincinnati and International Knee Documentation Committee patient outcome scores: a prospective study. Am J Sports Med. 2009;37(11):2151-7. [DOI] [PubMed] [Google Scholar]
- 23. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989(248):13-4. [PubMed] [Google Scholar]
- 24. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752-7. [DOI] [PubMed] [Google Scholar]
- 25. Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005;(436):30-9. [DOI] [PubMed] [Google Scholar]
- 26. Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18(1):13-44, v-vi. [DOI] [PubMed] [Google Scholar]
- 27. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-81. [PubMed] [Google Scholar]
- 28. Peterson L, Karlsson J, Brittberg M. Patellar instability with recurrent dislocation due to patellofemoral dysplasia. Results after surgical treatment. Bull Hosp Jt Dis Orthop Inst. 1988;48(2):130-9. [PubMed] [Google Scholar]
- 29. Minas T. A Primer in Cartilage Repair and Joint Preservation of the Knee. New York, NY: Elsevier; 2011. [Google Scholar]
- 30. Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42-55. [DOI] [PubMed] [Google Scholar]
- 31. Alparslan L, Minas T, Winalski CS. Magnetic resonance imaging of autologous chondrocyte implantation. Semin Ultrasound CT MR. 2001;22(4):341-51. [DOI] [PubMed] [Google Scholar]
- 32. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-9. [DOI] [PubMed] [Google Scholar]
- 33. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. [DOI] [PubMed] [Google Scholar]
- 34. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-7. [DOI] [PubMed] [Google Scholar]
- 35. Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36(12):2336-44. [DOI] [PubMed] [Google Scholar]
- 36. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16:83-86. [PubMed] [Google Scholar]
- 37. Jackson DW, Scheer MJ, Simon TM. Cartilage substitutes: overview of basic science and treatment options. J Am Acad Orthop Surg. 2001;9(1):37-52. [DOI] [PubMed] [Google Scholar]
- 38. Sgaglione NA, Miniaci A, Gillogly SD, Carter TR. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthroscopy. 2002;18(2 Suppl 1):9-32. [DOI] [PubMed] [Google Scholar]
- 39. Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325-31. [DOI] [PubMed] [Google Scholar]
- 40. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-8. [DOI] [PubMed] [Google Scholar]
- 41. Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P. Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med. 2012;40(1):58-67. [DOI] [PubMed] [Google Scholar]
- 42. Von Keudell A, Atzwanger J, Forstner R, Resch H, Hoffelner T, Mayer M. Radiological evaluation of cartilage after microfracture treatment: a long-term follow-up study. Eur J Radiol. 2012;81(7):1618-24. [DOI] [PubMed] [Google Scholar]
- 43. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(Suppl 2):25-32. [DOI] [PubMed] [Google Scholar]
- 44. Nho SJ, Foo LF, Green DM, Shindle MK, Warren RF, Wickiewicz TL, et al. Magnetic resonance imaging and clinical evaluation of patellar resurfacing with press-fit osteochondral autograft plugs. Am J Sports Med. 2008;36(6):1101-9. [DOI] [PubMed] [Google Scholar]
- 45. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 47. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-6. [DOI] [PubMed] [Google Scholar]
- 48. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. [DOI] [PubMed] [Google Scholar]
- 49. Pascual-Garrido C, Slabaugh MA, L’Heureux DR, Friel NA, Cole BJ. Recommendations and treatment outcomes for patellofemoral articular cartilage defects with autologous chondrocyte implantation: prospective evaluation at average 4-year follow-up. Am J Sports Med. 2009;37(Suppl 1):33S-41S. [DOI] [PubMed] [Google Scholar]
- 50. Farr J., 2nd Autologous chondrocyte implantation and anteromedialization in the treatment of patellofemoral chondrosis. Orthop Clin North Am. 2008;39(3):329-35, vi. [DOI] [PubMed] [Google Scholar]
- 51. Niemeyer P, Steinwachs M, Erggelet C, Kreuz PC, Kraft N, Köstler W, et al. Autologous chondrocyte implantation for the treatment of retropatellar cartilage defects: clinical results referred to defect localisation. Arch Orthop Trauma Surg. 2008;128(11):1223-31. [DOI] [PubMed] [Google Scholar]
- 52. Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37(6):1083-92. [DOI] [PubMed] [Google Scholar]
- 53. Gobbi A, Kon E, Berruto M, Francisco R, Filardo G, Marcacci M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34(11):1763-73. [DOI] [PubMed] [Google Scholar]