Abstract
PURPOSE
To identify focal lesions of elevated MRI T2 and T1ρ relaxation times in articular cartilage of an ACL-injured group using a novel cluster analysis technique.
MATERIALS AND METHODS
Eighteen ACL-injured patients underwent 3T MRI T2 and T1ρ relaxometry at baseline, six months and one year and six healthy volunteers at baseline, one day and one year. Clusters of contiguous pixels above or below T2 and T1ρ intensity and area thresholds were identified on a projection map of the 3D femoral cartilage surface. The total area of femoral cartilage plate covered by clusters (%CA) was split into areas above (%CA+) and below (%CA−) the thresholds and the differences in %CA(+ or −) over time in the ACL-injured group were determined using the Wilcoxon signed rank test.
RESULTS
%CA+ was greater in the ACL-injured patients than the healthy volunteers at six months and one year with average %CA+ of 5.2 ± 4.0% (p=0.0054) and 6.6 ± 3.7% (p=0.0041) for T2 and 6.2 ± 7.1% (p = 0.063) and 8.2 ± 6.9% (p = 0.042) for T1ρ, respectively. %CA− at six months and one year was 3.0 ± 1.8% (p > 0.1) and 5.9 ± 5.0% (p > 0.1) for T2 and 4.4 ± 4.9% (p > 0.1) and 4.5 ± 4.6% (p > 0.1) for T1ρ, respectively.
CONCLUSION
With the proposed cluster analysis technique, we have quantified cartilage lesion coverage and demonstrated that the ACL-injured group had greater areas of elevated T2 and T1ρ relaxation times as compared to healthy volunteers.
Keywords: quantitative MRI, cartilage, knee, ACL-injury, projection maps, cluster analysis
INTRODUCTION
Anterior cruciate ligament (ACL) ruptures have an annual incidence of 100,000 to 200,000 in the U.S. in individuals between 15–45 years old and have been shown to increase the risk of osteoarthritis (OA)1,2. Radiographic OA develops in as many as 50% of ACL-injured knees 10 to 20 years after reconstructive surgery and can only be detected once gross morphological abnormalities have occurred3–6. Ideally we would detect OA earlier in the disease process when treatments will likely be more effective. One early characteristic of OA is the depletion and disorganization of the articular cartilage extracellular matrix macromolecules, therefore, imaging techniques have been proposed and developed to detect these changes7–11. Changes in quantitative MRI parameters have been shown at six months and one-year post ACL injury21, making this population important for developing quantitative measures sensitive to these pre-radiographic OA matrix changes.
Advanced MRI techniques offer quantitative assessment of macromolecular changes to the cartilage matrix7–11. T2 and T1ρ relaxation time mapping have demonstrated some ability to track cartilage quality and have been used to study OA9–13. T2 measures the transverse relaxation time and is used to assess the water and collagen content in the cartilage14,15,18,19 while T1ρ, or relaxation in the rotating frame, probes the slow-motion interaction between the motion-restricted water molecules and large macromolecules and is used to assess glycosaminoglycan (GAG) content9,12,13,17. Both relaxation parameters have been shown to increase in the ACL-injured population, 1–2 years after reconstructive surgery20–22.
Previous studies have shown great potential for using quantitative imaging measures to track cartilage health but technical challenges still limit their widespread adoption. Existing segmentation techniques are time-consuming and often prevent use of all of the data. As a result, most studies, especially for 2D imaging, examine only a single slice from the lateral and medial compartments of the knee, often neglecting the trochlear region23,24. The choice of slice and definition of sub-regions within each slice varies across investigations, making it hard to compare data across time points or between studies. Averaging within regions or entire segmented slices is another common practice that leads to loss of spatial localization. Magic angle effects25 cause increased T2 in the posterior condyles and trochlear region and can erroneously increase the mean of a region if not considered. If areas of increased T2 relaxation time due to magic angle effects are included in one groups ROI and not another, reported means cannot be compared. Analysis methods that include the whole 3D dataset and take into consideration magic angle effects will allow for identification of focal defects and better comparisons over time and across populations.
Few studies implement advanced processing techniques that look at the heterogeneity of the cartilage quantitative data, full 3D analysis as well as registration for within population and across time-point comparisons. Texture, laminar and Z-score analyses have been used to examine the spatial distribution and heterogeneously quantitative data within the cartilage, showing that the natural heterogeneity of quantitative T2 and T1ρ values in healthy cartilage, typically increases with OA26,27,28–32. These processing techniques have shown promise but to date few investigations look at the full cartilage plate, therefore missing important regions of degeneration, such as the trochlea30,33. One challenge to carrying out 3D comparisons is the registration of data between time-points and individuals. One group has proposed a voxel-based relaxometry technique33 that addresses this issue. This approach was shown to be more sensitive to local patterns in T1ρ relaxation time than traditional ROI-based approaches. Another potential approach, which has been used to study variations in 3D cartilage thickness34,35, is to use projection maps; these maps could also be used for registration between time-points and for isolating heterogeneity and focal defects. Capturing as much information about pathological changes and improving the detection of these changes is very important for identifying early cartilage degeneration and monitoring therapy.
Focal defects in cartilage are an important pathological change in OA that has received relatively little attention in the quantitative cartilage MRI literature; most work to date has reported mean changes or general heterogeneity. This is important because we know that later in the OA disease process both general cartilage thinning and focal defects are observed36. The aim of this work was therefore to develop a new cluster analysis method that identifies focal cartilage lesions and evaluate it as a method to identify differences in 3D T2 and T1ρ relaxation time mapping data between ACL-injured and healthy individuals.
METHODS
Study Population
All scans were performed in the sagittal plane using two MR750 3T scanners (GE Healthcare, Waukesha, WI) and a volume-transmit, 8 channel-receive knee coil (Invivo Inv., Gainesville, FL). Eighteen ACL-injured patients were scanned at baseline (8–87 days post-surgery), six months post baseline and one-year post baseline37. Six healthy volunteers were scanned at baseline, one-day post baseline, and one-year post baseline to determine the reproducibility of the technique and to set the thresholds used for comparing groups. A radiologist with seventeen years of experience (GEG) excluded any healthy volunteer with cartilage defects. Informed consent was obtained and the study was conducted in accordance with the guidelines of the institutional review board.
Imaging Protocol and Quantitative Mapping
T1ρ relaxation times were estimated using a T1ρ magnetization-prepared pseudo-steady-state 3D fast spin echo (FSE) sequence (CubeQuant)38. Acquisition parameters: 500 Hz spin-lock pulse frequency, repetition time (TR) = 1228 ms, 90° flip angle, partial k-space acquisition using 0.5 averages, a resolution of 0.5 mm × 0.625 mm × 3 mm, 62.5kHz bandwidth, four spin-lock time (TSL) durations of 1 ms, 10 ms, 30 ms, and 60 ms, and a total scan time of 5:49 min. T1ρ relaxation time mapping was carried out by doing a pixel-wise mono-exponential fit to the series of varying TSL images using the equation S(TSL) ~ exp(−TSL/T1ρ) within packaged freeware (OsiriX, Pixmeo Sarl, Bern, Switzerland)39.
T2 relaxation times were estimated using a modified, quantitative 3D double-echo in steady-state sequence (DESS) technique40. 3D DESS is an established T2 mapping sequence used and validated in numerous studies24,40,41,42. The sequence acquired an echo on either side of an unbalanced spoiler gradient. The first echo has T1/T2 contrast (S+) and the second (S−) has T2 and diffusion contrast. The sequence was run twice in succession, first with a small diffusion spoiler gradient area (34.66 ms*mT/m on all three axes) and a large flip angle (35°) (DESS Low/S1), and second with a large diffusion spoiler gradient area (138.4 ms*mT/m on all three axes) and small flip angle (18°) (DESS Hi/S2). This results in four images per subject. Specific parameters include: echo times (TE1) of 9 ms (S+) and TE2 of 43 ms (S−) (TE2 is the time between the RF pulse from the prior repetition and the echo signal), TR = 26 ms, 1 average, a resolution of 0.625 mm × 0.62 mm × 3 mm, a bandwidth of 32.5kHz and a total scan time of 9:40 min (for both scans). We determined the T2 relaxation time in a voxel-wise manner using three calculated signal ratios (S1−/S1+, S2−/S2+, and S1+/S2+) and a dictionary created based on the signal model proposed by Wu and Buxton42. The dictionary was created once for specified imaging parameters, a range of possible T1 and T2 relaxation times and apparent diffusion coefficients (processing time: < 1min). It was then searched for the T2 relaxation time that minimizes the sum of squared error between the three signal ratios of the measured and modeled values. This analysis was carried out using a custom plugin (OsiriX).
MRI Post-processing
Registration
Many of the sagittal scans acquired had some degree of femoral internal or external rotation, which creates difficulties in registration between time points. Therefore, the 3D image data was reformatted to create registered, anatomically based sagittal images. To accomplish this, a line was drawn through the most posterior points of the medial and lateral femoral condyles. The plane perpendicular to this line was considered the anatomically based sagittal plane. The images were reformatted in OsiriX into this new plane using cubic-spline interpolation (Fig. I-ii).
Figure I.
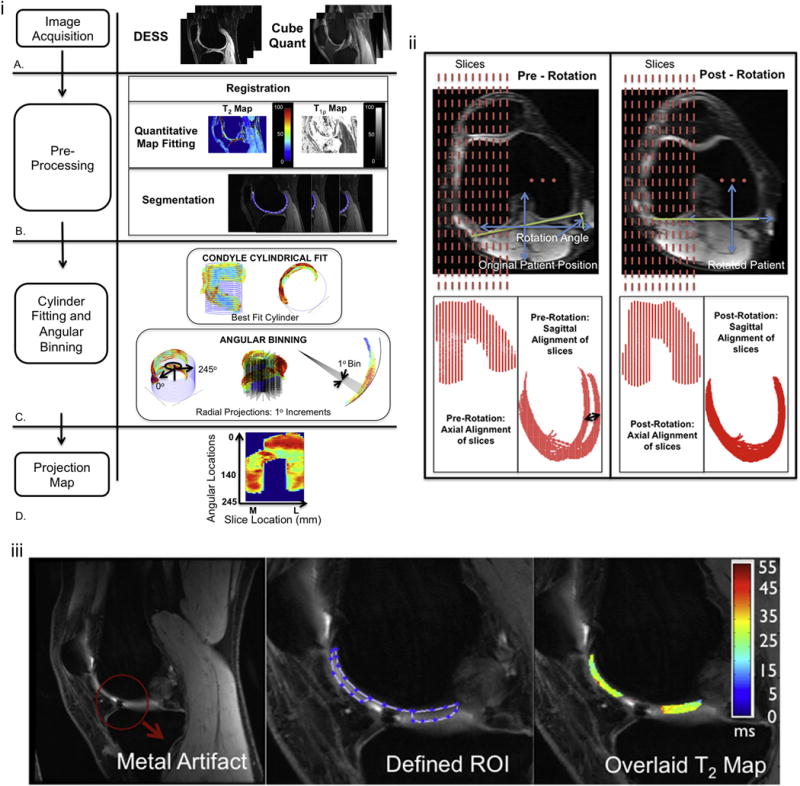
i. The methods flow chart shows the image acquisition using the DESS and Cubequant sequences (A), the pre-processing steps involving registration, quantitative map fitting and 3D manual segmentation (B) and the best fit cylinder and unwrapping of the cartilage using radial projection lines (C) to create a projection map plotting the angular location of the cartilage against the slice width in mm (D).
ii. Registration: Axial rotation and re-slicing of the knee to define the anatomically based sagittal plane perpendicular to the tangential line through the most posterior points of the femoral condyles and slices of equal longitudinal coverage across the knee. Pre-rotation sagittal images demonstrate the misalignment of the posterior condyles (black arrow) that is corrected after rotation.
iii. Manual Segmentation: Sample ACL-injured patient with a metal artifact in one of the slices. During segmentation, sufficient distance to the artifact was left so that no signal pileup or void was captured.
A custom software package (Matlab, the Mathworks, Natwick, MA) was developed to carry out the modules below:
Segmentation
Slice-by-slice manual segmentation was performed on the T1ρ-weighted images with spin-lock time (TSL = 10ms) and on the mean of the magnitude DESS Low images (segmentation time: ~90min/knee). These images provided the best anatomic contrast for segmentation. Binary masks created from the segmented data were superimposed onto their respective parameter maps for the extraction of the full volume quantitative T2 and T1ρ relaxation times (Fig. I-iB). Some ACL-injured patient data had metal artifacts due to surgical hardware; in such cases, special care was taken during segmentation to ensure that the distance to the artifact was sufficient and no signal void or pileup was captured (Fig. I-iii).
Projection Map
Projection maps were created for visualization and subsequent cluster analysis. First, the bone-cartilage interface was extracted from each segmented slice and collapsed into a single sagittal plane. A circle was fit to the data using a least-squares approach and the center and radius of this circle were used to create a cylinder that was fit through the femoral condyles (Fig. I-iC). In the original segmented data, the most anterior proximal point was identified in each slice and then rays were drawn at 1° increments from this point to 245°, thereby creating 245 angular bins (Fig. I-iC). This range encompasses all the cartilage across our two groups. Pixel data from cartilage that fell within the bin were averaged. In the case were pixels fell within multiple bins, an area-based weighted-average was used. Projection maps were created by plotting angular bin versus slice number (Fig. I-iD). To obtain isotropic projection maps, interpolation in the slice direction using a 1×10 interpolation factor was performed. A 1×5 low pass, blurring filter was then used to mitigate the effect of noise in the angular bin direction.
Difference Maps
Difference maps were created by subtracting projection maps at different time points. Difference maps decreased magic angle effects still present after the registration step at a band of about ±10° degrees around a ±54° angle from the static magnetic field (46° and 144° in the projection maps). Because there were slight differences in the segmentation of the cartilage edges, any pixel without a corresponding pixel at another time point were removed by erosion using a 7 pixel wide disk. Metal artifacts were manually excluded to reduce through-slice signal pile-up or void artifacts.
Cluster Analysis
Cluster analysis was used to identify focal lesions in the cartilage plates of the ACL-injured group. We classified clusters as either increased or decreased by setting two thresholds – intensity and size. For both T2 and T1ρ relaxation times, thresholds for increased and decreased clusters were set at +2σ, where σ is the mean standard deviation of the healthy groups’ difference map (Fig. II-B). A cluster was defined as a contiguous set of pixels above or below these thresholds, separated by a maximum of one pixel, corner or edge wise. A histogram of cluster areas within the healthy subjects for both T2 and T1ρ was plotted. Percentile values including 80th, 85th, 90th and 95th were explored and we chose to report clusters above the 85th percentile (12.4mm2) as representative of change (Fig. II-C). While the selection of a cluster area threshold is somewhat arbitrary, the 85th percentile appeared to remove noise but still identified focal defects. A metric termed percent cluster area (%CA) was defined as the percent area of the projection map covered by the identified clusters. This was further classified into %CA+ and %CA−; %CA+ is the area covered by clusters with values above the intensity threshold, while %CA− is area covered by clusters with values below the intensity threshold. %CA+ and %CA− were the primary outcome measures of this study (Fig. II-C).
Figure II.
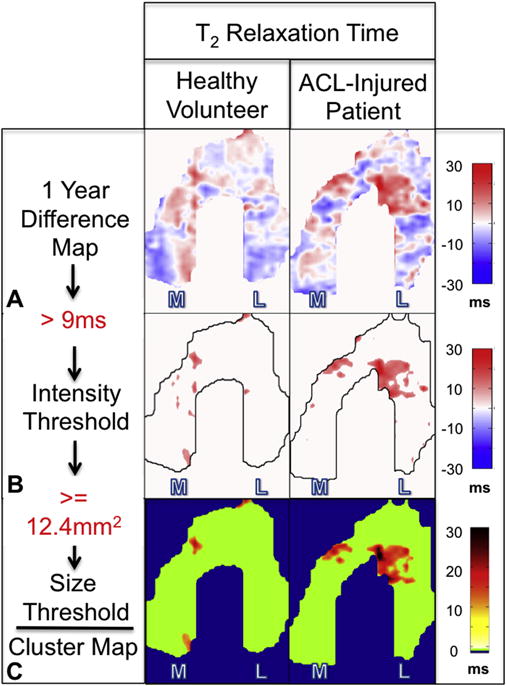
Cluster map pipeline showing the difference maps (A) used with the determined intensity (B) and area (C) thresholds to create the final cluster map.
Statistical Analysis
The %CAs calculated from the one-year difference map of the healthy subjects were compared to the %CAs calculated from the six month and one-year difference maps of the ACL-injured subjects. A one-tail, two-sample Wilcoxon signed-rank test was used for comparing the healthy and ACL-injured subjects while a one-tail, one-sample Wilcoxon signed-rank test was used for within ACL-injured subject comparisons. We used a one-tail test because we expect that the %CA’s within the ACL-injured population will be greater than those observed within the healthy subjects and we expect that these values will increase over time. This analysis was carried out for both %CA+ and %CA−, of both T2 and T1ρ relaxation time values. Statistical significance was set at p < 0.05 and analysis was performed using packaged software (R package stats, R Foundation for Statistical Computing, Vienna, Austria). Non-parametric analysis was chosen due to the positive skew of the patient data (which violates the assumption of normal distribution) and small sample size.
Short-term (between day 1 and day 2) and long-term (between day 1 and year 1) intra-subject variability as well as intra- (2 repeats) and inter- (2 observers) observer variability measurements were performed on the healthy subject data. Using the difference maps, the pixel-wise and global percent coefficient of variation of the root mean square error (%CVRMSE)44 were calculated.
To quantify all sources of variability and to properly identify their origin, we analyzed two aspects of intra-session variability: a) consecutive scans without repositioning and b) intrinsic MRI noise. For the first calculation, five additional healthy subjects were scanned twice consecutively and using the same segmentation and projection map technique the %CVRMSE was determined. For the second calculation, we determined the base level variability introduced to our projection maps due to scanner noise. This consisted of adding repeated measures of appropriately scaled and correlated noise to our acquired data to simulate back-to-back scanning without motion or other variations45. First, noise-only data was acquired by switching off the RF excitation in the MRI sequence. These acquired data was used to calculate the covariance matrix that described the Gaussian noise and coupling between the coil elements. We then synthesized Gaussian noise with the same determined statistic and added it to our acquired images. For each synthesis, a projection map was created and the mean CVRMSE% was calculated for ten back-to-back pairs.
Sensitivity analysis was performed on the healthy subject data to determine the effects of the different sizes of the condyles as well as bone shape changes over time. The radius of each determined best-fit cylinder was varied by ±5% and projection maps were created. The CVRMSE% was then calculated for each change.
RESULTS
Patient Characteristics and Qualitative Comparisons
Fourteen ACL-injured patients (12 male, 2 female, mean age 32.5 ± 9.8 years, mean height = 1.77 ± 0.1, mean weight = 81.5 ± 9.4, mean BMI = 26.1 ± 2.7 kg/m2) were included for the final T2 analysis and seventeen ACL-injured patients (13 male, 4 female, mean age 32.9 ± 9.5 years, mean height = 1.75 ± 0.1, mean weight = 82.2 ± 13.4, mean BMI = 26.7 ± 4.3 kg/m2) for the final T1ρ analysis. Five healthy volunteer (4 male, 1 female, mean age 26.6 ± 2.4 years, mean height = 1.79 ± 0.0, mean weight = 74.5 ± 7.6, mean BMI = 23.3 ± 2.4 kg/m2) were included for the reproducibility measurements, setting the thresholds and the between group comparisons. One healthy volunteer had to be excluded due to the presence of a cartilage lesion. Motion artifacts excluded three subjects from the ACL-injured T2 group and incomplete longitudinal data excluded one subject from the T1ρ group.
Intra- and inter-observer reproducibility (CVRMSE%)
All intra- and inter-observer mean global reproducibility measures were less that 9%. The short- and long-term mean intra-subject global CVRMSE% were less than 9% while the intra- and inter-observer mean global CVRMSE% were less than 6% (Table. I). Mean pixel-wise CVRMSE% were less than 15% for the short and long-term intra-subject measures, while the intra- and inter-observer reproducibility were less than 10%.
Table I.
Intra-subject (short and long term), intra-observer (short term) and inter-observer (short term) RMSE Coefficient of Variation (%) of T2 and T1ρ measurements.
| Volunteer | Intra-subject Short-term | Intra-subject Long-term | Intra-observer Short-term | Inter-observer Short-term |
|---|---|---|---|---|
| T2 Measurements (%CVRMSE) | ||||
| GLOBAL MEAN | 5.2 (± 0.9) | 9.0 (± 2.8) | 3.5 (± 1.5) | 5.3 (± 1.2) |
| GLOBAL RANGE | [4.1–6.3] | [6.6–13.7] | [2.0–5.2] | [4.1–6.6] |
| PIXEL-WISE MEAN | 10.8 (± 1.0) | 14.6 (± 3.0) | 5.8 (± 1.8) | 9.3 (± 2.9) |
| PIXEL-WISE RANGE | [9.2–11.8] | [11.8–19.6] | [4.0–8.3] | [6.5–11.1] |
| T1ρ Measurements (%CVRMSE) | ||||
| GLOBAL MEAN | 6.6 (± 3.2) | 5.3 (± 1.1) | 2.3 (± 0.5) | 3.6 (± 0.5) |
| GLOBAL RANGE | [3.6–10.4] | [4.3–6.9] | [1.6–2.8] | [2.8–4.1] |
| PIXEL-WISE MEAN | 12.2 (± 2.7) | 12.3 (± 2.4) | 5.0 (± 1.6) | 7.1 (± 0.9) |
| PIXEL-WISE RANGE | [9.2–15.0] | [9.5–15.3] | [3.1–7.5] | [5.6–7.9] |
Intra-session variability
All intra-session variability measures due to consecutive scans and noise were less that 6% for global CVRMSE% and less than 11% for pixel-wise CVRMSE%. The mean pixel-wise CVRMSE% for consecutive scans of five healthy subjects was 8.3 ± 1.7% for T2 and 6.8 ± 1.1% for T1ρ with their corresponding global CVRMSE% of 4.5 ± 1.8% for T2 and 3.6 ± 1.7% for T1ρ. With added MRI noise and the same segmentation applied to the generated images, the global CVRMSE% was 0.66% and 0.53% and the pixel-wise CVRMSE% was 2.1% and 1.5%, for T2 and T1ρ, respectively. Simulated cylinder radius changes of up to ±5% resulted in global CVRMSE% of less than 1%.
Cluster Analysis
The ACL-injured patients had higher %CA+ for both T2 and T1ρ relaxation times at six months and one year than the healthy volunteers at one year. Using the healthy volunteer data, thresholds for %CA+ and %CA− were identified as area greater than 12.4mm2 (Fig. II-C) and value +/−9.0ms and +/−10.8ms for T2 and T1ρ, respectively (Figs. III-B–C and V). For T2, the six-month (p=0.0054) and one year (p=0.0041) %CA+ of the ACL-injured group were statistically higher than the one-year %CA+ of the healthy group. For T1ρ, a statistical significant difference was observed at one-year between the ACL-injured and the healthy group (p=0.042) (Fig. III). In the ACL-injured group, there was an increasing trend from six months to one year, in mean %CA+, for both T2 (5.2 ± 4.0% to 6.6 ± 3.7%) and T1ρ (6.2 ± 7.1% to 8.2 ± 6.9%), which, for the number of individuals tested, was not statistically significant (p > 0.05).
Figure III.
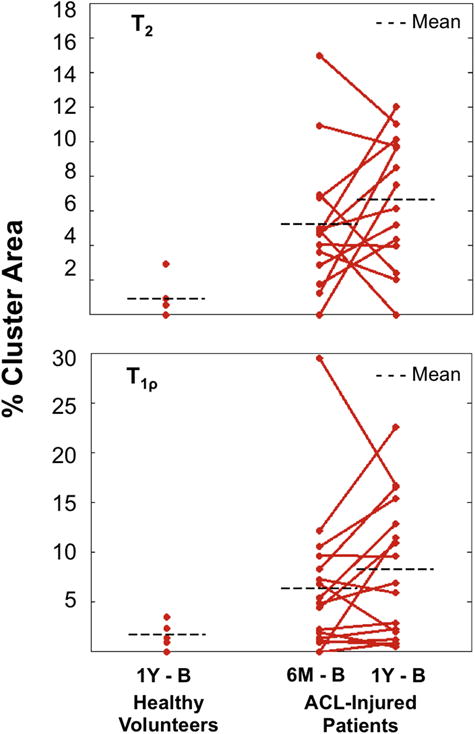
% Cluster Areas of projection maps covered by clusters in the ACL-injured population for both T2 and T1ρ as compared to the healthy volunteers. A T2 elevation threshold of +9ms and a T1ρ elevation threshold of +10.8ms was applied at 6 months and 1 year with individual %CA’s shown in 14 (T2) and 17 (T1ρ) ACL-injured patients and averaged %CA’s shown in both populations.
Overall, for the number of individuals tested, no statistically significant differences in %CA− between the injured and healthy groups (p > 0.1) and over time within the injured group (p > 0.1) were observed. The T2 %CA− of the ACL-injured group at both six months and one year was comparable to the T2 %CA− of the healthy group (mean %CA−: 3.0 ± 1.8% & 5.9 ± 5.0% vs. 4.4 ± 4.3%, respectively). ACL-injured patients had higher %CA− at six months and one year for T1ρ than the healthy volunteers (mean %CA−: 4.4 ± 4.9% & 4.5 ± 4.6% vs. 1.9 ± 2.2%, respectively).
DISCUSSION
In this study, we showed differences in T2 and T1ρ %CAs at six months and one year between the ACL-injured patients and the healthy volunteers. Increasing trends in %CA+ of the ACL-injured group were observed although, over this short time period, they did not reach statistical significance. This novel cluster analysis technique appears to be a useful tool for identifying and tracking focal regions of elevated quantitative MRI values in the ACL-injured population.
The spatial distribution of T2 and T1ρ relaxation times has been studied using texture and laminar analysis and reports have shown variations in healthy volunteers (up to 5ms from the deep to the superficial layers and between compartments8,29) that increase with OA26,27. Previous studies have been predominantly limited to one to three slices, usually in the medial and lateral condyles, which are often then subdivided into compartments23,24. The voxel-based relaxometry technique33 has shown the importance of full 3D analysis by assessing T1ρ z-score voxel based patterns of cartilage data that is morphed onto a common template. Our 3D cluster analysis technique on the other hand, identifies focal regions of elevated or decreased quantitative metrics on individual projection maps of averaged pixel data within angular bins. Threshold values of approximately 10ms for both T2 and T1ρ, help in identifying substantial and important focal degenerative lesions rather than describing the heterogeneity. Cluster analysis has been used successfully in functional MRI (fMRI) of the brain to identify activated regions from statistical parametric maps43; however, to our knowledge, no previous study has applied this approach to quantitative cartilage data. Combining these two approaches may prove promising.
We calculated six types of variability and found that our global CVRMSE% are comparable to those reported in literature and are lower than changes seen in osteoarthritic cartilage (5ms11). For T2, global CVRMSE% of 5.2% for short-term and 9.0% for long-term are similar to previously reported measurements of between 4–14%25,47,48. For T1ρ, intra-subject short-term global CVRMSE% of 6.6% and long-term global CVRMSE% of 5.3% fall within reported values of 1.7–8.7% in healthy volunteer studies24,46,48,49. The intra-session variability demonstrates that there is some baseline lower bound noise introduced by the scanner and the reported values of 2.1% for T2 and 1.5% for T1ρ are similar to literature findings for the two sequences of interest24. The implication of this finding is that we would expect an underlying contribution of noise to CV% measurements in addition to error due to segmentation, patient motion, registration and other post-processing steps. Overall, our projection map produced similar variability values24,46–49 as previous studies that looked at the T2 and T1ρ quantitative measures.
The proposed cluster analysis uses the difference maps from the healthy population to set the intensity threshold and cluster size across populations. The difference maps were key in addressing magic angle effects that could contribute to erroneously identifying regions of elevated T2 and, to a lesser extent, T1ρ relaxation times. Selecting the 85th percentile cluster size of 12.4mm2 allowed for characterizing focal lesions by mitigating the effects of noise such as single voxel changes, but still capturing focal lesions. Statistically significant differences were also observed at percentile values of 80, 90 and 95. We were able to demonstrate changes over time within both T2 and T1ρ relaxation time maps despite our limited healthy volunteer population size. These thresholding values could be better characterized in the future by tracking particular clusters over time and quantifying the size and intensity changes of these clusters.
Our finding of elevated %CA+ in the ACL population, as compared to our healthy volunteers, is similar to those of previous studies20–22,37. Changes did not occur across all patients and were not always consistent when comparing between T2 and T1ρ. With OA changes presenting in approximately 50% of the ACL-injured population3–6, we would only expect early changes in a subset of our population. Another interesting feature was the presence of decreased cluster areas in T2 and T1ρ relaxation times over time. Although calculated changes were not statistically significant between groups, the mean %CA− was higher in the ACL-injured group for T1ρ. These identified clusters may be worth tracking over a longer period of time.
Our proposed visualization and cluster analysis tool can provide a standardized method of capturing and quantifying changes in quantitative MRI metrics; however, there are some limitations of this study. Motion that could not be corrected using rigid body registration, excluded data from further analysis. Segmentation was performed twice to account for any motion in between scans, however this added to the manual processing time. In addition, registration utilized interpolation, which may introduce some blurring; however, we expect that the threshold choices in our analysis help mitigate introduced errors. Although the cylinder was fit to the cartilage-bone boundary, the condyles of the lateral and medial side are not the same size and some studies have shown that there are changes in bone shape as early as three months after injury with statistical significant changes observed over five years in the ACL-injured population50. However, simulations carried out to understand the potential effect of the difference in condyle size and bony changes found that CVRMSE% in the projection maps was less than 1%. Over longer periods of time, bone shape changes should be monitored. Some areas of cartilage were not segmented due to metal-induced artifacts and this lead to incomplete cartilage plates (< 15% removed) with possible clusters being missed. However, this was not of great concern, as complete cartilage would only increase the differences observed. Finally, no pre-surgery baseline data was collected for this study, however it is unclear what the effect of post-surgery edema will have on quantitative cartilage values.
In conclusion, we have demonstrated that our cluster analysis technique can identify focal changes in the ACL-injured population over time. Our results show promise for detecting and tracking early degenerative changes in a robust, objective manner. This tool may further help with understanding the cartilage degeneration process and serve in monitoring the effectiveness of therapeutic approaches for OA.
Supplementary Material
Electronic Appendix Figure. The difference maps and cluster projection maps of the T2 and T 1ρ relaxation times of two patients demonstrate the changes in the 3D cartilage volume at 6 months and 1 year, as compared to baseline. The two patients had an ACL-tear and subsequent ACL-reconstruction. (a) The difference maps demonstrate a focal change in quantitative T2 and T 1ρ values with the subsequent cluster maps while (b) demonstrates a diffused change in quantitative T2 and T 1ρ values with the subsequent cluster maps.
Acknowledgments
The authors gratefully acknowledge Weitian Chen for technical assistance during the CubeQuant T1rho acquisitions, Bragi Sveinsson for his assistance with the qDESS computations, Dr. Jarrett Rosenbery for his help with the statistical design and analysis and Dr. Jason Dragoo and Hillary Braun for help with recruiting the ACL-injured patients.
Role of the funding source
This project was supported by the following: the National Institutes of Health (Grant Numbers R01-EB002524, K24-AR062068, and R01-AR0063643, P41-EB015891), the Arthritis Foundation, the Stanford Diversifying Academia, Recruiting Excellence Graduate Research Fellowship and GE Healthcare. The study sponsors had no involvement in the study design, collection, analysis, or interpretation of data, nor in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
The authors contributed to this manuscript in the following ways: Conception and design (UDM, BAH, GEG, EJM); Analysis and interpretation of data (UDM, CDJ, BS, BAH, GEG, EJM); Drafting of the article (UDM, EJM); obtaining of funding (BAH, GEG, EJM); Collection and assembly of data (UDM, CDJ). All authors critically revised the manuscript and gave final approval of the article. UDM, BAH, GEG and EJM take responsibility for the integrity of the manuscript as a whole (emily.mcwalter@usask.ca)
Conflict of Interest
Uchechukwuka D. Monu has no conflicting interests. Caroline D. Jordan has no conflicting interests. Bonnie L. Samuelson has no conflicting interests. Brian A. Hargreaves receives research or institutional support from GE Healthcare and NIH, and receives patent royalties from GE Healthcare, Siemens and Philips. Garry E. Gold receives research or institutional support from GE Healthcare, the NIH, and provides consulting services to Boston Scientific. Emily J. McWalter has no conflicting interests.
References
- 1.Centers for Disease Control & Prevention, National Center for Health Statistics. National hospital discharge survey: annual summary 1996 [monograph on the Internet] Atlanta, GA: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 2.Øiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 4.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Hart C, van den Bekerom M, Patt T. The occurrence of osteoarthritis at a minimum of ten years after reconstruction of the anterior cruciate ligament. J Orthop Surg Res. 2008;3:24. doi: 10.1186/1749-799X-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 7.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388e94. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 8.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546e50. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 9.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547e53. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 10.Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, Hellio Le Graverand-Gastineau MP, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15:1225e34. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789e97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvvuri U, Kudchodkar S, Reddy R, et al. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10:838–44. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 13.Regatte RR, Akella SV, Borthakur A, et al. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17:114–21. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 14.Nishioka J, Hirose J, Nakamura E, et al. T1ρ and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35:147–55. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 15.Wong CS, Yan CH, Gong NJ, Li T, Chan Q, Chu YC. Imaging biomarker with T1ρ and T2 mappings in osteoarthritis - in vivo human articular cartilage study. Eur J Radiol. 2013;82(4):647–50. doi: 10.1016/j.ejrad.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Tsushima H, Okazaki K, Takayama Y, Hatakenaka M, Honda H, Izaawa T, et al. Evaluation of cartilage degradation in arthritis using T1ρ magnetic resonance imaging mapping. Rheumatol Int. 2012;32(9):2867–75. doi: 10.1007/s00296-011-2140-3. [DOI] [PubMed] [Google Scholar]
- 17.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38(6):863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 18.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology. 2000;214(1):259–66. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 19.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43(11):782–8. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2–initial experience with 1-year follow-up. Radiology. 2011;258(2):505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage Morphology and T1rho and T2 Quantification in ACL-reconstructed Knees: A 2-year Follow-up. Osteoarthritis Cartilage. 2013;21(8):1058–1067. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klocke NF, Amendola A, Thedens DR, Williams GN, Luty CM, Martin JA, et al. Comparison of T1ρ, dGEMRIC, and quantitative T2 MRI in preoperative ACL rupture patients. Acad Radiol. 2013;20(1):99–107. doi: 10.1016/j.acra.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan CD, McWalter EJ, Monu UD, Watkins RD, Chen W, Bangerter NK, et al. Variability of CubeQuant T1ρ, quantitative DESS T2, and cones sodium MRI in knee cartilage. Osteoarthritis Cartilage. 2014;22:1559–1567. doi: 10.1016/j.joca.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodwin DW, Wadghiri YZ, Dunn JF. Micro-imaging of articular cartilage: T2, proton density, and the magic angle effect. Acad Radiol. 1998;5:790–798. doi: 10.1016/s1076-6332(98)80264-x. [DOI] [PubMed] [Google Scholar]
- 26.Blumenkrantz G, Dunn TC, Carballido-Gamio J, Link TM, Majumdar S. Spatial Heterogeneity of Cartilage T2 in Osteo-arthritic Patients. Boston, MA: OARSI; 2005. [Google Scholar]
- 27.Carballido-Gamio J, Stahl R, Gabrielle B, Adan R, Sharmila M. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36:4059–67. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls –data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13(5):R153. doi: 10.1186/ar3469. Epub 2011 Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 30.Carballido-Gamio J, Link TM, Majumdar S. New techniques for cartilage magnetic resonance imaging relaxation time analysis: texture analysis of flattened cartilage and localized intra- and inter-subject comparisons. Magn Reson Med. 2008;59:1472–1477. doi: 10.1002/mrm.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Pai A, Blumenktrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61(6):1310–8. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Munoz T, Hellio Le Graverand-Gastineau M-P, et al. The feasibility of characterizing the spatial distribution of cartilage T2 using texture analysis. Osteoarthritis Cartilage. 2008;16(5):584–590. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedoia V, Li X, Su F, Calixto N, Majumdar S. Fully automatic analysis of the knee articular cartilage T1<rho> relaxation time using voxel-based relaxometry. J Magn Reson Imaging. 2016;43:970–980. doi: 10.1002/jmri.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhtar S, Poh CL, Kitney RI. An MRI derived articular cartilage visualization framework. Ostoearthritis Cartilage. 2007;15:1070–85. doi: 10.1016/j.joca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Duryea J, Iranpour-Boroujeni T, Collins JE, Vanwynngaarden C, Guermazi A, Katz JN, et al. Local-Area Cartilage Segmentation (LACS), A Semi-Automated Novel Method of Measuring Cartilage Loss in Knee Osteoarthritis. Arthritis Care Res. 2014;66(10):1560–1565. doi: 10.1002/acr.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 37.Jordan CD. PhD Thesis. Stanford University; 2013. Evaluation and Application of Novel Tools for Muskuloskeletal and Body MRI. [Google Scholar]
- 38.Chen W, Takahashi A, Han ET. 3D quantitative imaging of T1rho and T2 (Abstract) Proc Annual Meeting ISMRM. 2011;231 [Google Scholar]
- 39.Rosset OsiriX: An Open-Source Software for Navigating in Multidimensional DICOM Images. J Digit Imaging. 2004 Sep;17(3):205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T(2) and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med. 2012;67:1086e96. doi: 10.1002/mrm.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matzat SJ, McWalter EJ, Kogan F, Chen W, Gold GE. T2 Relaxation time quantitation differs between pulse sequences in articular cartilage. J Magn Reson Imaging. 2015;42:105–113. doi: 10.1002/jmri.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsch GH, Mamisch TC, Zak L, et al. Morphological and biochemical T2 evaluation of cartilage repair tissue based on a hybrid double echo at steady state (DESS-T2d) approach. J Magn Reson Imaging. 2011;34:895–903. doi: 10.1002/jmri.22677. [DOI] [PubMed] [Google Scholar]
- 43.Mezer A, Yovel Y, Pasternak O, Gorfine T, Assaf Y. Cluster analysis of resting-state fMRI time series. NeuroImage. 2009:1117–1125. doi: 10.1016/j.neuroimage.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Glüer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5:262e70. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 45.Peter K, Elliot RM. Image Reconstruction in SNR Units: A General Method for SNR Measurement. Magn Reson Med. 2005;54(6):1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298e307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258(3):832e42. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Wyatt C, Rivoire J, Han ET, Chen W, Schooler J, et al. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: repeatability and diurnal variation. J Magn Reson Imaging. 2014;39(5):1287e93. doi: 10.1002/jmri.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298e307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunter DJ, Lohmander LS, Makovey J, Tamez-Pena J, Totterman S, Schreyer E, Frobell RB. The Effect of Anterior Cruciate Ligament Injury on Bone Curvature: Exploratory Analysis in the KANON Trial. Osteoarthritis Cartilage. 2014;22(7):959–968. doi: 10.1016/j.joca.2014.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic Appendix Figure. The difference maps and cluster projection maps of the T2 and T 1ρ relaxation times of two patients demonstrate the changes in the 3D cartilage volume at 6 months and 1 year, as compared to baseline. The two patients had an ACL-tear and subsequent ACL-reconstruction. (a) The difference maps demonstrate a focal change in quantitative T2 and T 1ρ values with the subsequent cluster maps while (b) demonstrates a diffused change in quantitative T2 and T 1ρ values with the subsequent cluster maps.


