Abstract
Intracellular retinal iron accumulation has been implicated in the pathogenesis of age-related macular degeneration (AMD), the leading cause of irreversible blindness among individuals over the age of 50. Ceruloplasmin/hephaestin double knockout mice (Cp/Heph DKO) and hepcidin knockout mice (Hepc KO) accumulate retinal iron and model some features of AMD. Two canonical pathways govern cellular iron import – transferrin-bound iron import and non-transferrin bound iron import. In Cp/Heph DKO and Hepc KO iron-loaded retinas, transferrin-bound iron import is downregulated. Despite this effort to reduce cellular iron burden, iron continues to accumulate in these retinas in an age-dependent manner. Quantitative RT-PCR and Western analysis were used to quantify the expression of three ferrous iron importers, Dmt1, Zip8, and Zip14, in wild-type (Wt), Cp/Heph DKO, and Hepc KO retinas. Zip8 and Zip14 protein levels were analyzed using Western analysis in mice injected intravitreally with either apo- or holo-transferrin to elucidate one possible mechanism of Zip14 regulation in the retina. Both zip8 and zip14 were expressed in the mouse retina. Paradoxically, protein levels of non-transferrin bound iron importers were upregulated in both Cp/Heph DKO and Hepc KO retinas. Intravitreal holo-transferrin injection decreased Zip 14 protein levels. These data indicate that Zip8 and Zip14 may take up increasing amounts of non-transferrin bound iron in these two mouse models of retinal iron accumulation. Their upregulation in these already iron-loaded retinas suggests a vicious cycle leading to toxicity.
Keywords: Zip8, Zip14, retina, iron, age-related macular degeneration (AMD), Ceruloplasmin, Hephaestin, Hepcidin
1. Introduction
Although the retina requires iron, retinal iron flux must be tightly regulated to prevent catalysis of Fenton chemistry and ensuing oxidative stress. Iron is not excreted from the body, therefore it accumulates in tissues, including the retina, leading to age-related oxidative injury (Song and Dunaief, 2013). Ceruloplasmin/hephaestin double-knockout mice (Cp/Heph DKO) and hepcidin knockout mice (Hepc KO) accumulate intracellular retinal iron in an age-dependent manner, exhibit photoreceptor degeneration, and model some features of age-related macular degeneration (AMD), the leading cause of irreversible blindness among individuals over the age of 50. The mechanism of iron accumulation in the retinas of these knockout mice, despite the downregulation of transferrin-bound iron-importer, transferrin receptor (TfR), is not presently understood (Hadziahmetovic et al., 2008; 2011; Hahn et al., 2004).
Iron import relies on two canonical pathways: transferrin-bound iron (TBI) import and non-transferrin bound iron (NTBI) import. TBI uptake is mediated by transferrin (Tf), an extracellular ferric iron carrier protein, and TfR. Tf bound to two ferric iron atoms (holo-Tf) binds to TfR and the complex is endocytosed. The resulting endosome is acidified promoting ferric iron dissociation from the Tf-TfR complex. Endosomal ferric iron is reduced by a ferrireductase to ferrous iron, which can then be exported from the endosome into the cytoplasm by a ferrous iron transporter – predominantly divalent metal transporter 1 (Dmt1) (Rouault and Cooperman, 2006; Song and Dunaief, 2013). It is important to note that there are two transferrin receptors, TfR1 and TfR2. TfR1 is a high affinity receptor while TfR2 is a lower affinity receptor. TfR2 has previously been shown to bind human hemochromatosis protein (HFE) on its intracellular side. Binding of holo-Tf to TfR2 results in a structural shift in the TfR2 protein, resulting in the release of HFE into the cytosol. This allows HFE to regulate other iron homeostasis proteins (Goralska et al., 2009). In contrast to TBI, NTBI import machinery brings labile, extracellular ferrous iron directly across the plasma membrane without moving through the Tf-TfR endosomal complex. TBI and NTBI importers function at different pH levels which optimizes their performance in the acidified endosome and plasma membrane respectively.
Dmt1 is believed to be the predominant ferrous iron transporter in TBI import, as it functions optimally at the low pH found in the acidified endosome (Liuzzi et al., 2006; Skjørringe et al., 2015; Wang et al., 2012). There are four isoforms of Dmt1 mRNA, two of which contain a 3’ iron-responsive element (IRE) (Skjørringe et al., 2015). IREs are non-coding sequences located in either the 5’ or 3’ untranslated region (UTR) of mRNA. Iron regulatory protein (Irp) can bind either the 5’ or the 3’ IRE with very different effects. At the 3’ UTR, Irp binding increases mRNA stability. At the 5’ IRE, Irp sterically hinders translation by blocking elongation factor binding near the start codon. Irp’s ability to bind mRNA, in either the 5’ or 3’ UTR, is mediated by intracellular iron concentrations. Labile iron binds Irp, preventing Irp-IRE interaction (Zhang et al., 2014). Dmt1 mRNA contains a 3’ IRE, and thus experiences an increase in mRNA stability in iron-deficient conditions. The inverse is also true – when cells are iron loaded, Irp is bound to iron, not the 3’ UTR, and Dmt1 translation decreases due to decreased mRNA stability (Theil, 2015).
Zip8 and Zip14 function optimally at physiological pH (7.4) and are thus implicated in uptake of NTBI from blood and extracellular fluids (Liuzzi et al., 2006; Wang et al., 2012), although Zip14 has also been shown to participate in a limited capacity in TBI export from endosomes (Zhao et al., 2010). Zip8 and Zip14 belong to the LIV-1 subfamily of the Zrt- and Irt-like protein family and show 50% primary amino acid sequence homology, with 90% homology in the proposed divalent metal ion pore (Wang et al., 2012). Existing work on iron transporters within the LIV-1 subfamily has largely centered on Zip14. Zip14 is negatively regulated by HFE at the post-translational level, decreasing Zip14 protein half-life from 11 hours to 7.5 hours in HepG2 cells (Gao et al., 2008). In hepatocytes, increased intracellular iron results in increased Zip14 protein levels (Nam et al., 2013) as iron inhibits membrane extraction of internalized Zip14, preventing proteasomal degradation (Zhao et al., 2014). Lipopolysaccharide and interleukin-6 exposure lead to increased zip14 gene expression in vitro and in vivo, suggesting a role for Zip14-mediated divalent metal transport during periods of inflammation (Liuzzi et al., 2006). Zip14 serves as one link between chronic inflammation and iron accumulation, two of the factors implicated in the pathogenesis of age-related macular degeneration (Song and Dunaief, 2013). In the liver and pancreas, Zip14 is essential for NTBI uptake and is necessary for hepatic iron accumulation in another mouse model of organ-specific iron accumulation, HFE KO (Jenkitkasemwong et al., 2015). Zip8 cell-surface expression is also enhanced in iron-loaded rat hepatoma cells (Wang et al., 2012), although the mechanism has not yet been elucidated.
Cellular iron export is mediated by ferroportin (Fpn), a transmembrane iron transporter that cooperates with ferroxidases ceruloplasmin (Cp) and hephaestin (Heph) to export intracellular ferrous iron and convert it to the ferric state. Ferroportin is regulated by trans-acting factors, including the peptide hormone hepcidin (Hepc). Hepc binds Fpn, triggering its internalization and degradation. In the retina, Hepc production is upregulated locally by inflammation and elevated iron levels (Song and Dunaief, 2013).
In this study we test the hypothesis that Zip14, and perhaps Zip8, may be upregulated in iron loaded retinas, possibly contributing to retinal iron overload in these mice. We show that Zip8 and Zip14 are differentially regulated in two mouse models of retinal iron accumulation, Cp/Heph DKO and Hepc KO mice. Both Zip8 and Zip14 protein levels are increased in the Cp/Heph DKO retinas. However, Zip8, but not Zip14 protein levels, are increased in Hepc KO retinas. We identify transferrin saturation as a possible mediator of this differential regulation in these two models of retinal iron accumulation and demonstrate that Zip14, but not Zip8, levels can be modulated by intravitreal injection of holo-Tf. The upregulation of one or both importers in iron-loaded retinas suggests a role for Zip8 and Zip14 in the progressive retinal iron accumulation observed in Cp/Heph DKO and Hepc KO mice.
2. Materials and methods
2.1 Animals
C57BL/6J mice carrying a hypomorphic mutation in the Heph gene (Hephsla/sla or Hephsla/Y), and a targeted null mutation in the Cp gene (Cp−/−) were generated as previously published (Hahn et al., 2004) and are referred to herein as Cp/Heph double-knockout mice (Cp/Heph DKO). Cp/Heph DKO and were sacrificed at 7 months of age as previous reports have shown retinal iron overload by this age (Hahn et al., 2004). Hepc KO mice were generated as previously published (Lesbordes-Brion et al., 2006a) and were sacrificed at 13.5 months of age in line with previous reports which show retinal iron accumulation by this age (Hadziahmetovic et al., 2011). IL6−/− mice (B6.129S2-IL6tm1Kopf/J) were obtained from the Jackson Laboratory (Bar Harbor, ME) and are referred to herein as IL6 KO mice. Retinas from Zip14−/− mice were generously provided by Dr. Robert Cousins (University of Florida), and are referred to herein as Zip14 KO mice (Aydemir et al., 2012). Age, gender, and strain-matched Wt mice were used as controls. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Gene expression of Dmt1, Zip8, and Zip14 in the neurosensory retina was analyzed using quantitative real-time PCR as previously published (Hadziahmetovic et al., 2011). Gene expression assays (TaqMan; Applied Biosystems, Foster City, CA) were used for PCR analysis. Probes used were Zip8 (zip8, Mm00470855_m1), Zip14 (slc39a14, Mm01317439_m1), and Dmt1 (dmt1-1, 5′-TGCGGAAGCTAGAAGCATTT and 5′-CCACAGCCAGTGTCGAGTTA; dmt1-2, 5′-ACAGCTTCCCTTTGCTCTCA and 5′-CCAACCAACGGTTGAGTCAT; dmt1–3,4, 5′-CGCCCAGATTTTACACAGTG and 5′-AAGCTTCACTACCTGCACAC). Eukaryotic 18S rRNA (Hs99999901_s1) served as an internal control. RT-PCR (TaqMan; Applied Biosystems) was performed on a commercial sequence detection system (ABI Prism 7500; Applied Biosystems). All reactions were performed in biological and technical triplicates.
2.3 Whole Cell Protein Extraction and Western Blotting
Whole retina protein lysates were extracted using Laemmli SDS lysis buffer supplemented with protease/phosphatase inhibitor mixture and PMSF (Cell Signaling Technology). Lysates were treated and run as described previously (Li et al., 2015). Rabbit anti-Dmt1 (Alpha Diagnostic International NRAMP24-A), rabbit anti-Zip8 (ThermoScientific PA5-21073), rabbit anti-Zip14 (ThermoScientific PA5-21077), and rabbit anti-alpha tubulin (ThermoScientific PA5-22060) primary antibodies were used in combination with secondary antibody IRDye 680RD donkey anti-rabbit (LI-COR P/N 926-68072). Imaging was done using GE Amersham Imager 600. ImageJ software was used for band densitometry.
2.4 Intravitreal Injection
Intravitreal injections on IL6 KO mice were performed as described previously (Hadziahmetovic et al., 2011). The right eye of each animal was injected with 2µL of 1.2 mM holo-Tf and the left eye was injected with 2µL of 1.2 mM apo-Tf as a control (Millipore, Billerica, MA). Mice were euthanized 24 hours post-injection and retinas were collected.
2.5 Statistical Analysis
Mean ± SEM was calculated for each group. Student’s two-group, two-tailed t-test was used for statistical analysis of relative mRNA and band densitometry levels. Unpaired t-tests were used for all analysis except in the case of IL6 KO mice injected with either holo-Tf or apo-Tf. Each mouse’s left eye was injected with apo-Tf while the right eye was injected with holo-Tf. Thus we used a paired t-test. All statistical analyses were performed using GraphPad Prism 7.0 (San Diego, CA, USA).
3. Results
3.1 Changes in Retinal mRNA levels of Dmt1, Zip8 and Zip14 in Cp/Heph DKO Mice
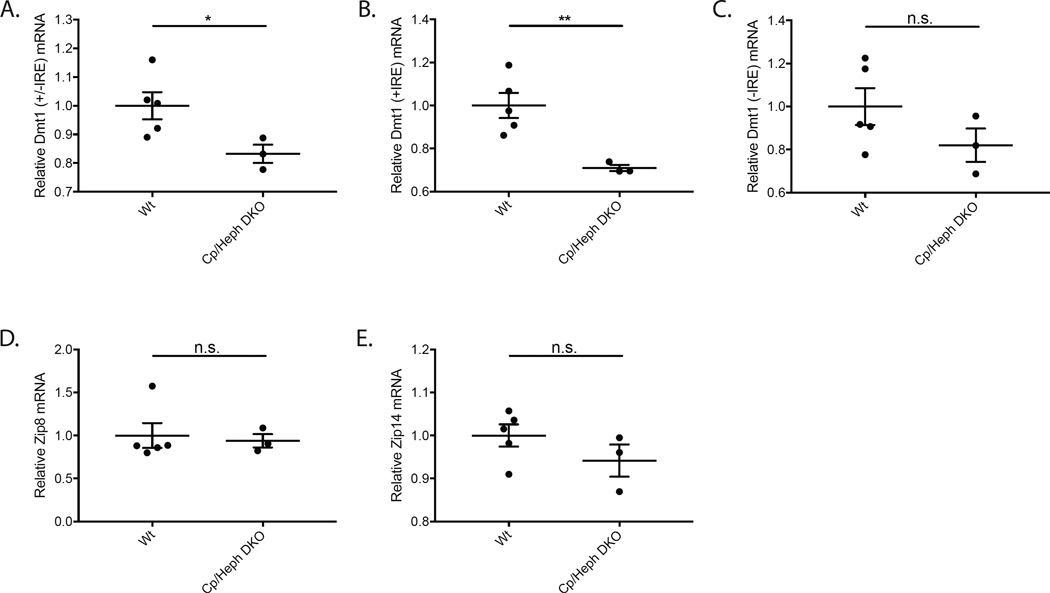
Gene expression of ferrous iron importers was analyzed using qPCR in the neurosensory retinas of 7 month old Cp/Heph DKO mice relative to age-, strain- and sex-matched Wt control mice. The total level of all Dmt1 mRNA (+/−IRE) decreased (Fig. 1A). The levels of the two Dmt1 mRNA isoforms with an IRE (+IRE), measured using the same probe, decreased (Fig 1B). mRNA levels of the two isoforms of Dmt1 mRNA lacking an IRE (−IRE), measured using the same probe, were not significantly different (Fig. 1C). Retinal Zip8 and Zip14 mRNA levels did not change (Fig. 1D–E). It has been previously reported that Zip8 cell-surface expression is increased in response to intracellular iron loading (Wang et al., 2012). Similarly, Zip14 protein has been shown to be upregulated in iron-loaded cells (Nam et al., 2013). This suggests modulation of Zip8 and Zip14 by iron accumulation may occur on the post-transcriptional level.
Figure 1. Quantitative PCR showing relative mRNA levels of ferrous iron importers in Cp/Heph DKO retinas compared to Wt controls.
Scatterplots showing relative mRNA levels in Cp/Heph DKO mice and age-, strain-, and sex-matched Wt controls measured by qPCR. Total Dmt1 (+/−IRE) mRNA levels were lower in Cp/Heph DKO mice (A). mRNA levels of the two Dmt1 (+IRE) isoforms were significantly lower in Cp/Heph DKO mice (B). Dmt1 (−IRE) mRNA levels were not significantly different in the two genotypes (C).The mRNA levels of Zip8 and Zip14 were not significantly changed between the Wt and Cp/Heph DKO retinas (D–E). Numbers represent mean values (±SEM). Wt controls (n=5) and Cp/Heph DKO neural retinas (n=3). * p < 0.05, ** p < 0.01.
3.2 Changes in Dmt1, Zip8, and Zip14 protein levels in Cp/Heph DKO Mice
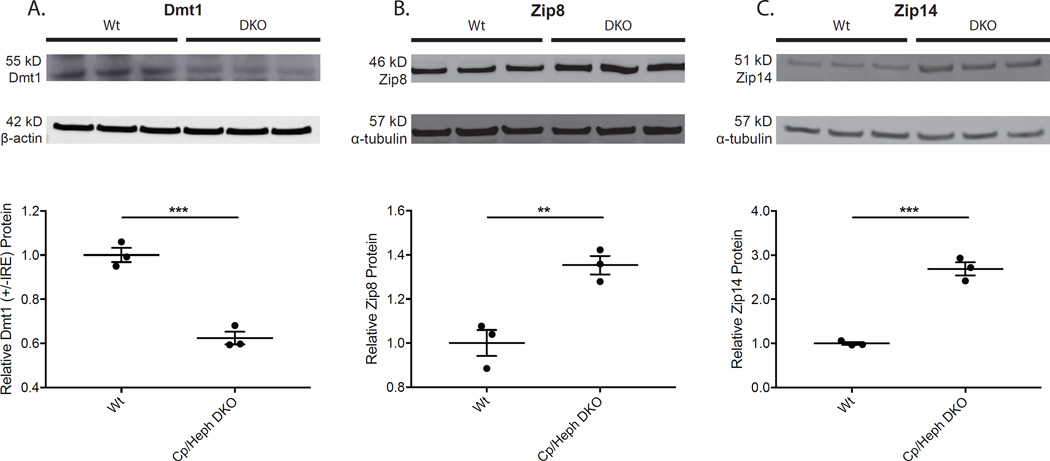
To test for post-transcriptional regulation of ferrous iron importers, semi-quantitative Western analysis was performed in 7 month old Cp/Heph DKO mice relative to age-, strain- and sex-matched Wt control mice. Dmt1 levels decreased (Fig. 2A). The decrease in Dmt1 protein level in Cp/Heph DKO retinas is consistent with the decrease in Dmt1 (+IRE) mRNA levels seen in qPCR analysis (Fig. 1B). Thus, in high intracellular iron conditions, total Dmt1 protein decreases, as does the level of TfR protein (Hadziahmetovic et al., 2008), indicating that TBI import is diminished. This suggests that progressive iron accumulation observed in Cp/Heph DKO retinas (Hahn et al., 2004) is not a result of TBI import.
Figure 2. Western analysis showing levels of ferrous iron importers in Cp/Heph DKO compared to Wt neural retina.
Relative protein levels in Cp/Heph DKO and Wt mouse neural retinas were evaluated using Western analysis. Dmt1 protein levels decreased (A), while Zip8 (B) and Zip14 (C) levels increased in Cp/Heph DKO retinas. Western analysis for Dmt1 – 55 kD (A), Zip8 – 46 kD (B), and Zip14 – 51 kD (C) on neural retinas from 7 month old age-, strain-, and sex-matched Wt controls (n=3) and Cp/Heph DKO mice (n=3). Loading control α-tubulin (57 kD) or β-actin (42 kD) bands are shown below each set of lanes. Plots of band densitometry normalized to loading control calculated using Image J software. Numbers represent mean values (±SEM). Wt controls (n=3) and Cp/Heph DKO neural retinas (n=3). ** p < 0.01, *** p < 0.001.
Western analysis from the same retinal protein extracts showed that Zip8 and Zip14 protein levels increased in Cp/Heph DKO mice (Fig. 2B–C) despite no change in Zip8 and Zip14 mRNA levels in Cp/Heph DKO retinas relative to Wt controls (Fig. 1D–E). Specificity of the 51 kD Zip14 band was confirmed in retinas from Zip14 KO mice (data not shown). This suggests that retinal Zip8 and Zip14 are post-transcriptionally regulated by iron in Cp/Heph DKO mice. A post-transcriptional mechanism of Zip14 upregulation has previously been demonstrated in hepatocyte cell culture in which iron prevents membrane extraction and subsequent degradation of Zip14 (Zhao et al., 2014). To determine if these post-transcriptional regulatory effects of iron on Zip8 and Zip14 are consistent across different transgenic mouse models of retinal iron loading, we tested mRNA and protein levels of Dmt1, Zip8, and Zip14 in Hepc KO mice as well.
3.3 Changes in Retinal mRNA levels of Dmt1, Zip8 and Zip14 in Hepc KO Mice
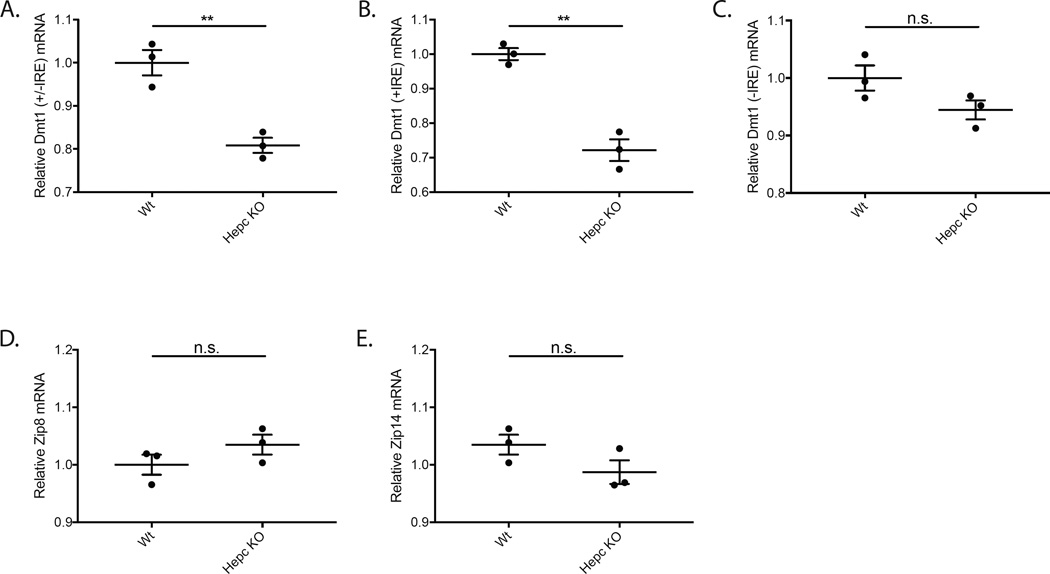
Regulation of Dmt1 mRNA in Hepc KO neurosensory retina mirrored our findings in the Cp/Heph DKO retina. Total Dmt1 (+/−IRE) mRNA in the Hepc KO retina decreased relative to age-, strain-, and sex-matched Wt controls (Fig. 3A). Levels of the two Dmt1 mRNA isoforms with an IRE (+IRE) decreased (Fig. 3B). Levels of the two isoforms of Dmt1 mRNA lacking IREs (-IRE) were not significantly different (Fig. 3C). Retinal Zip8 and Zip14 mRNA levels did not change in 13.5 month old Hepc KO mice relative to age-, strain- and sex-matched Wt control mice (Fig. 3D–E), mimicking the trend observed in Cp/Heph DKO retinas (Fig. 1D–E). These data show that in this second model of retinal iron accumulation Zip8 and Zip14 are not regulated by retinal iron on the transcriptional level.
Figure 3. Quantitative PCR showing relative mRNA levels of ferrous iron importers in Hepc KO retinas compared to Wt controls.
Scatterplots showing relative mRNA levels in Hepc KO mice and age-, strain-, and sex-matched Wt controls measured by qPCR. Total Dmt1 (+/−IRE) mRNA levels were lower in Hepc KO mice (A). mRNA levels of the two Dmt1 (+IRE) isoforms were significantly lower in Hepc KO mice (B). Dmt1 (−IRE) mRNA levels were not significantly different in the two genotypes (C). The mRNA levels of Zip8 and Zip14 were not significantly changed between the Wt and Hepc KO retinas (D–E). Numbers represent mean values (±SEM). Wt controls (n=3) and Hepc KO neural retinas (n=3). ** p < 0.01.
3.4 Changes in Dmt1, Zip8, and Zip14 protein levels in Hepc KO Mice
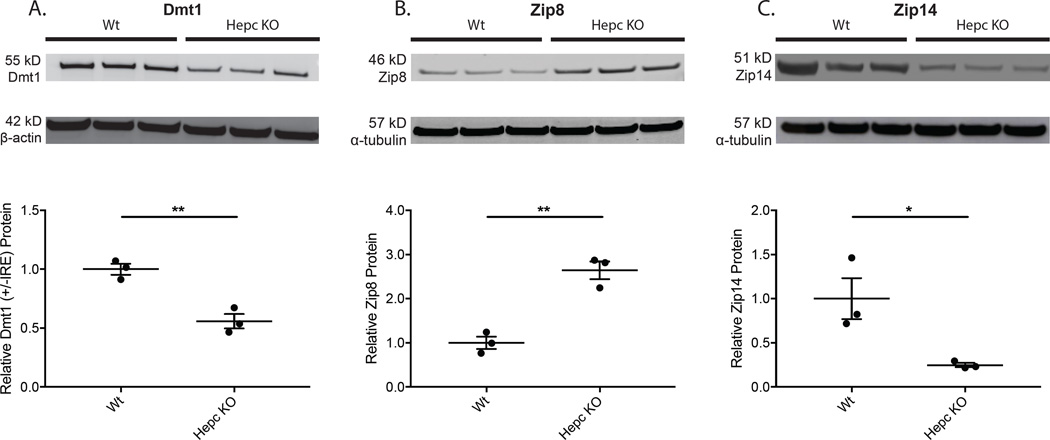
Semi-quantitative Western analysis showed that Dmt1 levels decreased in Hepc KO retinas relative to age-, strain-, and sex-matched Wt controls (Fig. 4A), consistent with the regulation of Dmt1 (+IRE) mRNA isoforms by the Irp-IRE regulatory axis. This observation reaffirms the data from Cp/Heph DKO iron-loaded retinas which showed a marked decrease in Dmt1 (+IRE) mRNA (Fig. 1A–B) and Dmt1 protein (Fig. 2A). These data, together with our prior observation of decreased TfR in the neural retinas of Hepc KO mice (Hadziahmetovic et al., 2011), suggest that progressive retinal iron accumulation in Hepc KO mice is not a result of TBI import.
Figure 4. Western analysis of neural retinas showing levels of ferrous iron importers in Hepc KOs compared to Wt.
Relative protein levels in Hepc KO and Wt mouse neural retina were evaluated using Western analysis. Dmt1 protein levels decreased (A), while Zip8 (B) levels increased in Hepc KO retinas. Interestingly, Zip14 levels decreased (C). Western analysis for Dmt1 – 55 kD (A), Zip8 – 46 kD (B), and Zip14 – 51 kD (C) on neural retinas from 13.5-month-old Wt (n=3) and Hepc KO mice (n=3). Loading control α-tubulin (57 kDa) or β-actin (42 kD) bands are shown below each set of lanes. Plots of band densitometry normalized to loading control calculated using Image J software. Numbers represent mean values (±SEM). Wt controls (n=3) and Hepc KO neural retinas (n=3). * p < 0.05, ** p < 0.01.
Western analysis showed increased retinal Zip8 protein levels in Hepc KO mice (Fig. 4B), similar to the result in Cp/Heph DKO retinas (Fig. 2B). In contrast to the results from Cp/Heph DKO retinas (Fig. 2C), Zip14 protein levels decreased in Hepc KO retinas (Fig. 4C). Cp/Heph DKO mice lack ferroxidases ceruloplasmin and hephaestin. These mice do not efficiently export iron from the gut into the blood, due to lack of hephaestin’s ferroxidase function. Thus, blood iron and transferrin saturation levels are low (ZL Harris, personal communication). In contrast to the Cp/Heph DKO transgenic mice, Hepc KO mice lack hepcidin, the hormone responsible for the degradation of Fpn, and thus have increased levels of Fpn (Nemeth et al., 2004). As a result, Hepc KO mice have increased levels of extracellular ferric iron and higher transferrin saturation relative to Wt mice (Mastrogiannaki et al., 2012) and Cp/Heph DKO mice. To determine if Zip14 levels are sensitive to transferrin saturation, potentially implicating transferrin saturation in the differential regulation of Zip14 in our two transgenic mouse retinas, we injected mice with apo- or holo-Tf in each eye and measured Zip8 and Zip14 protein levels.
3.5 Changes in levels of Zip8 and Zip14 in High Transferrin Saturation Conditions
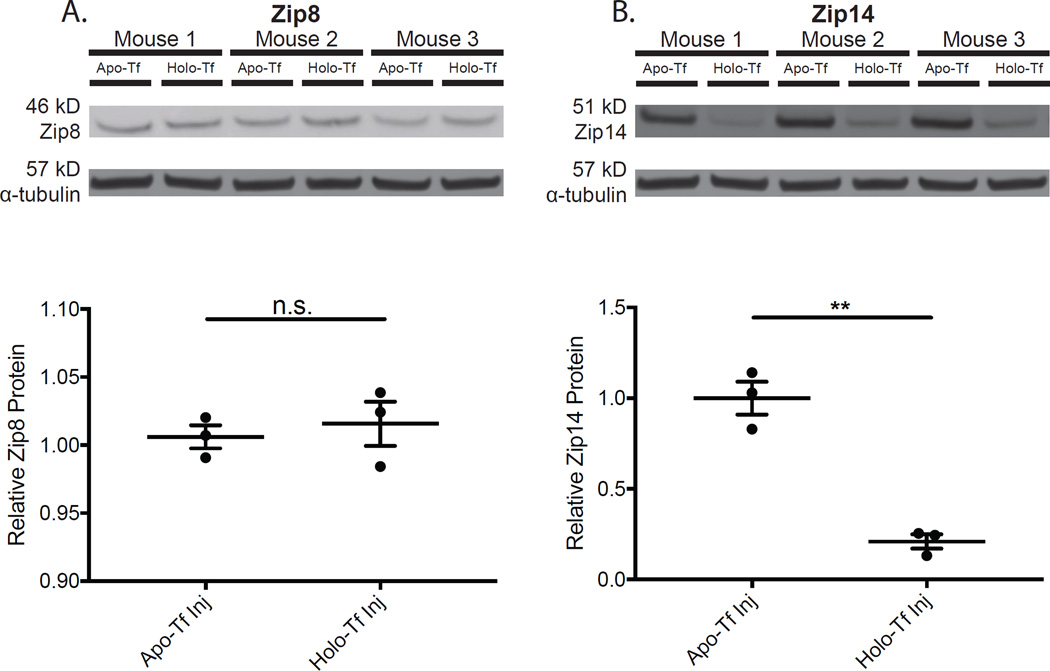
To test the effect of holo-Tf on Zip8 and Zip14, IL6 KO mice were injected intravitreally with apo- or holo-Tf to determine whether increased levels of holo-Tf had a regulatory effect on Zip8 and Zip14. We used IL6 KO mice for these intravitreal injection experiments because needle-injury in the mouse eye upregulates IL6 (Hadziahmetovic et al., 2011), which in turn upregulates Zip14 mRNA (Liuzzi et al., 2005). Western analysis revealed that Zip8 protein levels were unchanged in the holo-Tf injection condition compared to apo-Tf injection (Fig. 5A). Zip14 levels were decreased in the holo-Tf injection condition (Fig. 5B), suggesting that high transferrin saturation conditions may contribute to the decrease in Zip14 protein observed in Hepc KO retinas (Fig. 4C).
Figure 5. Western analysis of IL6 KO neural retinas showing levels of Zip8 and Zip14 in response to intravitreal injection of Holo-Transferrin.
Relative Zip8 and Zip14 protein levels in IL6 KO neural retinas treated with Holo- or Apo-Transferrin. Zip14, not Zip8, protein levels were modulated by Holo-Tf. Zip8 protein levels were unchanged after Holo-Tf injection compared to Apo-Tf injection (A) while Zip14 levels decreased in the retinas of IL6 KO eyes injected with Holo-Tf compared to Apo-Tf injection (B). Western analysis for Zip8 – 46 kD (A) and Zip14 – 51 kD (B) on neurosensory retinas from IL6 KO mice injected with Apo-Tf (n=3) or Holo-Tf (n=3). Loading control α-tubulin (57 kDa). Plots of band densitometry normalized to loading control calculated using Image J software. Numbers represent mean values (±SEM). ** p < 0.01.
3.6 In silico analysis of Zip8 and Zip14 mRNA expression in different regions and cell types of the retina
Attempted immunolocalization of Zip8 and Zip14 in the retina resulted in non-specific labeling. To determine which retinal cell types express Zip8 and Zip14, we utilized in silico analysis of existing retinal and RPE/choroid gene expression databases. These showed Zip8 and Zip14 expression in the mouse neurosensory retina (NSR) (Hadziahmetovic et al., 2012), retinal pigment epithelium (RPE) (Hadziahmetovic et al., 2012), retinal ganglion cells (Blackshaw et al., 2004), photoreceptors (Blackshaw et al., 2004), neurosensory retina vascular endothelial cells (I Benedicto, G Lehmann, E Rodriguez-Boulan, Personal Communication), choroidal vascular endothelial cells (I Benedicto, G Lehmann, E Rodriguez-Boulan, Personal Communication), and Müller cells (E Macosko, Personal Communication) (Table 1).
Table 1. In silico analysis of Zip8 and Zip14 mRNA expression in different regions and cell types of the mouse retina.
Analysis of several databases shows expression of Zip8 and Zip14 across the neurosensory retina (NSR), retinal pigment epithelium (RPE), and choroid. Each “X” indicates expression of both Zip8 and Zip14 in the indicated tissue or cell type. Zip8 and Zip14 mRNA is detected in vascular endothelial cells of the choroid and neural retina. Within the NSR, Zip8 and Zip14 is expressed in retinal ganglion cells, Müller cells, and photoreceptors.
| Adult NSR |
Adult RPE | Choroid - Vascular Endothelial Cells |
NSR - Vascular Endothelial Cells |
Ganglion Cells | Photoreceptors | Müller Cells | |
|---|---|---|---|---|---|---|---|
| Mouse Retina SAGE Library1 |
X | X | X | ||||
| Rodriguez-Boulan Lab2 | X | X | |||||
| Dunaief Lab3 | X | X | |||||
| McCarroll Lab4 | X | X | X | X | X |
- Mouse retina was isolated, and serial analysis of genomic expression (SAGE) libraries derived from retinal tissue were constructed, as described previously (Blackshaw et al., 2004);
- Choroid and NSR vascular endothelial cells were isolated by cell sorting after endothelial cell labeling (Nolan et al., 2013). RNA was extracted immediately after cell sorting and used for RNAseq analyses (I Benedicto, G Lehmann, E Rodriguez-Boulan, Personal Communication);
– Microarray analysis of the NSR and RPE transcriptome (Hadziahmetovic et al., 2012);
– Drop-seq analysis of P14 mouse NSR was used to define the transcriptome of each NSR cell type (E Macosko, Personal Communication).
4. Discussion
Zip8 and Zip14, recently shown to import iron into cells, are expressed in the retina. These two ferrous iron importers and a third, Dmt1, are all regulated by iron, but the direction of regulation and responsiveness to different forms of iron is unique for each protein. Protein levels of all three ferrous iron importers, Dmt1, Zip8, and Zip14, were altered in Cp/Heph DKO retinas and Hepc KO retinas compared to age-, strain-, and sex-matched Wt controls. Dmt1 mRNA and protein levels decreased in both Cp/Heph DKO (Fig. 1A, 2A) and Hepc KO (Fig. 3A, 4A) retinas. Zip8 and Zip14 mRNA levels (Fig. 1D–E, Fig. 3D–E) did not change in response to retinal iron loading in either transgenic model. Although the quantity of Zip8 protein levels increased in both models (Fig. 2B, 4B), Zip14 protein levels were regulated differently in the two models –increased in the Cp/Heph DKO retina (Fig. 2C) but significantly lower in Hepc KO retinas compared to Wt controls (Fig. 4C). To explain this difference in regulation between Zip8 and Zip14, we performed intravitreal injections of either apo- or holo-Tf and showed that Zip14, but not Zip8, protein levels decrease in response to increased retinal Holo-Tf (Fig. 5).
Cp/Heph DKO mice have impaired ferroxidase function and suffer from deficient intestinal dietary iron uptake, low blood iron levels, and anemia. Despite low blood iron levels and down-regulation of TBI import proteins, TfR and Dmt1, Cp/Heph DKO mice exhibit age-dependent intracellular iron accumulation throughout the neural retina (Hadziahmetovic et al., 2008). To identify iron importers that could be responsible for this accumulation we measured retinal mRNA and protein levels of Dmt1, Zip8, and Zip14 – comparing retinal extracts from Wt and Cp/Heph DKO mice. We found that Zip8 and Zip14, previously studied in the liver, are also expressed in the retina. The protein levels of these two ferrous iron importers, but not Dmt1, increased in Cp/Heph DKO retinas (Fig. 2). This upregulation could increase import of labile, ferrous iron into cells of the neural retina (Fig. 6B). This postulate is consistent with previous work which demonstrated that hepatic iron loading in HFE KO mice is dependent upon Zip14 (Jenkitkasemwong et al., 2015). Zip14 may contribute to the retinal iron accumulation characteristic of Cp/Heph DKO retinas, in a manner similar to that seen in HFE KO liver (Fig. 6B).
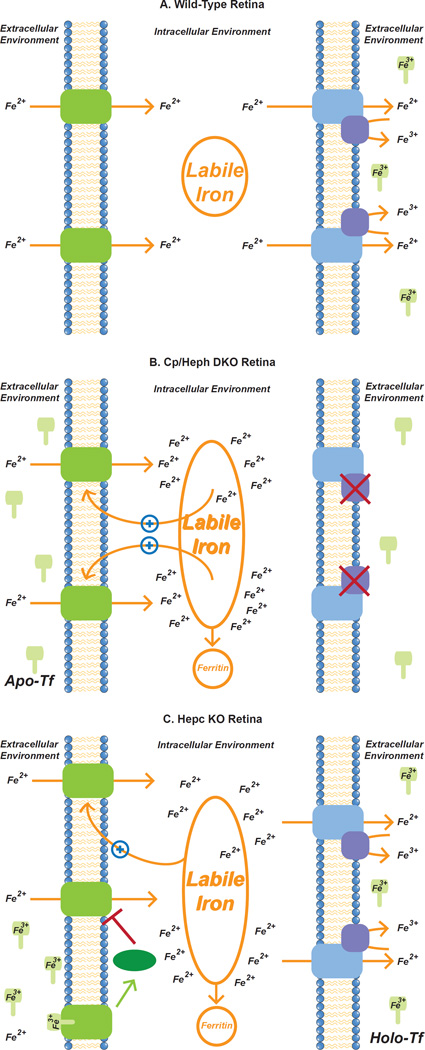
Figure 6. Potential role of Zip8 and 14 in retinal iron import in Wt, Cp/Heph DKO and Hepc KO retinas.
Wt retina allows ferrous iron import across cell membranes through Zip8 and Zip14 while simultaneously exporting labile intracellular iron from the intracellular to the extracellular environment via ferroportin (Fpn). On the extracellular side, ferrous iron is oxidized by Cp and/or Heph and then bound by transferrin, which sequesters extracellular iron and prevents catalysis of the Fenton reaction (A). The hypothesized goal of this system is to sequester extracellular ferrous iron intracellularly within ferritin or extracellularly within transferrin. Sequestration prevents the formation of dangerous reactive oxygen species and subsequent deleterious effects while meeting the metabolic iron needs of the cells (Song and Dunaief, 2013). In the Cp/Heph DKO retina, impaired iron export, via mutations in ferroxidases Cp and Heph, results in the build-up of intracellular labile iron levels leading to previously documented increased ferritin levels (Hadziahmetovic et al., 2008). This increase in the amount of labile intracellular iron delays extraction and proteasomal degradation of Zip14 as shown previously (Zhao et al., 2014). We propose that Zip8 levels may increase in Cp/Heph DKO retinas by the same mechanism. This could initiate a positive feedback loop that leads to increased intracellular iron accumulation, eventually exceeding the ability of ferritin to sequester the iron (B). Hepc KO mice also exhibit intracellular retinal iron loading. Based on the data presented herein on the differential regulation of Zip14 in Cp/Heph DKO and Hepc KO retinas, we propose that high Holo-Tf levels in Hepc KO retinas lead to decreased levels of Zip14 protein without affecting Zip8 protein levels. Zip8 levels increase due to inhibition of membrane extraction by iron as proposed in our model of Cp/Heph DKO retinas. We suggest that in the retina, the mechanism for Zip14 degradation may be modulated by HFE. HFE has previously been shown to decrease Zip14 half-life in human hepatoma cells (Gao et al., 2008) and is expressed in the retina (Gnana-Prakasam et al., 2010) (C). Zip8 and Zip14 could play a significant role in retinal iron accumulation via a positive feedback loop whereby intracellular iron delays Zip14, and possibly Zip8, membrane extraction and degradation.
In order to better understand the role of iron in the increase of Zip8 and Zip14 protein we measured retinal mRNA and protein levels of Dmt1, Zip8, and Zip14 in another transgenic model of retinal iron accumulation, Hepc KO (Fig. 3–4). Consistent with results from Cp/Heph DKO, Dmt1 protein levels decreased (Fig. 4A) and Zip8 levels increased (Fig. 4B) in Hepc KO retinas. Interestingly, Zip14 levels decreased (Fig. 4C) in the Hepc KO retina. Since Cp−/− mice have low serum transferrin saturation (Harris et al., 1999; Patel et al., 2002) and mice with decreased hepcidin expression mice have high serum transferrin saturation (Fleming et al., 2011; Lesbordes-Brion et al., 2006b; Nicolas et al., 2001), we tested the role of transferrin saturation in regulation of Zip8 and Zip14. To accomplish this we injected IL6 KO mice intravitreally with either apo- or holo-Tf in each eye and measured changes in Zip8 and Zip14 protein levels (Fig. 6C). In these retinas Zip8 levels were insensitive to holo-Tf (Fig. 5A), while Zip14 levels decreased dramatically (Fig. 5B). HFE, sequestered by TfR2 at moderate and low levels of transferrin saturation, can be released at high transferrin saturation levels (Feder et al., 1998) and decrease Zip14 half-life from 11 hours to 7.5 hours in HepG2 cells (Gao et al., 2008). Thus it is likely that HFE, which is expressed in the retina (Gnana-Prakasam et al., 2010), is responsible for the decrease in Zip14 protein observed in high holo-Tf conditions (Fig. 6C). This mechanism provides an explanation for the decrease in Zip14 observed in Hepc KO retinas. Retinal cells that constitute the blood-retinal barrier (retinal vascular endothelium) and RPE, as well as cells inside the blood-retinal barrier are likely to be exposed to holo-Tf. High transferrin saturation has been observed in Hepc KO serum, and is also likely to occur in the neural retina, since transferrin is present in the retina and vitreous (Song and Dunaief, 2013). Additionally, Fpn, present at higher levels due to the absence of hepcidin, can transport more iron across the blood-retinal barriers (Theurl et al., 2015).
We hypothesize that the upregulation of Zip8 and Zip14 protein in Cp/Heph DKO (Fig. 2B–C) and Zip8 in Hepc KO (Fig. 4B) retinas is due to increased intracellular labile iron accumulation which inhibits membrane extraction of Zip14 as described previously (Fig. 6B) (Zhao et al., 2014). Given the high degree of amino acid homology between Zip8 and Zip14 (Wang et al., 2012), it is possible that Zip8 is regulated by iron in the same manner as Zip14. This is consistent with the prior observation that cell-surface expression of Zip8 is enhanced in iron-loaded rat hepatoma cells (Wang et al., 2012).
In silico analysis revealed that Zip8 and Zip14 are expressed in retinal vascular endothelial cells and Müller cells. If the broader trends in retinal Zip8 and Zip14 regulation are maintained within these particular cell types, then Zip8 and Zip14 within these cells may be responsible for the progressive accumulation of retinal iron in these transgenic models despite already toxic amounts of retinal iron. In Cp/Heph KO mice, NTBI may be taken up by Zip8 and/or Zip14 on vascular endothelial cells, exported as ferrous iron by Fpn, then imported directly into adjacent Müller cells by Zip8 or Zip14 without being oxidized or binding to transferrin. Once inside the Müller cell, iron can be redistributed within the retina (Theurl et al., 2015).
Zip8 and Zip14 appear to display a positive feedback loop behavior with labile intracellular iron in mouse models of retinal iron accumulation. In this proposed pathway (Fig. 6B–C), ferrous iron imported through Zip8 and Zip14 delays their degradation. This pathway may be a key component of the iron buildup seen in Cp/Heph DKO and Hepc KO retinas, and could play a role in the progressive iron accumulation seen in human AMD retinas. It is also possible that Zip8 and Zip14 are both critical to the mechanism of retinal degeneration seen in the Wong et al. (2007) intravitreal labile iron injection study. This positive feedback loop may exist to promote anti-microbial iron sequestration, as it prioritizes the sequestration of labile, unbound iron which could be more easily taken up by invading pathogens (Hood and Skaar, 2012).
Zip8 and Zip14 appear to be modulators of retinal iron accumulation. Given the importance of iron accumulation in AMD, further study on the role of the Zip8-Zip14-iron regulatory axis in mediating intracellular iron accumulation of the retina is warranted, particularly the development of additional knockout mouse models including a Cp/Heph/Zip8/Zip14 KO mouse and a Hepc/Zip8 KO mouse. Zip8 and Zip14 may provide a viable future therapeutic target for stemming toxic iron accumulation in human retinas.
Highlights.
Upregulation of Zip8 and Zip14 may contribute to retinal iron overload.
Zip8 and/or Zip14 protein is upregulated in mouse models of retinal iron overload.
Zip8 and Zip14 are regulated on the post-transcriptional level in iron loaded retinas.
Zip14 but not Zip8 protein levels are sensitive to intraocular holo-transferrin injection.
Acknowledgments
Funding from NIH/NEI EY015240, Research to Prevent Blindness, the F.M. Kirby Foundation, a gift in memory of Lee F. Mauger, MD, and the Paul and Evanina Bell Mackall Foundation Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: J.S., S.G., Y.S., D.S., and M.H. performed the experiments. J.S. and J.L.D. analyzed data. J.S. and J.L.D drafted and revised the manuscript.
References
- Aydemir TB, Chang S-M, Guthrie GJ, Maki AB, Ryu M-S, Karabiyik A, Cousins RJ. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia) PLoS ONE. 2012;7:e48679. doi: 10.1371/journal.pone.0048679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho S-H, Yung R, Asch E, Ohno-Machado L, Wong WH, Cepko CL. Genomic Analysis of Mouse Retinal Development. PLoS Biol. 2004;2:e247–e221. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JN, Penny DM, Irrinki A, Lee VK, Lebrón JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RE, Feng Q, Britton RS. Knockout mouse models of iron homeostasis. Annu. Rev. Nutr. 2011;31:117–137. doi: 10.1146/annurev-nutr-072610-145117. [DOI] [PubMed] [Google Scholar]
- Gao J, Zhao N, Knutson MD, Enns CA. The Hereditary Hemochromatosis Protein, HFE, Inhibits Iron Uptake via Down-regulation of Zip14 in HepG2 Cells. Journal of Biological Chemistry. 2008;283:21462–21468. doi: 10.1074/jbc.M803150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnana-Prakasam JP, Martin PM, Smith SB, Ganapathy V. Expression and function of iron-regulatory proteins in retina. IUBMB Life. 2010 doi: 10.1002/iub.326. NA–NA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goralska M, Ferrell J, Harned J, Lall M, Nagar S, Fleisher LN, McGahan MC. Iron metabolism in the eye: A review. Experimental Eye Research. 2009;88:204–215. doi: 10.1016/j.exer.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziahmetovic M, Dentchev T, Song Y, Haddad N, He X, Hahn P, Pratico D, Wen R, Harris ZL, Lambris JD, Beard J, Dunaief JL. Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD. Investigative Ophthalmology & Visual Science. 2008;49:2728–2736. doi: 10.1167/iovs.07-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziahmetovic M, Kumar U, Song Y, Grieco S, Song D, Li Y, Tobias JW, Dunaief JL. Microarray Analysis of Murine Retinal Light Damage Reveals Changes in Iron Regulatory, Complement, and Antioxidant Genes in the Neurosensory Retina and Isolated RPE. Investigative Ophthalmology & Visual Science. 2012;53:5231–5241. doi: 10.1167/iovs.12-10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziahmetovic M, Song Y, Ponnuru P, Iacovelli J, Hunter A, Haddad N, Beard J, Connor JR, Vaulont S, Dunaief JL. Age-Dependent Retinal Iron Accumulation and Degeneration in Hepcidin Knockout Mice. Invest. Ophthalmol. Vis. Sci. 2011;52:109–110. doi: 10.1167/iovs.10-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proceedings of the National Academy of Sciences. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nature Reviews Microbiology. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkitkasemwong S, Wang C-Y, Coffey R, Zhang W, Chan A, Biel T, Kim J-S, Hojyo S, Fukada T, Knutson MD. SLC39A14 Is Required for the Development of Hepatocellular Iron Overload in Murine Models of Hereditary Hemochromatosis. Cell Metabolism. 2015:1–14. doi: 10.1016/j.cmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesbordes-Brion J-C, Viatte L, Bennoun M, Lou D-Q, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006a;108:1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- Lesbordes-Brion J-C, Viatte L, Bennoun M, Lou D-Q, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006b;108:1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- Li Y, Song D, Song Y, Zhao L, Wolkow N, Tobias JW, Song W, Dunaief JL. Iron-induced Local Complement Component 3 (C3) Up-regulation via Non-canonical Transforming Growth Factor (TGF)-β Signaling in the Retinal Pigment Epithelium. J. Biol. Chem. 2015;290:11918–11934. doi: 10.1074/jbc.M115.645903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Delga S, Deschemin J-C, Vaulont S, Peyssonnaux C. Deletion of HIF-2α in the enterocytes decreases the severity of tissue iron loading in hepcidin knockout mice. Blood. 2012;119:587–590. doi: 10.1182/blood-2011-09-380337. [DOI] [PubMed] [Google Scholar]
- Nam H, Wang CY, Zhang L, Zhang W, Hojyo S, Fukada T, Knutson MD. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica. 2013;98:1049–1057. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien J-P, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S. Brain Iron Metabolism. Seminars in Pediatric Neurology. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Skjørringe T, Burkhart A, Johnsen KB, Moos T. Divalent metal transporter 1 (DMT1) in the brain: implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Frontiers in Molecular Neuroscience. 2015;8 doi: 10.3389/fnmol.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Dunaief JL. Retinal iron homeostasis in health and disease. Front Aging Neurosci. 2013;5:1–13. doi: 10.3389/fnagi.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil EC. IRE mRNA riboregulators use metabolic iron (Fe2+) to control mRNA activity and iron chemistry in animals. Metallomics. 2015;7:15–24. doi: 10.1039/c4mt00136b. [DOI] [PubMed] [Google Scholar]
- Theurl M, Song D, Clark E, Sterling J, Grieco S, Altamura S, Galy B, Hentze M, Muckenthaler MU, Dunaief JL. Mice with hepcidin-resistant ferroportin accumulate iron in the retina. The FASEB Journal. 2015:1–11. doi: 10.1096/fj.15-276758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 Is an Iron and Zinc Transporter Whose Cell-surface Expression Is Up-regulated by Cellular Iron Loading. Journal of Biological Chemistry. 2012;287:34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D-L, Ghosh MC, Rouault TA. The physiological functions of iron regulatory proteins in iron homeostasis - an update. Front Pharmacol. 2014;5:124. doi: 10.3389/fphar.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like Protein 14 (ZIP14) Promotes the Cellular Assimilation of Iron from Transferrin. Journal of Biological Chemistry. 2010;285:32141–32150. doi: 10.1074/jbc.M110.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Zhang AS, Worthen C, Knutson MD, Enns CA. An iron-regulated and glycosylation-dependent proteasomal degradation pathway for the plasma membrane metal transporter ZIP14. Proceedings of the National Academy of Sciences. 2014;111:9175–9180. doi: 10.1073/pnas.1405355111. [DOI] [PMC free article] [PubMed] [Google Scholar]