INTRODUCTION
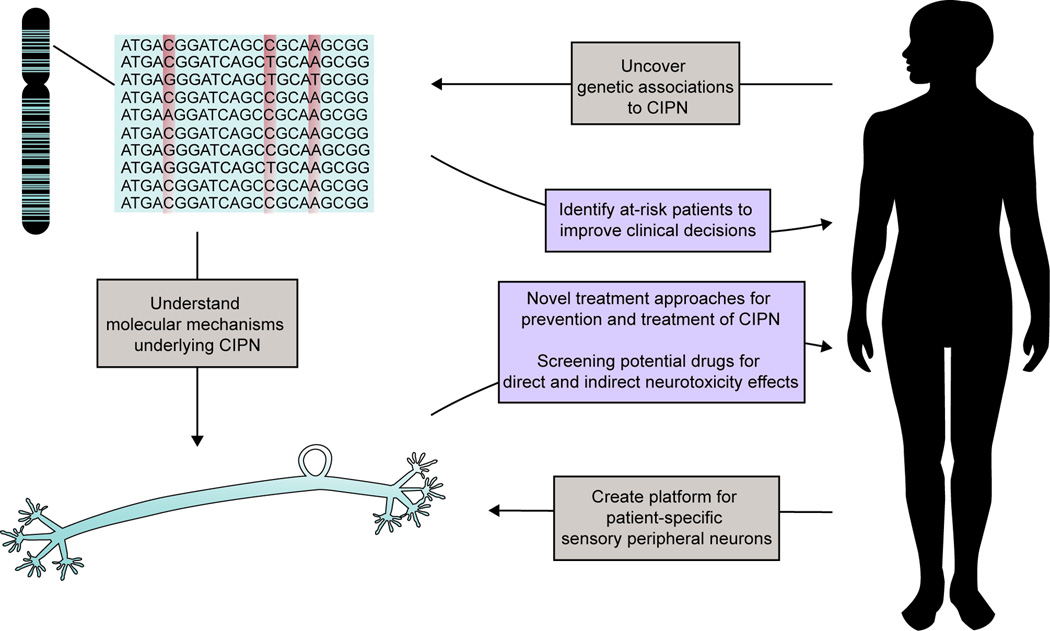
Chemotherapy-induced peripheral neuropathy (CIPN) is a common dose-limiting toxicity experienced in 30–40% of patients undergoing treatment with various chemotherapeutics, including taxanes, vinca alkaloids, epothilones, proteasome inhibitors and thalidomide. Importantly, CIPN significantly impacts a patient’s quality of life. Recent genetic association studies are enhancing our understanding of CIPN pathophysiology and serve as a foundation for identification of genetic biomarkers to predict toxicity risk and for the development of novel strategies for prevention and treatment (Figure 1).
Figure 1.
The precision medicine approach incorporates both genome-wide association studies and functional models to understand the molecular mechanisms underlying CIPN with the goal of discovering novel strategies to prevent and treat this dose-limiting toxicity. The identification of genetic biomarkers will enable screening for CIPN risk, thereby improving clinical decisions. In vitro models to study CIPN have the potential to inform how patient susceptibility develops, identify novel therapeutic strategies for treatment and prevention, and test new chemical entities for liability for this toxicity.
CHARACTERISTICS OF CIPN
Chemotherapy-induced nerve damage is predominantly a sensory neuropathy that manifests as numbness/tingling and burning sensations in a “glove and stocking” distribution, where the longest axons and most distal nerves are first affected.1 Debilitating CIPN symptoms that cause sensitivity to extreme temperatures and significantly interfere with daily activities, such as writing, dressing or even walking, can linger for up to two years and may never completely reverse in severe cases.1, 2 Clinical risk factors for developing CIPN include pre-existing neuropathy (e.g., diabetic neuropathy), prior treatment with neurotoxic agents, and high-exposure dosing regimens; however, these factors alone do not account for the interindividual variation in CIPN observed clinically. As the number of cancer survivors continues to increase, the importance of CIPN on long-term quality of life has gained recognition. Despite efforts to identify therapeutic approaches to combat CIPN, there are currently no clinical recommendations for prevention and only a moderate recommendation for the use of duloxetine in the treatment of CIPN.1 The development of clinically effective therapies to prevent and/or treat CIPN is limited by our current understanding of the pathophysiological mechanisms behind this toxicity. It is still unclear what underlying genes or genetic networks determine risk of CIPN and how they manifest the toxicity. Advances in genomic and stem cell technologies provide new approaches to address current gaps in our knowledge of drug-induced peripheral neuropathy. Below we discuss the application of genomics for discovering the pathophysiological mechanisms governing CIPN and the potential for a precision medicine-based approach in its prevention and/or treatment.
GENETIC ASSOCIATION STUDIES PROVIDE CLUES TO THE MOLECULAR MECHANISMS UNDERLYING CIPN
Advances in genome-wide approaches allow the capture of novel associations in an unbiased manner, interrogating both direct and indirect genetic effects on CIPN. While sample size limitations preclude the identification of specific genetic variants that are strongly associated with CIPN, the power of genome-wide association studies (GWAS) lies in the identification of critical genes important in the pathophysiology underlying this toxicity. Although clinical presentation of CIPN is relatively consistent among chemotherapies, recent genetic findings suggest that the predisposition and development of neurotoxicity may involve drug specific genetic networks. Further studies are needed to understand whether there may also be molecular mechanisms underlying CIPN that are shared across drugs.
Novel genes found from GWAS on CIPN have alluded to the importance of biological pathways in peripheral nerve damage and repair (summarized in Supplemental Table 1). A GWAS on oxaliplatin-induced neuropathy implicated genes relating to nerve development and neuron extension (FOXC1, ITGA1) and pain signaling neurotransmitters (TAC1).2 The first GWAS on paclitaxel-induced peripheral neuropathy identified three novel genes important in neurite growth during development (EPHA5, FZD3) and in regulation of actin in filopodia/lamellipodia formation (FGD4).3 Importantly, EPHA5 and related EPHA receptors were independently identified by multiple laboratories as critical genes for paclitaxel-induced CIPN. A recent GWAS of docetaxel-induced peripheral neuropathy identified a gene implicated in neurodegeneration (VAC14; Supplemental Table 1); a gene related to cellular structure (CEP72) was identified in a GWAS for vincristine-induced neurotoxicity.2 The implication of genes with known functions in nerve damage and repair is mirrored in the connection between rare congenital peripheral neuropathies and more common drug-induced neuropathies. In these cases, individuals with common genetic variants in Charcot-Marie-Tooth disease genes responsible for neuron morphogenesis (ARHGEF10, FGD4) only develop neuropathy symptoms when exposed to chemotherapeutics.2
Genetic association studies have also provided evidence that the cellular response to cytotoxic agents is involved in the development of CIPN. Genes related to apoptosis (ALOX12, CASP9) and inflammation (CTLA4) have been implicated in bortezomib-induced neuropathy.2 Genetic association data support the proposed mechanism that platinum agent-induced neuronal damage is initiated by the accumulation of platinum adducts in the dorsal root ganglion (DRG), a cluster of sensory nerve cell bodies that relays signals from peripheral neurons to the spinal cord.4 Platinum-induced neuropathy ensues as a result of inefficient cellular defenses to DNA damage/oxidative stress that inhibit proper neuronal regeneration.2
A significant challenge for all pharmacogenetic studies is finding appropriately sized and phenotyped populations for replication. As a result, the translation of the GWAS findings described here for the prediction of CIPN in patients will require further validation. Nonetheless, these findings have begun to distinguish the different genetic networks that influence the development of CIPN, providing a foundation to translate genetic associations into effective therapeutic strategies for the prevention and/or treatment of this dose-limiting toxicity.
FUNCTIONAL MODELS OF CIPN
The application of genomic technologies has highlighted the genetic complexity underlying this drug-induced toxicity; robust CIPN model systems are now needed to investigate how chemotherapeutics induce peripheral nerve degeneration and how genetic variations affect such response to chemotherapy stress. Rodent models have revealed key morphological changes in the affected nerves that are now hallmarks of CIPN, including the loss of intraepidermal nerve fiber endings, preferential sensory axonal degeneration, mitochondrial vacuolation, and varying degrees of demyelination.2 Although animal models are necessary for investigating potential neuroprotective treatments, they are not particularly efficient for multi-gene validation and network characterization. Studies in cultured DRG neurons and human neuroblastoma cell lines have suggested possible molecular mechanisms that cause CIPN, including drug transporters controlling neuronal drug exposure, ion channels involved in thermal sensitivity/insensitivity, calcium signaling responsible for maintaining mitochondrial function, mitochondrial DNA stability and response to oxidative stress.2 It is still unclear, however, which mechanisms are the primary cause for pathogenesis of CIPN and which are secondary cellular responses after onset of peripheral degeneration.
The inability to readily obtain and examine human sensory neurons that truly embody appropriate responses to chemotherapy exposure limits our ability to define the molecular mechanisms important in drug-induced sensory peripheral neuropathy. The application of human induced pluripotent stem cell (iPSC) technology holds the potential for rapid advances in understanding CIPN on a molecular level, including the investigation of how patient-specific genetic variation determines CIPN predisposition and screening of potential neuroprotective targets in a high throughput manner. The development of human iPSC-derived sensory peripheral neurons is underway, and such models are expected to more faithfully mimic the target tissue, giving us better insight on the functionality of identified genes on neuronal responses to chemotherapy. Recent work in iPSC-derived central neurons has demonstrated sensitivity to chemotherapy exposure that can be modulated by genetic disruption of genes previously associated with CIPN (e.g., TUBB2A and VAC14)2, 5, setting a precedent for how patient-specific genetic variations can be investigated. iPSC-derived sensory neurons can reveal genetic signatures present only under chemotherapy exposure, further enhancing our comprehension of how human peripheral neurons respond after chemotherapy exposure and distinguishing which genetic networks contribute to the primary mechanisms of CIPN. Furthermore, these systems may enhance drug development efforts by providing better prediction of the efficacy of CIPN-targeted therapies and the propensity for new therapeutic agents for CIPN-like toxicities. With the development of appropriate in vitro tools, we will be able to build our knowledge regarding the molecular mechanisms of CIPN development and thereby expand possible targets for its prevention and treatment.
PERSPECTIVES/FUTURE
Genome-wide association studies have revealed the polygenic nature of CIPN and suggest a critical role for peripheral nerve repair pathways in determining sensitivity to chemotherapeutic agents. As genomic sequencing technologies advance and become more affordable, and standard neurotoxicity assessments become readily available, novel clinically relevant genetic associations will be uncovered and currently reported associations will be validated. There remains a need for more in vitro tools to extend genetic findings into molecular mechanisms that can be exploited for novel therapies against CIPN. Human iPSC-derived sensory neurons hold great promise for understanding the genes and pathways underlying the development of drug-induced peripheral neuropathy, defining the contribution of genetic variation to the toxicity, and screening for drug targets. Overall, we have only begun to address the current gaps in our understanding of CIPN, but efforts to uncover underlying genetic drivers and establish robust model systems are critical steps for a precision medicine approach to prevent and treat this complex drug-induced toxicity.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grant R01 CA192156 and a grant from the Breast Cancer Research Foundation. K.C. was supported in part by NIH grant T32 GM007175.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
Supplementary Materials
Supplementary Table 1. Summary of genes identified rom recent genetic association studies of CIPN.
REFERENCES
- 1.Hershman DL, et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers?: American Society of Clinical Oncology Clinical Practice Guideline. 2014 doi: 10.1200/JOP.2014.001776. [DOI] [PubMed] [Google Scholar]
- 2.Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol. Oncol. 2016;140:176–183. doi: 10.1016/j.ygyno.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin RM, et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin. Cancer Res. 2012;18:5099–5109. doi: 10.1158/1078-0432.CCR-12-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprowl JA, et al. Oxaliplatin-induced neurotoxicity is dependent on the organic cation transporter OCT2. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11199–11204. doi: 10.1073/pnas.1305321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler HE, Wing C, Delaney SM, Komatsu M, Dolan ME. Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS One. 2015;10:e0118020. doi: 10.1371/journal.pone.0118020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.