Abstract
Chronic food restriction potentiates behavioral and cellular responses to drugs of abuse and D-1 dopamine receptor agonists administered systemically or locally in the nucleus accumbens (NAc). However, the alterations in NAc synaptic transmission underlying these effects are incompletely understood. AMPA receptor trafficking is a major mechanism for regulating synaptic strength, and previous studies have shown that both sucrose and d-amphetamine rapidly alter the abundance of AMPA receptor subunits in the NAc postsynaptic density (PSD) in a manner that differs between food-restricted and ad libitum fed rats. The present study examined whether food restriction, in the absence of reward stimulus challenge, alters AMPAR subunit abundance in the NAc PSD. Food restriction was found to increase surface expression and, specifically, PSD abundance, of GluA1 but not GluA2, suggesting synaptic incorporation of GluA2-lacking Ca2+-permeable AMPARs (CP-AMPARs). Naspm, an antagonist of CP-AMPARs, decreased the amplitude of evoked EPSCs in Nac shell, and blocked the enhanced locomotor response to local microinjection of the D-1 receptor agonist, SKF-82958, in food-restricted, but not ad libitum fed, subjects. Although microinjection of the D-2 receptor agonist, quinpirole, also induced greater locomotor activation in food-restricted than ad libitum fed rats, this effect was not decreased by Naspm. Taken together, the present findings are consistent with synaptic incorporation of CP-AMPARs in D-1 receptor expressing medium spiny neurons in NAc as a mechanistic underpinning of the enhanced responsiveness of food-restricted rats to natural rewards and drugs of abuse.
Keywords: neuroplasticity, reward, dieting, addiction
Graphical abstract
Increased surface expression and postsynaptic density abundance of GluA1, but not GluA2, in the nucleus accumbens of food-restricted (FR) relative to ad libitum fed (AL) rats, suggest synaptic insertion of Ca2+-permeable AMPARs. Electrophysiological support for this conclusion is provided, as are pharmacological results indicating involvement of these AMPARs in the enhanced behavioral response of food-restricted rats to D-1, but not D-2, dopamine receptor stimulation.
Introduction
Maintenance of energy homeostasis in an ecology of food scarcity is facilitated by neural and behavioral adaptations that increase the probability of food acquisition and consumption. Yet, food restriction driven by sociocultural pressures, cosmetic or health concerns, is a common practice (Kruger et al., 2004; Bish et al., 2005). Under these circumstances, the physiology of food scarcity may be induced within an environment that is abundant with food, associated cues, and proxies for food in the form of drugs that target the same neural substrate (Di Chiara 2005; Volkow et al., 2008). It is therefore not surprising that dieting is a risk factor for binge pathology (Stice et al., 2008), leads to rebound weight gain exceeding the weight lost (Mann et al., 2007; Pietilanen et al., 2012), and increases risk of initiating and relapsing to drug abuse (Krahn et al., 1992; Cochrane et al., 1998; Austin & Gortmaker, 2001; Cheskin et al., 2005; Seo & Jiang, 2012) Moreover, the prevalence of drug abuse among individuals with eating disorders is disproportionately high (Jonas et al., 1987; Pisetsky et al., 2008; Root et al., 2010). The causal relation between food restriction and binge eating, drug reward sensitivity, and incentive effects of drug-associated cues and contexts have been confirmed in animal models (Carroll et al., 1979; Hagan & Moss, 1997; Cabeza de Vaca & Carr, 1998; Zheng et al. 2012; D'Cunha et al., 2013).
Mechanistic studies suggest that effects of food restriction (FR) on responsiveness to drugs of abuse and associated environments are mediated by stable neuroadaptations rather than time since the last meal or circulating levels of metabolic hormones (Zheng et al., 2013). A variety of changes have been observed in the mesoaccumbens “reward” pathway, including decreased ventral tegmental tyrosine hydroxylase mRNA and protein levels (Pan et al., 2006), decreased basal extracellular (Pothos et al., 1995) and evoked release (Stouffer et al., 2015) of dopamine (DA), and decreased mRNA levels of NAc neuropeptides known to be synthesized by D-1 receptor-expressing medium spiny neurons (MSNs) (Haberny & Carr, 2005). These findings suggest that FR is accompanied by DA conservation. Yet, within NAc, increased intracellular signaling, gene expression, and behavior in response to D-1 receptor stimulation suggest compensatory upregulation (Carr et al., 2003; Haberny et al., 2004; Haberny & Carr; 2005a), with potential to enhance the effects of reward stimuli that induce extracellular DA concentrations adequate to stimulate the relatively low affinity D-1 receptor (Gerfen & Surmeier, 2011; Richfield et al., 1989).
One downstream effect of D-1 receptor stimulation is phosphorylation of the glutamatergic AMPA receptor GluA1 at Ser845. Relative to ad libitum fed (AL) subjects, FR subjects display increased pSer845-GluA1 in response to injection of a D-1 receptor agonist, d-amphetamine, sucrose consumption, and exposure to a cocaine-paired environment (Carr et al., 2010; Liu et al., 2011; Peng et al., 2014). This is significant from standpoints of neuronal excitability and behavior. Phosphorylation at Ser845 increases peak current and channel open probability (Roche et al., 1996; Banke et al., 2000), facilitates trafficking of GluA1-containing AMPARs to the cell surface (Shi et al., 2001; Esteban et al., 2003; Man et al., 2007), and stabilizes membrane Ca2+-permeable AMPARs (CP-AMPARs) (He et al., 2009). Indeed, sucrose consumption and d-amphetamine administration were shown to increase the abundance of AMPAR GluA1 and GluA2 in the NAc postsynaptic density (PSD) with a greater effect in FR than AL rats (Peng et al., 2011; Peng et al., 2014). Yet, these same studies suggested that FR, in the absence of reward stimulus challenge, may increase GluA1 but not GluA2 in the PSD. Specifically, control FR subjects handled and given access to water rather than sucrose, or injected intraperitoneally with saline-vehicle rather than amphetamine, displayed elevated levels of GluA1 relative to AL subjects. The purpose of the present study was therefore to test the hypothesis that FR induces synaptic incorporation of CP-AMPARs. This particular response to FR would be of high interest, considering that these receptors are relatively rare (Reimers et al., 2011), are driven into the synaptic membrane as a homeostatic response to deprivation of excitatory input in vitro (Thiagarajan et al., 2005), and mediate synaptic strengthening and behavior formation in vivo (Rumpel et al., 2005; Whitlock et al., 2006). To test the hypothesis, diet groups receiving no unusual stimulation prior to brain harvesting were compared for AMPAR surface expression, subunit protein levels in the NAc PSD, and whole cell indicators of calcium signaling and calcineurin, as they have been implicated in GluA1 trafficking in cultured neurons. Next, electrophysiolgical methods were used to substantiate biochemical findings suggestive of FR-induced insertion of GluA2-lacking AMPARs. Finally, locomotor activation elicited by D-1 and D-2 receptor stimulation in NAc was challenged with the selective antagonist of CP-AMPARs, Naspm. The electrophysiological and microinjection studies focused on the NAc shell, based on the critical involvement of this region in reward-related learning (Gambarana et al., 2003) and reinforcing effects of abused drugs (Ikemoto, 2007).
Materials and Methods
Subjects and food restriction
All subjects were male Sprague–Dawley rats (Taconic Farms, Germantown, NY, USA) weighing 350–400 g at the time of arrival in the central animal facility where they were housed in individual plastic cages with free access to standard pelleted lab chow (Rodent Diet #5001, Lab Diet, St. Louis, MO) and water unless otherwise noted. The animal room was maintained on a 12-h light/dark cycle, with lights on at 0600 h. Half the subjects in each experiment were placed on a chronic food restriction regimen whereby daily food allotment was initially limited to 10 g of chow, delivered at 1700 h, until a 20% decrease in body weight was attained. Attainment of the target body weight typically took 15-20 days. Daily feeding was then titrated to clamp body weight at that value for 1-2 additional weeks until brain harvesting in the biochemistry and electrophysiology experiments, or 1 week until initiation, and an additional 3 weeks through completion, of the behavioral experiment.
All experimental procedures were approved by the New York University School of Medicine Institutional Animal Care and Use Committee and were performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 85-23). All efforts were made to minimize animal suffering, to reduce the number of animals used and to utilize alternatives to in vivo techniques.
Brain harvesting and sample preparation
Subjects were sacrificed between 1000 and 1200 h by brief exposure to CO2 and decapitation by guillotine. Brains were rapidly extracted. For protein cross-linking, NAc was rapidly dissected on ice and minced with a razor blade. The sample from one hemisphere was added to an eppendorf tube containing ice-cold artificial CSF spiked with cross-linking reagent, bis (sulfosuccinimidyl) suberate (BS3) (Pierce, Rockford, IL), while the contralateral sample from each subject was placed in a tube without the BS3 cross-linker. For subcellular fractionation, NAc and caudate-putamen (CPu) were dissected on ice and bilateral samples from three rats per treatment condition were pooled in separate tubes for fractionation, yielding a total of six tubes per brain region for the FR and AL diet conditions.
Protein cross-linking and Western blotting to measure cell surface expression
Analysis of cell surface and intracellular AMPARs was achieved by using a BS3 cross-linking method previously described by Boudreau and Wolf (2005). Minced NAc tissue of one hemisphere was incubated with or without 2 mM BS3 cross-linker for 30 min at 4°C, end-over-end rotation. Reaction was terminated by the addition of 100 mM glycine for 10min at 4°C followed by a 2 min centrifugation to pellet tissue. Supernatant was removed and pelleted tissue was sonicated in ice-cold lysis buffer (25 mM HEPES, pH 7.4, 500 mM NaCl, 2 mM EDTA, 1 mM DTT, 1 mM PMSF, 20 mM NaF, 0.1% NP-40, 1 μM okadaic acid, 1 mM Na3VO4, and 1X protease inhibitors). Total lysates were spun at 20,000 × g for 2 min at 4°C, aliquoted and stored at −80°C until use. Samples (30 μg) were separated by electrophoresis on precast 4-20% SDS-PAGE gels (Lonza, Rockland, ME) and transferred to a PVDF membrane (PerkinElmer, Boston, MA) for 2.5 hr at 100V. Membranes were blocked for 60 min with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) and incubated overnight at 4°C with mouse monoclonal anti-GluA1 (1:1000; MAB2263, Millipore, Temecula, CA), rabbit polyclonal anti-GluA2 (1:1000; PA1-4659, Thermo Scientific, Rockford, IL), or mouse monoclonal anti-α-tubulin (1:10,000; T6199, Sigma-Aldrich, St. Louis, MO) diluted in Odyssey Blocking Buffer with 0.2% Tween-20. Blots were stripped and reprobed in reverse order with GluA1 or GluA2 antibody. Detection of band densities was optimal with chemiluminescent detection rather than the Li-COR infrared imaging system because of less background signal and signal amplification with the use of horseradish peroxidase-labelled secondary antibodies (Thermo Scientific, Rockford, IL; 1:6000). Immunoblots were developed with Supersignal West Pico and Femto Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) using a film developer. Band densities were quantified using Image Studio Lite Ver 5.0 following software instructions (LI-COR, Lincoln, NE). Samples (±BS3 or +BS3 alone) were run at least three times and averaged across runs. Changes in AMPAR surface and intracellular band densities were normalized to tubulin levels and expressed as the percentage of AL control (−BS3 for ±BS3 gels or +BS3 for +BS3 gels) on each gel.
Subcellular fractionation and Western blotting to measure PSD abundance
Protease inhibitor cocktail and phenylmethanesulfonyl fluoride (PMSF) were added to 0.32 M sucrose solution containing 1 mM NaHCO3, 1 mM MgCl 2, and 0.5 mM CaCl2 (Solution A). Brain tissue was rinsed, homogenized, and subsequently diluted to 10% weight/volume in Solution A. After being well mixed, 50 μl of the whole-cell homogenates were stored at −80 °C until use.
The whole-cell homogenate was centrifuged at 2000 g for 10 min, after which intact cells and nuclei formed a pellet at the bottom of the tube. The supernatant was saved, and the pellet was resuspended in Solution A. The homogenate was again centrifuged at 1400 g for 10 min. The supernatant was collected and combined with the previously collected supernatant. They were centrifuged together at 1400 g for 10 min and then at 13,800 g for 30 min. The pellet was collected and homogenized in 0.32 M sucrose solution containing 1 mM of NaHCO3, protease inhibitor cocktail, and PMSF (Solution B). This homogenate was placed on a sucrose gradient and centrifuged for 2-h at 82,500 g. The synaptosomal layer was collected from the interface of the 1 and 1.2 M sucrose layers. The sample was then resuspended in Solution B and centrifuged at 82,500 g for 45 min. After centrifugation, the upper liquid was discarded, and the pellet was resuspended in a solution of 25 mM Tris, at pH 7.4.
To isolate the PSD fraction, an equal volume solution containing 1% Tritron X-100, 0.32 M sucrose, and 12 mM Tris, at pH 8.1 was added to the resuspended sample. The mixture was then rocked at 4 °C for 15 min, followed by centrifugation at 13,800 g for 30 min. After centrifugation, the upper liquid was discarded, and the pellet was resuspended in a solution of 25 mM Tris, at pH 7.4 with 2% SDS and stored at −80 °C until use.
This method of PSD purification yields a fraction enriched in proteins that are preferentially localized or novel to the PSD (Jordan et al., 2004). To validate the purity of our PSD fraction and rule out contamination of endosomal membranes where AMPARs are interchanged, we probed for the early endosomal marker, rab5.
Proteins (6–10 μg/lane) were separated by electrophoresis on precast 4–12 % sodium dodecyl sulfate polyacrylamide gels (Lonza, Rockland, ME). Precision Plus Protein standard molecular weight markers (Bio-Rad) were loaded to estimate the size of the target proteins and to ensure complete transfer of proteins from gel to membrane. Proteins were electrophoretically transferred to Protran nitrocellulose membranes (Whatman, Mobile, AL) for 1.75 h at a constant voltage of 100 V. Membranes were blocked for 60 min with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) with shaking at room temperature. Membranes were then probed overnight at 4 °C using primary antibodies for target proteins or the protein loading control, α-tubulin. Primary antibodies were diluted in Odyssey Blocking Buffer with 0.2 % Tween 20. After probing with primary antibodies and washing with TBST buffer (3×5 min), membranes were incubated for 1 h at room temperature with IRDye 680RD Goat anti-Mouse IgG (H+L) and IRDye 800CWGoat anti-Rabbit IgG (H+L) (1:16, 000; LI-COR Biosciences) in Odyssey Blocking Buffer with 0.2 % Tween 20. Proteins were visualized using an Odyssey CLx infrared imaging system, and bands were quantified using the Image Studio software (LI-COR Biosciences). At least 3 gels were run to obtain averaged results for each target protein in each diet group and separate gels were run for NAc and CPu. Target proteins were normalized to total tubulin in the corresponding lane. Primary antibodies used included mouse monoclonal anti-GluA1 (1:1000; MAB2263, Millipore, Temecula, CA), rabbit polyclonal anti-phospho-Ser845-GluA1 (1:1000; AB5849, Millipore), rabbit polyclonal anti-GluA2 (1:1000; PA1-4659, Thermo Scientific, Rockford IL), mouse monoclonal anti-CaMKII-α (1:1000; 05-532, Millipore), rabbit polyclonal anti-CaMKII-β(1:1000; ab34703, Abcam, Cambridge, MA), rabbit polyclonal anti-calcineurin (1:10,000; ab3673, Abcam), rabbit polyclonal anti-rab5 (1:1,000; ab18211, Abcam), and mouse monoclonal anti-α-tubulin (1:10,000; T6199, Sigma-Aldrich, St. Louis, MO).
Electrophysiology
Rats were deeply anaesthetized with ketamine (100 mg/kg, i.p.), decapitated, and the brain quickly placed into ice-cold dissection buffer containing (in mM): 75 sucrose, 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2 6 H2O, 25 NaHCO3, 10 dextrose, bubbled with 95% O2 / 5% CO2 (pH 7.4). Coronal slices of the nucleus accumbens (300 μm) were prepared with a vibratome (Leica, VT1200S), placed in room temperature artificial cerebrospinal fluid (ACSF, in mM: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgSO4 7H2O, 26 NaHCO3, and 10 dextrose) for <30 min; then warmed and kept at 33-35°C for >1 hr before use. For experiments, slices were transferred to the recording chamber and superfused (2.0–2.5 ml min−1) with oxygenated ACSF at 33-35°C containing 50 μM picrotoxin, to block inhibitory transmission, to isolate EPSCs.
Somatic whole-cell recordings were made from shell region medium spiny neurons in voltage-clamp with a Multiclamp 700B amplifier (Molecular Devices) using IR-DIC video microscopy. Patch pipettes (4-6 MΩ) were filled with intracellular solution (in mM: 125 Cs-gluconate, 2 CsCl, 5 TEA-Cl, 4 Mg-ATP, 0.3 GTP, 10 phosphocreatine, 10 HEPES, 0.5 EGTA, and 3.5 QX-314). Data were filtered at 2 kHz, digitized at 10 kHz, and analyzed with Clampfit 10 (Molecular Devices). Extracellular stimulation (0.01-1 ms, 5-150 μA, 0.2 Hz) was applied with a small glass bipolar electrode 0.05-0.5 mm from the recording electrode. After >5 min of baseline recording, a solution containing the GluA2-lacking, CP-AMPAR antagonist Naspm (200 μM) was superfused for 10 min. Changes in excitatory postsynaptic current (EPSC) amplitude were measured before and after drug application at a holding potential of −70. Spontaneous EPSCs in these recordings were detected automatically with a template EPSC, and the average of all spontaneous event amplitudes and rates during the entire duration of each recording was computed. TTX was not included in the bath for these measurements.
Cannula implantation and intracerebral microinjection
Rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and stereotaxically implanted with two chronically indwelling guide cannulas (26 ga; Plastics One, Roanoke, VA) which were placed bilaterally 2.0 mm dorsal to injection sites in the NAc medial shell (1.6 mm anterior to bregma; 2.1 mm lateral to the sagittal suture, tips angled 8° toward the midline, 5.8 mm ventral to skull surface). Cannulas were permanently secured to the skull by flowing dental acrylic around them and four jeweler's screws
Solutions were loaded into two 30 cm lengths of PE-50 tubing attached at one end to 25-μl Hamilton syringes filled with distilled water and at the other end to 31-gauge injector cannulas, which extended 2.0 mm beyond the implanted guides. The syringes were mounted on the twin holders of a Harvard 2272 microliter syringe pump which delivered the 0.5 μl injection volumes over a period of 100 s. One minute following completion of injections, injector cannulas were removed from guides, stylets were replaced, and animals were returned to test chambers where the post-injection locomotor activity test began 5-min after completion of the microinjections.
Histology
Upon completion of behavioral testing, rats were euthanized with CO2 and decapitated. Brains were removed and fixed in 10% buffered formalin for at least 48 hr. Frozen coronal sections, 40 μm thick, were cut on a Reichert-Jung Cryostat, thaw-mounted on gelatin coated glass slides and stained with cresyl violet. Microinjection sites were determined by visual inspection of sections under an Olympus SZ40 microscope. Data obtained from subjects that did not have cannulas accurately placed bilaterally in the NAc shell, or on the shell/core or shell/olfactory tubercle border, were eliminated from the analysis. Accordingly, two subjects from each of the diet groups were removed from the study.
Drugs
SKF-82958 (Sigma-Aldrich) was dissolved in a small volume of DMSO and diluted with sterile 0.9% saline (final DMSO concentration 5%) and microinjected bilaterally in a dose of 2.0 μg or 5.0 μg. Quinpirole (Sigma-Aldrich) was dissolved in sterile 0.9% saline and microinjected bilaterally in a dose of 2.0 μg or 5.0 μg. 1-Naphthylacetyl spermine (Naspm; Sigma-Aldrich) was dissolved in sterile 0.9 % saline and microinjected bilaterally in a dose of 25.0 μg alone or in combination with SKF-82958 or quinpirole. Drug doses were selected based on their previous effectiveness in differentiating AL and FR subjects in studies of electrical brain stimulation reward and/or pilot testing (Carr et al., 2010, Peng et al., 2014).
Locomotor activity testing
Following recovery from surgery, twelve of the twenty four subjects to be tested for effects of the D-1 receptor agonist, SKF-82958, were assigned to the food restriction regimen. During the 3-4 week period required for attainment and stabilization of target body weight, subjects in both diet groups were habituated to handling, transport between vivarium and laboratory, gentle restraint on a foam cushion and a mock microinjection procedure. In the week preceding the experiment, subjects underwent four habituation sessions in the locomotor activity test chamber, on separate days, which were identical to actual test sessions in format and duration, with the exception that mock intracerebral microinjections were made.
Activity was monitored in a Lucite test chamber (25.4 × 30.5 × 30.5 cm) with a textured plastic floor. Automated data collection was accomplished by 12 infrared photo beam detectors along the length of the test chamber plus 12 beams on the vertical axis (Accuscan, Columbus, OH). Each test session began with a 15 min pre-injection measurement of horizontal activity and stereotypy in the test chamber, followed by removal and intracerebral microinjection, which was followed by a 30 min post-injection measurement.
Over a 3 week period, subjects underwent 6 test sessions. In the first two test sessions, conducted 3-4 days apart, subjects received bilateral microinjection of SKF-82958 (2.0 μg) or SKF-82958 in solution with 1-naphthylacetyl spermine (Naspm; 25.0 μg; Sigma-Aldrich) with counterbalancing of treatment order. In the second set of two test sessions subjects received bilateral microinjection of SKF-82958 at the higher dose of 5.0 μg with or without Naspm (25.0 μg). Again, treatment order was counterbalanced and sessions were conducted 3-4 days apart. No effects of vehicle or Naspm alone were expected based on previous NAc shell microinjection studies (Carr et al., 2010; Peng et al., 2014), however, one week after completion of testing with SKF-82958, two additional sessions were run in which subjects were microinjected with Naspm (25.0 μg) or vehicle (5% DMSO in sterile 0.9% saline).
Following observation that Naspm reversed the enhanced responses of FR rats to microinjection of SKF-82958, a new group of 8 AL and 9 FR rats were prepared and tested for the effects of the D-2 receptor agonist, quinpirole, at doses of 2.0 and 5.0 μg, with and without Naspm (25.0 μg).
Statistical analysis
Western blot and electrophysiological results were analyzed by t-tests for independent or matched samples as appropriate. Behavioral results were analyzed by 3-way or 2-way mixed design ANOVA.
Data obtained in this study and summarized in Results are available at: https://figshare.com/s/11e39d2364e5f57018e1.
Results
Food restriction increases surface expression of GluA1
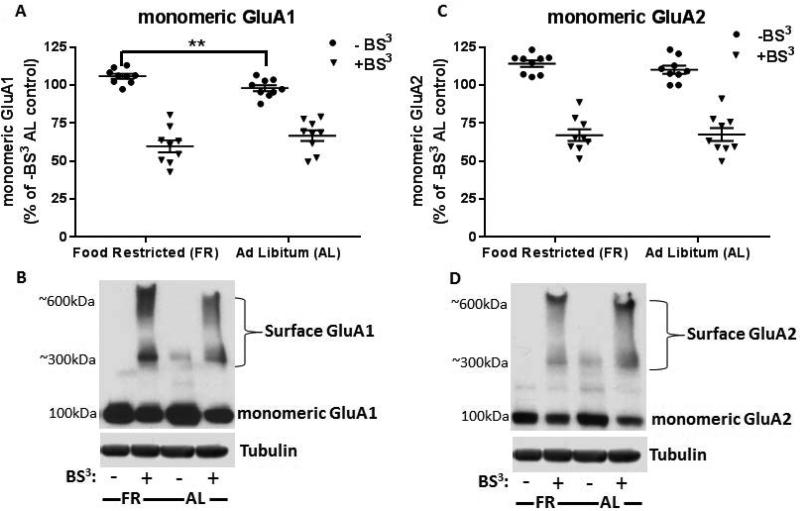
In the absence of BS3 cross-linker, immunoblot analysis of AMPARs in FR rats revealed a small increase in the level of the monomeric form of GluA1 (9.5 ± 1.6%, t(16)=3.08, p<.01) but not GluA2 (t(16)=1.17, p=.26), when compared to AL fed rats (Fig 1A and C). BS3 treatment of paired, bilateral NAc led to the detection of high-molecular weight species, indicative of surface AMPAR expression, with a corresponding decrease of intracellular (I) AMPARs (Fig 1B and D).
Figure 1.
Effect of chronic food restriction (FR) on AMPAR subunit expression and distribution in nucleus accumbens (NAc) as assessed by BS3 cross-linking studies. A and C: Quantification of monomeric AMPAR proteins, normalized to α-tubulin, revealed an increase in uncrosslinked (−BS3) monomeric GluA1 (A) but not GluA2 (C) with no change following BS3 cross-linking (+BS3) when compared to ad libitum (AL) fed control. B and D: Shown are representative immunoblots depicting the levels of uncrosslinked, monomeric AMPARs (total) while cross-linked AMPARs were observed as both high-molecular weight bands of 300- 600kDA (surface) and unmodified, monomeric bands of 100kDA (intracellular). Data obtained from individual subjects are expressed in comparison to the normalized control and displayed with mean ± s.e.m, n=9 per group, **p<.01.
Detection of surface AMPARs with BS3 treatment revealed the presence of an approximate 600kDA and 300kDA band, the latter conflicting with previously reported findings (Boudreau & Wolf, 2005). However, Lv XF et al. (2015) reported the formation of multiple high-molecular weight aggregates suggesting that slight differences in methodology, including differences in primary antibodies employed, may yield detection of various quaternary structures.
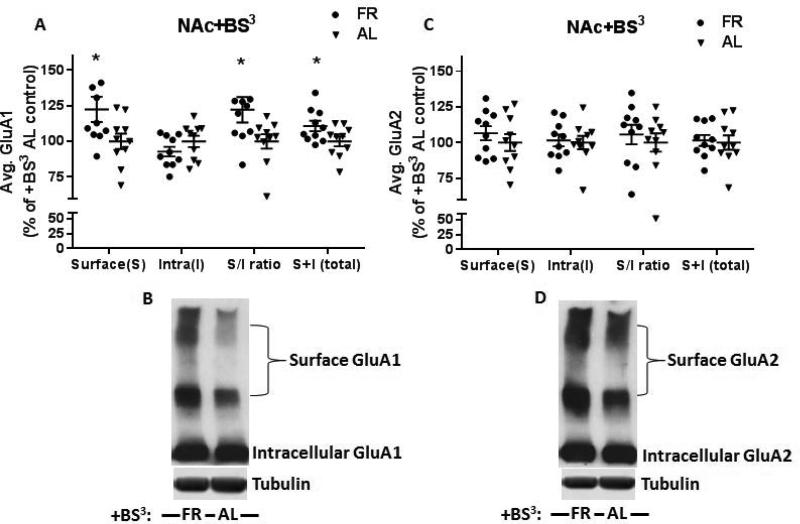
Further evaluation of BS3 cross-linked samples suggested a trend of decreased intracellular GluA1 in FR rats (9.9 ± 3.7%, p=.19) with a significant increase in surface expression (S) of GluA1 (22.5 ± 7.9%, t(18)=2.16, p<.05) but not GluA2 (t(18)=0.85, p=.41) in FR rats relative to AL rats (Fig 2A and C). Increased surface expression (S) of GluA1 was associated with a significant increase in surface/intracellular (S/I) ratio (22.2 ± 8.0%, t(18)=2.17, p<.05) while S/I ratio of GluA2 was unchanged (t(18)=.86, p=.40). Moreover, total AMPAR expression (surface + intracellular) again showed a slight increase in GluA1 (10.7 ± 3.5%, t(18)=2.1, p<.05) with no change in GluA2 (t(18)=.24, p=.81). Together, these findings suggest that the FR-induced increase in surface GluA1 reflects a shift of GluA1 from intracellular to surface compartments, and is associated with a slight increase in total GluA1.
Figure 2.
BS3 cross-linking of NAc from FR and AL rats revealed differential distribution of AMPARs in surface (S) and intracellular (I) compartments. A and C: Quantification of GluA1 (A) showed increased surface (S) and total (S+I) expression with a concomitant increase in surface/intracellular (S/I) ratios. No significant change in intracellular (I) pools was observed in FR rats when compared to AL control. Band densities are normalized to α-tubulin and expressed as percentage of AL control. Evaluation of GluA2 (C) revealed no change in surface (S), intracellular (I), surface/intracellular (S/I) ratio, or total (S+I). B and D: Shown are representative immunoblots of BS3 cross-linked samples. Data obtained from individual sujects are expressed in comparison to the normalized control and displayed with mean ± s.e.m., n=10 per group, *p<.05.
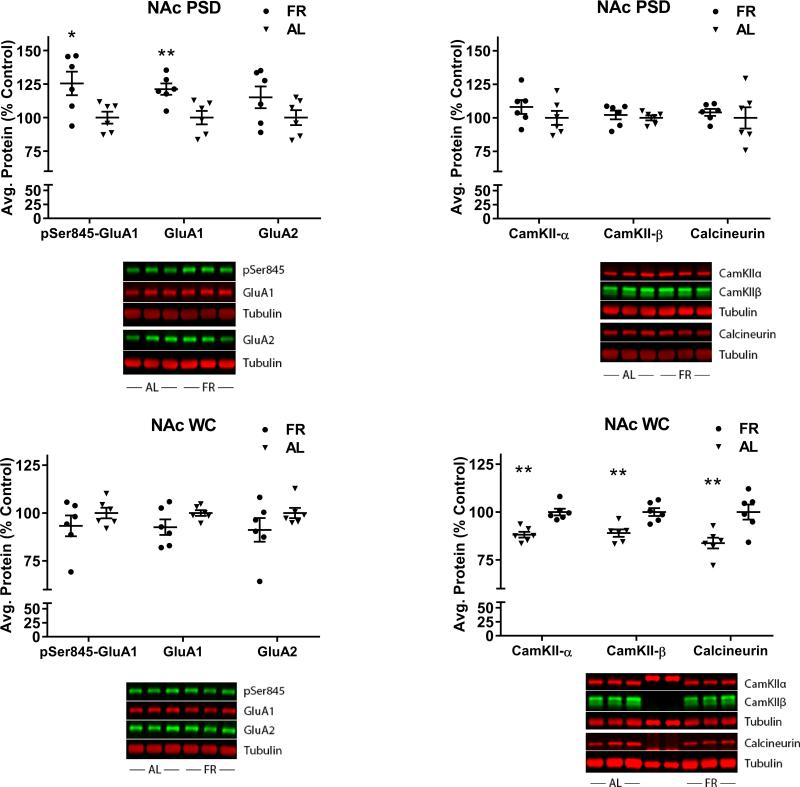
Food restriction increases GluA1 abundance in the PSD
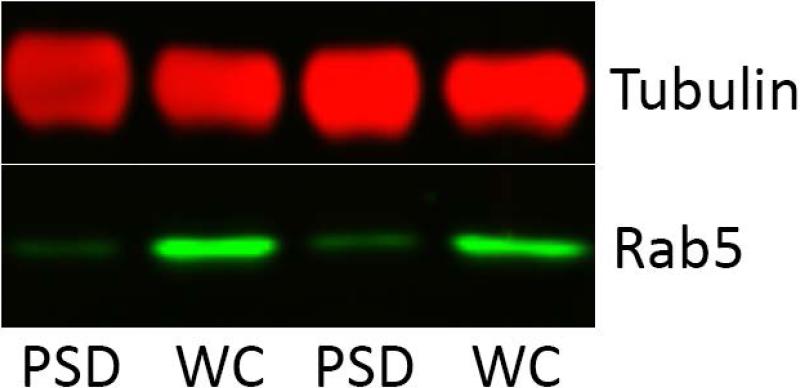
The method of subcellular fractionation used in this study is a standard protocol that yields a fraction enriched in proteins preferentially localized or novel to the PSD, and in which endosomes are disrupted by detergent, solubilizing endosomal AMPA receptors (Jordan et al., 2004). Purity of the current PSD fraction was confirmed by probing for the endosomal marker, rab5, and demonstrating little or no contamination (Fig 3).
Figure 3.
Whole cell homogenate (WC) and postsynaptic density fractions (PSD) of nucleus accumbens were probed for the endosomal marker rab5 to confirm purity of the PSD fraction.
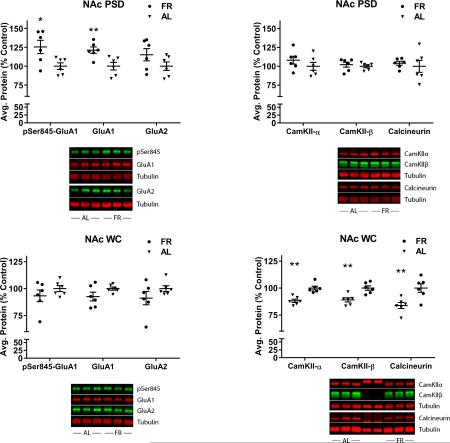
In the NAc PSD, FR rats displayed elevated levels of GluA1 (t(10)=3.49, p=.005) and pSer845-GluA1 (t(10)=2.9, p=.015), but not GluA2 (t(10)=1.54, p=.15), relative to AL rats (Fig 4, top left). None of these proteins differed between diet groups in the whole cell homogenate (GluA1: t(10)=1.71, p=.11; pSer845-GluA1: t(10)=1.1, p=.29; GluA2: (t(10)=1.31, p=.22) (Fig 4, bottom left). The discrepancy from the slight increase in total GluA1 seen in BS3 cross-linked tissue (above) may result from differences in sample processing, particularly solubilization of the receptor and the centrifugation step to pellet insoluble material. Consequently, the case for FR altering synthesis or degradation of AMPARs is equivocal, while a FR-induced increase in synaptic incorporation and Ser845 phosphorylation of GluA1 is supported.
Figure 4.
Effects of chronic food restriction (FR) on abundance of pSer845-GluA1, GluA1, GluA2, CaMKII-α and –β, and calcineurin in the postsynaptic density (PSD) and whole cell homogenate (WC) fractions of nucleus accumbens (NAc). Following densitometry, intensities of bands corresponding to the target protein for each sample were divided by the intensities of the corresponding α-tubulin bands. Data obtained from individual samples are expressed in comparison to the normalized control and displayed with mean ± s.e.m. Representative immunoblots are included beneath each graph. n=18 per group, with three NAc pooled per tube for fractionation. FR increased pSer845-GluA1 and GluA1 in the PSD (top left) but not the WC (bottom left). FR decreased CaMKII-α and –β, and calcineurin in the WC (bottom right) but not the PSD (top right). *p<.05; **p<.01.
In the NAc whole cell homogenate (WC), FR rats displayed lower levels of CaMKII-α (t(10)=5.06, p=.00004), CaMKII-β (t(10)=3.91, p=.003), and calcineurin (t(10)=3.34, p=.007) relative to AL rats (Fig 4, bottom right). None of these differences between diet groups were seen in the PSD fraction (CaMKII-α (t(10)=1.1, p=.30; CaMKII-β (t(10)=0.6, p=.58; calcineurin (t(10)=0.5, p=.63) (Fig 4, top right). These results suggest that FR is associated with decreased Ca2+ signaling and decreased level of the phosphatase that dephosphorylates pSer845-GluA1.
The differences between diet groups seen in the NAc PSD were not seen in the CPu PSD (not shown; GluA1: t(10)=1.8, p=.10; pSer845-GluA1: t(10)=0.7, p=.49). Nor were the effects of FR on CaMKII-α or calcineurin in NAc seen in the CPu WC (CaMKII-α (t(10)=0.87, p=.40; calcineurin (t(10)=1.56, p=.15). Unlike NAc, in which FR decreased levels of CaMKII-β, levels were increased in CPu (t(10)=2.67, p=.023).
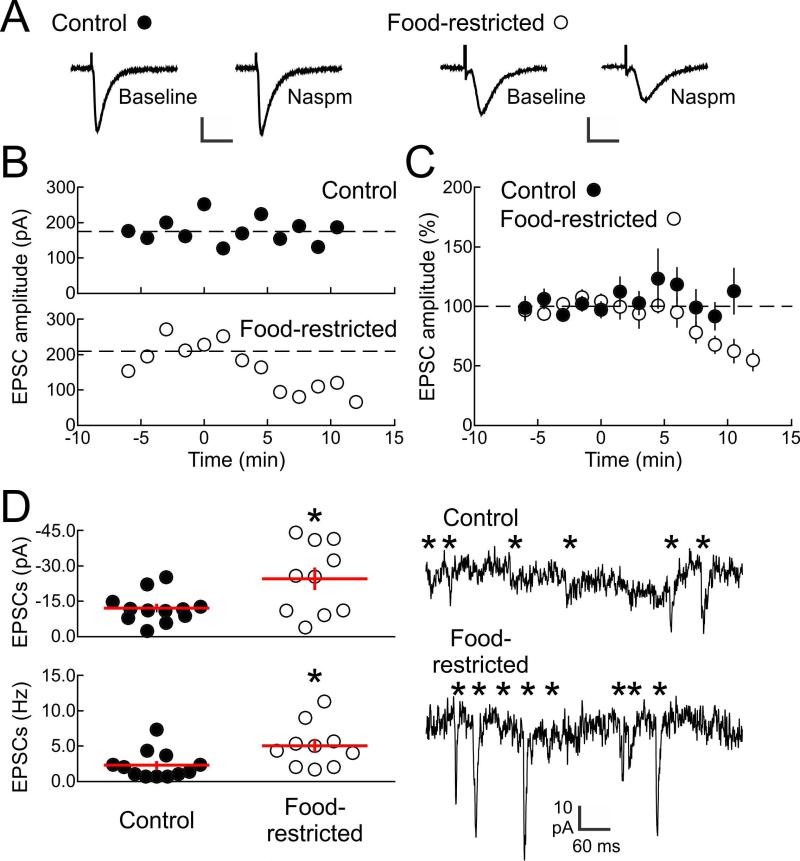
Food restriction induces CP-AMPAR incorporation
Previous studies indicate that various changes in physiological state can lead to upregulation of synaptic CP-AMPARs in NAc core neurons (Goffer et al., 2013; Tukey et al., 2013). Here we investigated whether FR increases CP-AMPAR incorporation in NAc shell neurons, based on the critical involvement of this region in reward-related learning (Gambarana et al., 2003) and reinforcing effects of abused drugs (Ikemoto, 2007). Whole-cell recordings were made from neurons in NAc shell brain slices from either FR or AL rats. EPSCs were isolated with picrotoxin (50 μM) to block inhibitory transmission and evoked via extracellular stimulation. For each cell, the average reduction in EPSC amplitude over a 3-10 minute period after Naspm washin was compared to baseline. EPSCs in neurons from FR rats were much more sensitive to Naspm washin (200 μM) than EPSCs recorded from neurons of AL animals. Specifically, EPSC amplitude was unaffected by Naspm in slices from AL animals (t(8)=0.12, p=.9) but reduced in slices from FR animals (t(9)=3.2, p=.01; Fig 5B). Summary data across all cells, expressed as percentage of control amplitude, indicated that EPSCs from AL animals were unaffected by Naspm (t(11)=0.73, p=.47), while EPSCs from FR animals were reduced compared to their own baseline (t(6)=2.7, p=.02) and compared to AL animals (t(17)=2.1, p=.033; Fig 5C). When spontaneous activity in these cells was examined, events recorded from FR animals had higher amplitudes (t(20)=6.8, p<.0001; Fig 5D, top) and were more frequent (t(20)=4.8, p=.0001; Fig 5D, bottom) than in cells from AL animals. However, Naspm washin did not significantly decrease spontaneous EPSC parameters in FR subjects (frequency t(8)=1.39, p=.20; amplitude (t(8)=1.01, p=.38).
Figure 5.
Incorporation of CP-AMPARs into NAc shell excitatory synapses after food restriction. A: Example evoked EPSCs from NAc shell neurons of AL control (left, scale: 30 ms, 100 pA) and FR (right, scale: 20 ms, 250 pA) rats. EPSCs were isolated in picrotoxin (50 μM) and measured before and after Naspm washin (200 μM). B: Time course of Naspm washin for example cell from AL control animal (top, filled circles) and FR animal (bottom, open circles). EPSCs from AL animal were unaffected by Naspm 3+ min after washin (baseline amplitude: 174.1±9.9 pA, amplitude 3+ minutes after Naspm washin: 176.3±13.2 pA, t(8)=0.12, p=.9), while EPSCs from FR animal were reduced (baseline amplitude: 208.4±24.6 pA, amplitude 3+ minutes after Naspm washin: 117.1±16.4 pA, t(9)=3.2, p=.01). Naspm washin occurred at time 0. C: Summary data across all cells showing that EPSCs from AL animals were unaffected by Naspm 3+ min after washin (109.1±12.4% of control amplitude, n=12 neurons, t(11)=0.73, p=.47), while EPSCs from FR animals were reduced (70.6±11.0% of control amplitude, n=7 neurons, t(6)=2.7, p=.02 compared to baseline; t(17)=2.1, p=.033 compared to neurons from AL animals). Naspm washin occurred at time 0. D: Analysis of spontaneous EPSCs recorded in AL and FR animals. Top left: summary of mean spontaneous EPSC amplitudes (AL: −12.1±1.8 pA, FR: −24.6±4.8 pA, t(20)= 6.8, *p<.0001). Bottom left: summary of mean spontaneous EPSC frequencies (AL: 2.3±0.6 Hz, FR: 5.0±1.0 Hz, t(20)=4.8, *p=.0001). Right: example recordings of spontaneous EPSCs. * individual detected spontaneous events.
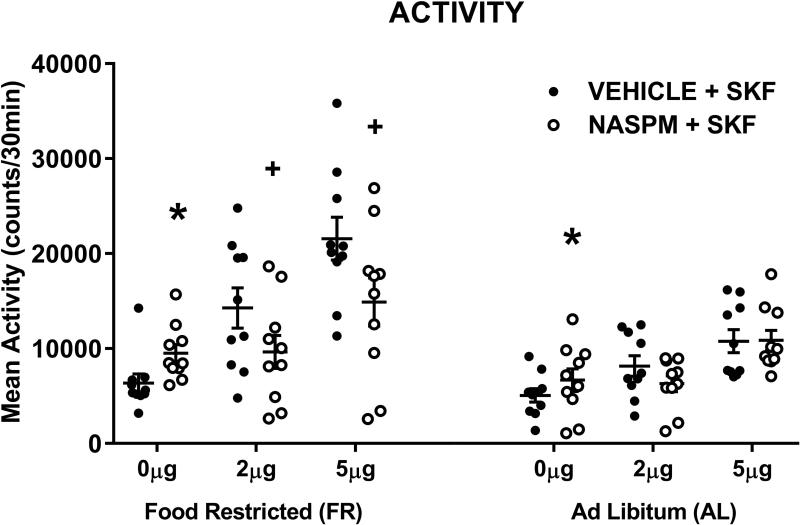
CP-AMPARs mediate enhanced D-1 receptor-stimulated locomotor activity in food-restricted rats
In the 15-min monitoring/habituation periods that preceded each intracranial injection and behavioral test session, there were no differences in locomotor activity across test days (F5,90=0.5, p=.77), between diet groups (F1,18=1.78, p=.2), or interactions between test day and diet (F5,90=0.25, p=.93). The locomotor-activating effect of SKF-82958 was dose-related (FDose;1,18=44.3, p<.0001), and greater in FR than AL rats (FDiet; 1,18 =10.58, p=.004). Naspm decreased the response to SKF-82958 (FNaspm;1,18=11.06, p=.009), with a significant interaction between Naspm treatment and diet (FNaspm x Diet;1,18=5.73, p=.028), reflecting the greater blocking effect of Naspm in FR relative to AL rats (Fig 6, top). Estimation of marginal means indicated that activity scores of FR rats in response to both doses of SKF-82958 in the absence of Naspm were outside the 95% confidence interval for scores obtained in the presence of Naspm. This was not the case for either dose of SKF-82958 in AL rats. Analysis of the follow-up test in which Naspm was compared to vehicle microinjection indicated that Naspm increased locomotor activity (F1,18=27.65, p<.0001) with no effect of diet (F1,18=1.7, p=.21) and no interaction between microinjection treatment and diet (F1,18=1.05, p=.32).
Figure 6.
Effects of SKF-82958, microinjected in nucleus accumbens shell, in the absence and presence of Naspm (25.0 μg), on locomotor activity in food-restricted (FR) and ad libitum fed (AL) rats. Data from individual subjects are displayed with mean (± s.e.m.) counts shown for the 30-min post-injection tests. * Naspm increased locomotor activity relative to saline vehicle, p<.0001; + Naspm decreased SKF-induced locomotor activity, p<.01. n=10 per diet group.
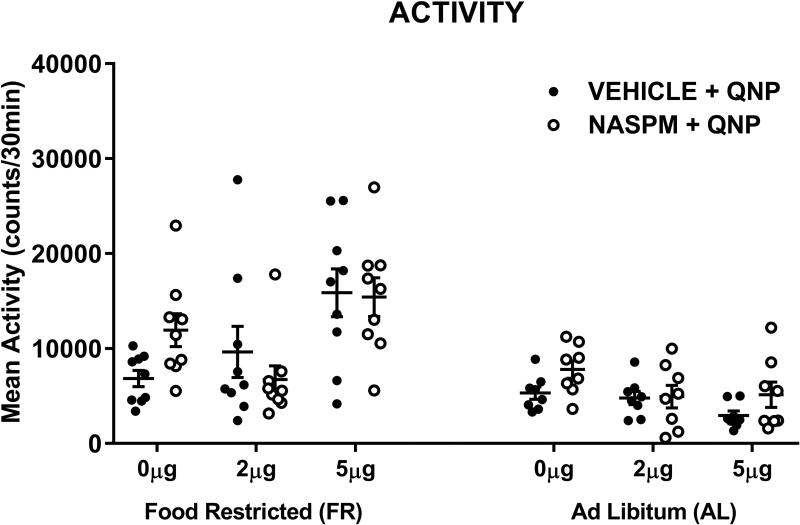
Following completion of the experiment just described, a control experiment, with new subjects, was conducted to test whether Naspm also decreases behavioral responsiveness to D-2 receptor stimulation (Fig 7). The locomotor-activating effect of quinpirole (top) was dose-related (FDose;1,15 =8.97, p<.01), and greater in FR than AL rats (FDiet;1,15=16.54, p<.001). There was no main effect of Naspm (FNaspm;1,15=0.07) and no interaction between Naspm treatment and diet (FNaspm x Diet;1,15=2.1, p=.17).
Figure 7.
Effects of quinpirole, microinjected in nucleus accumbens shell, in the absence and presence of Naspm (25.0 μg), on locomotor activity in food-restricted (FR) and ad libitum fed (AL) rats. Data obtained from individual subjects are displayed with mean (± s.e.m.) counts shown for the 30-min post-injection tests. Naspm did not affect quinpirole-induced locomotor activity. n=8 (AL) and 9 (FR) per diet group.
Discussion
Results of the present study confirm the prediction that FR increases surface expression and, specifically, the abundance of GluA1, but not GluA2, in the NAc PSD. The finding that FR also increased pSer845-GluA1 in the PSD is significant in several respects. Phosphorylation at Ser845 increases GluA1 peak current and channel open probability (Roche et al., 1996; Banke et al., 2000; Goel et al.,2011), is a necessary step in the synaptic trafficking of GluA1 (Shi et al, 2001; Esteban et al., 2003; Man et al., 2007; Goel et al.,2011), maintains surface stability of CP-AMPARs (He et al., 2009), and is a requirement for synaptic incorporation of GluA1 during both LTP (Esteban et al., 2003; Oh et al., 2006) and homeostatic synaptic scaling (Goel et al., 2011; Kim & Ziff, 2014). Thus, a sustained increase in pSer845 may be instrumental in trafficking and maintaining the increased abundance of GluA1 in the PSD of FR rats.
The present electrophysiological results strengthen interpretation of the biochemical results as reflecting synaptic incorporation of GluA2-lacking, CP-AMPARs. In cultured neurons deprived of excitatory input, the amplitude and frequency of spontaneous mEPSCs are increased (Thiagarajan et al., 2002, 2005), and are sensitive to Naspm (Thiagarajan et al. 2005; Sutton et al., 2006). Here, Naspm markedly and selectively decreased the amplitude of evoked EPSCs in MSNs of FR rats. FR was also shown to increase the amplitude and frequency of spontaneous EPSCs recorded from NAc medium spiny neurons (MSNs). However, these changes in spontaneous activity were not significantly decreased by Naspm. The absence of Naspm effect on amplitude is somewhat surprising given that the density of CP-AMPARs should contribute to this amplitude. This result could indicate that additional mechanisms play a role at the level of synapses recruited during spontaneous versus evoked activity. For example, the synapses activated during spontaneous release but not those activated during evoked release could incorporate more Naspm-insensitive AMPA channels in FR rats. In support of this possibility, differential modulation of evoked and spontaneous activity have previously been reported (Scanziani et al., 1992; Owen et al., 2013).
Together with the biochemical data, the electrophysiological findings suggest that CP-AMPARs are absent from the PSD under basal conditions but are incorporated during FR. Moreover, they seem to account for the enhanced behavioral response of FR subjects to D-1 receptor stimulation. The selective effect of Naspm on the otherwise enhanced D-1 receptor agonist-induced locomotor activation in FR rats adds to previous findings that Naspm blocked the enhanced rewarding effects of drugs microinjected into the NAc shell of FR rats (Carr et al., 2010; Peng et al., 2014). The finding that Naspm, alone, moderately increased locomotor activity across diet groups is suggestive of a nonspecific effect; importantly it did not obscure the strong and selective decreasing effect of Naspm on locomotor activity induced by D-1 receptor stimulation in FR rats.
In addition to LTP, in which CP-AMPARs are incorporated into the synaptic membrane during the initial stage of targeted synaptic strengthening (Plant et al., 2006; Yang et al., 2010), more widespread synaptic incorporation of CP-AMPARs occurs in response to suppression of excitatory input to neurons in culture (Thiagarajan et al., 2005, 2007; Lee, 2012; Kim & Ziff, 2014) and cortical and brainstem sensory neurons in vivo (Goel et al. 2011; Jitsuki et al. 2011; Sarin et al., 2012). This homeostatic synaptic scaling is triggered by decreased Ca2+ signaling (Thiagarajan, 2002; Kim & Ziff, 2014), and decreased activity of the Ca2+-dependent phosphatase, calcineurin, which normally dephosphorylates pSer845-GluA1 and enables endocytosis. The resultant stabilization of pSer845-GluA1 increases synaptic expression of CP-AMPARs (Kim & Ziff, 2014). There is an interesting parallel in the current observations of decreased whole cell levels of CaMKII-α, CaMKII-β, and calcineurin, with increased pSer845-GluA1 in the PSD. In a prior study it was found that brief restraint combined with intracerebroventricular injection of either saline or D-1 receptor agonist produced greater phosphorylation of CaMKII in NAc of FR as compared to AL rats (Haberny & Carr, 2005b). The decreased levels of total CaMKII under basal conditions along with increased stimulus-induced phosphorylation suggest compensatory upregulation of the stimulated response. Thus, the present findings, in combination with key findings in the literature, suggest a mechanistic scheme to be investigated in continuing molecular studies. Specifically, the D-1 receptor, which regulates Ca2+ influx to MSN dendritic spines via NMDA and L-type Ca2+ channels (Moyer et al., 2007), may be in a predominant low affinity state relative to D-2 receptors (Richfield et al., 1989), and therefore subnormally stimulated by the low tonic extracellular (Pothos et al., 1995) and evoked DA release (Stouffer et al., 2015) in FR subjects. A compensatory synaptic incorporation of CP-AMPARs in D-1 receptor-expressing MSNs would be enabled, at least in part, by decreased calcineurin and increased pSer845-GluA1 in response to decreased Ca2+ signaling. Behaviorally, the consequence would be increased incentive effects of food, drugs and cues that stimulate a DA surge. An important caveat to this speculative scheme is that phosphorylation and synaptic incorporation of GluA1 is regulated by calcineurin anchored to the postsynaptic scaffold protein, AKAP150 (Sanderson et al., 2012). In the present study, differences in calcineurin levels between diet groups were seen in the whole cell but not the PSD fraction where calcineurin is mediating these key regulatory activities.
Considering that food is the most essential and frequently consumed natural reward, particularly among rats that are individually housed in a predictable environment, it may be speculated that current observations reflect a homeostatic response to sustained reward deprivation. Interestingly, four other conditions associated with increased synaptic abundance of CP-AMPARs in NAc are cocaine abstinence (Boudreau & Wolf, 2005; Conrad et al., 2008), amphetamine abstinence (Jedynak et al., 2015), chronic pain (Goffer et al., 2013) and, most recently, withdrawal from ‘junk food’ (Oginsky et al., 2016). In cocaine abstinence these receptors are directly involved in escalation of cocaine-seeking and relapse (Conrad et al., 2008), while in chronic pain they ameliorate concomitant behavioral depression (Goffer et al., 2013). Chronic FR, psychostimulant withdrawal, junk food withdrawal, and chronic pain may all be viewed as aversive or reward-deficiency states and the resultant upregulation of AMPAR trafficking in NAc may be a common homeostatic response that facilitates resolution or restoration of motivational-affective balance. This hypothesis becomes especially plausible if increased CP-AMPAR abundance is associated with D-1 receptor-expressing MSNs. Optogenetic and chemogenetic analyses indicate that excitation of D-1 receptor-expressing MSNs increases behavioral activation and reward, while excitation of D-2 receptor-expressing MSNs exerts opposite effects (Ferguson et al., 2011; Lobo et al., 2011; Yawata et al., 2012). The present finding that Naspm decreased behavioral activation induced by D-1, but not D-2, receptor agonist microinjection in NAc shell provides support for the idea that insertion of CP-AMPARs might occur selectively in D-1 receptor-expressing MSNs. At the same time, the enhanced, but Naspm-insensitive, behavioral response to quinpirole in FR rats indicates that there is likely more than one neuroadaptation contributing to the net effect of FR on DA transmission and behavior. The recently demonstrated increase in D-2 receptor protein expression in NAc synaptoneurosomes of FR rats points to a potential mechanistic basis of the enhanced response to quinpirole (Jones et al., 2016).
In summary, it is proposed that increased synaptic incorporation of CP-AMPARs during FR serves to enhance the behavioral response to acute reward stimuli. In addition, due to increased D-1 receptor signaling and Ca2+-dependent signaling via CP-AMPARs, reward stimulation may also have increased capacity to induce neuroplastic changes that ingrain the associated reward-directed behavior (Mangiavacchi & Wolf, 2004; Asrar et al., 2009; Witgen et al., 2010). In the wild, the adaptive value of upregulated reward learning and strengthening of food acquisition/consummatory behavior is clear. In an environment where severe dieting is voluntary or a component of an eating disorder, and there is occasional breakthrough gorging on palatable food, or use of pharmacological proxies, those behaviors may be subject to strengthening that endures, contributing to an increased vulnerability to overeating, bingeing, and/or abusing drugs.
Acknowledgements
This work was supported by DA003956 and DA036784 (K.D. C.) from the National Institute on Drug Abuse.
Footnotes
Author contributions J.O. and K.D.C. designed the overall study and drafted the manuscript; all authors contributed to the final manuscript text; J.O. conducted subcellular fractionation experiments and data analysis, with contributions from A.R.; S.C.V. designed behavioral experiments and analyzed data; R.K. conducted behavioral experiments; R.C.F. designed electrophysiology protocols; J.K.S. and I.C. performed electrophysiology studies and analyzed data; K.T.J. piloted and established the crosslink protocol, conducted experiments and analyzed data..
The authors declare no competing financial interests.
References
- Asrar S, Zhou Z, Ren W, Jia Z. Ca(2+) permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II. PLoSOne. 2009;4:e4339. doi: 10.1371/journal.pone.0004339. doi: 10.1371/journal.pone.0004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SB, Gortmaker SL. Dieting and smoking initiation in early adolescent girls and boys: a prospective study. Am. J. Public Health. 2001;91:446–450. doi: 10.2105/ajph.91.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW, 3rd, Khan LK. Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes. Res. 2005;13:596–607. doi: 10.1038/oby.2005.64. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J. Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neurosci. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci. 2000;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacol. 2005;179:430–436. doi: 10.1007/s00213-004-2037-x. [DOI] [PubMed] [Google Scholar]
- Cochrane C, Malcolm R, Brewerton T. The role of weight control as a motivation for cocaine abuse. Addict. Behav. 1998;23:201–207. doi: 10.1016/s0306-4603(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cunha TM, Sedki F, Macri J, Casola C, Shalev U. The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacol. 2013;225:241–250. doi: 10.1007/s00213-012-2810-1. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: A case of homology? Physiol. Behav. 2005;86:9–10. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nature Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarana C, Masi F, Leggio B, Grappi S, Nanni G, Scheggi S, De Montis MG, Tagliamonte A. Acquisition of a palatable-food-sustained appetitive behavior in satiated rats is dependent on the dopaminergic response to this food in limbic areas. Neurosci. 2003;121:179–187. doi: 10.1016/s0306-4522(03)00383-x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Ann. Rev. Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat. Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y, Megill A, Takamiya K, Huganir RL, Lee HK. Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoSOne. 2011;6:e18264. doi: 10.1371/journal.pone.0018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffer Y, Xu D, Eberle SE, D’amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J. Neurosci. 2013;33:19034–19044. doi: 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberny S, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP Kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neurosci. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Comparison of basal and D-1 dopamine receptor agonist stimulated neuropeptide gene expression in caudate-putamen and nucleus accumbens of ad libitum fed and food-restricted rats. Molec. Brain Res. 2005a;141:121–127. doi: 10.1016/j.molbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated calcium-calmodulin kinase II and NMDA receptor/extracellular signal-regulated kinase 1/2-mediated cyclic amp response element-binding protein phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci. 2005b;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int. J. Eat. Dis. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc. Natl. Acad. Sci. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain. Res. Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak J, Hearing M, Ingebretson A, Ebner S, Kelly M, Fischer RA, Kourrich S, Thomas MJ. Cocaine and amphetamine induce overlapping but distinct patterns of AMPAR plasticity in nucleus accumbens medium spiny neurons. Neuropsychopharmacol. 2015;41:464–476. doi: 10.1038/npp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, Kessels HW, Takahashi T. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JM, Gold MS, Sweeney D, Pottash AL. Eating disorders and cocaine abuse: a survey of 259 cocaine abusers. J. Clin. Psychiatry. 1987;48:47–50. [PubMed] [Google Scholar]
- Jones KT, Woods C, Zhen J, Antonio T, Carr KD, Reith MEA. Effects of diet and insulin on dopamine transporter activity and expression in rat caudateputamen, nucleus accumbens, and midbrain. J. Neurochem. 2016 doi: 10.1111/jnc.13930. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol. Cell. Proteomics. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- Kim S, Ziff EB. Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biol. 2014;12:e1001900. doi: 10.1371/journal.pbio.1001900. doi: 10.1371/journal.pbio.1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. J. Subst. Abuse. 1992;4:341–353. doi: 10.1016/0899-3289(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Kruger J, Galuska DA, Serdula MK, Jones DA. Attempting to lose weight: specific practices among U.S. adults. Am. J. Prev. Med. 2004;26:402–406. doi: 10.1016/j.amepre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lee HK. Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Front. Mol. Neurosci. 2012;5:17. doi: 10.3389/fnmol.2012.00017. doi: 10.3389/fnmol.2012.00017. eCollection 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zheng D, Peng XX, Cabeza de Vaca S, Carr KD. Enhanced cocaine-conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluA1 in food-restricted rats. Brain Res. 2011;1400:31–41. doi: 10.1016/j.brainres.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. doi: 10.3389/fnana.2011.00041. eCollection 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X-F, Sun L-L, Cui C-L, Han J-S. NAc shell Arc/Arg3.1 protein mediates reconsolidation of morphine CPP by increased GluR1 cell surface expression: Activation of ERK-coupled CREB is required. J. Neuropsychopharmacol. 2015;18:1–10. doi: 10.1093/ijnp/pyv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad. Sci. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Mann T, Tomiyama J, Westling E, Lew A-M, Samuels B, Chatman J. Medicare's search for effective obesity treatments: Diets are not the answer. Amer. Psychol. 2007;62:220–233. doi: 10.1037/0003-066X.62.3.220. [DOI] [PubMed] [Google Scholar]
- Moyer JT, Wolf JA, Finkel LH. Effects of dopaminergic modulation on the integrative properties of the ventral striatal medium spiny neuron. J. Neurophysiol. 2007;98:3731–3748. doi: 10.1152/jn.00335.2007. [DOI] [PubMed] [Google Scholar]
- Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR. Eating ‘junk food’ produces rapid and long-lasting increases in. NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacol. 2016 doi: 10.1038/npp.2016.111. doi: 10.1038/npp.2016.111. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Berman Y, Haberny LY, Meller E, Carr KD. Synthesis, protein levels and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res. 2006;1122:135–142. doi: 10.1016/j.brainres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X-X, Cabeza de Vaca S, Ziff E, Carr KD. Involvement of nucleus accumbens AMPA receptor trafficking in augmentation of d-amphetamine reward in food-restricted rats. Psychopharmacol. 2014;231:3055–3063. doi: 10.1007/s00213-014-3476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X-X, Lister A, Rabinowitsch A, Kolaric R, Cabeza de Vaca S, Ziff EB, Carr KD. Episodic sucrose intake during food restriction increases synaptic abundance of AMPA receptors in nucleus accumbens and augments intake of sucrose following restoration of ad libitum feeding. Neurosci. 2015;295:58–71. doi: 10.1016/j.neuroscience.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X-X, Ziff EB, Carr KD. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011;65:1024–1031. doi: 10.1002/syn.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietilainen KH, Saarni SE, Kaprio J, Rissanen A. Does dieting make you fat? A twin study. Int. J. Obes. 2012;36:456–464. doi: 10.1038/ijo.2011.160. [DOI] [PubMed] [Google Scholar]
- Pisetsky EM, Chao YM, Dierker LC, May AM, Striegel-Moore RH. Disordered eating and substance use in high-school students: results from the Youth Risk Behavior Surveillance System. Int. J. Eat. Disord. 2008;41:464–470. doi: 10.1002/eat.20520. [DOI] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal longterm potentiation. Nat. Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J. Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neurosci. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Root TL, Pinheiro AP, Thornton L, Strober M, Fernandez-Aranda F, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, Klump KL, La Via M, Mitchell J, Woodside DB, Rotondo A, Berrettini WH, Kaye WH, Bulik CM. Substance use disorders in women with anorexia nervosa. Int. J. Eat. Disord. 2010;43:14–21. doi: 10.1002/eat.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, Chick WS, Dell'Acqua ML. AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J. Neurosci. 2012;32:15036–15052. doi: 10.1523/JNEUROSCI.3326-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin RM, Mowery TM, Garraghty PE. AMPA receptor subunit expression in the cuneate nucleus of adult squirrel monkeys after peripheral nerve injury. Neurosci. Lett. 2012;516:193–196. doi: 10.1016/j.neulet.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gähwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Seo D-C, Jiang N. Associations between smoking and severe dieting among adolescents. J. Youth Adolesc. 2009;38:1364–1373. doi: 10.1007/s10964-009-9421-0. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Stice E, Davis K, Miller NP, Marti NC. Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. J. Abnorm. Psychol. 2008;117:941–946. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer M, Woods C, Patel J, Lee C, Witkovsky P, Bao L, Machold R, Jones K, Cabeza de Vaca S, Reith M, Carr KD, Rice M. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nature Commun. 2015 Oct 27;6:8543. doi: 10.1038/ncomms9543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. Alpha- and beta-CaMKII: Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacol. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Tukey D, Ferreira J, Antoine S, D'Amour J, Ninan I, Cabeza de Vaca S, Incontro S, Horwitz J, Hartner D, Guarini C, Khatri L, Goffer Y, Xu D, Titcombe R, Khatri M, Marzan D, Mahajan S, Wang J, Froemke R, Carr KD, Aoki C, Ziff E. Sucrose ingestion induces rapid AMPA receptor trafficking. J. Neurosci. 2013;33:6123–6132. doi: 10.1523/JNEUROSCI.4806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Phil. Trans. Royal Soc. Brit. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, Saab F, Tonegawa S, Heinemann SF, O'Dell TJ, Fanselow MS, Vissel B. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoSOne. 2010;5 doi: 10.1371/journal.pone.0012818. doi: 10.1371/journal.pone.0012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Zhou Q. Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc. Natl. Acad. Sci. U S A. 2010;107:11999–12004. doi: 10.1073/pnas.0913004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc. Natl. Acad. Sci. 2012;109:12764–12769. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Cabeza de Vaca S, Carr KD. Food restriction increases acquisition, persistence and drug prime-induced expression of a cocaine-conditioned place preference in rats. Pharmacol. Biochem. Behav. 2012;100:538–544. doi: 10.1016/j.pbb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Liu S, Cabeza de Vaca S, Carr KD. Effects of time of feeding on psychostimulant reward, conditioned place preference, metabolic hormone levels, and nucleus accumbens biochemical measures in food-restricted rats. Psychopharmacol. 2013;227:307–320. doi: 10.1007/s00213-013-2981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]