Abstract
Background
Severe obesity in adolescence is associated with reduced life expectancy and impaired quality of life. Long-term benefits of conservative treatments in adolescents are limited, while short-term outcomes of adolescent bariatric surgery are promising. This study aimed to report 5-year outcomes following Roux-en-Y gastric bypass (RYGB) in adolescents, compared with conservatively treated adolescents and adults undergoing RYGB.
Methods
A nationwide prospective non-randomised controlled study of adolescents (13–18 years) with severe obesity undergoing RYGB, a matched adolescent control group undergoing conservative treatment, and an adult comparison group undergoing RYGB. The primary outcome measure was change in weight over 5 years. Multilevel mixed-effect regression models were used to assess longitudinal changes. Healthcare usage was analysed with linear regression together with nonparametric bootstrapping.
Findings
Eighty-one adolescents with baseline age 16·5 years (SD 1·2), weight 132·8 kg (SD 22·1) and body mass index (BMI) 45·5 kg/m2 (SD 6·1) underwent RYGB. Five-year weight change was −36·8 kg (95% CI −40·9 to −32·8) resulting in a BMI reduction of 13·1 kg/m2, although weight loss <10% occurred in 11%.
Comorbidities and cardiovascular risk factors resolved in 74–100%: type 2 diabetes (3/3), disturbed glucose homeostasis (18/21), dyslipidaemia (43/52), elevated blood pressure (11/12), inflammation (hs-CRP ≥ 2 mg/L; 45/61) and elevated liver enzymes (19/19), each comparing favourably with adolescent controls at 5 years.
Functional (SF-36) and obesity-specific (OP-14) quality of life improved in the adolescent RYGB group (mean difference 4·2, p=0·006 and −9·9 p=0·009). Twenty RYGB participants (25%) underwent additional abdominal surgery for complications of surgery or rapid weight loss, 72% demonstrated some nutritional deficiency, and healthcare consumption increased. Mean BMI increased in control adolescents (3·3 kg/m2, 95% CI 1·9 to 4·8), while BMI change in adults was similar to surgical adolescents (mean difference 0·8 kg/m2, 95% CI −1·1 to 2·8). Twenty adolescent controls (25%) underwent bariatric surgery within 5 years.
Interpretation
Adolescents with severe obesity undergoing RYGB experienced substantial weight loss over 5 years, alongside improvements in comorbidities, risk factors and quality of life. Surgical intervention was, however, associated with additional surgical interventions and nutritional deficiencies. Non-surgical treatment was associated with weight gain and 25% underwent bariatric surgery within 5 years.
Keywords: Adolescent, bariatric surgery, gastric bypass, obesity, surgery, long-term effects
Introduction
Severe obesity in adolescence is a life-threatening and life-shortening disease,1,2 leading to a multitude of other diseases.3,4 As the mean age of obesity onset has decreased,5 the onset of related diseases, most notably type 2 diabetes (T2DM), has shifted increasingly toward childhood.6 T2DM is markedly more aggressive when occurring in childhood,6 and obesity increases cardiovascular risk factors in childhood,7 leading to a poor prognosis in this group,8,9 with few effective therapeutic options available.10
The prevalence of adolescent obesity has now reached between 5% and 10% among developed countries.5,11,12 Non-surgical programmes remain the cornerstone of treatment of adolescents with severe obesity, although their effect is limited and insufficient for long-term reduction of obesity-related health hazards.13 However, surgery is increasingly being recommended14 and performed,15 and robust outcomes have been reported up to three years after surgery.16–18
This study reports outcomes over 5 years in adolescents following Roux-en-Y gastric bypass (RYGB) or conservative treatment in a Swedish nationwide prospective non-randomised controlled study, with an additional matched adult comparison group undergoing RYGB.
Materials and methods
Study design
The Adolescent Morbid Obesity Surgery (AMOS) study is a Swedish nationwide prospective, non-randomised controlled study.17 The study was conducted according to the Declaration of Helsinki with the approval of the Gothenburg regional ethics committee (523–04).
Participants
1. Adolescents treated with Roux-en-Y gastric bypass (RYGB)
All eligible adolescents presenting with severe obesity to three specialised paediatric obesity treatment units were offered assessment for surgery upon fulfilling inclusion criteria. This represented 100 patients, of whom 19 declined surgery and the remaining 81 adolescents ultimately underwent RYGB. Eligibility criteria were: age 13–18 years, BMI ≥ 40, or ≥35 kg/m2 with comorbidity (e.g. T2DM, dyslipidaemia, metabolic syndrome), pubertal Tanner stage >III, height growth velocity beyond peak, and at least 1 year in a formal, conventional weight loss programme. Major exclusion criteria included severe psychiatric disorder, ongoing drug abuse, obesity secondary to brain injury, and syndromic or monogenic obesity (the melanocortin 4 receptor was sequenced in >50% of patients based upon clinical suspicion). Recruitment occurred between 2006 and 2009 (Fig. S1).17
2. Adolescents receiving conventional treatment
A matched conservatively treated adolescent control group was identified from the Swedish Childhood Obesity Treatment Register (BORIS13), ensuring the date of surgery was within 1 month of baseline weight for the corresponding control patient. Sequential matching of individuals ensured that the mean values of matching variables (baseline BMI, age and sex) in the control group moved closer to the mean values within the surgical group as much as was possible with each additional control patient. This registry did not include detailed formal data regarding individuals’ compliance with conventional treatment.
3. Adults treated with gastric bypass
Adults aged 35–45 years with severe obesity (adult group) undergoing RYGB were matched by BMI and sex to adolescents undergoing surgery, and the same inclusion and exclusion criteria as adolescents were used.17
Treatments
The laparoscopic RYGB incorporated an ante-colic, ante-gastric Roux-en-Y construction with a linearly stapled gastro-jejunostomy,19 without closure of mesenteric windows. All adolescent and adult operations were performed at Sahlgrenska University Hospital, Gothenburg, by either of two experienced adult bariatric surgeons, assisted by a paediatric surgeon. Surgical treatment of adults was delivered by the same team in an identical setting in order to maximise comparability. The control group underwent individualised treatment according to Swedish standards.17 Within the pragmatic study design, conventional treatment was non-standardised, but was delivered as an individualised treatment by the multidisciplinary team (MDT) and focused on behaviour change.13,20,21
Clinical measurements
The primary outcome was change in weight across 5 years. Secondary outcomes included detailed anthropometry, biochemistry, quality of life evaluation and clinical outcomes.17
The term disturbed glucose homeostasis was adopted in response to incomplete data regarding fasting plasma glucose, and was defined by adding a fasting capillary glucose criterion, i.e. ≥6·1 mmol/L but <7·0 mmol/L (≥100 but <110 mg/dL), to the American Diabetes Association (ADA) definition of impaired fasting glucose, or prediabetes,22 i.e. the absence of medication use for DM with fasting plasma glucose ≥5·5 mmol/L but <7 mmol/L (≥100 mg/dL but <126 mg/dL), or HbA1c of ≥39 mmol/mol (≥5·7%) but <45 mmol/mol (<6·5%). T2DM and its remission were also diagnosed according to ADA definitions, remission determined using the criteria FBG <7·0 mmol/L (<126 mg/dL), HbA1C <45 mmol/mol (<6·5%), fasting capillary glucose <6·1 mmol/L (<110 mg/dL) in the absence of diabetes medication.22 All other definitions and remission criteria are provided in the web additional material.
Follow-up
Adolescent surgical patients were assessed before surgery and postoperatively at 2 and 6 months, 1, 2 and 5 years. Body weight, height, blood pressure, biochemical analyses and quality of life assessment were performed preoperatively and at 1, 2 and 5 years after surgery. Information regarding use of drugs or alcohol was sought from participants and caregivers at recruitment. Surgical adolescents were prescribed a daily multivitamin and mineral supplement (including 200 micrograms of folic acid), as well as additional vitamin B12 (cobalamin 1 mg /day), and calcium carbonate/ vitamin D (1 g/ 800 IU /day) tablets. Females also received iron (Fe2+ 100 mg /day) supplementation.
In the adolescent control group, weight and height were measured and registered at baseline and after 1, 2 and 5 years. At 5 years the control group was invited to a study visit for biochemistry and quality of life data collection.
Between years 2 and 5, adolescents were predominantly followed up in the community. In accordance with Swedish convention, systematic medical treatment for cardiovascular risk factors in youth, such as dyslipidaemia or hypertension, was not common practice.
In the adult group, weight and height were measured and registered prospectively at inclusion and 1 year postoperatively. Two- and 5-year weight data were drawn from community healthcare centre measurements, where available, and self-reported measurements otherwise.
Blood sampling and handling have been described in detail previously.17
Health-related quality of life
A Swedish version of Short Form-36 Health Survey v2 (SF-36), validated for use in adolescents, was used to measure health-related quality of life.23 The Obesity-related Problems scale (OP-14) was used to assess psychosocial problems related to weight and body shape.24
Adverse events
Thirty-day surgical complications data in the surgical group were assessed at the 2-month follow-up visit and thereafter prospectively recorded in the electronic case record file. A complementary retrospective survey of medical records was conducted to capture missing data up to 5-year follow-up. In addition, data on inpatient care (admissions and hospital days) and hospital-based outpatient care visits were retrieved from the nationwide National Patient Register, and prescription drug costs from the Prescribed Drug Register.
Statistical analysis
Descriptive statistics are given as means with standard deviations (SD). Multilevel mixed-effect regression models were fitted to the data to assess longitudinal changes. In the analyses, observations were considered nested within persons, and standard errors were calculated by taking into account the repeated measurements. Changes over time are expressed with 95% confidence intervals (CI). The underlying assumptions for the mixed-models were evaluated through analyses of the residuals.
Among control crossovers the last observation was carried forward for anthropometric data and crossovers were excluded from analysis for all other variables. Sex- and age-adjusted mean differences for 5-year accumulated hospital days, visits for outpatient care, and prescription drug costs were estimated using linear regression with 95% CIs generated by nonparametric bootstrapping not requiring additional assumptions.
All p-values are two-tailed and p<0·05 was considered statistically significant. Statistical analyses were carried out using the Stata statistical package 12·1 (Stata-Corp. 2011, Stata Statistical Software: Release 12, College Station, TX, USA; StataCorp LP).
Role of the funding source
Funders of the study did not contribute to the study design, the collection, analysis or interpretation of data, or manuscript writing.
Results
Baseline characteristics
Baseline details are given in Table 1 and Table 2. At inclusion, participants in the surgical group were older and had significantly higher BMI than the control group. The proportion of males was 44% in the control group and 35% in both surgical groups (non-significant). Mean age in the adult group was 39·7 years.
Table 1.
Baseline and 5-year characteristics.
| RYGB Adolescents | Control Adolescents | Gastric Bypass Adults | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 5 years | Baseline | 5 years | Baseline | 5 years | |
| Number | ||||||
| Total | 81 | 81 | 80 | 72 | 81 | 71 |
| Male | 28 | 28 | 35 | 30 | 28 | 23 |
| Female | 53 | 53 | 45 | 42 | 53 | 48 |
|
| ||||||
| Age (years) | ||||||
| Total | 16·5 (1·2) | 21·9 (1·2) | 15·8 (1·2) | 20·9 (1·3) | 39·7 (2·9) | 44·7 (2·9) |
| Male | 16·6 (1·3) | 22·0 (1·4) | 15·9 (1·2) | 21·0 (1·2) | 40·2 (3·5) | 45·2 (3·5) |
| Female | 16·5 (1·1) | 21·9 (1·1) | 15·7 (1·3) | 20·8 (1·3) | 39·5 (2·6) | 44·5 (2·6) |
|
| ||||||
| Height (cm) | ||||||
| Total | 171 (9) | 172 (9) | 171 (9) | 173 (10) | 171 (0) | 171 (0) |
| Male | 178 (10) | 180 (9) | 178 (8) | 180 (8) | 182 (0) | 182 (0) |
| Female | 167 (6) | 168 (6) | 166 (8) | 167 (8) | 166 (0) | 166 (0) |
|
| ||||||
| Weight (kg) | ||||||
| Total | 133 (22) | 96 (22) | 124 (21) | 124 (32) | 127 (20) | 90 (18) |
| Male | 147 (23) | 109 (26) | 135 (20) | 132 (27) | 142 (17) | 102 (17) |
| Female | 125 (17) | 89 (17) | 115 (17) | 118 (34) | 120 (17) | 85 (16) |
|
| ||||||
| BMI (kg/m2) | ||||||
| Total | 45·5 (6) | 32·3 (6) | 42·2 (5) | 41·7 (10) | 43·5 (5) | 31·0 (6) |
| Male | 46·7 (6) | 33·3 (7) | 43·0 (5) | 40·8 (8) | 43·1 (6) | 31·1 (6) |
| Female | 44·8 (6) | 31·8 (6) | 41·6 (5) | 42·3 (12) | 43·7 (5) | 31·0 (6) |
RYGB, Roux-en-Y gastric bypass (RYGB); BMI, body mass index. Data are presented as mean (standard deviation).
Table 2.
Anthropometric, biochemical and blood pressure data at baseline and 5 years.
| Panel A | RYGB adolescents | Control adolescents | RYGB vs. Control adolescents | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw data | Within group (RYGB Adolescents) mixed-model change | Raw data | Between group mixed-model difference | |||||||||
| Baseline | 5 years | Baseline to 5 years | 5 years | 5 years | ||||||||
| Variable | Mean (SD) | n | Mean (SD) | n | Mean change | 95% CI | p- value | Mean (SD) | n | Mean difference | 95% CI | p-value |
| Height (cm) | 170·8 (9·3) | 81 | 172·3 (9·4) | 81 | 1·48 | 0·9 to 2·1 | <0·001 | 173·0 (10·0) | 53 | −0·75 | −4·2 to 2·7 | 0·666 |
| Weight (kg) | 132·8 (22·1) | 81 | 96·0 (22·2) | 81 | −36·8 | −40·9 to −32·8 | <0·001 | 133·3 (28·9) | 53 | −37·21 | −46·4 to − 28·0 | <0·001 |
| BMI (kg/m2) | 45·5 (6·1) | 81 | 32·3 (6·3) | 81 | −13·14 | −14·5 to −11·8 | <0·001 | 44·6 (9·5) | 53 | −12·26 | −15·2 to − 9·3 | <0·001 |
| HbA1c (mmol/mol) | 35·1 (3·9) | 80 | 33·5 (3·8) | 65 | −1·56 | −2·5 to −0·6 | 0·002 | 35·3 (10·6) | 37 | −1·8 | −5·4 to 1·8 | 0·32 |
| Fasting plasma glucose (mmol/L) | 5·1 (0·5) | 80 | 4·8 (0·4) | 36 | −0·33 | −0·5 to −0·1 | 0·001 | 5·2 (0·7) | 18 | −0·45 | −0·8 to −0·1 | 0·009 |
| Fasting capillary glucose (mmol/L) | 5·6 (0·5) | 78 | 5·2 (0·5) | 73 | −0·35 | −0·5 to −0·22 | 0·001 | 5·8 (2·4) | 16 | −0·6 | −1·8 to 0·6 | 0·34 |
| Fasting plasma insulin (pmol/L) | 216·7 (122·4) | 79 | 65·0 (34·2) | 75 | −151·42 | −173·3 to − 129·5 | <0·001 | 182·8 (122·6) | 37 | −117·81 | −158·3 to − 77·3 | <0·001 |
| Triglycerides (mmol/L) | 1·3 (0·6) | 80 | 0·9 (0·3) | 76 | −0·39 | −0·5 to −0·3 | <0·001 | 1·4 (0·8) | 41 | −0·47 | −0·7 to −0·2 | <0·001 |
| LDL (mmol/L) | 2·6 (0·7) | 81 | 2·2 (0·7) | 76 | −0·46 | −0·6 to −0·3 | <0·001 | 3 (0·8) | 41 | −0·88 | −1·2 to −0·6 | <0·001 |
| HDL (mmol/L) | 1·1 (1·1) | 81 | 1·6 (0·5) | 75 | 0·49 | −0·4 to 0·6 | <0·001 | 1·0 (0·3) | 42 | 0·55 | 0·4 to 0·7 | <0·001 |
| Systolic blood pressure (mmHg) | 124·6 (12·3) | 78 | 113·2 (10·7) | 72 | −11·55 | −14·0 to −9·1 | <0·001 | 121·4 (11·4) | 40 | −8·18 | −12·5 to − 3·8 | <0·001 |
| Diastolic blood pressure (mmHg) | 76·9 (9·8) | 78 | 69·4 (9·9) | 72 | −7·4 | −10·2 to −4·6 | <0·001 | 77·7 (10·0) | 40 | −8·28 | −12·2 to − 4·4 | <0·001 |
| hsCRP (mg/L) | 7·2 (5·9) | 75 | 1·8 (2·2) | 77 | −5·41 | −7·4 to −3·5 | <0·001 | 7·9 (6·9) | 39 | −6·09 | −8·3 to −3·9 | <0·001 |
| ALT (μkat/L) | 0·6 (0·4) | 80 | 0·3 (0·2) | 76 | −0·35 | −0·4 to −0·3 | <0·001 | 0·4 (0·3) | 42 | −0·16 | −0·3 to −0·1 | <0·001 |
| AST (μkat/L) | 0·5 (0·2) | 80 | 0·4 (0·2) | 76 | −0·09 | −0·1 to −0·0 | 0·002 | 0·4 (0·2) | 41 | −0·04 | −0·1 to 0·0 | 0·25 |
| Haemoglobin (g/L) | 139·3 (12·3) | 78 | 127·7 (17·4) | 77 | −11·69 | −15·6 to −7·8 | <0·001 | 141·9 (14·4) | 42 | −14·17 | −20·1 to − 8·3 | <0·001 |
Per protocol data (crossovers excluded). RYGB, Roux-en-Y gastric bypass; SD, standard deviation; n, number of patients; CI, confidence interval; BMI, body mass index; HbA1c, glycated haemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; ALT, alanine transaminase; AST, aspartate transaminase.
Psychosocial impairment, such as depressive or anxiety disorder, was common in the surgical group and a neuropsychiatric diagnosis was present in 31% of subjects (specific diagnoses unavailable). Sixteen percent had previously demonstrated self-destructive behaviour. Forty-one percent had previously been treated in a paediatric psychiatry outpatient department.
Follow-up rates
The follow-up rate was 100% in the surgical group, 90% (72/80) in the control group and 88% (71/81) in the adult group at 5 years. The follow up rate of our cohorts in national health care registries was 100%.
Weight outcomes
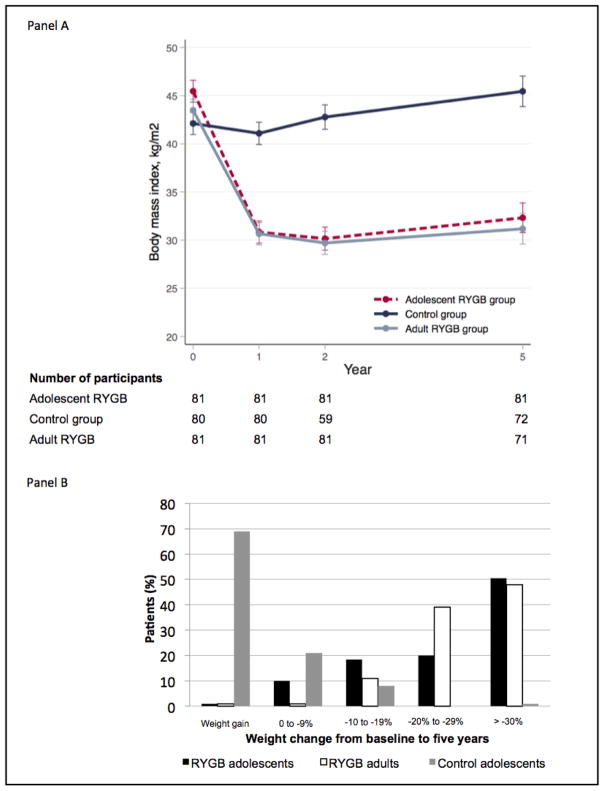
Anthropometric changes are given in Table 1 and Table 2. Mean BMI change across 5 years was −13·1 kg/m2 (95% CI −14·5 to −11·8) in the surgical group, +3·3 kg/m2 (95% CI +1·1 to +4·8) in the control group, and −12·3 kg/m2 (95% CI −13·7 to −10·9) in the adult group. The proportion of participants reaching a BMI <35 kg/m2was 72% (surgical), 7% (control), and 76% (adult) respectively. Thirty-seven percent of surgical group patients no longer had obesity (BMI<30), 3% in the control group, and 40% in the adult group. The majority of adolescent and adult surgical group patients achieved ≥20% total body weight loss (69% and 85% respectively), while a majority (69%) of control patients gained weight (Fig. 1b). Suboptimal weight loss was more common among adolescents than adults (p=0·035, Fig. 1b). Mean weight-regain between a nadir, observed at 2 years, and follow-up at 5 years, was similar in the operated adolescents and adults (Fig. 1a).
Figure 1. Body mass index (panel A) and weight (panel B) change from baseline to 5 years.
Control adolescent data are presented using the last observation before surgery carried forward for patients who underwent surgery within the follow-up period.
Twenty patients (25%) in the control group underwent bariatric surgery between follow-up years 2 and 5, having reached adult eligibility. This group had a median weight gain of 19·6 kg (range −1·1 to 53·5) from baseline until undergoing surgery, compared to a 7·3 kg (range −26·8 to 60·4) increase in control adolescents not undergoing surgery over 5 years.
Cardiometabolic risk factors
Longitudinal metabolic changes are reported for the surgical group alongside 5-year cross-sectional values for control participants in Table 2 and Table S1 (web additional material).
Glucose homeostasis
All measures of glucose homeostasis improved across 5 years (Table 2). At baseline, three patients (4%) had TD2M, all of whom were in remission 5 years after surgery. A disturbed glucose homeostasis was observed at baseline in 22 individuals (27%), which normalised in 18 patients (86%), although two new cases occurred after 5 years, resulting in a total of six cases (8%) at 5 years after surgery (Table 3). Fasting plasma insulin levels decreased markedly from 216·7 to 65·0 pmol/L (Table 2). Meanwhile, in the control group, the prevalence of disturbed glucose homeostasis was 16% at 5 years, and one new case of T2DM was observed (Table 3).
Table 3.
Prevalence and remission of CV risk factors at baseline and 5 years.
| Panel B | RYGB Adolescents | p-value | Control Adolescents | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline, n | 5 years | Resolution§ | RYGB Baseline vs. 5 years | 5 years | RYGB vs. Controls at 5 years | ||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |||
| T2DM | 3/81 | 3·7 (0·8 to 10·4) | 0/79 | 0·0 (0·0 to 4·6) | 3/3 | 100·0 (29·2 to 100·0) | 0·250 | 1/44 | 2·3 (0·1 to 12·0) | 0·372 |
| Disturbed glucose homeostasis | 22/81 | 27·2 (17·9 to 38·2) | 6/79 | 7·6 (2·8 to 15·8) | 18/21* | 85·7 (63·7 to 97·0) | 0·001 | 7/44 | 15·9 (6·6 to 30·1) | 0·098 |
| Elevated HbA1c | 10/80 | 12·5 (6·2 to 21·8) | 6/65 | 9·2 (3·5 to 19·0) | 5/8* | 62·5 (24·5 to 91·5) | 0·727 | 6/37 | 16·2 (6·2 to 32·0) | 0·345 |
| Impaired fasting plasma glucose | 16/80 | 20·0 (11·9 to 30·4) | 0/36 | 0·0 (0·0 to 9·7) | 13/13* | 100·0 (75·3 to 100·0) | 0·003 | 2/18 | 11·1 (1·4 to 34·7) | 0·107 |
| Elevated fasting plasma insulin | 56/79 | 70·9 (59·6 to 80·6) | 3/76 | 3·9 (0·8 to 11·1) | 49/52* | 94·2 (84·1 to 98·8) | <0·001 | 17/37 | 45·9 (29·5 to 63·1) | <0·001 |
| Dyslipidaemia | 56/81 | 69·1 (57·9 to 78·9) | 11/76 | 14·5 (7·5 to 24·4) | 43/52* | 82·7 (69·7 to 91·8) | <0·001 | 30/41 | 73·2 (57·1 to 85·8) | <0·001 |
| Elevated LDL | 13/81 | 16·0 (8·8 to 25·9) | 0/76 | 0·0 (0·0 to 4·7) | 13/13 | 100·0 (75·3 to 100·0) | <0·001 | 9/41 | 22·0 (10·6 to 37·6) | <0·001 |
| Elevated triglycerides | 25/80 | 31·3 (21·3 to 42·6) | 0/76 | 0·0 (0·0 to 4·7) | 22/22* | 100·0 (84·6 to 100·0) | <0·001 | 10/41 | 24·4 (12·4 to 40·3) | <0·001 |
| Low HDL | 41/81 | 50·6 (39·3 to 61·9) | 11/75 | 14·7 (7·6 to 24·7) | 28/37* | 75·7 (58·8 to 88·2) | <0·001 | 27/42 | 64·3 (48·0 to 78·4) | <0·001 |
| Elevated blood pressure | 12/78 | 15·4 (8·2 to 25·3) | 2/72 | 2·8 (0·3 to 9·7) | 12/12 | 100·0 (73·5 to 100·0) | 0·013 | 4/39 | 10·3 (2·9 to 24·2) | 0·182 |
| Elevated systolic blood pressure | 11/78 | 14·1 (7·3 to 23·8) | 0/72 | 0·0 (0·0 to 5·0) | 11/11 | 100·0 (71·5 to 100·0) | 0·001 | 2/39 | 5·1 (0·8 to 17·3) | 0·121 |
| Elevated diastolic blood pressure | 4/78 | 5·1 (1·4 to 12·6) | 2/72 | 2·8 (0·3 to 9·7) | 4/4 | 100·0 (39·8 to 100·0) | 0·688 | 4/39 | 10·3 (2·9 to 24·2) | 0·182 |
| Elevated hsCRP | 65/75 | 86·7 (76·8 to 93·4) | 19/77 | 24·7 (15·6 to 35·8) | 45/61* | 73·8 (60·9 to 84·2) | <0·001 | 32/39 | 82·1 (66·5 to 92·5) | <0·001 |
| Elevated liver enzymes | 25/81 | 30·9 (21·1 to 42·1) | 4/76 | 5·3 (1·5 to 12·9) | 23/25 | 92·0 (74·0 to 99·0) | <0·001 | 8/44 | 18·2 (8·2 to 32·7) | 0·030 |
| Elevated ALT | 24/80 | 30·0 (20·3 to 41·3) | 2/76 | 2·6 (0·3 to 9·2) | 23/24 | 95·8 (78·9 to 99·9) | <0·001 | 7/42 | 16·7 (7·0 to 31·4) | 0·010 |
| Elevated AST | 9/80 | 11·3 (5·3 to 20·3) | 4/76 | 5·3 (1·5 to 12·9) | 9/9 | 100·0 (66·4 to 100··0) | 0·267 | 3/41 | 7·3 (1·5 to 19·9) | 0·695 |
| Anaemia | 8/78 | 10·3 (4·5 to 19·2) | 25/77 | 32·5 (22·2 to 44·1) | 5/7* | 71·4 (29 to 96·3) | 0·002 | 3/42 | 7·1 (1·5 to 19·5) | 0·001 |
Per protocol data (crossovers excluded). RYGB, Roux-en-Y gastric bypass; SD, standard deviation; n, number of patients; CI, confidence interval; T2DM, type 2 diabetes mellitus; HbA1c, glycated haemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; AST, aspartate transaminase; ALT, alanine transaminase.
Definitions: T2DM, fasting plasma glucose ≥7 mmol/L, or HbA1c ≥45 mmol/mol); disturbed glucose homeostasis, fasting plasma glucose ≥5·5 mmol/L but <7 mmol/L, HbA1c ≥39 mmol/mol but <45 mmol/mol, or fasting capillary glucose ≥6·1 mmol/L but <7·0 mmol/L; elevated HbA1c, ≥39 mmol/mol; impaired fasting plasma glucose, ≥5·6 mmol/L; elevated fasting plasma insulin, ≥139 pmol/L; dyslipidaemia, elevated LDL or triglycerides, or low HDL; elevated LDL, if <21 years ≥3·37 mmol/L, if ≥21 years ≥4·14 mmol/L; elevated triglycerides, if <21 years ≥1·47 mmol/L, if ≥21 years ≥2·26 mmol/L; elevated HDL, if <21 years ≤1·04 mmol/L, if ≥21 years males ≤1·04 mmol/L, females ≤1·29 mmol/L; elevated blood pressure, elevated systolic or diastolic blood pressure; elevated systolic and diastolic blood pressure, if <18 years ≥95th percentile for age, sex and height, if ≥18 years systolic ≥140 mmHg or diastolic ≥90 mmHg; elevated hsCRP, ≥2mg/L; elevated liver enzymes, elevated AST or ALT; elevated ALT, ≥0.7 μkat/L; elevated AST, ≥0.7 μkat/L; anaemia, haemoglobin males <110 g/L, females <100 g/L;
see supplementary material for definition of resolution;
number in resolution calculation lower than baseline denominator owing to missing data.
Lipids
There were 56 cases (69%) of dyslipidaemia at baseline, decreasing to 11/76 (15%) at 5 years. Notably, all cases of elevated low-density lipoprotein (LDL) or triglycerides resolved across 5 years (Table 3). The 5-year prevalence of dyslipidaemia in the control group was 73% (Table 3).
Blood pressure
Blood pressure was elevated in 12/78 (15%) participants at baseline and normalised in all 12 at 5 years, although two incident cases led to a prevalence of 3% (Table 3). The 5-year prevalence in the control group was 10% (Table 3).
Inflammation
Elevated high sensitivity C-reactive protein (hsCRP; ≥2 mg/L) was present in 87% (65/75) participants at baseline, reducing to 25% (19/77) across 5 years. In the control group hsCRP was elevated in 82% (32/39) at 5 years (Table 3).
Liver function
Elevated alanine transaminase levels were present in 25/81 (31%) surgical patients at baseline, normalising in 92% of cases (23/25) at 5-year follow-up, although there were two incident cases (Table 3). Elevated aspartamine transaminase levels, observed in 9/80 (11%), normalised in all cases across 5 years (Table 3). Alkaline phosphatase is included in Table S1 and Table S2 (web additional material).
Vitamins, minerals and general nutritional markers
At 5 years, 63% (46/73) in the surgical group and 57% in the control group (20/35) had vitamin D (25-OH D) insufficiency (<50 nmol/l; p=0.674; Table S2).
Low ferritin and/or iron levels, present in 24% (18/76) of the surgery participants at baseline, increased to 66% (51/77), compared with 29% (12/42) in the control group at 5 years (Table S2). One of 74 surgical participants (1%) had a low vitamin B12 level at baseline, increasing to 16/73 individuals (22%) at 5 years, when the prevalence was 6% (2/31) in the control group (Table S2).
The prevalence of anaemia (haemoglobin in females <120 g/dL; males <130 g/dL) in the surgical group rose from 10% (8/78) to 32% (25/77) across 5 years, while in the control group it was 7% (3/42) at 5 years (Table 3).
Quality of life
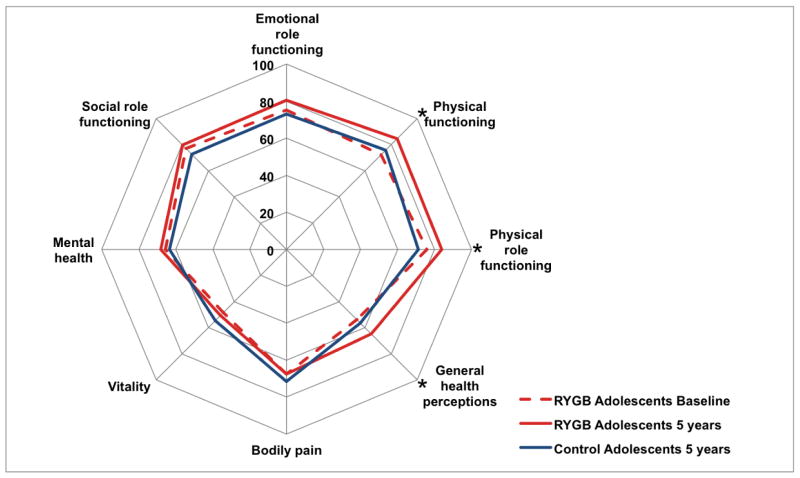
At 5-year follow-up, significant improvements were observed among adolescent surgical patients in the physical component summary score (Table S3) and in 3 of the 8 SF-36 health domains (Fig. 2, Table S3): physical functioning (mean change 13·5, 95% CI 8·1 to 19·0), physical role functioning (mean change 11·2, 95% CI 4·0 to 18·3) and general health perceptions (mean change 12·4, 95% CI 6·5 to 18·3), all of which are within the physical domain (Fig. 2). Physical role functioning was also significantly better among surgical group patients than controls (mean difference 13·5, 95% CI 2·2 to 24·8; Table S3). Weight-related psychosocial problems improved significantly across follow-up (mean difference −13·0, 95% CI −19·6 to −6·4).
Figure 2. Polar chart showing quality of life outcomes.
Data from SF-36 (short-form 36 questionnaire) scores. Asterisks indicate significant improvement between baseline and 5 years among RYGB adolescents.
Adverse events
Across 5 years, 20 patients (25%) in the surgical group underwent 21 additional abdominal surgical interventions, excluding plastic surgery (Table 4). Eleven procedures were for acute intestinal obstruction and nine for symptomatic gallstones. No deaths occurred across 5 years of follow-up. Some patients and their caregivers withheld information about substance misuse, even before surgery. We could not obtain valid data regarding adverse events and reoperation rates in the adult comparison group.
Table 4.
Adverse outcomes in adolescents following Roux-en-Y gastric bypass across 5 years.
| Panel A | ||
|---|---|---|
| Serious adverse events | n (%) | |
| All surgery | 20* (25) | |
| Laparoscopy | Small bowel obstruction§ | 11 (14) |
| Cholecystectomy | Gallstones | 9 (11) |
| Laparotomy | Severe abdominal pain | 1 (1) |
| Blood / iron transfusion | Severe anaemia^ | 2 (2) |
| Observation and investigation only | Abdominal pain | 9 (11) |
| Psychiatric assessment | Drug abuse# | 6 (7) |
| Panel B | |
|---|---|
| Other adverse outcomes | n (%) |
| Anaemia | 25/77 (32) |
| Low Vitamin D | 2/73 (3) |
| Low Vitamin B12 | 16/73 (22) |
| Low ferritin or iron | 51/77 (66) |
| Assessment by eating disorder team§ | 1/81 (1) |
Adverse outcomes among adolescents undergoing Roux-en-Y gastric bypass for severe obesity. Panel A – events involving admission to hospital; Panel B, events not requiring hospital admission.
21 procedures in 20 patients;
obstruction caused by internal herniation or adhesions;
narcotic abuse requiring medical referral or intervention;
anaemia requiring admission for iron therapy or blood transfusion;
individual was referred for assessment but was never diagnosed with an eating disorder; definitions and thresholds are provided within the supplementary data.
Healthcare use and medication
Over 5 years of follow-up and including the index hospitalisation, the surgical group accumulated a mean 16·1 hospital days, compared to 2·8 in the control group (mean difference 13·0, 95% CI 7·4 to 18·6). In-hospital days related to admissions for surgical procedures, including the index surgery, accounted for 6·5 days in the surgery group compared to 1·6 days in the control group (mean difference 5·0, 95% CI 2·7 to 7·2).
The number of outpatient visits was also higher in the surgical than the control group (14·6 vs. 10·0; mean difference 4·9, 95% CI 1·3 to 8·4).
Total prescription drug costs over 5 years were similar in the surgical and control groups ($2317 vs. $2701; mean difference −$611, 95% CI −3252 to 2030).
Discussion
Most adolescents undergoing RYGB for severe obesity in this study experienced substantial weight loss, metabolic improvement, reduction of the chronic inflammatory state and enhancement of quality of life, which remained 5 years after surgery. Concurrently, a control group undergoing conventional treatment experienced progressive weight gain.
RYGB resulted in a mean 29% weight loss after 5 years, which is comparable to the 28% reduction after three years reported in the Teen-LABS study.16 Rapid weight reduction during the first year was followed by modest weight regain between 2 and 5 years. The matched adult group, operated at the same centre, experienced a similar mean weight reduction. However, a greater variability in long-term weight outcome in adolescents, compared with adults, may indicate greater phenotypical heterogeneity and/or a greater need for postoperative support to optimise outcomes.
We and others have previously reported that metabolic risk factors and comorbid conditions improve markedly in adolescents 2 to 3 years after surgery.16–18 In this study we confirm that these positive trends remain after 5 years. We observed an amelioration of disturbed glucose homeostasis, dyslipidaemia and high blood pressure. We also found a substantial reduction in hsCRP following surgery, suggesting improvement of the chronic inflammatory state, which has been demonstrated to be a contributor to cardiovascular comorbidity development.25,26 At 5 years, metabolic risk factors, such as dyslipidaemia and elevated liver enzymes, were more prevalent in the control group than the surgical group, although direct comparison between the two adolescent groups was influenced by the crossover of participants to undergo RYGB during follow-up. Since individuals with the most severe weight gain underwent surgery during follow-up, the control group became progressively “healthier” across the follow-up period.
Gastric bypass surgery is associated with an inherent risk of developing vitamin and mineral deficiencies related to impairment of absorption and decreased food intake. Therefore, nutritional supplements were prescribed, according to Scandinavian clinical standards at that time. At 5 years after surgery we found a concerning prevalence of iron deficiency, associated low haemoglobin levels, and also vitamin D insufficiency. Poor compliance with supplementation may have contributed to this, as previously described. 17 This is an important area for improvement and recent guidance suggests adopting more aggressive supplementation, such as higher doses of vitamin D, as well as more effective compounds, such as calcium citrate rather than calcium carbonate. Regular access to long-term follow-up between 2 and 5 years may have ameliorated nutritional deficiencies.
Psychosocial impairment is highly prevalent in adolescents with severe obesity, 27 as was observed at baseline in this study. 17,28 We demonstrated improvement in obesity-related psychosocial problems in the surgical group over 5 years, as well as in generic self-reported quality of life, most notably in participants’ perceived general health and physical function. Improvements did not, however, occur across all aspects of quality of life, which should be communicated to patients and their families preoperatively to manage expectations. Specific attention must also be paid to identifying and helping individuals at risk of self-harm and suicide in this vulnerable group.
The accumulated in-hospital stay across 5 years was longer in the surgical group than the control group, which is in line with expectations given the primary procedure and incidence of complications and remedial interventions in the surgical group.29 Thus, the obesity-related comorbid diseases observed in control adolescents, did not lead to a greater need for in-hospital treatments within 5 years of follow-up. Despite including routine prescribed nutritional supplementation, the observed costs of medication were no greater in the surgical group than the control group.
The rate of additional procedures in the surgical group was higher than that reported within the Teen-LABS study,16 primarily due to a high rate of intra-abdominal herniation associated with non-closure of mesenteric defects. 30 Also contributing was a higher rate of cholecystectomy for gallstones in our study; a consequence of significant rapid weight loss.31 Rates of small bowel obstruction and cholecystectomy were, however, similar in Swedish adults undergoing the RYGB.30,32 Recent advances in practice have enabled reduction in the incidence of both internal herniation and gallstone formation by performing primary closure of mesenteric defects30 and administration of ursodeoxycholic acid prophylaxis,32 suggesting that the rate of additional surgery can be reduced by more than 50%. 30,32
The overall risk-benefit equation must, however, also take into account both the existing and imminent health implications in young persons with severe obesity and the failure of other therapies to achieve sustainable improvements.10,13 Although a small proportion of control adolescents succeeded in reaching normal weight across 5 years (3%), not only did the vast majority (90%) fail to achieve reversal of their obesity, but most (69%) actually gained weight. Delaying surgery thus represents an avoidable prolongation of exposure to cardiometabolic risk factors, with risk of development or progression of comorbid diseases.7
Strengths of this study include respectable rates of retention throughout follow-up, particularly considering the nature of an adolescent population and a 5-year follow-up period. Surgical procedures in adolescents and adults were carried out by surgeons in a single centre, using a standardised and well-recognised technique,19 refined over thousands of procedures in adults. The adult group experienced an almost identical treatment pathway, minimising bias related to the treatment. The Swedish healthcare registries guarantee an accurate quantification of postoperative healthcare and medication usage. Limitations include a non-randomised setting and a pragmatic, non-standardised conservative treatment. However, to the best of our knowledge, there is only one long-term study of a specific conservative treatment of obesity including adolescents, which achieved only modest weight loss and lost almost 40% to follow-up across 5 years.33 A randomised controlled trial would have reduced the potential for selection bias, however, in the absence of safety and efficacy data, we considered this design challenging. Many of the adult group weight data points were self-reported, although evidence in an adult bariatric population shows that this leads to under-reporting of weight by just 0.8 to 0.9 kg,34 allaying our concerns. There was also some attrition in our patient number regarding laboratory and quality of life measurements. A 25% crossover to surgery in the control group during follow-up limited the comparability of the adolescent groups. Due to the limited size of the study population, and therefore the low number of adverse events, adjustment was performed for age and sex alone. RYGB was the only surgical procedure performed as sleeve gastrectomy was novel at the time, although it has been used in later adolescent series.16,35 Finally, although this is a nationwide study, caution should be exercised in generalisation to other populations and regions.
Conclusion
RYGB results in substantial weight loss, frequent resolution of cardiometabolic comorbidity, and improvement in quality of life into the long-term in adolescents suffering from severe obesity. In contrast, non-surgical treatment led to further weight gain and one in four control adolescents underwent surgery during 5-year follow up. Surgical intervention was, however, associated with a high rate of additional surgical intervention and nutritional deficiencies.
The literature base now appears sufficiently mature to consider formal integration of bariatric surgery into treatment pathways for adolescents with severe obesity. However, we consider it crucial that adolescent bariatric surgery is performed within appropriate specialist multidisciplinary programmes, designed specifically to accommodate adolescent patients and provide long-term follow-up and support.
Future challenges include refining indications and contraindications, identifying ideal target age groups, and optimisation of postoperative support. We must also closely monitor for potential long-term adverse effects of surgery, across decades rather than years.
Supplementary Material
Research in context.
Evidence before this study
Before 2006 there was a large and growing body of evidence relating to bariatric surgery in adults, particularly using the Roux-en-Y gastric bypass (RYGB), but only limited experience from adolescents undergoing bariatric surgery.
We searched PubMed from 14 February, 1956 to 13 February, 2006, for “adolescent” OR “child*” AND “gastric bypass” AND “obesity”, with no restrictions on language. Of 246 items returned, we identified 6 relevant retrospective case series, including between 4 and 39 genetically normal patients undergoing RYGB, and dating as far back as 1975.
With increasing awareness of the dramatic health risks associated with severe obesity in the adolescent population, and limited success among non-surgical treatments, positive outcomes in adults prompted consideration of bariatric surgery in adolescents on a case-by-case basis in extreme circumstances. However, there was a paucity of prospective and systematic assessments of the risks and benefits in adolescents.
Within the limited existing case series, a mean BMI reduction of approximately 20 kg/m2 was reported at least 1 year after RYGB, but there were fewer than 45 patients with follow-up to 5 years or longer. Several adolescents demonstrated improvement in obesity-related metabolic axes, such as glucose homeostasis, lipids and blood pressure following surgery.
However, while these small series were certainly promising, inherent limitations rendered their results of limited reliability and generalisability. Most studies included a small number of participants and a retrospective design, many employing suboptimal methods of follow-up, without requiring clinic attendance, yet minor to moderate complications were relatively common.
Added value of this study
The added value of this prospective study predominantly lies in three areas. Firstly this study advances knowledge and understanding of the outcomes of RYGB among adolescents with severe obesity into the long-term, where previous prospective studies have reported outcomes up to 3 years after surgery thus far. Secondly, to the best of our knowledge, the present study is the first to concurrently examine a contemporary matched adolescent control group undergoing conventional treatment, and indeed a contemporary matched adult group undergoing RYGB, embedding the observed results within the context of the existing understanding of adult outcomes. Thirdly this study adds data from several national registries, expanding the outcomes reported in the literature to include healthcare consumption. We conclude, however, that there is a need to develop targeted strategies to reduce weight regain and avoid nutritional deficiencies in operated adolescents, and also to reduce the need for additional surgery.
Implications of all the available evidence
These long-term data extend knowledge beyond existing accumulated 2- and 3-year follow-up data from the US, Europe, Saudi Arabia and Australia, which have consistently supported the use of bariatric surgery in adolescents with severe obesity. Studies have shown that RYGB, sleeve gastrectomy and adjustable gastric banding are safe and effective in achieving and maintaining weight loss and significant metabolic health gains, often inducing remission of type 2 diabetes or prediabetes, dyslipidaemia and hypertension. The current evidence base, however, also highlights the challenges presented by performing bariatric surgery in adolescents.
We consider the literature base now sufficiently mature to consider formal integration of bariatric surgery into treatment pathways for adolescents with severe obesity. However, assessment of adolescents for surgery should be embedded within formal programmes incorporating all other available obesity treatments, led by a multidisciplinary team capable of conducting physical as well as psychosocial assessments of the individual patient. Provision must also be made for long-term follow-up and management, with concern for surgical and nutritional adverse events, avoidance of weight regain, and also for continuous psychological support when needed, knowing that this is a vulnerable patient group.
Acknowledgments
Funding: TO, EG, KJ, AJB, C-EF, CM, KE, PF, JD, GG, GM, JK, and SM were supported by grants from Vastra Gotaland Region, the Swedish Research Council (521-2012-319 and 2013-3770 [to GB]), the Swedish Governmental Agency for Innovation Systems (2013-01339), the Mrs Mary von Sydow Foundation, the Swedish Heart and Lung Foundation, the Swedish Childhood Diabetes Foundation, Stockholm County Council, and the National Board of Health and Welfare. TO and CM were supported by grants from the Swedish Order of Freemasons Children’s Foundation, Stiftelsen Goteborgs Barnhus, and Stiftelsen Allmanna Barnhuset. AJB has received funding from the Royal College of Surgeons of England in the form of a clinical research fellowship. MN was supported by a grant from the Swedish Research Council. MN and GB were supported by an award from the US National Institute of Diabetes, Digestive, and Kidney Diseases (National Institutes of Health; number R01DK105948). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor any other funding body.
We thank all participating adolescents and their families, as well as their health care staff throughout Sweden, who made this study possible. We would also like to thank Dr. Malin Werling and Prof. Hans Lönroth for their invaluable contributions.
Footnotes
Author contributions:
TO, CM and EG were involved in study conception, design, data collection and analysis, and writing the manuscript. AB, GB and MN were involved in study design, data collection and analysis, and writing the manuscript. CEF, JD, KE, PF, GG, KJ, JK and SM were involved in study design, data collection and writing the manuscript. MP was involved in study design, data analysis and writing the manuscript. All authors had full access to all data and approved the final manuscript.
Trial registration number – NCT00289705 - ClinicalTrials.gov
Conflicts of interest: TO reports receiving consulting fees for serving as a participant in the global advisory board and lecturing for Ethicon Endo surgery. TO has also received fees for lecturing for AstraZeneca and Sanofi. AJB reports receiving funding from the Royal College of Surgeons of England in the form of a clinical research fellowship. KJ reports receiving a lecturing fee from Nestlé. MN reports receiving consulting fees for participation in the scientific advisory committee of Itrim. Further, MN has received research grants from Pfizer, Cambridge Weight Plan, Novo Nordisk and Astra Zeneca; and lecture and consulting fees from Pfizer, Sanofi-Aventis, Roche and Strategic Health Resources. CM reports receiving consulting fees for participation in the scientific advisory committee of Itrim, Oriflame Wellnes and Sigrid Therapeutics AB. Further, CM has received research grants from Novo Nordisk. EG, CEF, JD, GB, KE, PF, GG, JK, SM, and MP report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neovius M, Sundstrom J, Rasmussen F. Combined effects of overweight and smoking in late adolescence on subsequent mortality: nationwide cohort study. Bmj. 2009;338:b496. doi: 10.1136/bmj.b496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dam RM, Willett WC, Manson JE, Hu FB. The relationship between overweight in adolescence and premature death in women. Annals of internal medicine. 2006;145(2):91–7. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 3.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. The New England journal of medicine. 2011;365(20):1876–85. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 4.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. The New England journal of medicine. 2015;373(14):1307–17. doi: 10.1056/NEJMoa1502821. [DOI] [PubMed] [Google Scholar]
- 5.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah AS, D’Alessio D, Ford-Adams ME, Desai AP, Inge TH. Bariatric Surgery: A Potential Treatment for Type 2 Diabetes in Youth. Diabetes care. 2016;39(6):934–40. doi: 10.2337/dc16-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beamish AJ, Olbers T. Bariatric and Metabolic Surgery in Adolescents: a Path to Decrease Adult Cardiovascular Mortality. Curr Atheroscler Rep. 2015;17(9):53. doi: 10.1007/s11883-015-0532-7. [DOI] [PubMed] [Google Scholar]
- 8.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. The New England journal of medicine. 2005;352(11):1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 9.Twig G, Yaniv G, Levine H, et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. The New England journal of medicine. 2016;374(25):2430–40. doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 10.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 11.Centre HSCI. Health Survey for England: Child Trend Tables. 2013. [Google Scholar]
- 12.Ogden CLCMKB, Flegal KM. Prevalence of obesity in the United States 2009–2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 13.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Archives of pediatrics & adolescent medicine. 2012;166(12):1103–8. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 14.Michalsky M, Reichard K, Inge T, et al. ASMBS pediatric committee best practice guidelines. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2012;8(1):1–7. doi: 10.1016/j.soard.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Paulus GF, de Vaan LE, Verdam FJ, Bouvy ND, Ambergen TA, van Heurn LW. Bariatric Surgery in Morbidly Obese Adolescents: a Systematic Review and Meta-analysis. Obesity surgery. 2015;25(5):860–78. doi: 10.1007/s11695-015-1581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. The New England journal of medicine. 2016;374(2):113–23. doi: 10.1056/NEJMoa1506699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olbers T, Gronowitz E, Werling M, et al. Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS) International journal of obesity. 2012;36(11):1388–95. doi: 10.1038/ijo.2012.160. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien PE, Sawyer SM, Laurie C, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. Jama. 2010;303(6):519–26. doi: 10.1001/jama.2010.81. [DOI] [PubMed] [Google Scholar]
- 19.Olbers T, Lonroth H, Fagevik-Olsen M, Lundell L. Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obesity surgery. 2003;13(3):364–70. doi: 10.1381/096089203765887679. [DOI] [PubMed] [Google Scholar]
- 20.Danielsson P, Bohlin A, Bendito A, Svensson A, Klaesson S. Five-year outpatient programme that provided children with continuous behavioural obesity treatment enjoyed high success rate. Acta paediatrica. 2016 doi: 10.1111/apa.13360. [DOI] [PubMed] [Google Scholar]
- 21.Nowicka P, Pietrobelli A, Flodmark CE. Low-intensity family therapy intervention is useful in a clinical setting to treat obese and extremely obese children. Int J Pediatr Obes. 2007;2(4):211–7. doi: 10.1080/17477160701379810. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taft C, Karlsson J, Sullivan M. Performance of the Swedish SF-36 version 2.0. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2004;13(1):251–6. doi: 10.1023/B:QURE.0000015290.76254.a5. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson J, Taft C, Sjostrom L, Torgerson JS, Sullivan M. Psychosocial functioning in the obese before and after weight reduction: construct validity and responsiveness of the Obesity-related Problems scale. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(5):617–30. doi: 10.1038/sj.ijo.0802272. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi S, Inoue N, Ohashi Y, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(8):1398–404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- 27.Herget S, Rudolph A, Hilbert A, Bluher S. Psychosocial status and mental health in adolescents before and after bariatric surgery: a systematic literature review. Obesity facts. 2014;7(4):233–45. doi: 10.1159/000365793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvholm K, Karlsson J, Olbers T, et al. Two-year trends in psychological outcomes after gastric bypass in adolescents with severe obesity. Obesity. 2015;23(10):1966–72. doi: 10.1002/oby.21188. [DOI] [PubMed] [Google Scholar]
- 29.Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. Jama. 2012;308(11):1132–41. doi: 10.1001/2012.jama.11792. [DOI] [PubMed] [Google Scholar]
- 30.Stenberg E, Szabo E, Agren G, et al. Closure of mesenteric defects in laparoscopic gastric bypass: a multicentre, randomised, parallel, open-label trial. Lancet. 2016;387(10026):1397–404. doi: 10.1016/S0140-6736(15)01126-5. [DOI] [PubMed] [Google Scholar]
- 31.Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. International journal of obesity. 2014;38(2):279–84. doi: 10.1038/ijo.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uy MC, Talingdan-Te MC, Espinosa WZ, Daez ML, Ong JP. Ursodeoxycholic acid in the prevention of gallstone formation after bariatric surgery: a meta-analysis. Obesity surgery. 2008;18(12):1532–8. doi: 10.1007/s11695-008-9587-7. [DOI] [PubMed] [Google Scholar]
- 33.Reinehr T, Kleber M, Lass N, Toschke AM. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. The American journal of clinical nutrition. 2010;91(5):1165–71. doi: 10.3945/ajcn.2009.28705. [DOI] [PubMed] [Google Scholar]
- 34.Christian NJ, King WC, Yanovski SZ, Courcoulas AP, Belle SH. Validity of self-reported weights following bariatric surgery. Jama. 2013;310(22):2454–6. doi: 10.1001/jama.2013.281043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Annals of surgery. 2012;256(2):266–73. doi: 10.1097/SLA.0b013e318251e92b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.