Abstract
Ca2+ signaling in astrocytes is considered to be mainly mediated by metabotropic receptors linked to intracellular Ca2+ release. However, recent studies demonstrate a significant contribution of Ca2+ influx to spontaneous and evoked Ca2+ signaling in astrocytes, suggesting that Ca2+ influx might account for astrocytic Ca2+ signaling to a greater extent than previously thought. Here, we investigated AMPA-evoked Ca2+ influx into olfactory bulb astrocytes in mouse brain slices using Fluo-4 and GCaMP6s, respectively. Bath application of AMPA evoked Ca2+ transients in periglomerular astrocytes that persisted after neuronal transmitter release was inhibited by tetrodotoxin and bafilomycin A1. Withdrawal of external Ca2+ suppressed AMPA-evoked Ca2+ transients, whereas depletion of Ca2+ stores had no effect. Both Ca2+ transients and inward currents induced by AMPA receptor activation were partly reduced by Naspm, a blocker of Ca2+-permeable AMPA receptors lacking the GluA2 subunit. Antibody staining revealed a strong expression of GluA1 and GluA4 and a weak expression of GluA2 in periglomerular astrocytes. Our results indicate that Naspm-sensitive, Ca2+-permeable AMPA receptors contribute to Ca2+ signaling in periglomerular astrocytes in the olfactory bulb.
It has become increasingly evident during the past decade that astrocytes are far more than supportive cells in the brain, but rather take active part in information processing such as synaptic transmission and synaptic plasticity1,2. Most of the functions attributed to astrocytes are governed by cytosolic Ca2+ signaling3,4. Astrocytes are equipped with a plethora of receptors for neurotransmitters, neuropeptides and growth factors, most of which are linked to Ca2+ release from internal stores5. Hence, it is generally accepted that internal Ca2+ release is the main player in glial cell physiology. Recent studies, however, challenge this notion and demonstrate pivotal roles of Ca2+ influx in spontaneous Ca2+ signaling and in astrocyte function such as neurovascular coupling6,7. Astrocytes express Ca2+-permeable ion channels such as ionotropic neurotransmitter receptors, transient receptor potential channels and store-operated Ca2+ channels that mediate Ca2+ influx from the extracellular space3,5,6,7. For example, Bergmann glial cells, specialized astrocytes in the cerebellum, possess ionotropic glutamate receptors of the AMPA type that consist of GluA1 and GluA4 subunits, but lack the GluA2 subunit and hence exhibit high Ca2+ permeability8,9. Artificial insertion of the GluA2 subunit renders AMPA receptors in Bergmann glial cells Ca2+-impermeable and results in retraction of glial processes from synapses of Purkinje cells and abnormal synaptic currents10. This phenotype was mimicked in glia-specific GluA1/GluA4 double knock-out mice, which additionally showed impairment in fine motor coordination, emphasizing a pivotal role of Ca2+-permeable AMPA receptors in neuron-glia interactions11. In other brain regions such as the thalamus, astrocytes possess AMPA receptors of different subunit composition including variable contribution of the GluA2 subunit to the channel assembly and hence display intermediate Ca2+ permeability12. In the hippocampus, NG2 glial cells express AMPA receptors, while astrocytes lack these receptors13,14,15,16.
In the olfactory bulb, activation of neurotransmitter receptors in astrocytes has been shown to result in Ca2+ transients17. The olfactory bulb is the first relay station of odor information processing and is targeted by axons of sensory neurons in the olfactory epithelium, the olfactory receptor neurons. Olfactory receptor neurons release glutamate and ATP in neuropilar regions called glomeruli18,19, where they stimulate Ca2+ signaling in periglomerular astrocytes by mGluR5 and P2Y1 receptors20,21. In addition, ATP is degraded to adenosine, acting on astrocytic A2A receptors21. Ca2+ increases in olfactory bulb astrocytes have been reported to release ATP from astrocytes and to trigger vasoresponses in blood vessels contacted by astrocytic end feet20,22,23,24. All Ca2+ responses to neurotransmitters measured in olfactory bulb astrocytes were mediated by Ca2+ release from internal stores, while Ca2+ influx from the extracellular space has not been demonstrated so far17. In the present study, we were interested whether olfactory bulb astrocytes express Ca2+-permeable AMPA receptors and whether these receptors are activated by glutamate released from olfactory receptor neurons. We studied Ca2+ responses and membrane currents in olfactory bulb astrocytes to application of AMPA and kainate, using Ca2+ imaging in brain slices and whole-cell patch-clamp recordings in acutely isolated astrocytes. Kainate-evoked membrane currents as well as Ca2+ transients induced by AMPA and electrical stimulation of olfactory receptor axons were partly reduced by Naspm (N-[3-[[4-[(3-aminopropyl) amino] butyl] amino] propyl]-1-naphthaleneacetamide trihydrochloride), an AMPA receptor blocker selective for GluA2-lacking, Ca2+-permeable AMPA receptors. Immunohistological staining revealed expression of GluA1, GluA2 and GluA4 in olfactory bulb astrocytes. The results indicate that olfactory bulb astrocytes possess both GluA2-containing and GluA2-lacking AMPA receptors, the latter being blocked by Naspm.
Results
Olfactory bulb astrocytes respond to AMPA application
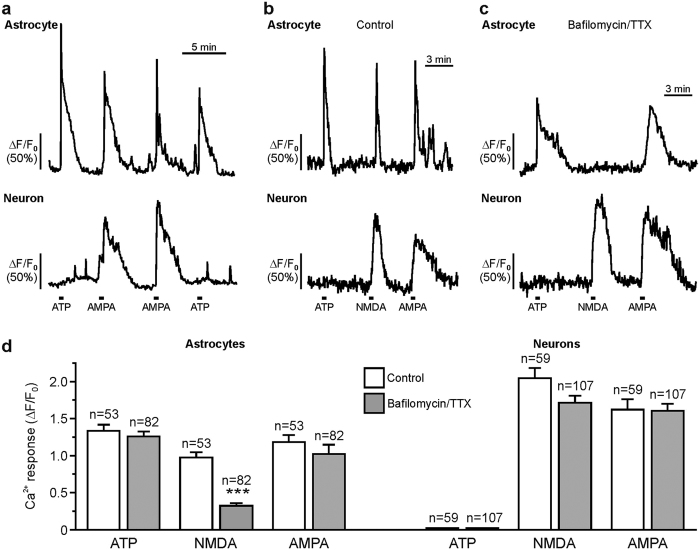
We were interested whether olfactory bulb astrocytes respond to bath application of AMPA with Ca2+ signaling. We used application of ATP to test the viability of the cells and to identify astrocytes in the glomerular layer. Olfactory bulb astrocytes express P2Y1 receptors and respond to bath application of ATP and ADP with Ca2+ transients, whereas olfactory bulb neurons do not21. In the present study, 100 μM ATP evoked a Ca2+-dependent increase in Fluo-4 fluorescence by 1.34 ± 0.08 ΔF/F0 (n = 53) in periglomerular astrocytes, while in neurons, ATP did not evoke Ca2+ signaling (Fig. 1a). 59.3% of ATP-sensitive astrocytes and all neurons also responded to bath application of 50 μM AMPA (Fig. 1a). The mean Ca2+ increase was 1.18 ± 0.09 ΔF/F0 (n = 53) in AMPA-responding astrocytes and 1.62 ± 0.14 ΔF/F0 (n = 59) in neurons. To test whether AMPA evoked Ca2+ transients in periglomerular astrocytes directly or via activation of neurons and subsequent neurotransmitter release, we suppressed vesicular neurotransmitter release by incubation of the brain slices with 10 μM bafilomycin A1 (duration 40 min) and added 1 μM tetrodotoxin (TTX) to the artificial cerebrospinal fluid (ACSF) (Fig. 1b and c). We used NMDA as a control of successful TTX/bafilomycin treatment, since direct NMDA-induced stimulation of astrocytes is negligible and hence NMDA-evoked Ca2+ transients in astrocytes are expected to be suppressed by TTX/bafilomycin22. 100 μM NMDA evoked an increase in Ca2+ of 0.97 ± 0.07 ΔF/F0 (n = 53) under control conditions, while the increase was significantly reduced to 0.32 ± 0.03 ΔF/F0 (n = 82; p < 0.001) by treatment with TTX/bafilomycin (Fig. 1d). In contrast to NMDA, both ATP and AMPA induced Ca2+ transients in astrocytes in the presence of TTX/bafilomycin that did not differ in amplitude compared to transients evoked in the absence of TTX/bafilomycin (Fig. 1b and c). The amplitudes of the Ca2+ transients in the presence of TTX/bafilomycin were 1.26 ± 0.07 ΔF/F0 (n = 82; ATP; p = 0.479) and 1.02 ± 0.13 ΔF/F0 (n = 82; AMPA; p = 0.318). The results indicate that ATP and AMPA directly induced Ca2+ transients in astrocytes. Therefore, we used bafilomycin A1 and TTX in all following Ca2+ imaging experiments (except for electrical stimulation of axons) to isolate the direct response in astrocytes.
Figure 1. Bafilomycin A1 and TTX fail to reduce AMPA-induced Ca2+ transients in periglomerular astrocytes.
(a) Ca2+ imaging traces of a single periglomerular astrocyte (upper trace) and neuron (lower trace) showing Ca2+ responses to ATP (100 μM) and AMPA (50 μM). (b) Ca2+ responses evoked by ATP (100 μM), NMDA (100 μM) and AMPA (50 μM) in the absence and (c) presence of TTX (1 μM) and bafilomycin A1 (10 μM). (d) Normalized and averaged amplitudes of Ca2+ transients evoked by ATP, NMDA and AMPA under control conditions (open bars) and in the presence of bafilomycin A1 and TTX (gray bars). ***p < 0.001.
Ca2+-permeable AMPA receptors mediate Ca2+ influx into periglomerular astrocytes
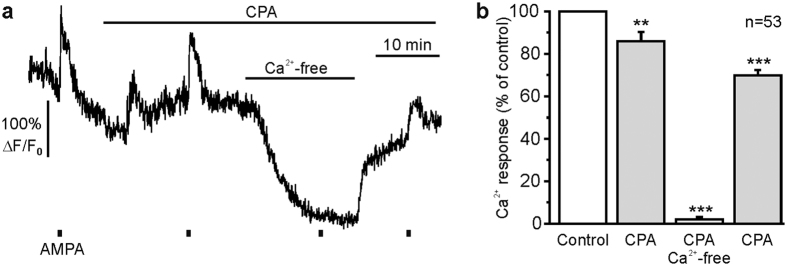
AMPA can activate AMPA receptors as well as kainate receptors25, both of which may trigger Ca2+ influx through the receptor channel itself. In addition, AMPA/kainate receptors have been shown to stimulate G protein-coupled pathways, including Ca2+ release from intracellular stores26,27,28. To test whether Ca2+ is released from internal stores, we applied AMPA (25 μM) after Ca2+ stores were depleted by incubation with 20 μM cyclopiazonic acid (CPA). As shown in Fig. 2, the AMPA-induced Ca2+ response in periglomerular astrocytes was only slightly reduced after Ca2+ store depletion. In Ca2+-free, EGTA-buffered ACSF, however, the Ca2+ response was entirely blocked (n = 53; p < 0.001), indicating that AMPA induced Ca2+ influx from the extracellular space.
Figure 2. AMPA-induced changes in intracellular Ca2+ are not dependent on intracellular Ca2+ stores.
(a) Ca2+ transient of a periglomerular astrocyte evoked by AMPA (25 μM) under control conditions, in the presence of CPA (20 μM), and in Ca2+-free saline in CPA. (b) CPA had only a small effect on AMPA-induced Ca2+ transients, while Ca2+ transients were entirely suppressed in Ca2+-free saline. **p < 0.01; ***p < 0.001.
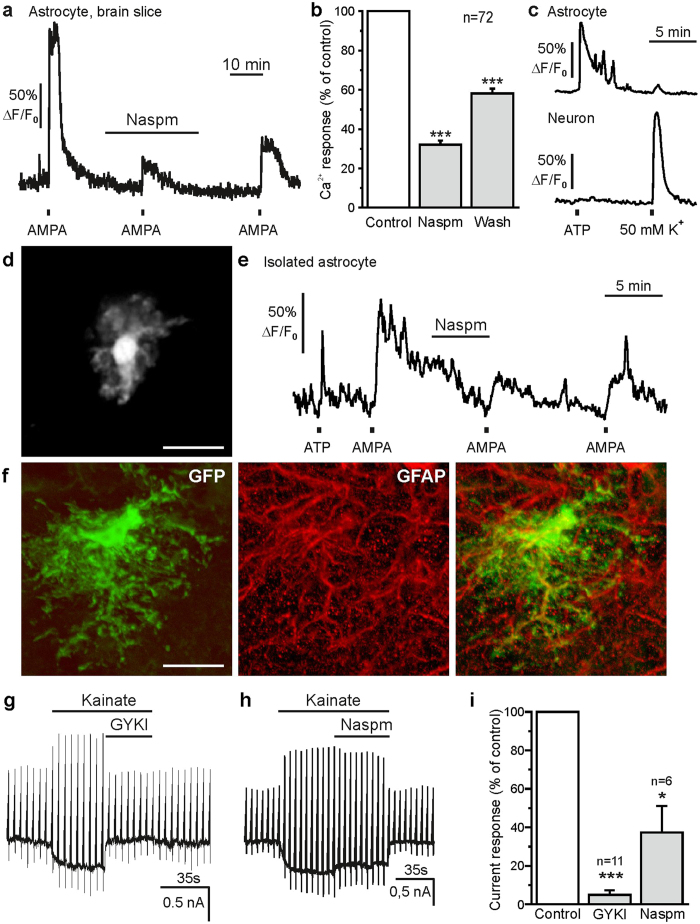
The AMPA-induced Ca2+ influx could be mediated by Ca2+-permeable AMPA receptors or by AMPA-evoked depolarization and subsequent activation of voltage-gated Ca2+ channels. To test whether AMPA evoked Ca2+ influx through Ca2+-permeable AMPA receptors we employed Naspm, a highly selective antagonist of GluA2-lacking, Ca2+-permeable AMPA receptors29,30. In the presence of 50 μM Naspm, AMPA-evoked Ca2+ transients were significantly reduced by 68.0 ± 2.2% (n = 72; p < 0.001) (Fig. 3a and b). In addition, depolarization of the cells by application of 50 mM K+ in the presence of bafilomycin A1 and TTX elicited only a small Ca2+ transient in astrocytes of 0.13 ± 0.09 ΔF/F0 (n = 16), leading to the conclusion that Ca2+ influx through voltage-gated Ca2+ channels is negligible (Fig. 3c). Hence, AMPA-evoked Ca2+ transients appear to be mainly mediated by Ca2+ influx through Ca2+-permeable AMPA receptors. We also used acutely isolated astrocytes to test the effect of Naspm on AMPA-evoked Ca2+ signaling. AMPA-evoked Ca2+ transients were significantly reduced by 50 μM Naspm (n = 31; p < 0.001), confirming the involvement of Ca2+-permeable AMPA receptors in Ca2+ signaling in olfactory bulb astrocytes (Fig. 3d and e). On average, 25 μM AMPA induced Ca2+ transients with an amplitude of 2.50 ± 0.50 ΔF/F0 (n = 31), which were reduced to 52.7 ± 5.3% by Naspm.
Figure 3. Naspm reduces AMPA receptor-induced Ca2+ transients and membrane currents in periglomerular astrocytes.
(a) Effect of Naspm (50 μM) on AMPA-induced Ca2+ transients after incubation of brain slices in bafilomycin A1 and TTX. (b) Naspm significantly reduced AMPA-induced Ca2+ transients in periglomerular astrocytes. Wash out of Naspm led to a significant recovery of the Ca2+ response. (c) Increasing external K+ to 50 mM evoked large Ca2+ transients in neurons, but only small Ca2+ rises in astrocytes, indicating lack of voltage-gated Ca2+ influx in astrocytes. (d) Fluo-4-loaded isolated astrocyte. Scale bar: 20 μm. (e) Effect of Naspm (50 μM) on AMPA-evoked Ca2+ transients in an isolated olfactory bulb astrocyte. (f) Immunostaining of an eGFP-positive periglomerular astrocyte in an hGFAP-eGFP mouse (anti-GFP, green) and colabeling of GFAP as a marker for astrocytes (anti-GFAP, red). Scale bar: 10 μm. (g) Whole-cell current trace of a dissociated astrocyte recorded in BaCl2 (100 μM), quinine (100 μM) and cyclothiazide (100 μM). Kainate (500 μM) evoked an inward current that was entirely blocked by GYKI 53655 (100 μM), but (h) was only partly reduced by Naspm (50 μM). (i) Normalized averaged effects of GYKI 53655and Naspm on kainate-induced currents.
We aimed to verify these results in electrophysiological experiments on acutely dissociated astrocytes from the olfactory bulb glomerular layer, using hGFAP-eGFP reporter mice to identify astrocytes (Fig. 3f). We applied 500 μM kainate in the presence of 100 μM cyclothiazide to induce AMPA receptor-mediated membrane currents with minimal desensitization12,31. We and others have previously shown that activation of AMPA/KA receptors in glial cells leads to Na+ influx that plugs K+ channels32,33. To avoid that the kainate-induced receptor currents were obscured by a simultaneous block of Kir channels, BaCl2 was applied. Kainate application evoked an inward current of 427.8 ± 190.3 pA (n = 15) (at −70 mV) corresponding to a current density of 16.0 ± 5.1 pA/pF (n = 15) (Fig. 3f). 100 μM GYKI 53655 (1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride), a selective AMPA receptor blocker barely acting on kainate receptors34, entirely inhibited the kainate-induced inward current (n = 11; p < 0.001), confirming the activation of AMPA receptors by kainate in our experiments (Fig. 3g and i). In contrast to GYKI 53655, Naspm (50 μM) had variable effects on the kainate-induced inward current. In 2 out of 8 experiments, Naspm did not affect the kainate-evoked inward current, while in one experiment, Naspm entirely blocked the kainate-induced inward current. In the remaining 5 experiments, Naspm reduced the current by different amounts. In Naspm-sensitive cells, the kainate-induced inward current was significantly reduced by Naspm to 37.4 ± 13.7% of the control (n = 6; p = 0.031) (Fig. 3h and i). These results show that AMPA induced an influx of Ca2+ into periglomerular astrocytes that in the majority (75%) of cells is Naspm-sensitive and thus mediated by Ca2+-permeable AMPA receptors.
Distribution of AMPA receptor subunits in the glomerular layer
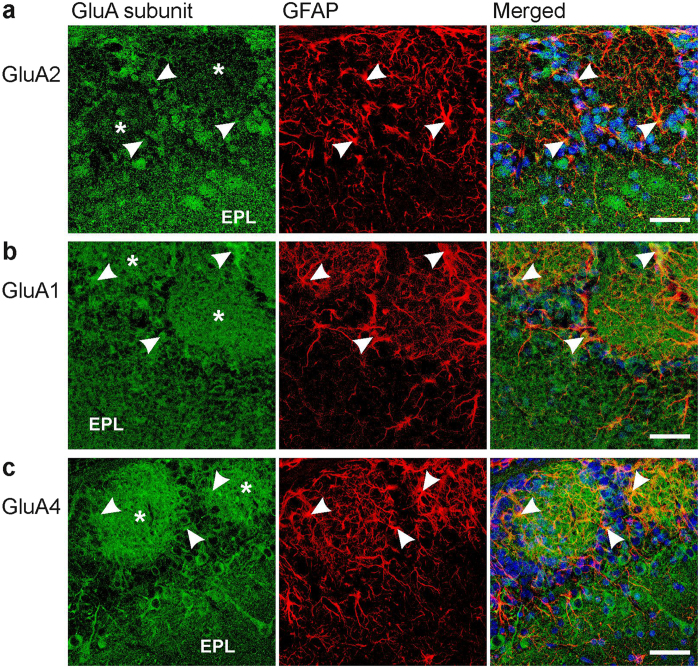
The Ca2+ permeability of AMPA receptors critically depends on the relative abundance of GluA2 within the channel complex14,35,36. Since our electrophysiological and Ca2+ imaging data suggest Ca2+-permeable AMPA receptors in periglomerular astrocytes, we investigated the cellular distribution of GluA2 in the olfactory bulb. GluA2 immunoreactivity was mainly found rather homogeneously in the external plexiform layer and in somata of periglomerular cells, whereas GluA2 immunoreactivity was much weaker in the synaptic neuropil in the core of the glomeruli (Fig. 4a). Only moderate colocalization with GFAP-positive periglomerular astrocytes was detected (Fig. 4a, merged image). We also investigated the distribution of GluA1 and GluA4. GluA3 was not investigated because it is barely expressed in the olfactory bulb37,38. GluA1 was widely distributed in the glomerular and the external plexiform layers, including the neuropil of glomeruli (Fig. 4b). Intense colocalization of GluA1 and GFAP was found in periglomerular astrocytes and their processes in the neuropil. GluA4 immunoreactivity resembled GluA1 immunoreactivity, with universal distribution in the external plexiform layer, the glomerular layer and the glomerular neuropil as well as clear colocalization with GFAP (Fig. 4c). We used cerebellar tissue of the same animals to verify specificity of antibody staining. The distribution of all three subunits investigated was in accordance with the distribution found in previous publications39,40,41,42; GluA1 was expressed in both neurons and glial cells, GluA2 exclusively in neurons and GluA4 predominantly in Bergmann glial cells (Supplementary Fig. S1).
Figure 4. Immunostaining of AMPA receptor subunits in the olfactory bulb.
(a) GluA2 immunoreactivity (green) was detected in the external plexiform layer (EPL) and in cell bodies surrounding glomeruli. Glomeruli are indicated by asterisks. Moderate GluA2 immunoreactivity was also found in astrocytes highlighted by GFAP immunoreactivity (red), as indicated by yellow pixels in the merged image. Arrows point to astrocyte structures that were colabeled with GluA immunoreactivity. Nuclei were stained with Hoechst 33342 (blue). (b) GluA1 and GFAP colocalization. (c) GluA4 and GFAP colocalization. Scale bars: 20 μm.
Endogenous glutamate release activates astrocytic Ca2+-permeable AMPA receptors
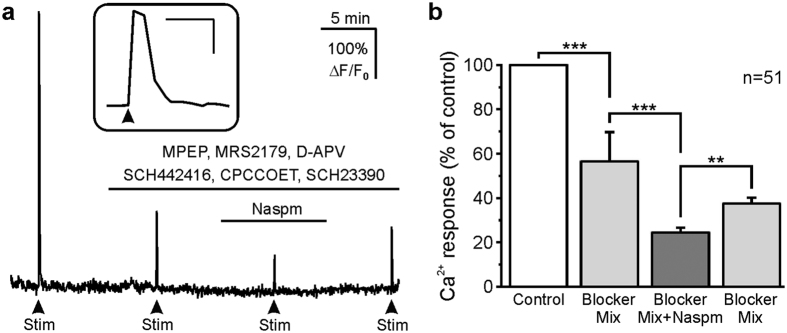
Olfactory receptor neurons (ORN) release glutamate from their axon terminals in the glomeruli, which evokes Ca2+ signaling in astrocytes18,20,23. We studied the effect of glutamate released upon electrical stimulation (10 Hz, 2 s) of ORN axons on astrocytic Ca2+ (Fig. 5). We crossbred Glast-CreERT2 and GCaMP6sfl/fl mice to receive mice in which the genetic Ca2+ indicator GCaMP6s is specifically expressed by astrocytes. In brain slices of these mice, ORN stimulation resulted in Ca2+ transients with a mean amplitude of 3.51 ± 0.25 ΔF/F0 (n = 51) in periglomerular astrocytes. We aimed to isolate the putative Ca2+ response induced by activation of Ca2+-permeable AMPA receptors in astrocytes by applying a mix of receptor blockers antagonizing metabotropic neurotransmitter receptors known to induce Ca2+ transients in olfactory bulb astrocytes. In addition, we reduced glutamatergic activation of neurons and hence indirect effects with D-APV (D-2-amino-5-phosphonovaleric acid). This blocker mix significantly reduced the stimulation-induced Ca2+ response in periglomerular astrocytes to 56.4 ± 3.0% of the control (n = 51; p < 0.001) (Fig. 5a). Addition of Naspm (50 μM) further decreased the amplitude of stimulation-induced Ca2+ transients to 24.4 ± 2.2% of the control (n = 51). Ca2+ transients in the presence of Naspm were significantly smaller as compared to Ca2+ transients in the presence of the mix of blockers without Naspm (p < 0.001), indicating that glutamate release from OSN axons triggers Ca2+ signaling in periglomerular astrocytes via Ca2+-permeable AMPA receptors.
Figure 5. Electrical stimulation of axons of olfactory receptor neurons evokes Naspm-sensitive Ca2+ transients in GCaMP6s-expressing astrocytes.
(a) Ca2+ transients evoked by electrical stimulation in the absence of receptor blockers, in the presence of a mix of receptor blockers (2 μM MPEP, antagonist of mGluR5; 30 μM MRS2179, P2Y1 receptor antagonist; 100 μM D-APV, NMDA receptor antagonist; 1 μM SCH442416, type 1 dopamine receptor antagonist; 100 μM CPCCOET, mGluR1 antagonist; 2 μM SCH23390, A2A receptor antagonist) and after addition of Naspm (50 μM). Inset: First Ca2+ response (control) at larger time scale. Inset scale bars: 20 s, 200% ΔF/F0. (b) Normalized averaged amplitudes of Ca2+ rises evoked by electrical stimulation.
Discussion
In the present study, we have investigated the role of AMPA receptors in Ca2+ signaling in periglomerular astrocytes of the olfactory bulb. Our results show that axonal stimulation activates AMPA receptor-mediated Ca2+ influx into periglomerular astrocytes. This Ca2+ influx was largely reduced by Naspm, a blocker of GluA2-lacking, Ca2+-permeable AMPA receptors29,30. Our antibody staining revealed expression of GluA1, GluA2 and GluA4 in periglomerular astrocytes, and patch-clamp recordings demonstrated only partial block of AMPA receptor-mediated inward currents by Naspm, suggesting that both GluA2-containing and GluA2-lacking AMPA receptors are expressed by periglomerular astrocytes.
Abundant expression of GluA1, GluA2 and GluA4 in the rodent olfactory bulb has been published before. GluA1, e.g., is mainly found in the external plexiform and the glomerular layers and is expressed by periglomerular neurons and mitral/tufted cells39,43,44,45. GluA2 has the highest expression of all GluA subunits in the olfactory bulb as assed by PCR36 and is located in mitral/tufted cells and granule cells40. GluA3 expression in the olfactory bulb is negligible, while GluA4 expression is moderate and GluA4 protein could be detected in the mitral cell layer, the external plexiform layer, the glomerular layer and the nerve layer38,40. However, all these studies focused on neurons, and none of them investigated the colocalization of the GluA subunits with astrocytes. We colabeled GluA immunostainings with an antibody against GFAP to highlight astrocytes in the glomerular layer. Colocalization of GluA subunits and GFAP was found for all subunits tested, with obvious colocalization for GluA1 and GluA4, but only moderate colocalization for GluA2. This suggests that only a fraction of AMPA receptors in periglomerular astrocytes comprise GluA2 subunits, while the remaining fraction of AMPA receptors lacks GluA2 subunits and hence is both Ca2+-permeable and Naspm-sensitive35,36,46. This is confirmed by the partial block of AMPA receptor currents by Naspm as measured in acutely dissociated, GFP-labeled astrocytes.
Several results indicate that in periglomerular astrocytes, AMPA directly gates Ca2+ influx. Firstly, AMPA-induced Ca2+ transients upon agonist bath application were not reduced by suppressing potential indirect effects (through neuronal transmitter release) with the sodium channel blocker TTX and bafilomycin, an inhibitor of vesicular H+ pumps required for filling synaptic vesicles with neurotransmitter molecules47. NMDA-evoked and high-K+-evoked Ca2+ transients in astrocytes, in contrast, were greatly reduced by TTX/bafilomycin, in line with the notion that olfactory astrocytes do not significantly express voltage-gated Ca2+ channels and NMDA receptors and hence high-K+-evoked and NMDA-evoked Ca2+ signaling was mainly due to neuronal transmitter release. Whether the remaining, TTX/bafilomycin-insensitive part of NMDA-evoked Ca2+ transients reflects insufficient efficacy of TTX/bafilomycin or expression of NMDA receptors in olfactory bulb astrocytes, as demonstrated for cortical astrocytes and for oligodendrocytes, remains to be shown48,49,50,51,52,53. In addition, TTX/bafilomycin-insensitive neurotransmitter release such as reversal of neurotransmitter uptake due to the NMDA-evoked increase in Na+ in neurons and subsequent activation of astrocytic receptors might also contribute to NMDA-evoked Ca2+-transients in astrocytes. Such TTX/bafilomycin-insensitive neurotransmitter release could contribute to astrocytic Ca2+ signalling not only upon application of NMDA, but also AMPA. However, it is very unlikely that this has a major contribution to the AMPA-evoked Ca2+ signaling, since the same mechanism is activated during NMDA and high K+ application, which produced only small Ca2+ transients in the presence of TTX/bafilomycin.
Secondly, AMPA receptor activation did only weakly evoke Ca2+ release from internal stores, but induced Ca2+ transients that mainly depended on the presence of Ca2+ in the bath solution, indicating Ca2+ influx. Besides Ca2+ influx through AMPA receptors, an increase in Ca2+ due to inhibition or reversal of Na+/Ca2+ exchanger (NCX) upon the AMPA-evoked Na+ increase in astrocytes might contribute to the Ca2+ transients triggered by AMPA application, as shown before for Na+ increases evoked by GABA transport into olfactory bulb and hippocampal astrocytes22,54. In olfactory bulb astrocytes, NCX-dependent Ca2+ increases were small yet sufficient to trigger Ca2+-induced Ca2+ release, which was abolished upon store depletion with CPA22. AMPA-evoked Ca2+ transients in the present study, in contrast, were only weakly affected by store depletion, indicating that they were not mainly mediated by NCX-dependent Ca2+-induced Ca2+ release.
Thirdly, our findings were confirmed with acutely dissociated cells excluding indirect effects through neuronal activation. The observation that AMPA receptor currents were sensitive to Naspm further substantiated a Ca2+ permeability of the receptors. It should be noted that during our patch-clamp experiments, AMPA receptor-evoked currents were isolated by blocking potassium conductances with Ba2+ and quinine, which affect membrane properties of astrocytes55,56 and suppress Na+-dependent block of K+ channels32,33; hence, in the absence of K+ channel blockers, AMPA receptor-evoked effects on membrane currents might be more complex than shown in our experiments. Importantly, we have demonstrated that Ca2+-permeable AMPA receptors of periglomerular astrocytes are activated through electrical stimulation of ORN axons. ORN axons release glutamate and ATP18,19,21,57, and electrical stimulation of ORN axons as well as odor stimulation of ORN has been shown to trigger Ca2+ signaling in periglomerular astrocytes by activation of mGluR5, P2Y1 and A2A receptors20,21. However, in the present study we have inhibited these receptors as well as dopamine receptors, which in the olfactory bulb are expressed by many neurons58,59,60, to reduce potential indirect effects via dopaminergic neurons. The stimulation-induced Ca2+ response in periglomerular astrocytes remaining in this blocker cocktail was sensitive to Naspm, indicating that this form of axon-glia interaction activates Ca2+-permeable AMPA receptors. Olfactory bulb astrocytes have been shown to mediate neurovascular coupling20,22,23 as well as release of glutamate, GABA and ATP affecting mitral cells and granule cells24,61. Accordingly, astrocytes might sense the level of axonal activity by gradual activation of Ca2+ influx through their AMPA receptors, thereby modulating gliotransmitter release and adapting local circulation and energy supply to the actual metabolic requirements. In addition, astrocytes are involved in the development of the glomeruli, and AMPA receptor-mediated astrocyte Ca2+ signaling might affect neurite growth and synaptogenesis62,63.
Material and Methods
Animals used for slice preparation
For Ca2+ imaging experiments, NMRI, GCaMP6sfl/fl and GLAST-CreETR2 mice of both genders at postnatal days 14 to 40 were used64,65. NMRI and GLAST-CreETR2xGCaMP6sfl/fl mice were raised in the animal facility at the University of Hamburg (Germany). hGFAP-eGFP mice66 used for electrophysiology and immunofluorescence staining were obtained from the animal facility at the University of Bonn Medical Center (Germany). All experiments were performed in accordance with EU and local animal welfare guidelines and were approved by the state’s animal welfare committee (GZ G21305/591-00.33; Behörde für Gesundheit und Verbraucherschutz, Hamburg, Germany). Olfactory bulbs were prepared and sliced (VT1200, Leica, Benzheim, Germany) in cooled, carbogen-gassed preparation solution and transferred to carbogen-gassed ACSF at 30 °C for recovery.
Solutions and chemicals
The following solutions were employed (molarity in mM), ACSF: 120 NaCl, 2.5 KCl, 1 NaH2PO4 × 2H2O, 26 NaHCO3, 2.8 D-(+)-glucose, 1 MgCl2, 2 CaCl2; Ca2+-free ACSF: 120 NaCl, 2.5 KCl, 1 NaH2PO4 × 2H2O, 26 NaHCO3, 2.8 D-(+)-glucose, 3 MgCl2, 0.5 EGTA; preparation solution for Ca2+ imaging: 83 NaCl, 1 NaH2PO4 × 2H2O, 26.2 NaHCO3, 2.5 KCl, 70 Sucrose, 20 D-(+)-glucose, 2.5 MgSO4 × 7 H2O; preparation solution for electrophysiology: 87 NaCl, 1.25 NaH2PO4 × 2H2O, 25 NaHCO3, 2.5 KCl, 7 MgCl2 × 6 H2O, 0.5 CaCl2 × 6 H2O, 60 Sucrose, 25 D-(+)-glucose (325 mOsm). ACSF, preparation solutions and Ca2+-free ACSF were continuously gassed with carbogen (95% O2, 5% CO2) to buffer the pH at 7.4 and to supply oxygen. Patch clamp-recording of isolated cells was performed in bath solution containing (in mM): 150 NaCl, 5 KCl, 2 MgCl2 × 6 H2O, 2 CaCl2 × 6 H2O, 10 D-(+)-glucose, 10 HEPES, pH 7.4, gassed with oxygen. The compounds amino-3-hydroxy-5-methyl-4-isoxazolephosphonic acid (AMPA), cyclothiazide, (E)-ethyl 1,1a,7,7a-tetrahydro-7-(hydroxyimino)cyclopropa[b]chromene-1a-carboxylate (CPCCOEt), 2-methyl-6-(phenylethynyl)pyridine (MPEP), 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS2179), 2-(2-furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine (SCH442416) and (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH23390) were obtained from Abcam (Cambridge, United Kingdom). Adenosine 5′-triphosphate (ATP), kainate, N-methyl-D-aspartic acid (NMDA) and papain was purchased from Sigma Aldrich (Taufkirchen, Germany), GYKI 53655 from Tocris (Bristol, UK). Bafilomycin A1 and CPA were acquired from Enzo Life Sciences (Lörrach, Germany). The reagents D-APV, Naspm and TTX were received from Alomone labs (Jerusalem, Israel). All reagents were stored as stock solutions corresponding to the manufacturer’s instructions and added to ACSF directly before the experiment.
Ca2+ imaging
Tissue slices were placed in a recording chamber and fixed with a platinum frame fitted with nylon strings. Slices were incubated with the membrane-permeable form of the Ca2+ indicator Fluo-4 (Fluo-4-AM; 2 μM in ACSF) made from a 4-mM stock solution (dissolved in DMSO and 20% pluronic acid) for 40 min. In most of the experiments, bafilomycin A1 (10 μM) was added to the Fluo-4-AM solution. In some experiments, GCaMP6s fluorescence was used as an indicator of Ca2+ concentration. Changes in intracellular Ca2+ levels in periglomerular astrocytes were recorded by confocal microscopy (C1 Eclipse, Nikon, Düsseldorf, Germany). An excitation laser wavelength of 488 nm and a frame rate of 0.3–0.5 fps were used. Drugs were administered via the perfusion system except for AMPA. The application solution containing AMPA was applied directly into the perfusion stream in the bath with a custom made application system to allow for a semi-fast application (within 3–5 s). The flow rate of the application system equaled the flow rate of the perfusion system, resulting in a 1:2 dilution of the agonist concentration as adjusted in the application solution.
For electrical stimulation of axons in tissue slices, a glass pipette with a resistance of 2–2.5 MΩ filled with ACSF was used. The pipette was placed on the surface of the olfactory nerve layer (ONL) comprising axons of olfactory receptor neurons and 250-μA stimuli were applied for 2 s at 10 Hz.
Isolation of periglomerular astrocytes
For tissue preparation, 300 μm thick sagittal slices from the olfactory bulb were prepared from hGFAP-eGFP mice (postnatal day 8–14) in ice cold, carbogen (95% O2/5% CO2) gassed ACSF supplemented with sucrose (preparation solution) using a vibratome (VT1200S, Leica, Nussloch, Germany). Slices were transferred for 15 min to warm (35 °C) preparation solution and then to standard ACSF (room temperature, 1 h). Cells were isolated using an enzymatic/mechanical approach13. Slices were incubated in ACSF supplemented with papain (1.5 mg/ml; 24 U/ml) (Sigma, Taufkirchen, Germany) and L-cysteine (0.35 mg/ml) (Sigma) (10 min) and continuously bubbled with carbogen. Subsequently, slices were transferred to the recording solution and the glomerular layer was dissected under a stereomicroscope (KL200, Zeiss, Jena, Germany). Cells were isolated in the recording chamber with tungsten needles and Pasteur pipettes, and allowed to settle for 15 min before analysis. Periglomerular astrocytes were identified by their green fluorescence and morphology. For Ca2+ imaging experiments, astrocytes were isolated from NMRI mice, seeded on concanavalin A-coated cover slipes, loaded with Fluo-4-AM (2 μM in ACSF) for 30–45 min and imaged as discribed for brain slices.
Electrophysiological recordings
Experiments on isolated cells employed a customized concentration clamp device connected to an EPC-7 amplifier and TIDA software (Heka Lambrecht, Germany) as described elsewhere13. Astrocytes were visualized with an inverted microscope (Axiovert 135, Zeiss) equipped with DIC and epifluorescence. Pipettes were manufactured from borosilicate glass (2–4 MΩ; Science Products, Hofheim, Germany) and filled with a solution containing (in mM): 130 KCl, 0.5 CaCl2, 2 MgCl2, 5 BAPTA, 10 HEPES, and 3 Na2-ATP, 0.05 spermine, pH 7.25. Currents were sampled at 0.1 to 30 kHz and filtered at 3 or 10 kHz. Holding potential was −70 mV. Input and series resistance were continuously checked by applying 10 mV test pulses. The liquid junction potential was not corrected for. Recordings were performed at room temperature. To separate the AMPA receptor conductance from simultaneously occurring changes in K+ conductance, drug application to isolated cells was performed in HEPES-buffered recording solution, supplemented with the K+ channel blockers quinine (100 μM) and BaCl2 (100 μM)33.
Data analysis
To analyze changes of the Ca2+ level in astrocytes, cell somata were marked as regions of interests (ROIs) using EZC1 Viewer software (Nikon). Cells located in the glomerular layer that showed a Ca2+ response to ATP were identified as periglomerular astrocytes21. To analyze changes of Ca2+ levels over time, Fluo-4 and GCaMP6s fluorescence intensity (F), respectively, was recorded throughout the experiment and normalized to the basal fluorescence intensity in absence of stimuli (F0). Changes in Ca2+ are given by ΔF/F0. All values are given as mean values ± standard error of the mean. Data for every set of experiment was acquired from at least three different animals. The assessment of statistical significance by comparing two means was done by Student’s t-test or, if applicable, one-way ANOVA with Fisher’s post-hoc test at an error probability p (*p < 0.05; **p < 0.01; ***p < 0.001).
Immunohistology
Immunohistological staining was performed on 100-μm thick sagittal slices of olfactory bulbs of NMRI and hGFAP-eGFP mice. For anti-GluA staining, cerebella of the same animals were used as a positive control to verify specific antibody staining. After dissection, olfactory bulbs and cerebella were stored and refrigerated in paraformaldehyde solution (PFA, 4%) in phosphate buffered solution (PBS) containing (in mM): 130 NaCl, 7 Na2HPO4, 3 NaH2PO4. Slices were cut with a vibratome (VT1000, Leica) and incubated with the primary antibodies anti-GluA1 (guinea pig; 1:200; Alomone Labs), anti-GluA2 (rabbit; 1:200; Millipore, Darmstadt, Germany), anti-GluA4 (rabbit; 1:200; Millipore), anti-GFAP (rabbit, 1:000, Dako, Hamburg, Germany), anti-GFAP (chicken; 1:500; Abcam) and anti-GFP (chicken, 1:500, SySy, Göttingen, Germany). Antibodies were diluted in 1% NGS, 0.05% TritonX100 in PBS. Subsequently, slices were incubated with secondary antibodies over night at room temperature. Secondary antibodies (1:1000 in PBS) used were: goat anti-rabbit Alexa Fluor 488 (Invitrogen Thermo Fisher, Darmstadt, Germany), goat anti-rabbit Alexa Fluor 555 (Invitrogen Thermo Fisher), goat anti-chicken Alexa Fluor 555 and goat anti-chicken Alexa 488 (Abcam), CF488A donkey anti-guinea pig (Sigma-Aldrich). Hoechst 33342 (5 μM; Molecular Probes, Eugine, USA) was used for nuclear staining. Stacks of confocal images were acquired (C1 Eclipse, Nikon) using a 40x/NA 1.3 oil immersion lens. The axial step size was 150 nm. Image stacks of GluA staining were deconvolved using Huygen’s software (SVI, Hilversum, Netherlands). Projections were made using Image J (NIH, Bethesda, USA) and adjusted for brightness and contrast.
Additional Information
How to cite this article: Droste, D. et al. Ca2+-permeable AMPA receptors in mouse olfactory bulb astrocytes. Sci. Rep. 7, 44817; doi: 10.1038/srep44817 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft (LO 779/10-1 to C.L., SPP1757: STE 552/5 to C.S., SPP1757: SE 774/6 to G.S.). The authors thank A.C. Rakete and A. Theil for technical support. The authors thank F. Kirchhoff and A. Scheller (Homburg, Germany) for providing transgenic mice.
Footnotes
The authors declare no competing financial interests.
Author Contributions D.D., G.S., C.S. and C.L. conceived and designed the experiments. D.D., G.S., L.S. and O.J. performed the experiments. D.D., L.S., O.J. and G.S. analyzed the data. D.D., G.S., C.S. and C.L. wrote the paper.
References
- Parpura V. et al. Glial cells in (patho)physiology. J. Neurochem. 121, 4–27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallérac G. & Rouach N. Astrocytes as new targets to improve cognitive functions. Prog. Neurobiol. 144, 48–67 (2016). [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Rodríguez J. J. & Parpura V. Calcium signalling in astroglia. Mol. Cell. Endocrinol. 353, 45–56 (2012). [DOI] [PubMed] [Google Scholar]
- Bazargani N. & Attwell D. Astrocyte calcium signaling: the third wave. Nat. Neurosci. 19, 182–189 (2016). [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Verkhratsky A. J. & Lohr C. Calcium signalling in glial cells. Cell Calcium 24, 405–416 (1998). [DOI] [PubMed] [Google Scholar]
- Rungta R. L. et al. Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia. 64, 2093–2103 (2016). [DOI] [PubMed] [Google Scholar]
- Mishra A. et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci., doi: 10.1038/nn.4428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Möller T., Berger T., Schnitzer J. & Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science 256, 1563–1566 (1992). [DOI] [PubMed] [Google Scholar]
- Burnashev N. et al. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science 256, 1566–1570 (1992). [DOI] [PubMed] [Google Scholar]
- Iino M. et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, 926–929 (2001). [DOI] [PubMed] [Google Scholar]
- Saab A. S. et al. Bergmann glial AMPA receptors are required for fine motor coordination. Science. 337, 749–753 (2012). [DOI] [PubMed] [Google Scholar]
- Höft S., Griemsmann S., Seifert G. & Steinhäuser C. Heterogeneity in expression of functional ionotropic glutamate and GABA receptors in astrocytes across brain regions: insights from the thalamus. Philos. Trans. R. Soc. Lond. B 369, 20130602 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G. & Steinhäuser C. Glial cells in the mouse hippocampus express AMPA receptors with an intermediate Ca2+ permeability. Eur. J. Neurosci. 7, 1872–1881 (1995). [DOI] [PubMed] [Google Scholar]
- Seifert G., Rehn L., Weber M. & Steinhäuser C. AMPA receptor subunits expressed by single astrocytes in the juvenile mouse hippocampus. Brain Res. Mol. Brain Res. 47, 286–294 (1997). [DOI] [PubMed] [Google Scholar]
- Bergles D. E., Jabs R. & Steinhäuser C. Neuron-glia synapses in the brain. Brain Res. Rev. 63, 130–137 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K. et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J. Neurosci. 23, 1750–1758 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr C., Grosche A., Reichenbach A. & Hirnet D. Purinergic neuron-glia interactions in sensory systems. Pflugers Arch. 466, 1859–1872 (2014). [DOI] [PubMed] [Google Scholar]
- Berkowicz D. A., Trombley P. Q. & Shepherd G. M. Evidence for glutamate as the olfactory receptor cell neurotransmitter. J. Neurophysiol. 71, 2557–2561 (1994). [DOI] [PubMed] [Google Scholar]
- Thyssen A. et al. Ectopic vesicular neurotransmitter release along sensory axons mediates neurovascular coupling via glial calcium signaling. Proc. Natl. Acad. Sci. USA 107, 15258–15263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold G. C., Albeanu D. F., Sato T. F. & Murthy V. N. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron 58, 897–910 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doengi M., Deitmer J. W. & Lohr C. New evidence for purinergic signaling in the olfactory bulb: A2A and P2Y1 receptors mediate intracellular calcium release in astrocytes. FASEB J 22, 2368–2378 (2008). [DOI] [PubMed] [Google Scholar]
- Doengi M. et al. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc. Natl. Acad. Sci. USA 106, 17570–17575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu Y. et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 18, 210–218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L. et al. Astroglial connexin 43 hemichannels modulate olfactory bulb slow oscillations. J. Neurosci. 35, 15339–15352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B. & Mulle C. Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology 34, 123–139 (1995). [DOI] [PubMed] [Google Scholar]
- Rozas J. L., Paternain A. V. & Lerma J. Noncanonical signaling by ionotropic kainate receptors. Neuron 39, 543–553 (2003). [DOI] [PubMed] [Google Scholar]
- Takago H., Nakamura Y. & Takahashi T. G protein-dependent presynaptic inhibition mediated by AMPA receptors at the calyx of Held, Proc. Natl. Acad. Sci. USA 102, 7368–7373 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Moreno A. & Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20, 1211–1218 (1998). [DOI] [PubMed] [Google Scholar]
- Koike M., Iino M. & Ozawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci. Res. 29, 27–36 (1997). [DOI] [PubMed] [Google Scholar]
- Tsubokawa H., Oguro K., Masuzawa T., Nakaima T. & Kawai N. Effects of a spider toxin and its analogue on glutamate-activated currents in the hippocampal CA1 neuron after ischemia. J. Neurophysiol. 74, 218–225 (1995). [DOI] [PubMed] [Google Scholar]
- Partin K. M., Patneau D. K., Winters C. A., Mayer M. L. & Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11, 1069–1082 (1993). [DOI] [PubMed] [Google Scholar]
- Borges K. & Kettenmann H. Blockade of K+ channels induced by AMPA/kainate receptor activation in mouse oligodendrocyte precursor cells is mediated by Na+ entry. J. Neurosci. Res. 42, 579–593 (1995). [DOI] [PubMed] [Google Scholar]
- Schröder W., Seifert G., Hüttmann K., Hinterkeuser S. & Steinhäuser C. AMPA receptor-mediated modulation of inward rectifier K+ channels in astrocytes of mouse hippocampus. Mol. Cell. Neurosci. 19, 447–458 (2002). [DOI] [PubMed] [Google Scholar]
- Paternain A. V., Morales M. & Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14, 185–189 (1995). [DOI] [PubMed] [Google Scholar]
- Jonas P., Racca C., Sakmann B., Seeburg P. H. & Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 12, 1281–1289 (1994). [DOI] [PubMed] [Google Scholar]
- Isaac J. T. R., Ashby M. C. & McBain C. J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54, 859–871 (2007). [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Hermans-Borgmeyer I., Hofer M., Ghosh P. & Heinemann S. Ionotropic glutamate-receptor gene expression in hypothalamus: localization of AMPA, kainate, and NMDA receptor RNA with in situ hybridization. J. Comp. Neurol. 343, 428–444 (1994). [DOI] [PubMed] [Google Scholar]
- Horning M. S. et al. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor subunit expression in rat olfactory bulb. Neurosci. Lett. 372, 230–234 (2004). [DOI] [PubMed] [Google Scholar]
- Petralia R. S. & Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J. Comp. Neurol. 318, 329–354 (1992). [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Wang Y. X., Mayat E. & Wenthold R. J. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J. Comp. Neurol. 385, 456–476 (1997). [DOI] [PubMed] [Google Scholar]
- Martin L. J., Blackstone C. D., Levey A. I., Huganir R. L. & Price D. L. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience 53, 327–358 (1993). [DOI] [PubMed] [Google Scholar]
- Douyard J., Shen L., Huganir R. L. & Rubio M. E. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J. Comp. Neurol. 502, 141–156 (2007). [DOI] [PubMed] [Google Scholar]
- Hamilton K. A. & Coppola D. M. Distribution of GluR1 is altered in the olfactory bulb following neonatal naris occlusion. J. Neurobiol. 54, 326–336 (2003). [DOI] [PubMed] [Google Scholar]
- Hamilton K. A. et al. Sensory deafferentation transsynaptically alters neuronal GluR1 expression in the external plexiform layer of the adult mouse main olfactory bulb. Chem. Senses 33, 201–210 (2008). [DOI] [PubMed] [Google Scholar]
- Montague A. A. & Greer C. A. Differential distribution of ionotropic glutamate receptor subunits in the rat olfactory bulb. J. Comp. Neurol. 405, 233–246 (1999). [DOI] [PubMed] [Google Scholar]
- Man H.-Y. GluA2-lacking, calcium-permeable AMPA receptors–inducers of plasticity? Curr. Opin. Neurobiol. 21, 291–298 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor E., Leventhal P. S. & Schaeffer S. F. Partial purification and characterization of the vacuolar H+-ATPase of mammalian synaptic vesicles. J. Neurochem. 55, 1663–1670 (1990). [DOI] [PubMed] [Google Scholar]
- Schipke C. G. Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. Faseb J(2001). [DOI] [PubMed] [Google Scholar]
- Salter M. G. & Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 438, 1167–1171 (2005). [DOI] [PubMed] [Google Scholar]
- Micu I. et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2006). [DOI] [PubMed] [Google Scholar]
- Káradóttir R., Cavelier P., Bergersen L. H. & Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U., Pankratov Y., Kirchhoff F., North R. A. & Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 26, 2673–2683 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palygin O., Lalo U., Verkhratsky A. & Pankratov Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium 48, 225–231 (2010). [DOI] [PubMed] [Google Scholar]
- Boddum K. et al. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat Commun. 7, 13572 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W., Shargool M. & Hertz L. Barium-induced inhibition of K+ transport mechanisms in cortical astrocytes - its possible contribution to the large Ba2+-evoked extracellular K+ signal in brain. Neuroscience 13, 945–949 (1984). [DOI] [PubMed] [Google Scholar]
- Afzalov R. et al. Low micromolar Ba2+ potentiates glutamate transporter current in hippocampal astrocytes. Front Cell Neurosci. 7, 135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger A., Deitmer J. W. & Lohr C. Axon-glia communication evokes calcium signaling in olfactory ensheathing cells of the developing olfactory bulb. Glia 55, 352–359 (2007). [DOI] [PubMed] [Google Scholar]
- Hökfelt T. et al. Histochemical support for a dopaminergic mechanism in the dendrites of certain periglomerular cells in the rat olfactory bulb. Neurosci. Lett. 1, 85–90 (1975). [DOI] [PubMed] [Google Scholar]
- Halasz N. et al. Transmitter histochemistry of the rat olfactory bulb. I. Immunohistochemical localization of monoamine synthesizing enzymes. Support for intrabulbar, periglomerular dopamine neurons. Brain Res. 126, 455–474 (1977). [DOI] [PubMed] [Google Scholar]
- Cave J. W. & Baker H. Dopamine systems in the forebrain. Adv. Exp. Med. Biol. 651, 15–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov A. S., Angulo M. C., Audinat E. & Charpak S. Target cell-specific modulation of neuronal activity by astrocytes. Proc. Natl. Acad. Sci. USA 103, 10058–10063 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar H. B., Purcell A. L. & Greer C. A. Glomerular formation in the developing rat olfactory bulb. J. Comp. Neurol. 413, 289–304 (1999). [PubMed] [Google Scholar]
- Bailey M. S., Puche A. C. & Shipley M. T. Development of the olfactory bulb: evidence for glia-neuron interactions in glomerular formation. J. Comp. Neurol. 415, 423–448 (1999). [PubMed] [Google Scholar]
- Chen T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T. et al. Inducible gene deletion in astroglia and radial glia–a valuable tool for functional and lineage analysis. Glia 54, 21–34 (2006). [DOI] [PubMed] [Google Scholar]
- Nolte C. et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33, 72–86 (2001). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.