Abstract
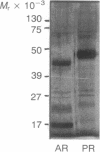
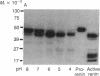
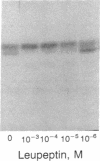
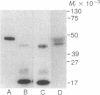
Using pure recombinant human prorenin as a substrate, we have identified an enzyme in human kidney that accurately processes prorenin to active renin (EC 3.4.23.15). In the crude homogenate, the predominant activity of this potential renin-processing enzyme (RPE) converted the Mr 47,000 inactive prorenin to Mr 44,000 active renin and had a pH optimum of approximately 6. The activity was blocked by cysteine protease inhibitors, but not by pepstatin, EDTA, or serine protease inhibitors. This RPE activity was not detected in a similarly prepared homogenate of human chorion decidua tissue, which produces primarily prorenin, or in human plasma. The activity was purified 100-fold by ammonium sulfate precipitation, p-chloromercuribenzoate affinity chromatography, and chromatofocusing. The partially purified enzyme has a Mr of approximately 27,000 and an isoelectric point in the pH 4.8-5.6 range. The activity in the purified RPE preparation had the same pH optimum as that in crude homogenate, cleaved the prosegment at the same site used by the kidney in vivo based on amino-terminal sequencing of the processed renin, and did not degrade prorenin or renin. These data suggest that the cysteine protease we have isolated is a candidate for authentic renal RPE.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Brennan S. O., Carrell R. W., Cousens L. S., Brake A. J., Barr P. J. Yeast KEX2 protease has the properties of a human proalbumin converting enzyme. Science. 1987 Jan 16;235(4786):348–350. doi: 10.1126/science.3541206. [DOI] [PubMed] [Google Scholar]
- Carilli C. T., Wallace L. C., Smith L. M., Wong M. A., Lewicki J. A. Semi-preparative purification of recombinant human renin and prorenin. J Chromatogr. 1988 Jul 1;444:203–208. doi: 10.1016/s0021-9673(01)94023-3. [DOI] [PubMed] [Google Scholar]
- Do Y. S., Sherrod A., Lobo R. A., Paulson R. J., Shinagawa T., Chen S. W., Kjos S., Hsueh W. A. Human ovarian theca cells are a source of renin. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1957–1961. doi: 10.1073/pnas.85.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Y. S., Shinagawa T., Tam H., Inagami T., Hsueh W. A. Characterization of pure human renal renin. Evidence for a subunit structure. J Biol Chem. 1987 Jan 25;262(3):1037–1043. [PubMed] [Google Scholar]
- Docherty K., Hutton J. C., Steiner D. F. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem. 1984 May 25;259(10):6041–6044. [PubMed] [Google Scholar]
- Fritz L. C., Arfsten A. E., Dzau V. J., Atlas S. A., Baxter J. D., Fiddes J. C., Shine J., Cofer C. L., Kushner P., Ponte P. A. Characterization of human prorenin expressed in mammalian cells from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4114–4118. doi: 10.1073/pnas.83.12.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen F. X., Devaux C., Guyenne T., Menard J., Corvol P. Multiple forms of human renin. Purification and characterization. J Biol Chem. 1979 Jun 10;254(11):4848–4855. [PubMed] [Google Scholar]
- Goldstone R., Horton R., Carlson E. J., Hsueh W. A. Reciprocal changes in active and inactive renin after converting enzyme inhibition in normal man. J Clin Endocrinol Metab. 1983 Feb;56(2):264–268. doi: 10.1210/jcem-56-2-264. [DOI] [PubMed] [Google Scholar]
- Hardman J. A., Hort Y. J., Catanzaro D. F., Tellam J. T., Baxter J. D., Morris B. J., Shine J. Primary structure of the human renin gene. DNA. 1984 Dec;3(6):457–468. doi: 10.1089/dna.1.1984.3.457. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Hui J., Zürcher-Neely H., Poorman R. A. A structural model to explain the partial catalytic activity of human prorenin. Am J Hypertens. 1989 May;2(5 Pt 1):367–380. doi: 10.1093/ajh/2.5.367. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hirose S., Kim S., Miyazaki H., Park Y. S., Murakami K. In vitro biosynthesis of human renin and identification of plasma inactive renin as an activation intermediate. J Biol Chem. 1985 Dec 25;260(30):16400–16405. [PubMed] [Google Scholar]
- Hobart P. M., Fogliano M., O'Connor B. A., Schaefer I. M., Chirgwin J. M. Human renin gene: structure and sequence analysis. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5026–5030. doi: 10.1073/pnas.81.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Miyazaki H., Hirose S., Hori H., Hayashi T., Kageyama R., Ohkubo H., Nakanishi S., Murakami K. Cloning and sequence analysis of cDNA for human renin precursor. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7405–7409. doi: 10.1073/pnas.80.24.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagami T., Okamoto H., Ohtsuki K., Shimamoto K., Chao J., Margolius H. S. Human plasma inactive renin: purification and activation by proteases. J Clin Endocrinol Metab. 1982 Oct;55(4):619–627. doi: 10.1210/jcem-55-4-619. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y., Saito M., Hori H., Poorman R. A., Yoshida S., Murakami K. Immunoaffinity purification of human prorenin produced in Chinese hamster ovary cells. Biochem Biophys Res Commun. 1988 Apr 29;152(2):849–856. doi: 10.1016/s0006-291x(88)80116-5. [DOI] [PubMed] [Google Scholar]
- Luetscher J. A., Kraemer F. B., Wilson D. M., Schwartz H. C., Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985 May 30;312(22):1412–1417. doi: 10.1056/NEJM198505303122202. [DOI] [PubMed] [Google Scholar]
- McIntyre G. D., Leckie B., Hallett A., Szelke M. Purification of human renin by affinity chromatography using a new peptide inhibitor of renin, H.77 (D-His-Pro-Phe-His-LeuR-Leu-Val-Tyr). Biochem J. 1983 May 1;211(2):519–522. doi: 10.1042/bj2110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Perrelet A., Vassalli J. D., Anderson R. G. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986 Dec;103(6 Pt 1):2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R. N. Cellular biology of the renin-angiotensin systems. Arch Intern Med. 1984 Oct;144(10):2037–2041. [PubMed] [Google Scholar]
- Saint-André J. P., Rohmer V., Pinet F., Rousselet M. C., Bigorgne J. C., Corvol P. Renin and cathepsin B in human pituitary lactotroph cells. An ultrastructural study. Histochemistry. 1989;91(4):291–297. doi: 10.1007/BF00493003. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H. Prorenin and other large molecular weight forms of renin. Endocr Rev. 1980 Fall;1(4):365–391. doi: 10.1210/edrv-1-4-365. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Do Y. S., Kjos S., Anderson P. W., Shinagawa T., Dubeau L., Hsueh W. A. Human decidua is a major source of renin. J Clin Invest. 1989 Jun;83(6):2085–2092. doi: 10.1172/JCI114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. E., Gounaris A. D., Haber E. Isolation of a renal thiol protease that activates inactive plasma renin. Clin Sci (Lond) 1979 Dec;57 (Suppl 5):93s–96s. doi: 10.1042/cs057093s. [DOI] [PubMed] [Google Scholar]
- Slater E. E., Strout H. V., Jr Pure human renin. Identification and characterization and of two major molecular weight forms. J Biol Chem. 1981 Aug 10;256(15):8164–8171. [PubMed] [Google Scholar]
- Soubrier F., Panthier J. J., Corvol P., Rougeon F. Molecular cloning and nucleotide sequence of a human renin cDNA fragment. Nucleic Acids Res. 1983 Oct 25;11(20):7181–7190. doi: 10.1093/nar/11.20.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Murakami K., Miyake Y. Activation of kidney prorenin by kidney cathepsin B isozymes. J Biochem. 1982 Jan;91(1):419–422. doi: 10.1093/oxfordjournals.jbchem.a133705. [DOI] [PubMed] [Google Scholar]
- Taugner R., Bührle C. P., Nobiling R., Kirschke H. Coexistence of renin and cathepsin B in epithelioid cell secretory granules. Histochemistry. 1985;83(2):103–108. doi: 10.1007/BF00495138. [DOI] [PubMed] [Google Scholar]
- Taugner R., Murakami K., Kim S. J. Renin activation in juvenile secretory granules? Immunocytochemical experiments with an antiserum directed against the prosegment of human renin. Histochemistry. 1986;85(2):107–109. doi: 10.1007/BF00491755. [DOI] [PubMed] [Google Scholar]
- Xiong W., Chao L., Chao J. Renal kallikrein mRNA localization by in situ hybridization. Kidney Int. 1989 Jun;35(6):1324–1329. doi: 10.1038/ki.1989.130. [DOI] [PubMed] [Google Scholar]
- Yokosawa H., Holladay L. A., Inagami T., Haas E., Murakami K. Human renal renin. Complete purification and characterization. J Biol Chem. 1980 Apr 25;255(8):3498–3502. [PubMed] [Google Scholar]