Abstract
Introduction: HIV infection is associated with vascular dysfunction and adverse cardiovascular outcomes. Our objective was to review the evidence regarding the clinical utility of endothelial activation and coagulation biomarkers for the prognosis of HIV-infected patients.
Methods: We searched PubMed and Embase for publications using the keywords “HIV” or “HIV infection” and “endothelium” or “coagulation”. We reviewed reference lists and hand-searched for additional relevant articles. All clinical studies that enrolled non-pregnant, HIV-infected adults, measured biomarkers reflecting endothelial activation or coagulation, and prospectively evaluated their associations with vascular dysfunction or clinical outcomes were included.
Results: Seventeen studies were identified that fulfilled the inclusion criteria, of which 11 investigated endothelial activation biomarkers and 12 investigated coagulation biomarkers. Biomarkers and outcomes varied widely across studies. Overall, published studies support an association between P-selectin and venous thromboembolism in HIV-infected patients, an association between tissue-type plasminogen activator and death, and associations between D-dimer and several clinical outcomes, including venous thromboembolism, cardiovascular disease, and all-cause mortality.
Conclusions: Several studies have demonstrated associations between biomarkers of endothelial activation and coagulation and clinically important outcomes in HIV-1 infection. Additional large-scale prospective investigations to determine the utility of endothelial activation and coagulation biomarkers for risk stratification and prediction of adverse outcomes are clearly warranted.
Keywords: HIV, biomarker, endothelium, coagulation, vascular dysfunction
Introduction
Since effective antiretroviral therapy (ART) became widely available, the risks for morbidity and mortality due to opportunistic infections have greatly decreased for persons living with HIV infection.1,2 Unfortunately, recent evidence shows that HIV-infected persons are at higher risk for cardiovascular, renal, and hepatic disease, despite effective ART.3-6 Increasing evidence points to chronic inflammation among individuals who develop HIV-related end-organ disease and other complications.7,8 Such inflammation may activate the coagulation cascade, leading to a pro-thrombotic tendency in HIV-infected persons that could lead to arterial or venous thromboembolism (VTE).9,10
Subclinical atherosclerotic disease and vascular dysfunction have also been identified in HIV-infected individuals.11 While traditional risk factors are still important, traditional risk assessments such as the Framingham Risk Score underestimate cardiovascular risk in HIV-infected persons.12,13 Inflammation, endothelial activation, and oxidative stress, all increased in HIV-1 infection, are known to be major driving forces for the initiation of coronary plaques, their progression to instability, and eventual plaque disruption.14-16 Indeed, several studies have demonstrated increased levels of biomarkers of endothelial activation (e.g., VCAM-1, ICAM-1, E-selectin) and coagulation (e.g., P-selectin, d-dimer, fibrinogen) in HIV-infected persons compared with healthy, uninfected controls.17-20
There are multiple mechanisms whereby HIV-1 proteins and antiretroviral drugs may lead to endothelial damage (reviewed in ref. 21). The HIV-1 envelope protein gp120 and the regulatory protein Tat are both associated with endothelial cell apoptosis and increased cellular adhesion molecules, adhesion, permeability, and reactive oxygen species (ROS). Tat is also associated with decreased endothelial relaxation, and increased monocyte chemoattractant protein-1, matrix metalloproteinase, chemotaxis, proliferation, and angiogenesis. Other accessory proteins may augment these effects: Nef and Vpr by increasing endothelial cell apoptosis, and Vpu by increasing expression of cellular adhesion molecules (Table 1 in ref. 21). Antiretroviral drugs may exacerbate HIV-1-related endothelial effects. Nucleoside or nucleotide reverse transcriptase inhibitors decrease mitrochondrial function, levels of reduced glutathione, and vasorelaxation, and increase ROS, vasoconstrictor release, endothelial proliferation, and vascular permeability. Protease inhibitors have been associated with decreased mitrochondrial function, endothelial nitric oxide synthase, vasorelaxation, and flow-mediated dilation, as well as increased ROS, mitochondrial DNA damage, vascular permeability, and carotid intima media thickness. There are no known endothelial effects of non-nucleoside reverse transcriptase inhibitors, integrase inhibitors, or entry inhibitors (Table 2 in ref. 21).
Because endothelial activation and coagulation may each play key mechanistic roles leading to adverse outcomes in HIV-infected patients, biomarkers of these processes may prove valuable for the diagnosis, prognosis, or risk stratification of HIV-infected patients. In addition, a better understanding of the mechanisms leading to HIV-1-related vascular dysfunction may lead to specific treatments aimed at reducing endothelial activation or coagulopathy. Our objective was therefore to review the evidence regarding the clinical utility of biomarkers of endothelial activation and coagulation for prognosis in HIV-infected patients.
Results
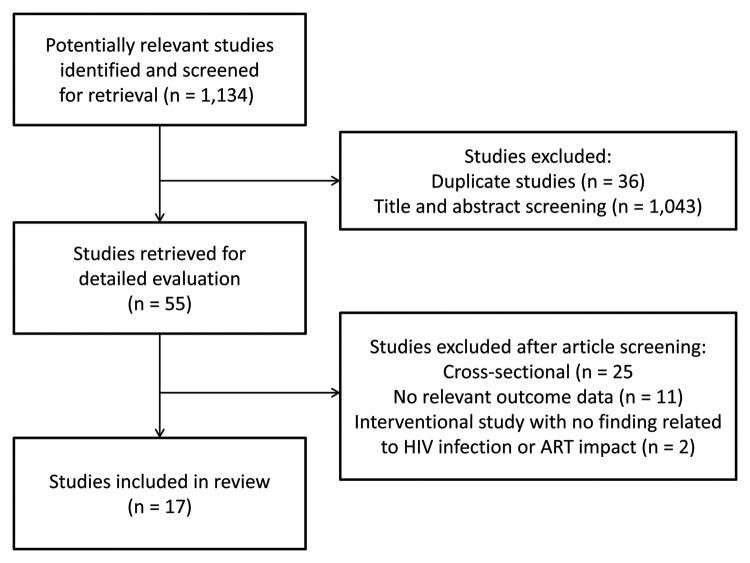
Our search identified 1134 unique articles (see Fig. 1). Of these, 55 studies met our initial screening criteria (i.e., studies of HIV-infected adults in which biomarkers were measured). After retrieval of the full-text publication, 38 studies were excluded for the following reasons: 25 studies were cross-sectional, 11 studies did not report a relevant outcome, and 2 studies were interventional trials: one study evaluated the short-term effect of vaccination on inflammatory biomarkers,22 and the other study examined the effect of telmisartan on blood pressure and proteinuria.23 The remaining 17 studies were included in our review. Four studies were randomized trials of interventions aimed at improving vascular function: salsalate,24 pentoxifylline,25 rosiglitazone,26 and NRTI-sparing vs. standard triple PI-based ART.27 All remaining studies were observational designs, two of which were secondary analyses of data collected during a prospective clinical trial.10,28
Figure 1. Study flow diagram.
Biomarkers of endothelial activation
We identified 11 studies investigating associations between biomarkers of endothelial activation and vascular dysfunction or clinical outcomes in HIV infection (see Table 1). Nine studies evaluated ICAM-1, 8 evaluated VCAM-1, 3 evaluated E-selectin, and one each evaluated ICAM-3, P-selectin, and VEGF, respectively. Two studies used nested case-control designs, and one used a retrospective case-control with prospective follow-up of cases. Five studies evaluated vascular function using measures including carotid intima-media thickness (c-IMT, n = 2), flow-mediated dilation (n = 2), arterial stiffness (n = 1), circulating endothelial cells (n = 1), finger arterial pulse wave amplitude (n = 1), and nitroglycerin-mediated dilation (n = 1). Six studies evaluated clinical outcomes, including CD4 count decline (n = 1), cytomegalovirus retinitis (n = 1), cardiovascular disease (CVD) events (n = 1), death (n = 1), Kaposi sarcoma (n = 1), and VTE (n = 1). The study of CVD events used a composite outcome including acute myocardial infarction, silent myocardial infarction, coronary revascularization, acute coronary syndrome, cerebrovascular accident, lower extremity revascularization, and sudden cardiac death.29
Table 1. Biomarkers of endothelial activation.
| Author | Year | Biomarkers | Study design | Patient population and follow-up | Outcomes studied | Finding |
|---|---|---|---|---|---|---|
| Zangerle43 | 1994 | ICAM-1 | Prospective | 47 HIV-infected adults followed for mean 12.7 mo (range, 8–16 mo) | CD4 decline | • No association between ICAM-1 levels and CD4 count decline |
| Greenwood44 | 1998 | ICAM-1, VCAM-1 | Prospective | 25 HIV-infected adults with and without CMV retinitis followed for up to 19 mo | CMV retinitis | • No difference in ICAM-1 or VCAM-1 levels between patients with and without retinitis • No difference in ICAM-1 or VCAM-1 levels between active flares and quiescent periods |
| Renwick45 | 2002 | VEGF | Prospective | 40 HIV-infected men followed in the Amsterdam Cohort Studies (follow-up time not reported) | Kaposi sarcoma | • No change in VEGF levels after HIV-1 or HHV-8 seroconversion • No difference in VEGF concentration in men who did or did not develop KS |
| Sipsas31 | 2003 | E-selectin, ICAM-1 | Prospective | 64 HIV-infected adults followed for median 46 mo (range, 2–78 mo) | Death | • Baseline levels of E-selectin and ICAM-1 higher in those who died • ICAM-1 associated with time to death, but not in multivariate analysis |
| van Vonderen27 | 2009 | ICAM-1, VCAM-1 | Prospective | 37 HIV-infected, ART-naïve men randomized to receive either AZT/3TC/LPV/r or NVP/LPV/r and followed for 24 mo | c-IMT, arterial stiffness | • Levels of VCAM-1 and ICAM-1 decreased in both groups during treatment • c-IMT increased and arterial stiffness decreased, with no difference between arms |
| Ford29 | 2010 | ICAM-1, VCAM-1 | Nested case-control within prospective NIH cohort | 52 HIV-infected adults with a CVD event (cases) and 102 matched controls followed for mean 8.9 and 8.4 y from ART initiation, respectively | CVD event (see text for details) | • Elevated VCAM-1 associated with CVD events, but association not significant in multivariable analysis |
| Gupta25 | 2010 | E-selectin, ICAM-1, VCAM-1 | Prospective | 9 HIV-infected adults not requiring ART, randomized to pentoxifylline or placebo, followed for 8 weeks | FMD, NTGMD | • Pentoxifylline reduced VCAM-1 levels and improved FMD over 8 weeks |
| Hileman24 | 2010 | ICAM-1, VCAM-1 | Prospective | 40 HIV-infected adults with virologic suppression on ART, randomized to salsalate or placebo, followed for 13 weeks | FMD | • Neither ICAM-1 nor VCAM-1 levels correlated with change in FMD • Change in FMD did not differ between arms |
| Francisci32 | 2011 | VCAM-1 | Prospective, with retrospective case-control component | 69 HIV-infected adults initiating ABC- or TDF-containing ART followed for 6–12 mo, 20 HIV-infected untreated controls, and 10 healthy controls | Finger arterial pulse wave amplitude, circulating endothelial cells | • HIV-infected adults had increased circulating endothelial cells and impaired tonometry • Endothelial function was worse in adults taking ABC than those taking TDF • VCAM-1 levels elevated in HIV infection, and decreased to a greater extent among adults treated with TDF |
| Musselwhite30 | 2011 | E-selectin, ICAM-1, ICAM-3, P-selectin, VCAM-1 | Nested case-control within prospective NIH cohort | 23 HIV-infected adults with VTE (cases) and 69 matched HIV-infected controls followed for median 6.9 and 7.2 y from ART initiation, respectively | VTE | • Increased P-selectin levels associated with VTE |
| Tungsiripat26 | 2011 | ICAM-1, VCAM-1 | Prospective | 71 HIV-infected adults with lipoatrophy on thymidine-sparing ART, randomized to rosiglitazone or placebo and followed for 48 weeks | c-IMT | • VCAM-1 levels decreased and c-IMT increased, with no difference between groups • Neither ICAM-1 nor VCAM-1 levels associated with c-IMT |
3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; AZT, zidovudine; c-IMT, carotid intima-media thickness; CMV, cytomegalovirus; CVD, cardiovascular disease; FMD, flow-mediated dilation; HIV, human immunodeficiency virus; KS, Kaposi sarcoma; LPV/r, boosted lopinavir; NIH, National Institutes of Health; NTGMD, nitroglycerin-mediated dilation; NVP, nevirapine; TDF, tenofovir; VEGF, vascular endothelial growth factor; VTE, venous thromboembolism
Only one study reported positive findings: in the study by Musselwhite et al., P-selectin levels were associated with VTE.30 Two studies reported associations that were not significant in multivariable analysis. In one, higher ICAM-1 levels were associated with shorter time to death.31 In the other, higher VCAM-1 levels were associated with CVD events.29 The results of three studies suggested an indirect association between endothelial activation biomarkers and outcomes. In the first, VCAM-1 and ICAM-1 decreased over 24 mo of ART, while c-IMT increased and arterial stiffness decreased.27 In the second, pentoxifylline reduced VCAM-1 levels and improved flow mediated dilation (FMD) over 8 weeks of follow-up.25 In the third, VCAM-1 levels decreased to a greater extent among participants taking tenofovir-containing regimens, in whom endothelial function also improved to a greater extent than among participants taking abacavir-containing regimens.32 In all three of these studies, no evaluation was presented to determine whether baseline biomarker levels predicted outcomes. Five studies demonstrated no difference between the outcome of interest and endothelial activation biomarkers.
Biomarkers of coagulation
We identified 12 studies investigating associations between biomarkers of coagulation and vascular dysfunction or clinical outcomes in HIV infection (see Table 2). d-dimer was evaluated in seven studies and VWF in six studies, while fibrinogen, PAI-1, prothombin fragment 1+2, and tissue factor were evaluated in two studies each. Thrombomodulin and tPA were each evaluated in only one of these 12 studies. One study conducted an extensive coagulation work-up including activated protein C sensitivity ratio, endogenous thrombin potential, protein C, prothrombin, PT, PTT, thrombin-antithrombin complex, total and free protein S, as well as d-dimer and VWF.33 Five studies used nested case-control designs. Four studies evaluated vascular function using measures including c-IMT (n = 3), arterial stiffness (n = 1), and FMD (n = 1). Eight studies evaluated clinical outcomes, including all-cause mortality (n = 3), CVD events (n = 1), deep venous thrombosis (n = 1), opportunistic infections (n = 1), and VTE (n = 1). One study evaluated HIV disease progression, defined as AIDS-defining illness, death, or a CD4 count <50 cells/μL.34
Table 2. Biomarkers of coagulation.
| Author | Year | Biomarkers | Study design | Patient population and follow-up | Primary outcome | Finding |
|---|---|---|---|---|---|---|
| Schved36 | 1992 | PAI-1, tPA, VWF | Prospective | 85 HIV-infected adults, 65 of whom were followed prospectively for a median 22 mo | Death | • Higher PAI-1, tPA, and VWF in advanced disease • Increased VWF and tPA in non-survivors • tPA and CD4 count independently predicted death |
| Aukrust34 | 2000 | VWF | Prospective | 43 HIV-infected adults followed for a median of 5 y (range, 3.8 to 6 y) and 19 healthy controls | Disease progression (see text for details) | • Marked rise in VWF associated with disease progression • Positive correlation between VWF levels and HIV-1 RNA |
| Hsue46 | 2004 | Fibrinogen | Prospective | 148 HIV-infected adults followed for 12 mo, and 63 healthy controls | c-IMT, c-IMT progression | • Higher fibrinogen levels and c-IMT in HIV-infected adults • No association between fibrinogen and c-IMT or c-IMT progression |
| Kuller10 | 2008 | d-dimer, prothrombin fragment 1+2 | Nested case control within the SMART study47 | 499 HIV-infected adults randomized to drug conservation or viral suppression strategies followed for 1 mo, with nested case-control comparing 85 adults who died to 170 matched HIV-infected controls (follow-up time not reported) | All-cause mortality | • Higher levels of d-dimer at study entry associated with increased risk of all-cause mortality • d-dimer levels associated with HIV-1 RNA levels, with both higher in the drug conservation group |
| Rodger28 | 2009 | d-dimer, prothrombin fragment 1+2 | Nested case control within the SMART study47 | 91 HIV-infected adults with opportunistic infections (cases) and 182 HIV-infected controls (follow-up time not reported) | Opportunistic infection | • Neither baseline d-dimer nor prothrombin fragment 1+2 predicted opportunistic infections |
| van Vonderen27 | 2009 | PAI-1, VWF | Prospective | 37 HIV-infected, ART-naïve men randomized to receive either AZT/3TC/LPV/r or NVP/LPV/r and followed for 24 mo | c-IMT, arterial stiffness | • VWF levels decreased in both groups during treatment • c-IMT increased and arterial stiffness decreased, with no difference between arms |
| Ford29 | 2010 | d-dimer, tissue factor | Nested case-control within prospective NIH cohort | 52 HIV-infected adults with a CVD event (cases) and 102 matched controls followed for mean 8.9 and 8.4 y from ART initiation, respectively | CVD event (see reference for details) | • Elevated d-dimer and tissue factor associated with CVD events • Only d-dimer independently associated with CVD events in multivariable analysis |
| Hileman24 | 2010 | d-dimer, fibrinogen | Prospective | 40 HIV-infected adults with virologic suppression on ART, randomized to salsalate or placebo, followed for 13 weeks | FMD | • Neither d-dimer nor fibrinogen levels correlated with change in FMD • Change in FMD did not differ between arms |
| Jong33 | 2010 | APCsr, d-dimer, endogenous thrombin potential, protein C, prothrombin, PT, PTT, thrombin-antithrombin complex, total and free protein S, VWF | Prospective | 123 HIV-infected adults initiating ART followed for a median 7.2 mo (± 1.6 mo) and 71 healthy controls | DVT | • No asymptomatic DVT in 57 HIV-infected adults tested • All biomarkers of coagulation except APCsr improved after ART initiation • Persistent differences with uninfected controls |
| Musselwhite30 | 2011 | d-dimer, tissue factor, thrombomodulin, VWF | Nested case-control within NIH cohort | 23 HIV-infected adults with VTE (cases) and 69 matched HIV-infected controls followed for median 6.9 and 7.2 y from ART initiation, respectively | VTE | • Increased d-dimer levels were associated with VTE |
| Tungsiripat26 | 2011 | VWF | Prospective | 71 HIV-infected adults with lipoatrophy on thymidine-sparing regimens, randomized to rosiglitazone or placebo, followed for 48 weeks | c-IMT | • VWF levels decreased and c-IMT increased, with no difference between groups • VWF levels not associated with c-IMT |
| Justice35 | 2012 | D-dimer | Nested case control followed in VACS | 1302 HIV-infected adults (follow-up time not reported) | Death | • D-dimer correlated with VACS Index, which was more predictive of mortality than any biomarker • Addition of D-dimer to the VACS Index improved classification by 7% |
3TC, lamivudine; AIDS, acquired immunodeficiency syndrome; APCsr, activated protein C sensitivity ratio; ART, antiretroviral therapy; AZT, zidovudine; c-IMT, carotid intima-media thickness; DVT, deep venous thrombosis; FMD, flow-mediated dilation; HIV, human immunodeficiency virus; LPV/r, boosted lopinavir; NVP, nevirapine; PAI-1, plasminogen activator-inhibitor 1; PT, prothrombin time; PTT, partial thromboplastin time; tPA, tissue-type plasminogen activator; VACS, Veterans Aging Cohort Study; VTE, venous thromboembolism; von Willebrand Factor, VWF.
Five studies demonstrated associations between endothelial activation and outcomes of interest, four of which involved d-dimer. In these four studies, d-dimer predicted all-cause mortality (n = 2),10,35 CVD events (n = 1),29 and VTE (n = 1).30 In the fifth positive study, tPA and CD4 count independently predicted death.36 Results from two studies suggested an indirect association between biomarkers and outcomes. In the first, a marked rise in VWF levels was associated with disease progression, with a positive correlation between VWF levels and HIV-1 RNA levels.34 There was not a direct evaluation of whether baseline VWF levels predicted disease progression. In the second study, VWF levels decreased while c-IMT increased and arterial stiffness decreased during ART.27 Again, there was not a direct evaluation of whether baseline VWF levels predicted vascular function. Four studies reported no association between coagulation biomarkers and outcomes of interest. One study identified no outcomes, and so had no power to determine associations.33
Discussion
We conducted a comprehensive systematic review of the clinical utility of biomarkers reflecting endothelial activation and coagulation in HIV-1 infection. Our objective in conducting this review has been to evaluate the status of work in this area and identify productive avenues for future research. Increased coagulation and endothelial activation biomarkers have been reported in a number of studies of HIV-infected adults, with decreases in these markers after ART initiation (reviewed in refs. 7, 21, and 37). However, only a small number of studies have evaluated the association of endothelial activation and coagulation with either vascular dysfunction (a surrogate marker of future adverse outcomes) or clinical outcomes in this patient population. Seventeen studies were identified that fulfilled the inclusion criteria, of which 11 investigated endothelial activation biomarkers and 12 investigated coagulation biomarkers. The biomarkers and outcomes studied varied widely. Sample sizes were relatively small, ranging from 9 to 499 HIV-infected participants, with the exception of one very large study of over 1300 participants.35
Our review of endothelial activation biomarkers identified the platelet activation biomarker P-selectin as having clinical utility for the diagnosis of VTE.30 Notably, P-selectin has been previously reported as a predictor of VTE in the general population,38 and increased plasma levels of P-selectin have been reported among pregnant HIV-infected women who developed preeclampsia, compared with those who did not.39 While higher ICAM-1 levels were associated with mortality in one study,31 and higher VCAM-1 levels with CVD events in another,29 neither finding was an independent predictor of outcomes. Other studies we identified failed to evaluate the prognostic value of endothelial activation biomarkers despite longitudinal follow-up.
Are biomarkers of endothelial activation therefore not useful for the prediction of outcomes other than VTE? We have recently published a prospective study of endothelial activation biomarkers in HIV-1 seroconverters that was not included in this review. In this study, we found that levels of ICAM-1 and VCAM-1 were persistently elevated from the date of HIV-1 acquisition and that plasma VCAM-1 levels measured during chronic infection were independently associated with time to HIV progression or death.40 Our search did not identify eligible studies of several other biomarkers in this category, including Ang-1, Ang-2, ADMA, and the soluble forms of endocan, endothelin-1, Flt-1, and Tie2 receptor. Investigation of these biomarkers is recommended as holding promise. Indeed, we have found in a small study of Kenyan women initiating ART that soluble ICAM-1 and plasma Ang-2 levels decreased after ART initiation, with concomitant increases in the beneficial protein Ang-1. Although both biomarkers predicted mortality after ART initiation in this cohort, Ang-2 had better predictive value.41
Our review of coagulation biomarkers showed that d-dimer is the most promising biomarker, given its associations with several clinical outcomes, including venous thromboembolism, cardiovascular disease, and all-cause mortality.10,29,30,35 Interestingly, d-dimer has been found to be a predictor of mortality in Crimean-Congo hemorrhagic fever,42 suggesting the possibility that this biomarker may have utility in other viral infections as well. In addition, tPA was identified as a potential biomarker for mortality in HIV-infected patients.36 Future studies of biomarkers in HIV-1 infection should include d-dimer and consider inclusion of tPA, in order to help establish the clinical utility of these biomarkers for risk stratification and the prediction of clinically relevant endpoints. Our search did not identify eligible studies of several other coagulation biomarkers, including ADAMTS13, factor VIII activity, soluble fibrin, and thrombospondin.
Our study has several limitations. First, our search strategy was broad, but we may have missed some articles in which the biomarkers were not discussed in terms of their effects on endothelial activation or coagulation. We tried to address this by adding searches of biomarker names and by hand-searching the reference lists of identified studies. Second, many publications were produced by single-center teams or were retrospective analyses of previously collected specimens and data, limiting the generalizability to other populations or jurisdictions. Third, the identified studies included a wide range of biomarkers, and did not always evaluate the association of biomarkers with the later development of outcomes. In addition, the selection of outcomes varied across studies, and their definitions may have differed. Finally, because many of the biomarkers studied are not standardized, available literature can only report similarities in the direction and relative magnitude of associations across studies. We were unable to identify a sufficiently large number of adequately powered studies with prospective designs, careful selection of biomarkers, and standardized outcomes to confirm conclusively whether any of these biomarkers have clinical utility in most HIV-infected patient populations at the present time.
Materials and Methods
Data sources
We systematically and inclusively identified all studies that reported data on biomarkers of: (1) endothelial activation (including angiopoietin-1 [Ang-1], angiopoietin-2 [Ang-2], asymmetric dimethylarginine [ADMA], and the soluble forms of endocan, endothelin-1, E-selectin, FMS-like tyrosine kinase-1 [sFlt-1], intercellular adhesion molecule 1 [ICAM-1], ICAM-3, P-selectin, Tie2 receptor, vascular cell adhesion molecule 1 [VCAM-1], and vascular endothelial growth factor [VEGF]), and (2) coagulation (including ADAMTS13, antithrombin, d-dimer, factor VIII activity, fibrinogen, plasminogen activator-inhibitor 1 [PAI-1], protein C, protein S, prothrombin, prothrombin fragment 1+2, soluble fibrin, thrombin, thrombomodulin, thrombospondin, tissue-type plasminogen activator [tPA], tissue factor, and von Willebrand factor [VWF]) in HIV-infected adult patients. We electronically searched PubMed (1950 to Week 27, 2012) and Embase (1980 to Week 27, 2012) databases for all pertinent articles published in English (see Table S1).
Study selection methods
Study selection was performed independently by two reviewers (RM and SMG), with disagreement resolved through arbitration by a third reviewer (WCL). A study was included if it met the following criteria:
(1) Inclusion of non-pregnant, HIV-infected adults, aged 18 y or older;
(2) Measurement of any known biomarker of endothelial activation and/or coagulation; and
(3) Measurement of vascular dysfunction (e.g., carotid intima-media thickness) or a clinical endpoint (e.g., all-cause mortality).
Studies that included only children (i.e., <18 y of age), case reports, case series, and studies of interventions in which only short-term outcomes were evaluated (e.g., changes in blood pressure) were excluded. We excluded cross-sectional studies and prospective studies that only evaluated changes in biomarker levels, usually after ART initiation. Conference abstracts and publications in languages other than English were also excluded, as we were not able to fully assess these studies.
Study data extraction and analysis
For each of the selected studies, we extracted the biomarkers evaluated, study design, patient population, duration of follow-up, and details of the outcomes and findings. Results were tabulated for each type of biomarker (i.e., endothelial activation and coagulation) and compared across studies where appropriate. Due to broad study heterogeneity and disparate outcomes, we did not attempt to numerically combine or perform a metaanalysis of study results.
Conclusions
This systematic review of the published literature demonstrates that several biomarkers reflecting endothelial activation and coagulation, including d-dimer, P-selectin, and tPA, may represent potentially useful biomarkers for the prediction of clinical outcomes in HIV-infected patients. The clinical utility of these biomarkers is limited by the paucity and inconsistency of available evidence, the lack of standardized approaches to biomarker testing and assessment, and the lack of prospective validation in representative patient populations. Due to the increased risk of morbid outcomes in HIV-infected patients, additional large-scale prospective investigations to determine the utility of the most promising endothelial activation and coagulation biomarkers to stratify risk and predict adverse outcomes are clearly warranted. Such research has the potential to elucidate mechanisms of endothelial injury and has the potential to identify specific treatments aimed at reducing endothelial activation or reducing risk of thrombosis.
Supplementary Material
Disclosure of Potential Conflicts of Interest
WCL is listed as a co-inventor on a patent applied for by the University Health Network (Toronto, ON Canada) to develop point-of-care tests for endothelial activation biomarkers in infectious diseases. All other authors report no conflict of interest.
Acknowledgments
Special thanks go to Sherry A Dodson, Clinical Librarian, University of Washington Health Sciences Library for her assistance with our search strategies. This work was supported by a New Investigator Award to SMG from the University of Washington Center for AIDS Research, which is funded by the National Institutes of Health (NIH) (P30 AI027757) and is supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Institute of Mental Health; National Institute on Drug Abuse; National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; and National Institute on Aging; by the NIH (AI-58698 and AI-38518); and by a Canada Research Chair in Infectious Diseases and Inflammation to WCL from the Canadian Institutes for Health Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Centers for Disease Control and Prevention (CDC). . HIV surveillance--United States, 1981-2008. MMWR Morb Mortal Wkly Rep 2011; 60:689 - 93; PMID: 21637182 [PubMed] [Google Scholar]
- 2.Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, et al. . The survival benefits of AIDS treatment in the United States. J Infect Dis 2006; 194:11 - 9; http://dx.doi.org/ 10.1086/505147; PMID: 16741877 [DOI] [PubMed] [Google Scholar]
- 3.Phillips AN, Neaton J, Lundgren JD. . The role of HIV in serious diseases other than AIDS. AIDS 2008; 22:2409 - 18; http://dx.doi.org/ 10.1097/QAD.0b013e3283174636; PMID: 19005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lifson AR, Belloso WH, Carey C, Davey RT, Duprez D, El-Sadr WM, et al. , INSIGHT Cause of Death Writing Group. . Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials 2008; 9:177 - 85; http://dx.doi.org/ 10.1310/hct0903-177; PMID: 18547904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG, Phillips AN. . HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009; 338:a3172; http://dx.doi.org/ 10.1136/bmj.a3172; PMID: 19171560 [DOI] [PubMed] [Google Scholar]
- 6.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. , Swiss HIV Cohort Study. . Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130 - 9; http://dx.doi.org/ 10.1093/cid/cir626; PMID: 21998280 [DOI] [PubMed] [Google Scholar]
- 7.Nixon DE, Landay AL. . Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS 2010; 5:498 - 503; http://dx.doi.org/ 10.1097/COH.0b013e32833ed6f4; PMID: 20978393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubé MP, Sattler FR. . Inflammation and complications of HIV disease. J Infect Dis 2010; 201:1783 - 5; http://dx.doi.org/ 10.1086/652751; PMID: 20446849 [DOI] [PubMed] [Google Scholar]
- 9.Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M, Swiss HIV Cohort Study. . Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis 2002; 185:456 - 62; http://dx.doi.org/ 10.1086/338572; PMID: 11865397 [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. , INSIGHT SMART Study Group. . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203; http://dx.doi.org/ 10.1371/journal.pmed.0050203; PMID: 18942885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. . Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS 2009; 23:1841 - 9; http://dx.doi.org/ 10.1097/QAD.0b013e32832d3b85; PMID: 19455012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law MG, Friis-Møller N, El-Sadr WM, Weber R, Reiss P, D’Arminio Monforte A, et al. , D:A:D Study Group. . The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med 2006; 7:218 - 30; http://dx.doi.org/ 10.1111/j.1468-1293.2006.00362.x; PMID: 16630034 [DOI] [PubMed] [Google Scholar]
- 13.Fourie C, van Rooyen J, Pieters M, Conradie K, Hoekstra T, Schutte A. . Is HIV-1 infection associated with endothelial dysfunction in a population of African ancestry in South Africa?. Cardiovasc J Afr 2011; 22:134 - 40; http://dx.doi.org/ 10.5830/CVJA-2010-056; PMID: 21713302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong EJ, Morrow DA, Sabatine MS. . Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines. Circulation 2006; 113:e72 - 5; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.105.595520; PMID: 16476853 [DOI] [PubMed] [Google Scholar]
- 15.Armstrong EJ, Morrow DA, Sabatine MS. . Inflammatory biomarkers in acute coronary syndromes: part II: acute-phase reactants and biomarkers of endothelial cell activation. Circulation 2006; 113:e152 - 5; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.105.595538; PMID: 16490825 [DOI] [PubMed] [Google Scholar]
- 16.Armstrong EJ, Morrow DA, Sabatine MS. . Inflammatory biomarkers in acute coronary syndromes: part III: biomarkers of oxidative stress and angiogenic growth factors. Circulation 2006; 113:e289 - 92; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.105.595546; PMID: 16505179 [DOI] [PubMed] [Google Scholar]
- 17.Calza L, Pocaterra D, Pavoni M, Colangeli V, Manfredi R, Verucchi G, et al. . Plasma levels of VCAM-1, ICAM-1, E-Selectin, and P-Selectin in 99 HIV-positive patients versus 51 HIV-negative healthy controls. J Acquir Immune Defic Syndr 2009; 50:430 - 2; http://dx.doi.org/ 10.1097/QAI.0b013e31819a292c; PMID: 19322038 [DOI] [PubMed] [Google Scholar]
- 18.Gattegno L, Bentata-Peyssare M, Gronowski S, Chaouche K, Ferriere F. . Elevated concentrations of circulating intercellular adhesion molecule 1 (ICAM-1) and of vascular cell adhesion molecule 1 (VCAM-1) in HIV-1 infection. Cell Adhes Commun 1995; 3:179 - 85; http://dx.doi.org/ 10.3109/15419069509081285; PMID: 8846020 [DOI] [PubMed] [Google Scholar]
- 19.Lichtner M, Cuomo MR, Rossi R, Strano S, Massetti AP, Mastroianni CM, et al. . Increased carotid intima media thickness is associated with depletion of circulating myeloid dendritic cells in HIV-infected patients on suppressive antiretroviral treatment. Atherosclerosis 2009; 204:e1 - 3; http://dx.doi.org/ 10.1016/j.atherosclerosis.2008.12.025; PMID: 19185298 [DOI] [PubMed] [Google Scholar]
- 20.Bernal E, Marín I, Muñoz A, Sabán J, Vicente-Vera T, Cano A. . High prevalence of subclinical atherosclerotic disease in Spanish HIV-infected patients with low cardiovascular risk. AIDS Patient Care STDS 2011; 25:269 - 72; http://dx.doi.org/ 10.1089/apc.2011.0014; PMID: 21466379 [DOI] [PubMed] [Google Scholar]
- 21.Kline ER, Sutliff RL. . The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J Investig Med 2008; 56:752 - 69; PMID: 18525451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlachopoulos C, Xaplanteris P, Sambatakou H, Mariolis E, Bratsas A, Christoforatou E, et al. . Acute systemic inflammation induced by influenza A (H1N1) vaccination causes a deterioration in endothelial function in HIV-infected patients. HIV Med 2011; 12:594 - 601; http://dx.doi.org/ 10.1111/j.1468-1293.2011.00935.x; PMID: 21645196 [DOI] [PubMed] [Google Scholar]
- 23.Ucciferri C, Falasca K, Mancino P, Di Iorio A, Vecchiet J. . Microalbuminuria and hypertension in HIV-infected patients: a preliminary study of telmisartan. Eur Rev Med Pharmacol Sci 2012; 16:491 - 8; PMID: 22696876 [PubMed] [Google Scholar]
- 24.Hileman CO, Carman TL, Gripshover BM, O’Riordan M, Storer NJ, Harrill DE, et al. . Salsalate is poorly tolerated and fails to improve endothelial function in virologically suppressed HIV-infected adults. AIDS 2010; 24:1958 - 61; http://dx.doi.org/ 10.1097/QAD.0b013e32833c3251; PMID: 20613460 [DOI] [PubMed] [Google Scholar]
- 25.Gupta SK, Johnson RM, Mather KJ, Clauss M, Rehman J, Saha C, et al. . Anti-inflammatory treatment with pentoxifylline improves HIV-related endothelial dysfunction: a pilot study. AIDS 2010; 24:1377 - 80; http://dx.doi.org/ 10.1097/QAD.0b013e3283396024; PMID: 20559042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tungsiripat M, El-Bejjani D, Rizk N, Dogra V, O’Riordan MA, Ross AC, et al. . Carotid intima media thickness, inflammatory markers, and endothelial activation markers in HIV Patients with lipoatrophy increased at 48 weeks regardless of use of rosiglitazone or placebo. AIDS Res Hum Retroviruses 2011; 27:295 - 302; http://dx.doi.org/ 10.1089/aid.2010.0187; PMID: 20969457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vonderen MG, Hassink EA, van Agtmael MA, Stehouwer CD, Danner SA, Reiss P, et al. . Increase in carotid artery intima-media thickness and arterial stiffness but improvement in several markers of endothelial function after initiation of antiretroviral therapy. J Infect Dis 2009; 199:1186 - 94; http://dx.doi.org/ 10.1086/597475; PMID: 19275490 [DOI] [PubMed] [Google Scholar]
- 28.Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, et al. , INSIGHT Strategies for Management of Antiretroviral Therapy (SMART) Study Group. . Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis 2009; 200:973 - 83; http://dx.doi.org/ 10.1086/605447; PMID: 19678756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. . Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010; 24:1509 - 17; http://dx.doi.org/ 10.1097/QAD.0b013e32833ad914; PMID: 20505494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musselwhite LW, Sheikh V, Norton TD, Rupert A, Porter BO, Penzak SR, et al. . Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS 2011; 25:787 - 95; http://dx.doi.org/ 10.1097/QAD.0b013e3283453fcb; PMID: 21412059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sipsas NV, Sfikakis PP, Touloumi G, Pantazis N, Choremi H, Kordossis T. . Elevated serum levels of soluble immune activation markers are associated with increased risk for death in HAART-naive HIV-1-infected patients. AIDS Patient Care STDS 2003; 17:147 - 53; http://dx.doi.org/ 10.1089/108729103321619755; PMID: 12737638 [DOI] [PubMed] [Google Scholar]
- 32.Francisci D, Falcinelli E, Belfiori B, Petito E, Fierro T, Baldelli F, et al. . Impact of tenofovir versus abacavir on HIV-related endothelial dysfunction. AIDS Patient Care STDS 2011; 25:567 - 9; http://dx.doi.org/ 10.1089/apc.2011.0184; PMID: 21851265 [DOI] [PubMed] [Google Scholar]
- 33.Jong E, Louw S, van Gorp EC, Meijers JC, ten Cate H, Jacobson BF. . The effect of initiating combined antiretroviral therapy on endothelial cell activation and coagulation markers in South African HIV-infected individuals. Thromb Haemost 2010; 104:1228 - 34; http://dx.doi.org/ 10.1160/TH10-04-0233; PMID: 20886182 [DOI] [PubMed] [Google Scholar]
- 34.Aukrust P, Bjørnsen S, Lunden B, Otterdal K, Ng EC, Ameln W, et al. . Persistently elevated levels of von Willebrand factor antigen in HIV infection. Downregulation during highly active antiretroviral therapy. Thromb Haemost 2000; 84:183 - 7; PMID: 10959687 [PubMed] [Google Scholar]
- 35.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. , VACS Project Team. . Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV?. Clin Infect Dis 2012; 54:984 - 94; http://dx.doi.org/ 10.1093/cid/cir989; PMID: 22337823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schved JF, Gris JC, Arnaud A, Martinez P, Sanchez N, Wautier JL, et al. . von Willebrand factor antigen, tissue-type plasminogen activator antigen, and risk of death in human immunodeficiency virus 1-related clinical disease: independent prognostic relevance of tissue-type plasminogen activator. J Lab Clin Med 1992; 120:411 - 9; PMID: 1517688 [PubMed] [Google Scholar]
- 37.Gresele P, Falcinelli E, Sebastiano M, Baldelli F. . Endothelial and platelet function alterations in HIV-infected patients. Thromb Res 2012; 129:301 - 8; http://dx.doi.org/ 10.1016/j.thromres.2011.11.022; PMID: 22192157 [DOI] [PubMed] [Google Scholar]
- 38.Rectenwald JE, Myers DD Jr., Hawley AE, Longo C, Henke PK, Guire KE, et al. . D-dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost 2005; 94:1312 - 7; PMID: 16411411 [DOI] [PubMed] [Google Scholar]
- 39.Suy A, Martínez E, Coll O, Lonca M, Palacio M, de Lazzari E, et al. . Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS 2006; 20:59 - 66; http://dx.doi.org/ 10.1097/01.aids.0000198090.70325.bd; PMID: 16327320 [DOI] [PubMed] [Google Scholar]
- 40.Graham SM, Rajwans N, Jaoko W, et al. . Endothelial activation biomarkers increase after HIV-1 acquisition: plasma VCAM-1 predicts disease progression. AIDS 2013; In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham SM, Rajwans N, Tapia KA, Jaoko W, Estambale BB, McClelland RS. . A prospective study of endothelial activation biomarkers, including plasma angiopoietin-1 and angiopoietin-2, in Kenyan women initiating antiviral therapy. BMC Infect Dis 2013; 13:263; http://dx.doi.org/ 10.1186/1471-2334-13-263; PMID: 23734875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozturk B, Tutuncu E, Kuscu F, Gurbuz Y, Sencan I, Tuzun H. . Evaluation of factors predictive of the prognosis in Crimean-Congo hemorrhagic fever: new suggestions. Int J Infect Dis 2012; 16:e89 - 93; http://dx.doi.org/ 10.1016/j.ijid.2011.06.005; PMID: 22154082 [DOI] [PubMed] [Google Scholar]
- 43.Zangerle R, Fuchs D, Sarcletti M, Gallati H, Reibnegger G, Wachter H, et al. . Increased concentrations of soluble tumor necrosis factor receptor 75 but not of soluble intercellular adhesion molecule-1 are associated with the decline of CD4+ lymphocytes in HIV infection. Clin Immunol Immunopathol 1994; 72:328 - 34; http://dx.doi.org/ 10.1006/clin.1994.1149; PMID: 7914841 [DOI] [PubMed] [Google Scholar]
- 44.Greenwood AJ, Hughes J, Wallace G, Seed P, Stanford MR, Graham EM. . Soluble intercellular adhesion molecule-1 (sICAM-1) and vascular cell adhesion molecule-1 (sVCAM-1) in patients with HIV/AIDS does not appear to correlate with cytomegalovirus retinitis. Int J STD AIDS 1998; 9:713 - 4; PMID: 9863589 [PubMed] [Google Scholar]
- 45.Renwick N, Weverling GJ, Brouwer J, Bakker M, Schulz TF, Goudsmit J. . Vascular endothelial growth factor levels in serum do not increase following HIV type 1 and HHV8 seroconversion and lack correlation with AIDS-related Kaposi’s sarcoma. AIDS Res Hum Retroviruses 2002; 18:695 - 8; http://dx.doi.org/ 10.1089/088922202760072302; PMID: 12167275 [DOI] [PubMed] [Google Scholar]
- 46.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, et al. . Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004; 109:1603 - 8; http://dx.doi.org/ 10.1161/01.CIR.0000124480.32233.8A; PMID: 15023877 [DOI] [PubMed] [Google Scholar]
- 47.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. , Strategies for Management of Antiretroviral Therapy (SMART) Study Group. . CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283 - 96; http://dx.doi.org/ 10.1056/NEJMoa062360; PMID: 17135583 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.