Abstract
Islet inflammation promotes β-cell loss and type 2 diabetes (T2D), a process replicated in Zucker Diabetic Fatty (ZDF) rats in which β-cell loss has been linked to cannabinoid-1 receptor (CB1R)–induced proinflammatory signaling in macrophages infiltrating pancreatic islets. Here, we analyzed CB1R signaling in macrophages and its developmental role in T2D. ZDF rats with global deletion of CB1R are protected from β-cell loss, hyperglycemia, and nephropathy that are present in ZDF littermates. Adoptive transfer of CB1R−/− bone marrow to ZDF rats also prevents β-cell loss and hyperglycemia but not nephropathy. ZDF islets contain elevated levels of CB1R, interleukin-1β, tumor necrosis factor-α, the chemokine CCL2, and interferon regulatory factor-5 (IRF5), a marker of inflammatory macrophage polarization. In primary cultured rodent and human macrophages, CB1R activation increased Irf5 expression, whereas knockdown of Irf5 blunted CB1R-induced secretion of inflammatory cytokines without affecting CCL2 expression, which was p38MAPKα dependent. Macrophage-specific in vivo knockdown of Irf5 protected ZDF rats from β-cell loss and hyperglycemia. Thus, IRF5 is a crucial downstream mediator of diabetogenic CB1R signaling in macrophages and a potential therapeutic target.
Introduction
Obesity is a risk factor for insulin resistance (IR), which can lead to progressive dysfunction and loss of pancreatic β-cells resulting in overt type 2 diabetes (T2D) (1,2), although β-cell dysfunction may arise independently, as it can precede the onset of IR (3). Adipose tissue inflammation plays a critical role in obesity-related IR (4), and a similar process associated with inflammatory cell infiltration in the endocrine pancreas has been linked to β-cell loss and the development of T2D (5).
Endocannabinoids (ECs) are endogenous ligands of cannabinoid receptors (cannabinoid-1 receptor [CB1R] and cannabinoid-2 receptor) that also mediate the effects of marijuana (6). The EC/CB1R system is overactive in obesity/metabolic syndrome (7,8), and blockade or genetic deletion of CB1R mitigates diet-induced obesity and its metabolic complications, including IR and T2D (reviewed by Mazier et al. [9]). CB1R blockade has similar beneficial effects in people with metabolic syndrome (10) or T2D (11) but can cause psychiatric side effects due to blocking CB1R in the central nervous system. ECs can inhibit hepatic insulin sensitivity via CB1R in the central nervous system (12) but can also inhibit insulin signaling directly via CB1R in adipose tissue (13), skeletal muscle (14), liver (15), and adipose tissue macrophages (16), and these latter targets account for the efficacy of peripherally restricted CB1R antagonists in mitigating IR (17,18).
Macrophage CB1Rs also play a prominent role in the progressive loss of β-cell function in Zucker Diabetic Fatty (ZDF) rats, a rodent model of T2D. The pancreatic islets of adult ZDF rats have reduced numbers of β-cells and are heavily infiltrated with proinflammatory macrophages expressing high levels of CB1R and the Nlrp3/Asc inflammasome (19). Peripheral CB1R blockade, macrophage depletion, or macrophage-specific knockdown of CB1R prevented these changes and preserved normoglycemia (19), which further illustrates the anti-inflammatory effect of CB1R blockade. Chronic CB1R blockade promotes a shift in the polarization of macrophages from proinflammatory to anti-inflammatory (19) and also reduces macrophage infiltration of diabetic islets by inhibiting the secretion of MCP-1 (or CCL2) (19,20). ZDF rats also develop severe diabetic nephropathy associated with a loss of glomerular podocytes without significant macrophage infiltration or increase in Nlrp3/ASC inflammasome expression in the kidney (21).
Together, these findings raise questions of whether there is an obligatory role for ECs in the development of T2D and its renal complication in the ZDF model. To this end, we have generated CB1R-deficient rats on a ZDF background (ZDF-Cnr1 rats) and analyzed glycemic functions and renal parameters, as well as their modulation by adoptive bone marrow (BM) transfer. Our results indicate the obligatory requirement for peripheral CB1Rs in both T2D and diabetic nephropathy, with CB1R in BM-derived cells required for β-cell loss and the development of hyperglycemia, but not for podocyte loss and the resulting nephropathy.
Interferon regulatory factor-5 (IRF5) was recently implicated in polarizing macrophages toward the inflammatory phenotype (22), whereas mice with global or macrophage-specific deletion of Irf5 that were maintained on a high-fat diet remain insulin sensitive and display beneficial expansion of subcutaneous adipose tissue (23). Because of the unexpected similar expansion of subcutaneous but not visceral fat tissue observed in ZDF-Cnr1 rats, we explored the involvement of IRF5 in β-cell loss via CB1R-mediated inflammatory signaling. Here we report that IRF5 mediates CB1R-induced cytokine secretion and the resulting β-cell loss, whereas CB1R-induced CCL2 production and macrophage transmigration is independent of IRF5 and involves activation of the α-isoform of p38 mitogen-activated protein kinase (p38MAPK).
Research Design and Methods
Animals
Animal protocols were approved by our institutional animal care and use committee. Male ZDF rats and their lean controls were obtained from Charles River Laboratories, housed individually under a 12-h light/dark cycle, and fed ad libitum a standard laboratory diet (STD; NIH-31 Rodent Diet).
Generation and Characterization of ZDF-Cnr1 Rats
A pair of zinc finger nucleases (Sigma-Aldrich, St. Louis, MO) was designed to cleave within the coding region of the Cnr1 gene, with the target site 5′-TACCACTTCATCGGCAGCctggcaGTGGCCGACCTCCTG-3′ (zinc finger nuclease binding site set in capital letters). Identified founders carried an 11–base pair (BP) deletion located between T17193 to C17203 in the genomic DNA sequence.
Genotyping
Cnr1 and Fa genes were amplified as described in Supplementary Table 2.
Drugs and Chemicals
JD5037 was synthesized and its pharmacological properties analyzed as described previously (18). CP-55,940 was obtained from the NIDA Drug Supply Program (Research Triangle Park, NC). N-arachidonoylethanolamine (or anandamide [AEA]), arachidonoyl-2′-chloroethylamine (ACEA) and SP600125 were purchased from Cayman (Ann Arbor, MI). SB202190 was from Calbiochem (La Jolla, CA). All other chemicals were from Sigma-Aldrich.
Serum and Urine Parameters
Blood glucose levels were determined using the Elite Glucometer (Bayer, Pittsburgh, PA). Serum alanine aminotransferase (ALT), aspartate aminotransferase, free fatty acid (FFA), total cholesterol, triglycerides, insulin, proinsulin, C-peptide, glucagon, leptin, and adiponectin were quantified as described previously (19). IGF-I content was quantified with a Mouse/Rat IGF-I ELISA Kit (R&D Systems, Minneapolis, MN), whereas growth hormone was detected using a Rat/Mouse ELISA Kit (Millipore, Billerica, MA). Serum and urine creatinine, urea, and albumin concentrations and glomerular filtration rate were determined as described previously (21).
Glucose Tolerance, Insulin Sensitivity Tests, HOMA-IR, and Glucose-Stimulated Insulin Secretion
Glucose tolerance and insulin sensitivity tests were performed as described previously (24). HOMA-IR was calculated as follows: fasting insulin (μU/mL) × fasting glucose (mg/dL)/405 (25). Glucose-stimulated insulin secretion (GSIS) was determined as described previously (19).
GTPγS Binding
[35S]GTPγS binding assay was performed as described (26).
Whole-Body Irradiation and BM Transfer
Whole-body irradiation was conducted as described previously (27). Six-week-old male ZDF rats received 1 Gy total body irradiation from a 137Cs source. BM cells from ZDF and ZDF-Cnr1 donor rats were obtained as described previously (27). After a 2-h rest after irradiation, recipient rats were injected with 108 BM cells via a tail vein. Animals were then rested for 2 weeks, and survivors with proper chimerism were used for experiments.
Determination of Chimerism
DNA was isolated from blood using a DNeasy kit (69504; Qiagen, Germantown, MD). Cnr1 expression was detected using the genotyping protocol.
Blood Cell Counts
Blood samples were analyzed in an automated Hemavet blood analyzer (Drew Scientific Group, Miami Lakes, FL).
Histology and Immunohistochemistry
Pancreas, kidney, and adipose tissue were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned (4-μm sections) onto glass slides. Renal and adipose tissue histology were evaluated after hematoxylin-eosin and periodic acid Schiff staining. The antibodies used are listed in Supplementary Table 3. Slides were revealed by using the appropriate Elite ABC HRP (horseradish peroxidase)/diaminobenzediene (DAB) system (Vector Laboratories), counterstained with hematoxylin-eosin (Gills Formula; Vector Laboratories), or by secondary antibodies coupled to Alexa Fluor 488, 555, or 647. DAB slides were analyzed using an Olympus BX41 microscope, whereas fluorescent staining was analyzed using a Zeiss LSM700 confocal microscope. Immunopositivity was quantified using ImageJ software. Adipocyte diameters were evaluated digitally in light microscopy images of adipose tissue sections (n = 6–7 pictures per animal, three animals per group) using ImageJ software.
TUNEL Staining
TUNEL staining in pancreatic islet was performed using the Click-it TUNEL Alexa Fluor Imaging Assay from ThermoFisher Scientific (C10245).
Isolation of Pancreatic Islets, β-Cell Mass
Islets were isolated, and β-cell mass was determined as described previously (19).
Islets Inflammation
Caspase-1 activity was determined using the Caspase-1 Assay Kit (Fluorometric) from Abcam (ab39412); interleukin (IL)-1β and tumor necrosis factor-α (TNF-α) proteins were measured using the Rat Quantikine ELISA Kit (R&D Systems).
Cell Culture
Human monocytic THP-1 cells were grown according to the recommendation of the American Type Culture Collection (ATCC). Cells were differentiated at 1.5–2 × 106 cells/well (35 mm) with 50 ng/mL phorbol myristic acid (P8139; Sigma-Aldrich) for 4 days. THP-1–derived macrophages were incubated in RPMI 1640 free of phorbol myristic acid, supplemented with 10% FBS (30–2020; ATCC), 50 μg/mL streptomycin, and 50 units/mL penicillin (30–2300; ATCC) for 24 h at 37°C, under an atmosphere of 5% CO2 in air prior to treatment. IL-1β, IL-18, IL-6, CCL2, and TNF-α protein concentrations were determined using ELISA kits from R&D Systems according to the manufacturer protocol.
Small Interfering RNA Gene Knockdown
Aliquots of peritoneally elicited macrophages (PEC) or THP-1 cells were exposed for 48 h to siRNA listed in Supplementary Table 4. Control siRNAs were designed using the C911 technique (28).
Preparation and In Vivo Administration of Glucan-Encapsulated siRNA Particles
Fluorescein isothiocyanate–labeled glucan shells were prepared as described previously (29), and 8-week-old ZDF rats were then injected intraperitoneally every other day for 12 days with 6.17 mg/kg glucan-encapsulated siRNA particles (GeRPs) loaded with 308 nmol/kg endoporter and 0.410 mg/kg siRNA (Supplementary Table 4).
Immunoblotting
Cells were treated with pertussis toxin (PTX) 100 ng/mL, SB202190 25 μmol/L, SP600125 25 μmol/L, JD5037 100 nmol/L, and AEA and ACEA 1–10 μmol/L. Western blotting analyses were conducted as described previously (30), and antibodies used are listed in Supplementary Table 5.
Real-Time PCR
Total RNA extraction, reverse transcription, and real-time PCR were performed as described previously (19).
Statistics
If not otherwise specified, values are expressed as the mean ± SEM. Data were analyzed by Student t test or by one-way ANOVA followed by the Tukey-Kramer post hoc test. Time-dependent variables were analyzed, and results in multiple groups were compared by two-way ANOVA followed by Bonferroni test. Significance was set at P < 0.05.
Results
Generation and Characterization of Cnr1−/− Rats on a ZDF Background
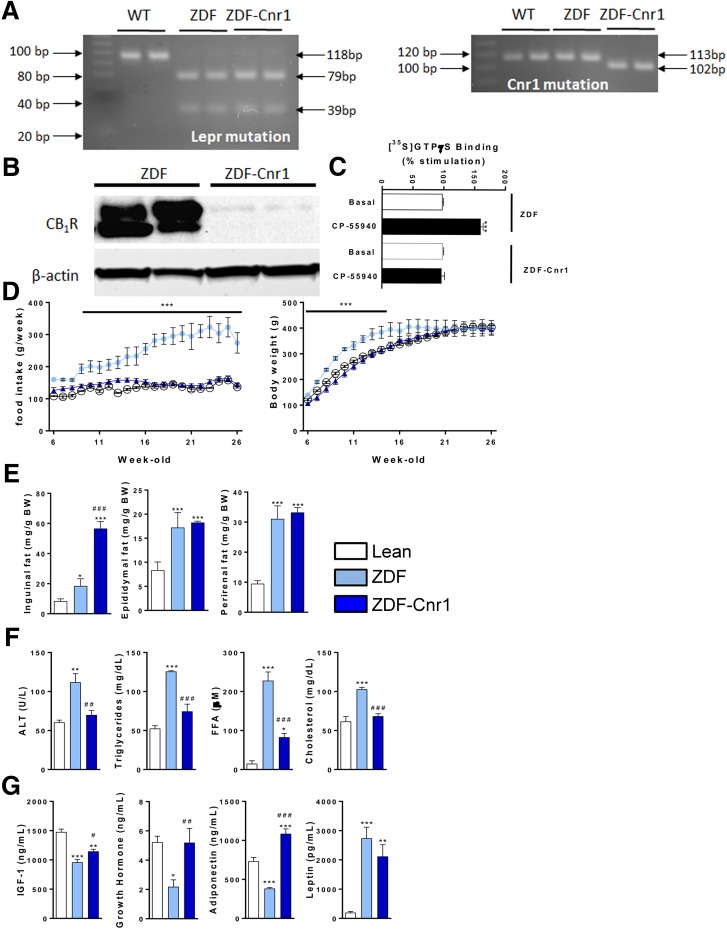
ZDF rats with a global knockout of CB1R (ZDF-Cnr1 rats) retained the mutation of the leptin receptor gene characteristic of Zucker fatty rats and ZDF rats and had an 11-BP deletion in the Cnr1 genomic DNA, as demonstrated by genotyping (Fig. 1A). Of note, the Cnr1 mutation led to a frameshift in the open reading frame resulting in a premature stop codon. As a result, CB1R protein was undetectable in the brain of ZDF-Cnr1 rats (Fig. 1B). In addition, the CB1R agonist CP-55940 increased [35S]GTPγS binding in plasma membranes prepared from ZDF but not from ZDF-Cnr1 brains (Fig. 1C), confirming the absence of CB1R signaling in the knockouts. ZDF-Cnr1 rats displayed lower food intake throughout the observation period and a slower rate of weight gain compared with their ZDF littermates but reached a similar body weight by the age of 26 weeks (Fig. 1D). Interestingly, the inguinal fat mass (often referred to as subcutaneous fat) was significantly larger in ZDF-Cnr1 than in ZDF rats, whereas the epididymal and perirenal fat pads, representing visceral fat, were of similar size (Fig. 1E). This was associated with a striking increase in the diameter of inguinal adipocytes in ZDF-Cnr1 versus ZDF rats, whereas an analogous difference in the size of perirenal adipocytes was much smaller (Supplementary Fig. 1).
Figure 1.
Characterization of ZDF-Cnr1 rats. A: Representative gel analysis for Lepr mutation and Cnr1 mutation. B: CB1R immunoblot from a ZDF vs. ZDF-Cnr1 brain. C: [35S]GTPγS binding assay using membranes from ZDF vs. ZDF-Cnr1 brains; columns and bars represent the mean ± SEM from five separate preparations. ***P < 0.005 relative basal activity. D: Daily food intake and body weight measurements from 6 to 26 weeks of age in lean controls, ZDF rats, and ZDF-Cnr1 rats. Points and bars represent the mean ± SEM from lean control (open circles), ZDF (light blue circles), and ZDF-Cnr1 (blue triangles) rats. E: Weight of inguinal, epididymal, and perirenal fat pads in lean (open columns, n = 10), ZDF (light blue, n = 10), and ZDF-Cnr1 rats (blue, n = 10). F: Serum ALT, triglyceride, FFA, and total cholesterol levels. G: Serum concentration of IGF-I, growth hormone, adiponectin, and leptin levels. Columns and bars represent the mean ± SEM. Significant differences from values in lean controls (*) or ZDF rats (#), * or #P < 0.05, ** or ##P < 0.01, *** or ###P < 0.001. BW, body weight; WT, wild type.
The elevated serum levels of ALT, triglycerides, FFAs, and total cholesterol in ZDF rats were normalized in ZDF-Cnr1 rats (Fig. 1F). Additionally, the absence of Cnr1 resulted in normalization of the reduced plasma levels of growth hormone and IGF-I observed in ZDF rats (Fig. 1G). The hypoadiponectinemia of ZDF rats was reversed beyond the levels in lean controls, whereas the hyperleptinemia was unaffected by the deletion of Cnr1 (Fig. 1G).
β-Cell Function Is Preserved and Hyperglycemia Prevented by Cnr1 Deletion
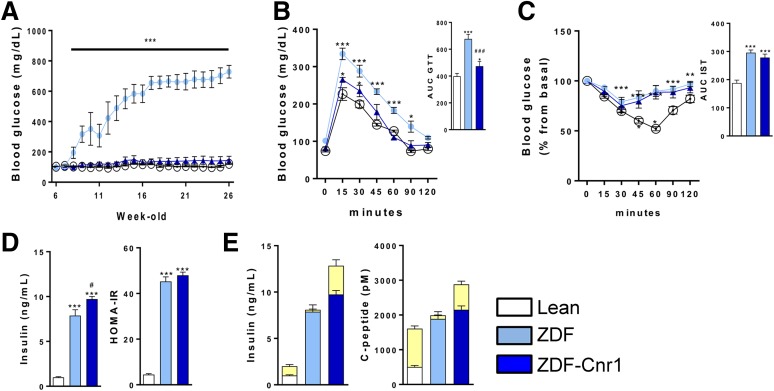
ZDF rats develop extreme hyperglycemia due to β-cell loss by the age of 12–14 weeks (19). In contrast, ZDF-Cnr1 rats remained euglycemic for up to 26 weeks of age (Fig. 2A). At 8 weeks (i.e., before hyperglycemia starts to develop in ZDF rats), ZDF-Cnr1 rats were more glucose tolerant than ZDF rats (Fig. 2B). In contrast, the two strains displayed equal IR in the insulin sensitivity test (Fig. 2C), as is also reflected by the similar degree of hyperinsulinemia and similar increases in HOMA-IR (Fig. 2D). Despite their similar IR, ZDF-Cnr1 rats have improved β-cell function, as reflected in the preserved GSIS, whereas ZDF rats were nonresponsive to a glucose challenge (Fig. 2E).
Figure 2.
Glycemic control and β-cell status in lean control, ZDF, and ZDF-Cnr1 rats. A: Evolution of blood glucose from 6 to 26 weeks of age in lean (open circles), ZDF (light blue circles), and ZDF-Cnr1 (blue triangles) rats. B: Glucose tolerance tests conducted at 8 weeks of age, as described in research design and methods. C: Insulin sensitivity tests (ISTs) at 8 weeks, as described in research and design methods. D: Serum insulin and HOMA-IR at 8 weeks. E: GSIS indicated by the yellow segments, determined at 8 weeks, as described in research design and methods. Columns and bars represent mean ± SEM (n = 8–10 rats/group). Significant difference from corresponding value in lean (white bars) (*P < 0.05, **P < 0.01, ***P < 0.001) or ZDF rats (light blue bars) (#P < 0.05, ###P < 0.001). AUC, area under the curve.
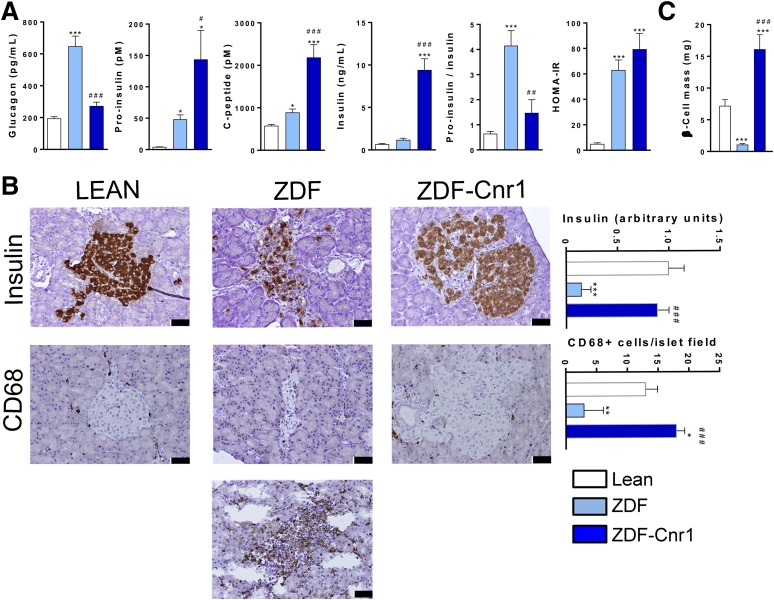
ZDF-Cnr1 rats exhibited normal plasma levels of glucagon in contrast to the hyperglucagonemia of ZDF rats (Fig. 3A), which may contribute to the improved glucose tolerance in the presence of IR in ZDF-Cnr1 rats. Also, plasma proinsulin, insulin, and C-peptide levels were higher whereas the proinsulin/insulin ratio was significantly lower in ZDF-Cnr1 than in ZDF littermates (Fig. 3A), which is indicative of improved β-cell function and survival. The protection of β-cells in ZDF-Cnr1 rats was further confirmed by the higher islet insulin content (Fig. 3B). Of note, the strong infiltration of islets with CD68+ macrophages previously observed in younger, 14-week-old ZDF rats (19) was absent by the age of 26 weeks (Fig. 3B), probably due to the nearly complete destruction of islets by this age. Nevertheless, in a few remaining identifiable islets we could still observe strong infiltration of CD68+ macrophages (Fig. 3B, third row). In contrast, ZDF-Cnr1 rats maintained an almost normal islet structure without prominent CD68+ macrophage infiltration and had significantly larger β-cell mass than ZDF littermates (Fig. 3C). The higher β-cell mass inversely correlated with β-cell death, as reflected by the increased number of TUNEL-positive cells in ZDF compared with ZDF-Cnr1 islets (Supplementary Fig. 2A).
Figure 3.
Hormonal parameters and β-cell status in lean, ZDF, and ZDF-Cnr1 rats. A: Serum hormone levels, proinsulin/insulin ratio, and HOMA-IR, determined at 26 weeks of age in lean (open), ZDF (light blue), and ZDF-Cnr1 rats (blue). B: Islet insulin and CD68+ cell content determined by immunohistochemistry after killing of the rats at 26 weeks. The bottom (seventh) panel illustrates a rare remaining islet heavily infiltrated by CD68+ cells. C: β-Cell mass, determined as described in research design and methods. Columns and bars are the mean ± SEM from 8–12 animals/group. Scale bar, 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001 compared to lean control; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to ZDF rats.
ZDF-Cnr1 Rats Are Protected From Diabetic Nephropathy
CB1R is a major effector in the development of diabetic nephropathy, and treatment of ZDF rats with a peripheral CB1R antagonist prevented or reversed this complication (21). Diabetic nephropathy, evident in 26-week-old ZDF rats by robust deterioration in renal parameters, was largely absent in ZDF-Cnr1 rats, as reflected in normalization of the albuminuria, glomerular filtration rate, and blood urea nitrogen (Supplementary Fig. 3A). Correspondingly, ZDF-Cnr1 rats were protected from podocyte loss, as demonstrated by Wilms tumor 1 immunostaining (Supplementary Fig. 3B). Furthermore, ZDF rats displayed glomerular enlargement and early mesangial matrix expansion that were absent in lean control or ZDF-Cnr1 rats. The pronounced albuminuria of ZDF rats was associated with prominent tubular protein resorption droplets and occasional tubular dilatation with proteinaceous casts. Again, no such changes were observed in ZDF-Cnr1 rats (Supplementary Fig. 3C).
CB1R in Myeloid Cells Drive β-Cell Loss and Hyperglycemia but Not Nephropathy or Dyslipidemia
Since macrophages are derived from BM myeloid precursors, we used the irradiation-BM transplantation (BMT) approach to test whether the lifelong β-cell protection in ZDF-Cnr1 rats is due to the absence of CB1R in macrophages.
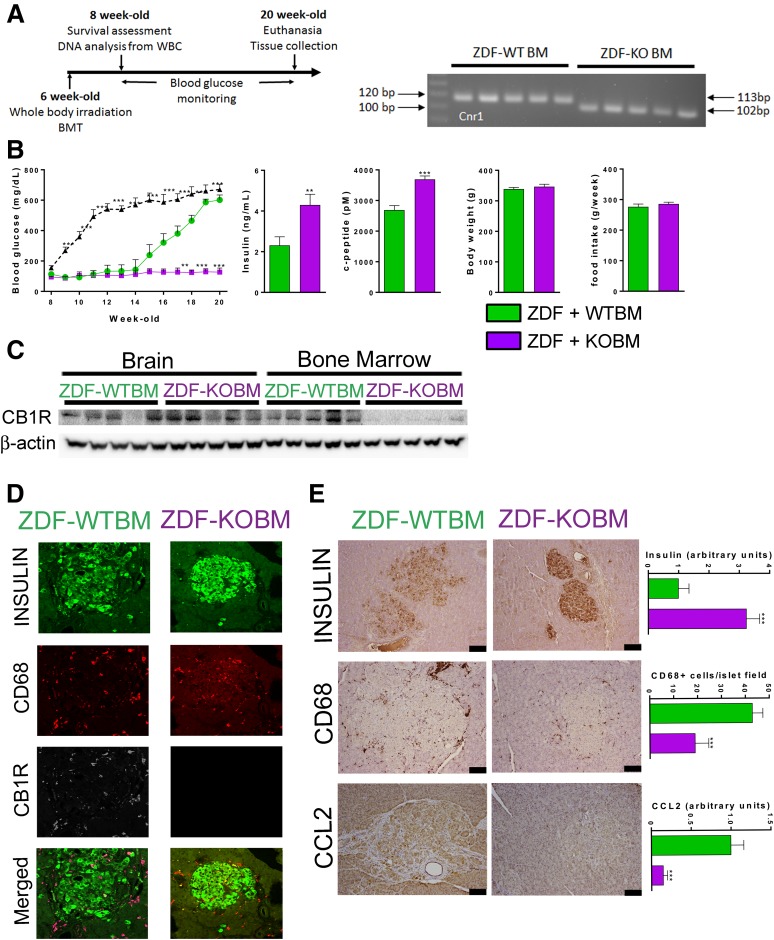
For total body radiation exposure from a 137Cs source, 1 Gy was the dose high enough to eliminate all circulating white blood cells without being lethal for at least 7 days after irradiation (Supplementary Fig. 4). Also, recipient rats needed at least 108 donor BM cells to ensure survival. Animals were therefore irradiated at 6 weeks of age and transplanted 2 h after irradiation with 108 BM cells pooled from three donor animals. Two weeks later, white blood cells were collected and DNA was extracted to verify the donor genotype. Five of seven recipients survived in each group, with all survivors achieving the expected chimerism (Fig. 4A).
Figure 4.
CB1R ablation in myeloid cells protects ZDF rats from hyperglycemia. A: Time line for whole-body irradiation/BM transfer; genotyping by agarose gel chromatography of white blood cells (WBCs) from ZDF rats receiving ZDF BM (WTBM) or ZDF-KO BM (KOBM), with the 113-BP and 102-BP amplicons indicating Cnr1+/+ and Cnr1−/− genotype, respectively. B: Evolution of blood glucose and serum levels of insulin and C-peptide along with body weight and mean weekly food intake at 20 weeks in ZDF rats receiving wild-type BM (green) or Cnr1−/− BM (purple). Filled triangles and the dashed line indicates blood glucose levels in nonirradiated ZDF rats. C: Western blot of CB1R protein in brain and BM cells after killing of the rats at 20 weeks. D: Fluorescence immunohistochemistry of insulin, CB1Rs, CD68, and colocalization analyzed by confocal microscopy. E: Increased insulin and decreased CD68 and CCL2 content of islets from ZDF rats receiving Cnr1−/− compared with wild-type ZDF BM. Columns and bars are the mean ± SEM from n = 5 animals/group. Scale bar, 100 μm. **P < 0.01, ***P < 0.001 relative to values in ZDF rats receiving wild-type BM (green columns).
BMT from ZDF donors to ZDF recipients tested for genotype-independent effects of irradiation and BMT. Recipients developed progressive hyperglycemia from 14 weeks on, reaching a plateau at 600 mg/dL by week 20 (Fig. 4B). The extent and rate of development of hyperglycemia were similar to those in control ZDF rats (dashed line), but its onset was delayed by 6 weeks, corresponding to the time it takes for the transferred cells to repopulate the irradiated BM and peripheral tissues (27). In contrast, ZDF rats receiving BM from ZDF-Cnr1 donors remained normoglycemic throughout the entire 20-week observation period and maintained higher plasma insulin and C-peptide levels compared with rats receiving ZDF BM (Fig. 4B). Interestingly, there was no difference in food intake or body weight between the two groups (Fig. 4B). CB1R protein was readily detectable postmortem in the BM of recipients of ZDF BM but was absent in recipients of ZDF-Cnr1 BM. As expected, CB1R was present at similar high levels in brain samples from both groups (Fig. 4C). Furthermore, CB1R highly colocalized with CD68+ macrophages in the islets of ZDF rats transplanted with ZDF BM, whereas there were fewer CD68+ cells and no detectable CB1Rs in islets of recipients of ZDF-Cnr1 BM (Fig. 4D). The absence of CB1Rs in hematopoietic cells was associated with higher pancreatic insulin content, normal pancreatic islet architecture, and reduced CD68+ macrophage infiltration, most likely due to reduced levels of the chemoattractant protein CCL2 (Fig. 4E). β-Cells in ZDF islets produce CCL2 (31,32) (Supplementary Fig. 2B), whereas the dramatically higher CCL2 expression in islets of ZDF rats transplanted with wild-type compared with Cnr1−/− BM (Fig. 4E) suggests that transmigrating macrophages contribute to CCL2 secretion, as also supported by the ability of cultured macrophages to secrete CCL2 (see below) (Figs. 5 and 6).
Figure 5.
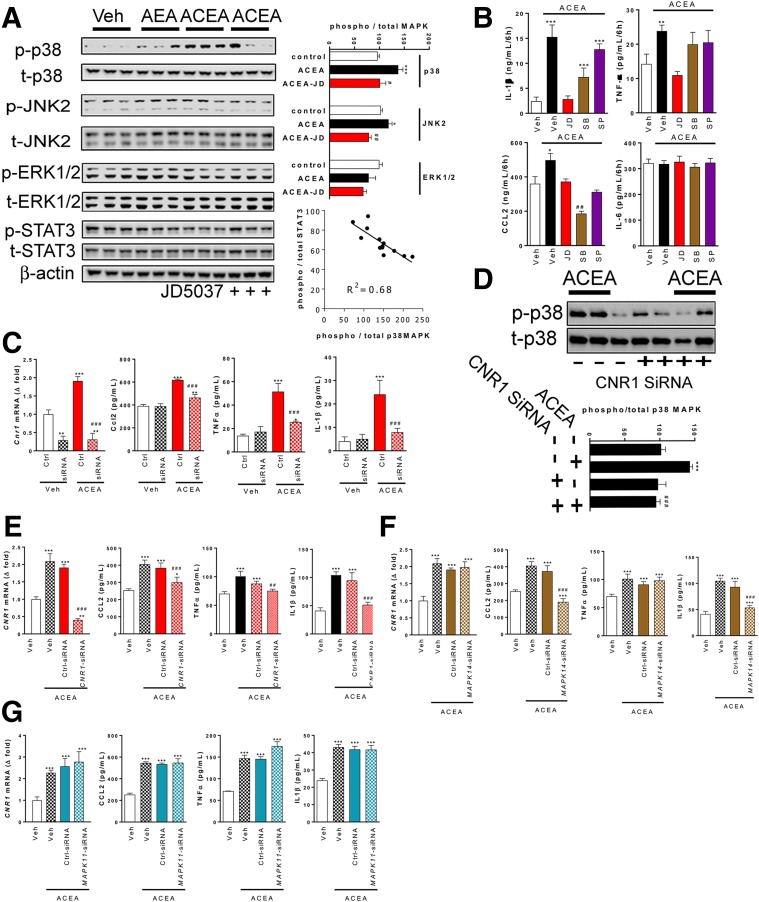
Role of MAP kinases in CB1R-induced chemokine and cytokine secretion by rat peritoneal macrophages (A–D) or THP-1 cells (E–G). A: Western blot analysis of phosphorylation of p38MAPK, JNK2, or ERK1/2 by 5 μmol/L AEA or 5 μmol/L ACEA in the presence or absence of 100 nmol/L JD5037 in three representative experiments; quantitation by densitometry. Bottom right: inverse relationship between ACEA-induced phosphorylation of p38MAPK and STAT3. B: Chemokine (CCL2) and cytokine secretion by 106 cells/well over 8 h in the presence of the indicated drugs. C: Effects of Cnr1 siRNA knockdown on Cnr1 mRNA and on chemokine and cytokine secretion induced by 5 μmol/L ACEA by aliquots of 106 rat peritoneal macrophages. D: siRNA-mediated knockdown of Cnr1 inhibits ACEA-induced p38MAPK phosphorylation. E: Experiments as in C, using human THP-1 cells. F: Effects of p38MAPKα knockdown on ACEA-induced chemokine and cytokine secretion by THP-1 cells. G: Effects of p38MAPKβ knockdown on the same parameters. *P < 0.05, **P < 0.01, ***P < 0.001 relative to vehicle; #P < 0.05, ##P < 0.01, ###P < 0.001 relative to ACEA-treated cells. Columns and bars are the mean ± SEM from four independent experiments. Ctrl, control.
Figure 6.
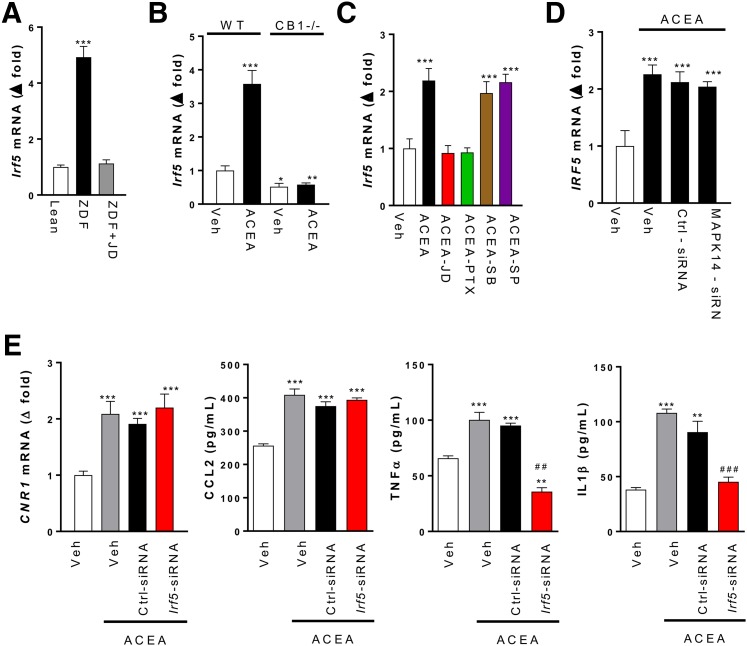
CB1R regulation of Irf5 expression and its role in β-cell loss in vivo. A: Irf5 mRNA levels in islets isolated from lean control (Ctrl) and ZDF rats treated for 4 weeks with vehicle (Veh) or 3 mg/kg/day JD5037. B: Irf5 mRNA levels in PECs isolated from wild-type or Cnr1−/− mice exposed to vehicle or 5 μmol/L ACEA for 24 h. C: Irf5 mRNA in rat PECs treated with vehicle or 5 μmol/L ACEA alone or in the presence of 100 nmol/L JD5037, 100 ng/mL PTX, 25 μg/mL SB202190, or 25 μg/mL SP600125. D: ACEA-induced increase in IRF5 expression is not affected by MAPK14 knockdown. E: Effects of Irf5 knockdown on Cnr1 mRNA and on chemokine and cytokine secretion induced by 5 μmol/L ACEA in aliquots of 106 rat peritoneal macrophages. Points/columns and bars are the mean ± SEM from six rats/group. Significant differences from values in Veh (*) or Veh + ACEA groups (#). *P < 0.05; **P < 0.01; ***P < 0.001; ##P < 0.01; ###P < 0.001.
In contrast, adoptive transfer of Cnr1−/− BM did not influence the dyslipidemia (Supplementary Table 2) or the development of diabetic nephropathy (Supplementary Fig. 5A), including the loss of Wilms tumor 1–positive podocytes (Supplementary Fig. 5B).
Distinct CB1R Signaling Pathways Involved in Chemokine and Inflammatory Cytokine Secretion by Macrophages
Next, we analyzed the CB1R signaling pathways that mediate the diabetogenic functions of macrophages (19). The stimulation of rat PECs with the physiological CB1R agonist AEA (5 μmol/L) or its stable analog ACEA (5 μmol/L) activated p38MAPK and c-Jun N-terminal kinase (JNK) 2 without any detectable change in extracellular signal–regulated kinase 1/2 (ERK1/2) phosphorylation (Fig. 5A). CB1R activation reduced STAT3 phosphorylation, a known corollary of M1 polarization (33), which was inversely related to p38MAPK activation (Fig. 5A). ACEA also induced the secretion of the cytokines IL-1β and TNF-α and the chemokine CCL2, but failed to affect IL-6 production (Fig. 5B). SB202190, a potent inhibitor of p38MAPKα and p38MAPKβ, suppressed the secretion of CCL2 below control levels but did not affect TNF-α secretion, and a partial reduction of CB1R-evoked IL-1β secretion was not statistically significant (Fig. 5B). The JNK1/2/3 inhibitor SP600125, while fully abolishing c-Jun phosphorylation (data not shown), was only effective in inhibiting the ACEA-evoked CCL2 secretion (Fig. 5B). The effects of ACEA on PEC secretory responses were fully abolished by the CB1R inverse agonist JD5037 (Fig. 5B) and were similarly inhibited by siRNA-mediated selective knockdown of Cnr1 (Fig. 5C).
In the macrophage-differentiated human monocytic cell line THP-1, silencing CNR1 abolished ACEA-induced p38MAPK activation (Fig. 5D) and TNF-α, IL-1β, and CCL2 secretion (Fig. 5E). Knocking down MAPK14 (encoding p38MAPKα), but not MAPK11 (encoding p38MAPKβ), completely blocked ACEA-induced secretion of CCL2 and significantly reduced the secretion of IL-1β but, again, failed to influence ACEA-induced TNF-α secretion (Fig. 5F and G). Thus, CB1R activation in macrophages engages p38MAPKα to induce CCL2, but not TNF-α, secretion and partially contributes to IL-1β secretion.
Prompted by the similar selective expansion of subcutaneous, but not visceral, fat tissue in ZDF-Cnr1 rats (Fig. 1E) and in mice deficient in the proinflammatory transcription factor Irf5 (see Introduction), we explored the involvement of IRF5 in CB1R-mediated inflammatory signaling and β-cell loss. Irf5 gene expression was robustly increased in pancreatic islets isolated from ZDF compared with lean rats, whereas there was no such increase in islets of ZDF-Cnr1 rats or in islets of ZDF rats chronically treated with JD5037 (Fig. 6A). The involvement of CB1R was further confirmed in PECs isolated from wild-type mice, exposure of which to 1 μmol/L ACEA induced a threefold to fourfold increase in Irf5 mRNA, with no such effect in PECs from Cnr1−/− mice (Fig. 6B). Furthermore, in rat PECs, 1 μmol/L ACEA increased Irf5 mRNA twofold, an effect blocked by JD5037 or PTX but not by SB202190 or SP600125 (Fig. 6C). Thus, CB1R regulates Irf5 expression via Gi/o-mediated inhibition of adenylate cyclase rather than activation of MAP kinases. In THP-1 macrophages, 1 μmol/L ACEA also caused an approximately twofold increase in IRF5 mRNA, and a similar effect was seen in THP-1 cells with siRNA-mediated knockdown of MAPK14, confirming the lack of involvement of p38MAPKα (Fig. 6D). SiRNA-mediated knockdown of Irf5 in rat PECs inhibited the ACEA-induced secretion of both TNF-α and IL-1β but had no effect on CCL2 secretion or Cnr1 expression (Fig. 6E), suggesting a requirement for IRF5 in CB1R-mediated cytokine production.
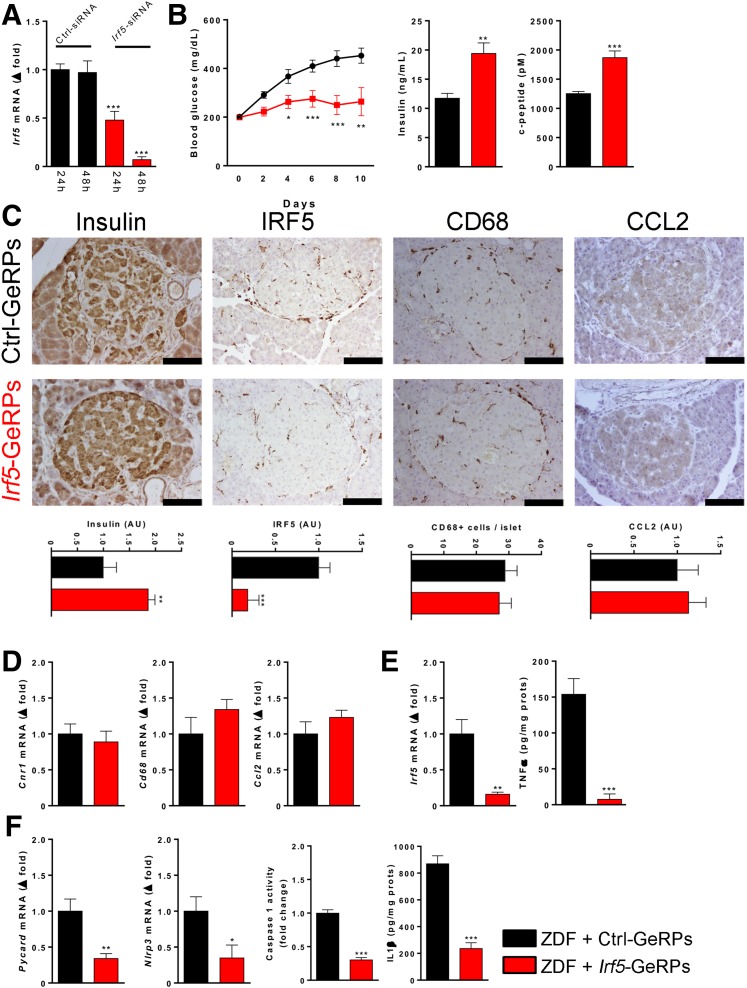
To explore the potential role of the CB1R/Gi/o/IRF5 signaling cascade in the loss of β-cell function in ZDF rats, 8-week-old ZDF rats were chronically treated with GeRP containing siRNA against rat Irf5, a technique that allows selective knockdown of target genes in phagocytic macrophages in vivo (19,34). We first optimized Irf5 siRNAs and selected one that yielded >90% knockdown of Irf5 gene expression in rat PECs after 48 h (Fig. 7A). ZDF rats were then treated intraperitoneally every other day for 12 days with GeRPs containing control siRNA or Irf5 siRNA. ZDF rats treated with control siRNA developed progressive hyperglycemia, whereas those receiving Irf5 siRNA maintained their pretreatment blood glucose level and also retained significantly higher plasma insulin and C-peptide levels (Fig. 7B). β-Cell protection was further indicated by the higher insulin content of islets from Irf5 siRNA-treated rats compared with control siRNA-treated rats (Fig. 7C). As expected, Irf5 knockdown significantly reduced Irf5 protein abundance in islets, whereas the number of CD68+ macrophages and CCL2 protein levels were similar in the two groups, indicating a lack of IRF5 involvement in CCL2 secretion (Fig. 7C). Similarly, Irf5 knockdown did not affect Cnr1, CD68, and Ccl2 mRNA levels in islets (Fig. 7D) but resulted in a >90% decrease in TNF-α content (Fig. 7E), accompanied by significant decreases in Pycard and Nlrp3 gene expression, caspase-1 activity, and IL-1β content (Fig. 7F). Furthermore, macrophage-specific in vivo knockdown of Irf5 resulted in an increased in CD3+ T lymphocytes (Supplementary Fig. 6A) associated with a decrease in TH1 markers (Supplementary Fig. 6B) and an increase in TH2 markers (Supplementary Fig. 6C), without a change in the TH17 response (Supplementary Fig. 6D). Additionally, Irf5 knockdown did not affect Il12p40 or Il23 expression but did increase Il10 expression (Supplementary Fig. 6E).
Figure 7.
Effects of GeRP-mediated knockdown of Irf5 in ZDF rats on islet protein and gene expression. A: In vitro validation in rat PECs of the Irf5 siRNA used in vivo, as shown in panel B. B: GeRP-mediated delivery of Irf5 siRNA (red), but not control siRNA (black), protects ZDF rats from hyperglycemia and the loss of β-cell function. C: Representative immunohistological stainings for insulin, IRF5, CD68, and CCL2 protein levels in islets from ZDF rats treated with Irf5 siRNA (red) vs. control siRNA (black). Scale bar, 100 μm. Quantitation by densitometry or immune-positive cell counts, as described in research design and methods. D: Lack of effect of in vivo knockdown of Irf5 on Cnr1, Cd68, and Ccl2 mRNA in isolated islets. E: Downregulation of Irf5 expression and TNF-α protein in islets by the same treatment. F: Downregulation of Pycard and Nlrp3 expression, caspase-1 activity, and IL-1β protein in islets of ZDF rats treated with Irf5 siRNA vs. control siRNA-containing GeRPs. Points/columns and bars are mean ± SEM values from six rats/group. *P < 0.05, **P < 0.01, ***P < 0.005 relative to the control siRNA-treated group. AU, arbitrary unit.
Discussion
Islet inflammation is a contributing factor to the progression of compensated IR into insulin-dependent T2D, and CB1R on proinflammatory macrophages play a prominent role in diabetic insulitis and loss of β-cell function in the ZDF model of T2D (19). To test the developmental role of CB1R in T2D, we generated CB1R-deficient rats on a ZDF background. Here we report that ZDF-Cnr1 rats are as hyperinsulinemic and insulin resistant as ZDF rats, but they are protected from the loss of β-cell function and consequently remain normoglycemic and are also protected from the associated nephropathy. Furthermore, adoptive transfer of ZDF-Cnr1 BM to ZDF recipients replicates the preservation of β-cells and normoglycemia seen in ZDF-Cnr1 rats, but does not mitigate the loss of glomerular podocytes and the associated nephropathy. This provides strong evidence that CB1Rs in BM-derived macrophages are both necessary and sufficient for the development of T2D but do not play a role in diabetic nephropathy in this model. Furthermore, we demonstrate for the first time the key role of IRF5, the master regulator of inflammatory polarization of macrophages (22), as a downstream signaling molecule mediating CB1R-induced cytokine release by macrophages in vitro and the diabetogenic effect CB1R activation in vivo.
ZDF-Cnr1 rats remain normoglycemic for up to 26 weeks of age and do not display the macrophage infiltration of islets and the associated loss of β-cell function observed in diabetic ZDF rats. The elevated levels of plasma FFA, TG, and total cholesterol, as well as the hypoadiponectemia are also normalized in ZDF-Cnr1 rats, which is in agreement with the documented role of CB1R in these metabolic disturbances (17). Finally, ZDF-Cnr1 rats are protected from diabetic nephropathy, as reflected by normal parameters of renal function.
Interestingly, the hyperphagia of ZDF rats is also prevented by the knockout of Cnr1, indicating that the hyperphagia in leptin deficiency requires intact CB1R signaling. The lower food intake of ZDF-Cnr1 rats was associated with a somewhat slower early weight gain (Fig. 1D), which could affect insulin sensitivity. However, Cnr1 deletion protected β-cells and preserved β-cell function without affecting the IR of ZDF rats. Furthermore, adoptive transfer of CB1R-deficient BM to ZDF rats prevented hyperglycemia and β-cell loss without affecting food intake or body weight (Fig. 4D). Thus, CB1R-mediated effects on β-cell function are unrelated to changes in food intake or body weight.
The absence of hyperglycemia and β-cell loss in ZDF rats transplanted with Cnr1−/− BM is not due to the process of BMT per se, because transferring wild-type ZDF BM to wild-type ZDF recipients did not prevent the full development of hyperglycemia. The protective effect of Cnr1−/− BMT is reminiscent of the similar effect of macrophage-specific in vivo knockdown of Cnr1 (19). The role of macrophage CB1R in β-cell loss is further supported by the protective effect of macrophage-specific knockdown of Irf5 and the regulation of Irf5 expression by CB1R. Thus, macrophage CB1Rs are both necessary and sufficient to account for the development of T2D in this model. However, we cannot exclude the possibility that glucotoxicity and lipotoxicity (35) also contribute to β-cell damage, either directly or by inducing the EC/CB1R system, as shown previously (19).
In contrast, the transfer of Cnr1−/− BM to ZDF rats did not mitigate diabetic nephropathy, indicating the involvement of a different cellular pool of CB1Rs. This is in agreement with the absence of significant infiltration of proinflammatory macrophages into glomeruli during the development of nephropathy in ZDF rats (21). A similar lack of involvement of proinflammatory macrophages has been indicated by the findings that selective deletion of Nlrp3 or caspase-1 expression in BM-derived cells failed to protect mice against the development of diabetic nephropathy, and the protective effect of the global deletion of Nlrp3 was not reversed by the transplantation of wild-type BM cells into Nlrp3-deficient mice (36).
The congruent effects of pharmacological blockade and genetic deletion of CB1Rs strongly support the diabetogenic role of increased CB1R activity. This is different from the situation in leptin-deficient ob/ob mice, in which germline deletion of Cnr1 aggravated rather than mitigated their glucose intolerance (37). However, ob/ob mice on a C57BL/6J background compensate for IR by β-cell proliferation and thus do not become overtly diabetic, so the key pathogenic process promoted by CB1R activity in ZDF rats was absent in the murine model. On the other hand, the deletion of Cnr1 in both models failed to reverse basal hyperinsulinemia and IR, which is likely related to the leptin-deficient state. Leptin receptors are present on β-cells where they mediate increased KATP channel activity (38), and their activation was reported to decrease basal, but not glucose-stimulated, insulin secretion (39).
Most importantly, we discovered that the transcription factor IRF5 is a key downstream mediator of CB1R-induced cytokine release by inflammatory macrophages and the resulting loss of β-cell function. First, Irf5 expression was robustly increased in the islets of ZDF compared with lean control rats. This increase was prevented by pharmacological blockade or genetic deletion of CB1R, which also prevented macrophage infiltration of islets, suggesting that Irf5 expression was induced in macrophages. This was then further confirmed by the CB1R agonist–induced increase in Irf5 expression in primary cultured mouse and rat macrophages or in THP-1 cells. This effect was PTX sensitive but unaffected by p38MAPK or JNK blockade or knockdown, suggesting that it resulted from Gi/o-mediated inhibition of adenylate cyclase. Furthermore, siRNA-mediated knockdown of Irf5 in macrophages blunted the CB1R agonist–induced increase in TNF-α and IL-1β secretion and Nlrp3 expression, indicating the obligatory role of IRF5 in proinflammatory cytokine release. The anti-inflammatory response to Irf5 knockdown also involved a TH2-type immune response, as indicated by the increase in CD3+ T lymphocytes and the increased expression of the TH2 markers Gata3 and Il4 and decreased expression of the TH1 marker Tbet in pancreatic islets. These changes are similar to those found in adipose tissue of mice with diet-induced obesity (23) and are consistent with the proposed role of a TH1-type inflammatory response in T2D and its renal complications (40,41). Finally, macrophage-specific in vivo knockdown of Irf5 in ZDF rats protected β-cells and prevented the development of hyperglycemia, similar to the earlier reported effects of chronic CB1R blockade or selective knockdown of Cnr1 in macrophages (19).
IRF5, originally discovered as a transcription factor induced by type I interferons during viral infections (42), was more recently identified as a master regulator of macrophage M1 polarization and a mediator of obesity-related adipose tissue inflammation and the resulting IR (23). The present findings establish that the G-protein–coupled receptor CB1R is an upstream regulator of Irf5 expression and point to a broader metabolic function of macrophage IRF5 as a key driver of diabetogenic insulitis and β-cell loss.
Unlike CB1R blockade or gene deletion, which also reduced CCL2 chemokine secretion and the resulting transmigration of macrophages into islets (19), the knockdown of Irf5 failed to influence these parameters. This suggested that CB1Rs signal via an alternative, IRF5-independent pathway to promote CCL2 secretion. Indeed, CB1R agonists activated p38MAPK and JNK but not ERK1/2 in rodent and human macrophages, and inhibitors of p38MAPK or JNK blocked the parallel increase in CCL2 secretion without affecting the increased secretion of TNF-α or IL-1β. The siRNA-mediated knockdown of p38MAPKα, but not p38MAPKβ, in THP-1 macrophages similarly inhibited CB1R-induced CCL2, but not TNF-α secretion, and partially inhibited IL-1β secretion. This supports earlier findings that CB1R promotes CCL2 secretion via p38MAPK (43), but documents for the first time show the selective role of the p38MAPKα isoform in this effect. Whereas the above findings indicate that inflammatory macrophages are an important source of CCL2, we could also detect this chemokine in β-cells, which is in agreement with evidence for CCL2 secretion by islet β-cells (31,32). It is possible that in the early stages of diabetic insulitis, β-cell–derived CCL2 initiates the transmigration of macrophages, which then amplify the production of this chemotactic signal. Another possible link between β-cells and macrophages is islet amyloid polypeptide, which is secreted by β-cells and stimulates inflammatory cytokine production by macrophages (44,45).
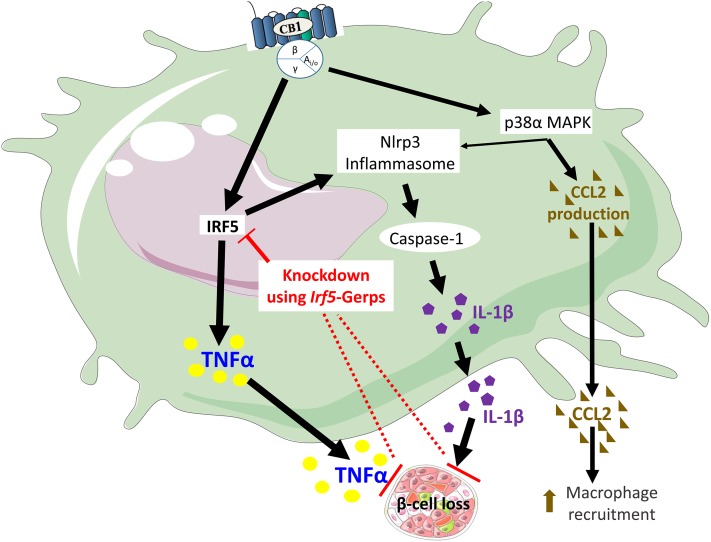
Together, the above findings demonstrate that activation of CB1Rs in proinflammatory M1 macrophages increases chemokine and cytokine secretion via distinct, partially overlapping signaling pathways, as illustrated in Fig. 8. The CB1R-induced secretion of CCL2, but not TNF-α, is mediated via the activation of p38MAPKα. On the other hand, the CB1R-induced secretion of TNF-α, but not CCL2, is mediated via IRF5, and both pathways contribute to increased IL-1β secretion, with IRF5 having a more dominant role. The obligatory role of IRF5 in the secretion of cytotoxic cytokines thought to drive β-cell loss (19) is highlighted by the protective effect of macrophage-specific in vivo knockdown of Irf5. These findings mark IRF5 as a potential therapeutic target in T2D.
Figure 8.
Schematic illustration of the proposed mechanism by which the activation of CB1Rs in macrophages leads to the loss of β-cell function. This figure was prepared using a template on the Servier medical art website (http://www.servier.com/Powerpoint-image-bank).
Supplementary Material
Article Information
Acknowledgments. The authors thank J.F. McElroy and R.J. Chorvat (Jenrin Discovery) for providing the CB1R antagonist JD5037, Peter Gao (National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health [NIAAA, NIH]) for advice on whole-body irradiation and BM transplantation, Dan Pare (National Institute of Allergy and Infectious Diseases, NIH) for his help in performing whole-body irradiation, J. Harvey-White (NIAAA, NIH) for technical assistance, and R. Kechrid (NIAAA, NIH) for assistance with the animal studies.
Funding. G.S. is supported by a János Bólyai Research Scholarship of the Hungarian Academy of Sciences. The work from the laboratory of M.P.C. was supported by a grant from the NIH (DK-103047). This study was supported by intramural NIH funds to G.K.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.J. designed the study, performed most of the experiments, designed and tested the GeRPs, analyzed the results, and wrote the manuscript. G.S. designed the study, performed Western blot analyses of macrophage signaling, analyzed the results, and wrote the manuscript. R.C. conducted radioligand binding assays. G.G. performed some of the whole-body irradiation and BM transfer experiments. D.J.H. conducted in vivo Irf5-silencing experiments. J.K.P. performed the morphometric analysis of adipocytes. S.N. prepared, provided, designed, and tested the GeRPs. Y.S. and M.P.C. prepared and provided the GeRPs. J.L. and Z.L. assisted with cell culture and PCR analyses. A.Z.R. performed the renal pathology analyses. G.K. designed the study, analyzed the results, and wrote the manuscript. All authors had access to the manuscript and agreed with the final version. G.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1199/-/DC1.
G.S. is currently affiliated with the Department of Physiology, Faculty of Medicine, Semmelweis University, Budapest, Hungary.
References
- 1.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 4.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 5.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 2007;56:2356–2370 [DOI] [PubMed] [Google Scholar]
- 6.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 2006;58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engeli S, Böhnke J, Feldpausch M, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005;54:2838–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 2013;17:475–490 [DOI] [PubMed] [Google Scholar]
- 9.Mazier W, Saucisse N, Gatta-Cherifi B, Cota D. The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metab 2015;26:524–537 [DOI] [PubMed] [Google Scholar]
- 10.Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study Group . Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005;353:2121–2134 [DOI] [PubMed] [Google Scholar]
- 11.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF; RIO-Diabetes Study Group . Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006;368:1660–1672 [DOI] [PubMed] [Google Scholar]
- 12.O’Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 2011;60:1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuya DT, Poletto AC, Freitas HS, Machado UF. Inhibition of cannabinoid CB1 receptor upregulates Slc2a4 expression via nuclear factor-κB and sterol regulatory element-binding protein-1 in adipocytes. J Mol Endocrinol 2012;49:97–106 [DOI] [PubMed] [Google Scholar]
- 14.Eckardt K, Sell H, Taube A, et al. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia 2009;52:664–674 [DOI] [PubMed] [Google Scholar]
- 15.Osei-Hyiaman D, Liu J, Zhou L, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 2008;118:3160–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranville A, Herling AW, Biemer-Daub G, Voss MD. Reversal of inflammation-induced impairment of glucose uptake in adipocytes by direct effect of CB1 antagonism on adipose tissue macrophages. Obesity (Silver Spring) 2010;18:2247–2254 [DOI] [PubMed] [Google Scholar]
- 17.Tam J, Vemuri VK, Liu J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 2010;120:2953–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam J, Cinar R, Liu J, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 2012;16:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med 2013;19:1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schäfer A, Pfrang J, Neumüller J, Fiedler S, Ertl G, Bauersachs J. The cannabinoid receptor-1 antagonist rimonabant inhibits platelet activation and reduces pro-inflammatory chemokines and leukocytes in Zucker rats. Br J Pharmacol 2008;154:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jourdan T, Szanda G, Rosenberg AZ, et al. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci U S A 2014;111:E5420–E5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 2011;12:231–238 [DOI] [PubMed] [Google Scholar]
- 23.Dalmas E, Toubal A, Alzaid F, et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat Med 2015;21:610–618 [DOI] [PubMed] [Google Scholar]
- 24.Godlewski G, Jourdan T, Szanda G, et al. Mice lacking GPR3 receptors display late-onset obese phenotype due to impaired thermogenic function in brown adipose tissue. Sci Rep 2015;5:14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care 1997;20:1087–1092 [DOI] [PubMed] [Google Scholar]
- 26.Iyer MR, Cinar R, Liu J, et al. Structural basis of species-dependent differential affinity of 6-alkoxy-5-aryl-3-pyridinecarboxamide cannabinoid-1 receptor antagonists. Mol Pharmacol 2015;88:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duran-Struuck R, Dysko RC. Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci 2009;48:11–22 [PMC free article] [PubMed] [Google Scholar]
- 28.Buehler E, Chen Y-C, Martin S. C911: a bench-level control for sequence specific siRNA off-target effects. PLoS One 2012;7:e51942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tesz GJ, Aouadi M, Prot M, et al. Glucan particles for selective delivery of siRNA to phagocytic cells in mice. Biochem J 2011;436:351–362 [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Zhou L, Xiong K, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 2012;142:1218–1228.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piemonti L, Leone BE, Nano R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes 2002;51:55–65 [DOI] [PubMed] [Google Scholar]
- 32.Taylor-Fishwick DA, Weaver J, Glenn L, et al. Selective inhibition of 12-lipoxygenase protects islets and beta cells from inflammatory cytokine-mediated beta cell dysfunction. Diabetologia 2015;58:549–557 [DOI] [PubMed] [Google Scholar]
- 33.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aouadi M, Tesz GJ, Nicoloro SM, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 2009;458:1180–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab 2012;15:518–533 [DOI] [PubMed] [Google Scholar]
- 36.Shahzad K, Bock F, Dong W, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int 2015;87:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Schmidt SF, Friedman JM. Developmental role for endocannabinoid signaling in regulating glucose metabolism and growth. Diabetes 2013;62:2359–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harvey J, McKenna F, Herson PS, Spanswick D, Ashford ML. Leptin activates ATP-sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. J Physiol 1997;504:527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida K, Murakami T, Mizuno A, Iida M, Kuwajima M, Shima K. Leptin suppresses basal insulin secretion from rat pancreatic islets. Regul Pept 1997;70:179–182 [DOI] [PubMed] [Google Scholar]
- 40.Zeng C, Shi X, Zhang B, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl) 2012;90:175–186 [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Xiao C, Wang P, et al. The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Hum Immunol 2014;75:289–296 [DOI] [PubMed] [Google Scholar]
- 42.Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem 2001;276:23382–23390 [DOI] [PubMed] [Google Scholar]
- 43.Han KH, Lim S, Ryu J, et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res 2009;84:378–386 [DOI] [PubMed] [Google Scholar]
- 44.Westwell-Roper C, Dai DL, Soukhatcheva G, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol 2011;187:2755–2765 [DOI] [PubMed] [Google Scholar]
- 45.Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 2010;11:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.