Abstract
Objectives/Hypothesis
A prototype tympanostomy tube, composed of (polybutyl/methyl methacrylate-co-dimethyl amino ethyl methacrylate (PBM)), was tested to (1) evaluate the effect of PBM tubes on rat dermis as a corollary for biocompatibility and (2) to observe the efficacy of dissolution with isopropyl alcohol (iPrOH) and ethanol (EtOH).
Subjects and Methods
A two-part study was conducted to assess biocompatible substance with inducible dissolvability as a critical characteristic for a newly engineered tympanostomy tube. First, tympanostomy tubes were inserted subcutaneously in 10 rats, which served as an animal model for biosafety and compared to traditional tubes with respect to histologic reaction. Tissue surrounding the PBM prototype tubes was submitted for histopathology and demonstrated no tissue reactivity or signs of major inflammation. In the second part, we evaluated the dissolvability of the tube with either isopropyl alcohol, ethanol, ofloxacin, ciprodex, water, and soapy water. PBM tubes were exposed to decreasing concentrations of iPrOH and EtOH with interval qualitative assessment of dissolution.
Results
(1) Histologic examination did not reveal pathology with PBM tubes; (2) Concentrations of at least 50% iPrOH and EtOH dissolve PBM tubes within 48 hours while concentrations of at least 75% iPrOH and EtOH were required for dissolution when exposure was limited to four 20-minute intervals.
Conclusion
PBM is biocompatible in the rat model. Additionally, PBM demonstrates rapid dissolution upon alcohol-based stimuli, validating the proof-of-concept of dissolvable “on-command” or biocommandible ear tubes. Further testing of PBM is needed with a less ototoxic dissolver and in a better simulated middle ear environment, before testing can be performed in humans.
Keywords: Biocommandability, dissolvable, myringotomy tube, tympanostomy tube, pressure equalization tube, grommet
Introduction
Otitis media is one of the most frequent pathologies in the pediatric population, surpassed only by upper respiratory tract infections. Each year in the United States, otitis media results in at least 16 million office visits[1] at a cost of at least 5 billion dollars[2]. Otitis media and the resulting middle ear effusion can cause significant hearing loss, speech delay, and predisposition to more severe ear infections, including mastoiditis[3][4].
Annually in the United States, approximately 1 million tympanostomy tubes are placed, making this the most common surgical procedure among otolaryngologists[5]. Ear tube surgery remains a highly effective treatment for chronic otitis media because it mitigates the middle ear inflammatory process, leads to prompt resolution of ear infections, and drains fluid that would otherwise cause hearing impairment.
While the placement of tympanostomy tubes is very safe, the procedure has known complications. The average duration of standard tympanostomy tubes placement is 12 to 16 months but can be highly variable. There are several factors that influence extrusion, including the tube size and diameter, severity of otitis media, volume of ear drainage, and tympanic membrane thickness [6]. These factors make it impossible to predict when tubes will fall out spontaneously and judgments on otoscopy are often erroneous. If the tubes do not fall out of the tympanic membrane after three years of observation, the child often undergoes a second surgery under general anesthesia to have the tubes removed. A study by Bhattacharyya found that 21,446 ear tubes needed to be removed surgically in 2006, or roughly 3.8% of tubes placed[7].
In addition to the unpredictability of extrusion, there is an increased risk of tympanic perforation with retained myringotomy tubes[6]. Short-term tubes have a perforation rate of only 2%, but tubes that remain for over 2 years can have a perforation rate of up to 13% [8].
Since their introduction by Armstrong in 1954[9] Armstrong, tympanostomy tubes have undergone relatively minor changes in design. Modifications made include different shapes, flanges design, diameter size, and polymers like Teflon and silicone. None of these modifications serve to address the complications noted above (retention and perforation) or change the fundamental matter of tympanostomy tubes being an implantable foreign body.
Recently, researchers have studied the concept of biodegradable ear tubes which begin the dissolution process at the moment of insertion [10][11]. Our dissolvable “biocommandible” ear tube (which maintains form and function until a trigger is applied), addresses the problems caused by the current generation of plastic tubes Figure 1. Since dissolvable ear tubes would not have to be surgically removed, there would be minimal to no risk of additional surgery and no need for general anesthesia to remove a tube that does not spontaneously extrude on its own. Also, a dissolvable tube would provide the potential benefit of lower perforation rates as compared to a tympanostomy tube that stays in too long while awaiting spontaneous extrusion.
Figure 1.
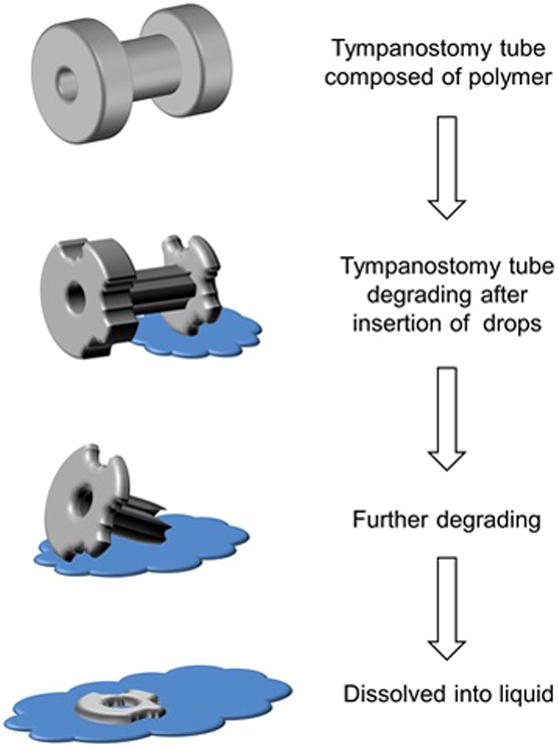
Diagram showing proof of concept ear tube made of bio-compatible material (PMB) that can retain its form and function in physiological conditions and quickly dissolve on contact with uniquely formulated ear drops.
Methods and Materials
There were two experiments completed in this study, which are detailed below: an animal based experiment to test the biocompatibility of the polymer used for the dissolvable ear tube and a bench analysis of the dissolvability profile of that polymer when exposed to alcohol, ofloxacin, ciprodex, water, and soapy water. Our protocol # 00030339 was approved by Children's Research Institute (CRI) and Institutional Animal Care and Use Committee.
Tympanostomy tube engineering
The polymer polybutyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) (PBM) available commercially as EUDRAGIT EPO® (Evonik Industries, Essen, Germany) was chosen for the tubes. PBM was selected because of its already established biocompatibility in the human model as a coating for enteric capsules and used for taste masking. Additional desirable qualities include water solubility in low pH environment (pH 1.0-4.0), meaning it is non-dissolvable or stable in most human implant environments; the ease at which it is dissolved in EtOH or iPrOH; and its ability to be easily formed into the desired structure with low-cost/low-tech equipment.
To validate this polymer for the application of dissolvable, “biocommandible” ear tubes, we molded structures similar in shape to the current flanged ear tube design. The ear tube shape was 3D printed to create a mold. From this positive mold, a negative mold was impressed in silicone. A small amount of PBM was introduced in the negative mold and kept in the oven at 150 degrees Celsius for 10 minutes, allowing the mold to fill with molten PBM. The mold was then cooled off and the ear tube extracted from the silicone. We then tested its stability in water, soapy water, ofloxacin, ciprofloxacin, and 70% ethanol water solution. Water as well as soap with water did not affect dissolution nor did ofloxin after 48 hours of direct exposure. Ciprofloxin was unable to evoke dissolution after 24 hours. As shown in Figure 2, the PBM polymer ear tube prototype is completely dissolved in a 50% to 70% ethanol water solution after 140 minutes, while remaining intact in water or mixture of soap and water.
Figure 2.
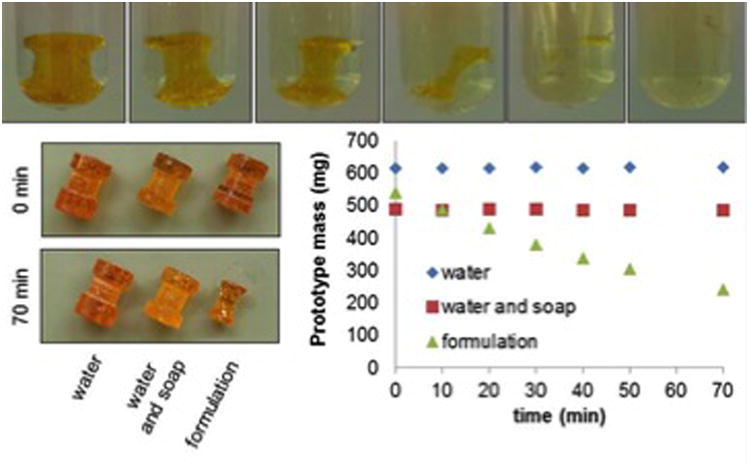
Dissolution of prototype; Bottom left: Before and after of prototypes in water, water/soap, and 70% ethanol; Bottom right: Mass versus time for prototypes soaked in water, water/soap, and 70% ethanol.
Miniaturization of the tubes was then achieved Figure 3. Using melted PBM, an extrusion technique was used to produce uniform two-millimeter (mm) diameter hollow bore tubes. To increase the structural integrity of the tubes, a plastisizer was added, which augments the tube's flexibility. Our animal studies utilized two novel dissolvable tubes featuring PBM in combination with Steryl alcohol and PBM in combination with Poly(ethyl acrylate-co-methyl methacrylate) 2:1 ®(PEM) (Evonik Industries, Essen, Germany). With validation from our preliminary study and experimental tympanostomy tubes, we were able to proceed with our biocompatibility and dissolution experimentations.
Figure 3.

Prototype tubes which were miniaturized and then placed into rat animal model.
Experiment 1- Biocompatibility experimentation
Ten Wistar rats (Rattus norvegius) weighing between 180 to 200 grams were purchased from Charles Rivers Laboratory (Wilmington, Massachusetts). The ten rats were divided into two groups: a control group (N=4) implanted with commercially available tympanostomy tubes and an experimental group (N=6). Within the experimental group the rats were subdivided further with three rats receiving implantation with a composite tube of 85% PBM with 15% PEM as a plasticizer (PBM-A) and three rats with tubes composed of 85% PBM with 15% Steryl alcohol (PBM-B). The tubes were then surgically inserted into the subdermis of the rats to test for biosafety/biocompatibilty and compare this to traditional tubes with respect to histologic reaction.
Throughout the course of the study the rats were monitored for signs of distress such as head pressing, ataxia, isolation, poor hygiene and porphyrin staining. The implantation site was inspected and observations were logged. Inspection occurred every two days for the first half of the experiment and then spaced out to every four days. Additionally, the rats were weighed as an objective measure of health. None of the subjects demonstrated ulceration or tumor at the implantation site nor did any have weight loss surpassing 20% of previously recorded weight.
At the 2-week interval, one rat from the control arm was selected for euthanasia along with two from the experimental group -- one each from the PBM-A and the PBM-B cohort. The implantation site was examined for abnormalities including ulcerations, inflammation, and tumor genesis. The pelts were immediately fixed in 10% formalin for at least 24 hours and then sectioned, processed, and paraffin-embedded. Slides were cut at 4 microns and stained using standard H&E protocols. Similarly, euthanasia of three additional animals occurred at the four-week interval with all remaining animals euthanized at six weeks, marking the conclusion of the study.
Experiment 2- Dissolution Study
The second portion of the experimental design was conducted to explore the dissolution of PBM tubes in the presence of alcohol. The first phase of bench testing assessed the efficacy of two alcohols, 100% isopropyl alcohol (iPrOH) and 95% ethanol (EtOH). These two alcohol compounds were chosen for their common availability. The alcohols were diluted with deionized water to concentrations of 100, 75, 50, 25, 10, and 5 percent and two milliliters (mL) of each concentration was dispensed in 2.5 mL microcentrifuge tubes.
Two mm segments of PBM-B grommets were placed into each concentration. Qualitative assessment and photo-documentation occurred every twenty minutes for the first hour, and then again at 24 and 48 hours. The results of this initial titration were utilized to plan the second phase of experimentation, which is described next.
In the second phase, concentrations that were able to disturb the mechanical integrity of PBM-B were instilled in 1 mL aliquots over 2 mm segments of PBM-B placed in well culture plates. The solutions were allowed 20 minutes of exposure time. After 20 minutes, any remaining fluid that was not evaporated was aspirated from the culture wells and the wells were placed in a fume hood to allow for further desiccation. This process was repeated a total of four times. The purpose of the design was to replicate one possible clinical application regimen where drops are instilled twice daily for two days. This set of procedures was repeated simultaneously with a second set of PBM-B tubes to facilitate comparative analysis and increase accuracy and confidence in the results. Qualitative assessment and photo-documentation occurred after each instillation of drops.
Results
Animal study
Throughout the duration of the study the observational logs did not reveal any signs of distress in any rat subjects either from the experimental group or control group. All rats continued to gain weight until the time of euthanasia (Figure 4). Histopathological sections from the experimental groups PBM-A (Figure 5-A) and PBM-B (Figure 5-B) show normal dermis, subcutaneous tissue, and muscle with no significant histopathology noted. On the other hand, sections from the control group showed granulation tissue formation (Figure 5-C). Though not intended as a measured outcome in the experimental design, careful gross observations of the experimental tubes were noted. Both experimental tubes performed similarly with no distinguishable difference when directly compared. In tubes explanted at two weeks, there were no observable differences between in vivo experimental tubes and de novo tubes aside from the development of an opaque patina. Experimental tubes retrieved at the end of the experiment demonstrated a slight compressibility along the long axis that was evidenced by a change in the lumen shape from circular to ovoid. Notwithstanding the change in shape, there was little appreciable difference in the integrity of the tubes as they still were easily palpated through the overlying soft tissue and maintained lumen integrity.
Figure 4.
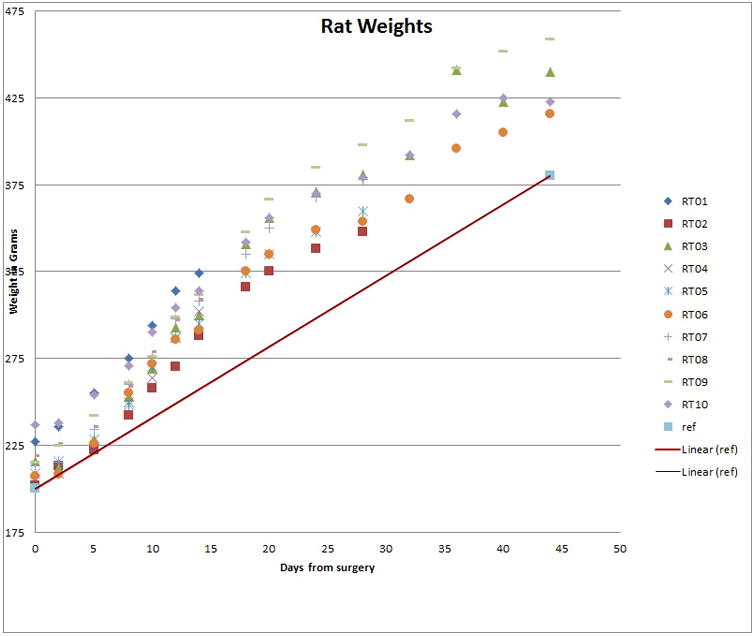
Recorded weights of rats in both experimental and control groups. All rats demonstrated a positive slope in weight gain.
Figure 5.
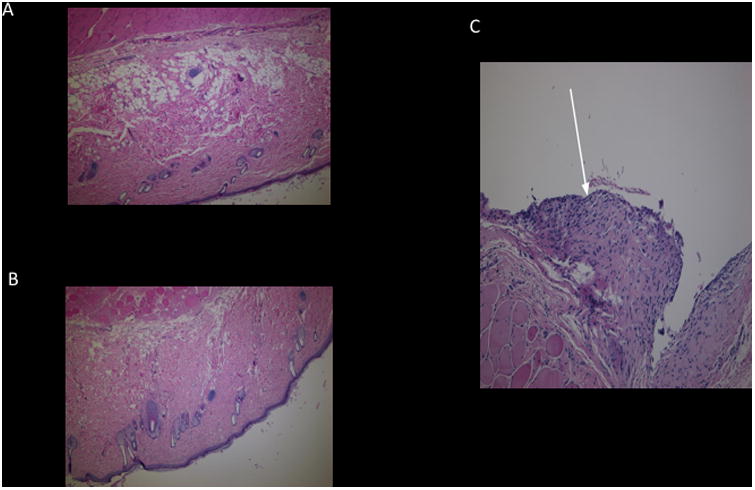
A) Histopathologic section with hematoxylin and eosin staining of dermis with surrounding tissue of rat implanted with experimental composite 85% PBM 15% PEM tube (PBM-A). No evidence of pathology or significant inflammation in the surrounding tissue. B) Histopathologic section with hematoxylin and eosin staining of dermis with surrounding tissue of rat implanted with experimental composite 85% PBM with 15% Steryl alcohol (PBM-B) with no evidence of pathology. C) Histopathologic section with hematoxylin and eosin staining of dermis with surrounding tissue of rat implanted with commercially available myringotomy tube. Arrow highlights areas of granulation tissue.
Benchtop dissolution studies
The results of the benchtop studies demonstrated the feasibility of dissolution of PBM-B tubes when exposed to iPrOH and EtOH as a stimulus at a concentration of >50% within 48 hours of exposure. In the first phase where PBM-B tubes were directly submerged in decreasing concentrations of 100% iPrOH and 95% EtOH after twenty minutes, undiluted EtOH and a concentration diluted by 25% was able to disturb the mechanical properties of PBM along with iPrOH diluted by 25%. Forty minutes of direct contact with undiluted iPrOH was able to cause dissolution of PBM-B tubes. At 60 minutes, iPrOH diluted by 50% caused dissolution, and at 24 hours, EtOH diluted by 50% dissolved the PBM-B tubes.
No further dilutions of iPrOH or EtOH were able to effectively cause dissolution within 24 hours of observation (Table 1). Nor were EtOH or iPrOH diluted by greater than 50% able to dissolve PBM-B tubes even with constant direct exposure. Therefore, in the ensuing experiment determined to consider the effect of time limited interrupted exposure of alcohol on the mechanical properties of PBM-B, only solutions diluted by less than 50% were utilized. In total, 100% iPrOH and 95% EtOH were tested undiluted and then when diluted by 25 and 50 percent. After the first administration of alcohol, iPROH both undiluted and diluted by 25 percent was able to actuate dissolution (Figure 6). A following administration allowed for effective dissolution utilizing undiluted 95% EtOH. A third administration yielded dissolution with EtOH diluted by 25%. With the remaining solutions of iPrOH and EtOH diluted by 50%, a fourth and final administration was not able to provoke dissolution. The results were identical between the sets two trials with PBM-B.
Table 1. Direct exposure of 85% PBM with 15% Steryl alcohol (PBM-B) to Isopropyl Alcohol and Ethanol.
| Alcohol Concentration % | 20 Minutes | 40 Minutes | 60 minutes | 24 Hours | 48 Hours | |
|---|---|---|---|---|---|---|
| 100 | iPrOH | + | Dissolved | --- | --- | --- |
| EtOH | Dissolved | --- | --- | --- | --- | |
| 75 | iPrOH | Dissolved | --- | --- | --- | --- |
| EtOH | Dissolved | --- | --- | --- | --- | |
| 50 | iPrOH | + | + | Dissolved | --- | --- |
| EtOH | + | + | + | Dissolved | --- | |
| 25 | iPrOH | + | + | + | + | + |
| EtOH | + | + | + | + | + | |
| 10 | iPrOH | + | + | + | + | + |
| EtOH | + | + | + | + | + | |
| 5 | iPrOH | + | + | + | + | + |
| EtOH | + | + | + | + | + | |
+ Undissolved ---Test not completed due to dissolution at previous earlier time interval
Figure 6.
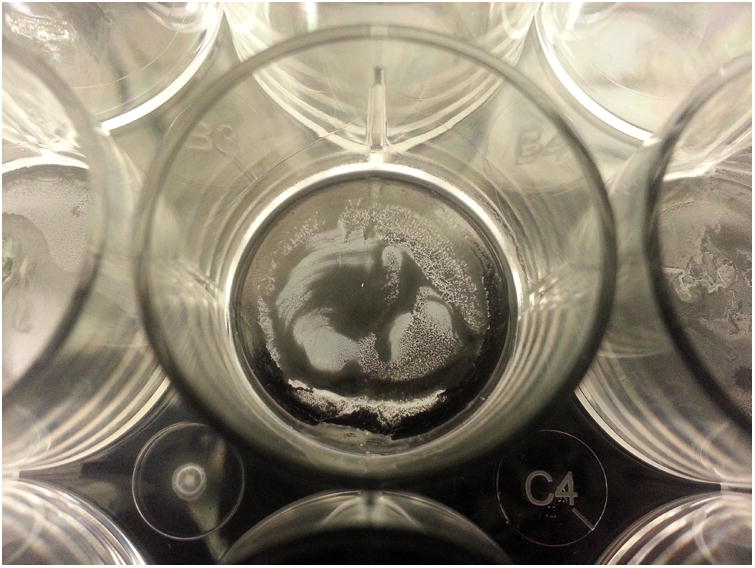
Representative example of tube dissolution (75% Isopropyl Alcohol First Administration)
Discussion
Biodegradable ear tubes have been studied by other researchers but none have created tubes that are “biocommandible” – i.e., dissolvable “on command” [10][11]. Rather, the other tubes, unlike those in this study, are designed to degrade naturally at pre-determined rates and begin to lose their mechanical integrity from the moment of insertion. Though progressive, the degradation rate is difficult to predict since the milieu of the middle ear varies with moisture or infection. Moreover, the tubes that have been developed thus far disintegrate well before the one year needed for typical treatment of otitis media.
Other researchers have attempted to design and fabricate dissolvable ear tubes. Sherman's group fabricated ear tubes from Calcium Alginate and tested the mechanical strength of their tubes compared to off the shelf tubes. They observed that exposure of alginate tubes to otological solutions for 24 hours resulted in degradation of their mechanical properties; Sherman's group has yet to test these ear tubes in living animals [11].
D'Eredita's group constructed ear tubes from poly-bis(ethylanate)phosphazene (PBE), tested them in guinea pigs and observed that there was neither infection nor an inflammatory reaction to the tube within the middle ear in any animal. At 30 days, 53% of the tubes had disintegrated spontaneously. At 60 days, only 25% of the tubes were still functioning [10]. They concluded that more research must be performed before these new PBE PE tubes can be considered for clinical use.
Notably, the degradation rate for the tubes examined by Sherman and D'Eredita appears unpredictable. Hence, the longevity of these tubes is inadequate, as degradation occurs well before the minimum period needed for proper treatment of otitis media and eustachian tube maturation. By contrast, our tubes maintain form and function and appear to degrade on command only at a time determined and prescribed by a physician.
In addition, because of their dissolvability, our biocommandible tubes will not have to be surgically removed and there would be reduced need for further procedures under general anesthesia for removal of prolonged and retained tubes. This is important since research suggests that excessive general anesthesia may affect learning in children under 3 years of age [12].
Finally, an ear tube that can be dissolved “on demand” would provide the potential benefit of lower perforation rates, particularly from tubes implanted over 24 months while awaiting spontaneous extrusion. Our design could remain for the desired duration that clinicians deem necessary for children to outgrow the otitis media prone time period. The clinician would then apply ear drops to patients as a simple medical procedure once they determine that the risk of ear infections has decreased, or that the child's eustachian tube has developed enough to adequately drain middle ear fluid on its own.
The results of this study further support the overall biocompatibility of PBM, which has previously been established as a coating for enteric tubes. All rats in the study demonstrated consistent weight gain until the time of euthanasia. Furthermore, the rats in the experimental group did not exhibit any signs of distress after the immediate post-operative period. Interestingly, histopathologic sectioning of the rats implanted with the PBM tubes did not reveal any abnormalities in contrast to granulation tissue that was seen surrounding the implantation site of commercially available tympanostomy tubes.
The benchtop dissolution studies provided proof of feasibility as well as a proof of concept for our biocommandible tubes. The dissolution of the tube material is expected to involve two processes: The first is diffusion of the alcohol solvent and the second is unraveling of the polymer chains. It is generally accepted that molecules of solvent interacts with functional groups and weaken the intermolecular forces that hold the chain together by replacing chain-chain bonds by chain-solvent bonds. These interactions may be of an electrostatic nature with nitrogen cations or H-bonds with hydroxyl groups present on the polymer chain. Once the solvent has replaced all of the chain-chain bonds, the structure of the polymer unravels.
Exposure directly to concentrations of at least 50% iPrOH and EtOH were able to effect dissolution of PBM. Furthermore, when administered in a simulated clinical application regimen both alcohols were able to effect dissolution, albeit at a higher concentration than direct exposure. Moreover, when the experimental polymers were exposed to materials ordinarily in the environment such as water, and soapy water, the tubes retained their mechanical integrity. The tubes were similarly resistant to degradation from ofloxin drops and ciprofloxin after 24hrs of direct exposure.
We recognize that our PMB prototype tube is still in its preclinical stages. The tube will require longer study periods in a better simulated environment. Our study only examined the tubes for 44 days and the feasibility of dissolving this tube in a real-life scenario is yet to be examined, however experiments with chinchillas are underway. Because of this concern about ototoxicity we have eliminated the need for any ethanol and have focused future studies exclusively on hydrogen peroxide, H2O2, which breaks down into water and oxygen, and also appears to degrade on PMB polymer in in just a few days.
The concept of “biocommandability” should not be limited to the application of tympanostomy tubes. For example, temporary steroid eluding bio-absorbable stents have recently been developed for use in sinus surgery [13]. We anticipate that with this “on-demand” technology, clinicians will have much better control of the life-span and functional period of the implant with a simple application of a special nasal spray that will trigger the stent to dissolve. In a similar vein, “biocommandability” is not limited to the field of otolaryngology. Across all medical specialties there are many examples where placement of temporary foreign bodies, whether it be nasolacrimal duct stents in ophthalmology, ureteral stents and catheters in urology, or esophageal stents in general surgery, could potentially benefit from controlled and directed degradation.
We recognize that our study design has intrinsic limitations that restrict its further generalizability. Our study design utilized the Wistar rat as the animal model and the squamous epithelium on their dorsum as a corollary for the human eardrum. In contrast, the chinchilla (Chinchilla lanigera) or Guinea pig (Cavia porcellus) animal model is widely utilized in otology research because the tympanic membrane and middle ear anatomy are akin to humans and we plan on using these animals in future studies[14]. Furthermore, the utilization of alcohol as the stimulus of dissolution is arguable. Ethanol has been previously reported as ototoxic in the animal models causing a decrease in cochlear microphonic and endocochlear potential in guinea pigs and chinchillas respectively [15]. Ohashi and colleagues, in their experimentation with vaporized isopropyl alcohol and guinea pig middle ear mucosa, found moderate deterioration of the ciliary activity and severe damage of epithelial cells with exposure to extremely high concentrations of isopropyl alcohol [16,17]. Because of this concern about ototoxicity we have focused future studies exclusively on hydrogen peroxide, H2O2, which breaks down into water and oxygen, and also appears to degrade on PMB polymer in rapid fashion.
We plan additional experimentation and exploration in this nascent field of “biocommandibilty”. Having shown lack of biologic reactivity in the rat model, we will proceed to our next experiment to place PBM myringotomy tubes in the tympanic membranes of chinchillas with hydrogen peroxide. In the interim, our benchtop studies to refine the dissolving stimulus will continue.
Conclusion
This study is an important first step is developing biocommandible ear tubes for the pediatric population. At present, there is no dissolvable “on command” surgical implant for the human body. To this end, this project is an advance towards an ideal tympanostomy tube; one constructed of a biocompatible polymer that dissolves rapidly when desired by the clinician in a predictable fashion after being exposed to a controlled stimulus.
Acknowledgments
We would like to acknowledge the contributions of Carolyn Cochenour, BBME with assistance in conceptualization and the engineering of the experimental tympanostomy tubes.
Financial disclosure: Dr. Brian Reilly received funding for this project through the NIH National Center for Advancing Translational Sciences (CTSI-CN) with Grant Award UL1TR000075 and KL2TR000076.
Footnotes
Conflict of Interest: Dr. Brian Reilly and Dr. Matthieu Dumont submitted for Dissolvable on-command implant patent US 20150057590A1 which is under review by the US Patent Office.
Level of Evidence: NA. Original Experiment
References
- 1.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 2.Gates G. Cost-effectiveness considerations in otitis media treatment. Otolaryngol Head and Neck Surg. 1996;114(4):525–30. doi: 10.1016/S0194-59989670243-7. [DOI] [PubMed] [Google Scholar]
- 3.Roberts J, Hunter L, Gravel J, et al. Otitis media, hearing loss, and language learning: controversies and current research. Journal Dev Pediatr. 2004;25(2):110–122. doi: 10.1097/00004703-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kangsanarak J, Fooanant S, Ruckphaopunt K, et al. Extracranial and intracranial complications of suppurative otitis media. Report of 102 cases. J of Laryngol Otol. 1993;107(11):999–1004. doi: 10.1017/s0022215100125095. [DOI] [PubMed] [Google Scholar]
- 5.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;28(11):1–25. [PubMed] [Google Scholar]
- 6.Weigel MT, Parker MY, Goldsmith MM, et al. A prospective randomized study of four commonly used tympanostomy tubes. Laryngoscope. 1989;99(3):252–256. doi: 10.1288/00005537-198903000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya N. Ambulatory pediatric otolaryngologic procedures in the United States: Characteristics and perioperative safety. Laryngoscope. 2010;120(4):821–825. doi: 10.1002/lary.20852. [DOI] [PubMed] [Google Scholar]
- 8.Paradise JL. On tympanostomy tubes: rationale, results, reservations, and recommendations. Pediatrics. 1977;60(1):86–90. [PubMed] [Google Scholar]
- 9.Armstrong BW. A new treatment for chronic secretory otitis media. AMA Arch Otolaryngol. 1954;59(6):653–654. doi: 10.1001/archotol.1954.00710050665001. [DOI] [PubMed] [Google Scholar]
- 10.D'Eredità R, Marsh RR, Lora S, Kazahaya K. A new absorbable pressure-equalizing tube. Otolaryngol Head Neck Surg. 2002;127(1):67–72. doi: 10.1067/mhn.2002.126722. [DOI] [PubMed] [Google Scholar]
- 11.Sherman EG, Antonelli PJ, Tran-Son-Tay R. Development of a calcium alginate tympanostomy tube. Laryngoscope. 2010;120(12):2473–2477. doi: 10.1002/lary.20981. [DOI] [PubMed] [Google Scholar]
- 12.Wilder RT, Flick JP, Sprung J, et al. Early Exposure to Anesthesia and Learning Disabilities in a Population-based Birth Cohort. Anesthesiology. 2009;110(4):796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murr AH, Smith TL, Hwang PH, et al. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent. Int Forum Allergy Rhinol. 2011;1(1):23–32. doi: 10.1002/alr.20020. [DOI] [PubMed] [Google Scholar]
- 14.Wang AY, Shen Y, Wang JT, et al. Animal models of chronic tympanic membrane perforation: A ‘time-out’ to review evidence and standardize design. Int J Pedtatric Otorhinolarynglol. 2014;78(12):2048–2055. doi: 10.1016/j.ijporl.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Morizono T, Sikora MA. Ototoxicity of ethanol in the tympanic cleft in animals. Acta Otolaryngol. 1981;92(1-2):33–40. doi: 10.3109/00016488109133235. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi Yoshihiro, et al. Acute effects of isopropyl alcohol exposure on the middle ear mucosa. Journal of Applied Toxicology. 1987;7:3. 205–211. doi: 10.1002/jat.2550070310. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi Yoshihiro, et al. Toxicity of isopropyl alcohol exposure on the nasal mucociliary system in the guinea pig. Environmental research. 1988;461:25–38. doi: 10.1016/s0013-9351(88)80056-2. [DOI] [PubMed] [Google Scholar]


