Abstract
Actigraphy has attracted much attention for assessing physical activity in the past decade. Many algorithms have been developed to automate the analysis process, but none has targeted a general model to discover related features for detecting or predicting mobility function, or more specifically, mobility impairment and major mobility disability (MMD). Men (N=357) and women (N=778) aged 70–89 years wore a tri-axial accelerometer (Actigraph GT3X) on the right hip during free-living conditions for 8.4±3.0 days. One-second epoch data were summarized into 67 features. Several machine learning techniques were used to select features from the free-living condition to predict mobility impairment, defined as 400 m walking speed <0.80 m/sec. Selected features were also included in a model to predict the first occurrence of MMD— defined as the loss in the ability to walk 400 meters. Each method yielded a similar estimate of 400 meter walking speed with a root mean square error of ~0.07 m/sec and an R-squared values ranging from 0.37–0.41. Sensitivity and specificity of identifying slow walkers was approximately 70% and 80% for all methods, respectively. The top five features, which were related to movement pace and amount (activity counts and steps), length in activity engagement (bout length), accumulation patterns of activity, and movement variability significantly improved the prediction of MMD beyond that found with common covariates (age, diseases, anthropometry etc.). This study identified a subset of actigraphy features collected in free-living conditions that are moderately accurate in identifying persons with clinically-assessed mobility impaired and significantly improve the prediction of MMD. These findings suggest that the combination of features as opposed to a specific feature is important to consider when choosing features and/or combinations of features for prediction of mobility phenotypes in older adults.
Keywords: aging, disability, physical activity, sedentary, machine learning, data mining
1. Introduction
Over the past decade, actigraphy monitors (e.g. accelerometers) have been widely used for monitoring physical activities (Aminian and Najafi 2004; Zijlstra and Aminian 2007). Improvements in technology now provide high-resolution multi-axis data that can accurately characterize movement and mobility (Lemoyne et al. 2008). Despite the accuracy in detecting ambulation (Pruitt et al. 2008), very few studies have expanded these data beyond physical activity ascertainment. This is partly due to the complex nature of these data that often requires non-traditional methods to process and extract meaningful information. Machine learning techniques have become popular for this purpose, but they have typically been applied to characterizing physical activity type and intensity (Kwapisz et al. 2011; Lakka et al. 2003). Characterizing specific features of the actigraphy signal could have broader applications for identifying mobility issues in older adults regardless of the types and intensity of physical activity. One could envision using body worn actigraphs for quantifying the degree and quality of movement patterns, similar to the way in which a heart rate Holter monitor evaluates cardiac function in free-living conditions.
Multi-axis accelerometers are currently being used to detect gait quality in overt disease conditions such as Parkinson’s and when attempting to characterize fall events (Cancela et al. 2011). Results from these studies suggest that data from multidimensional accelerometers are correlated with the severity of mobility disturbances both in the laboratory (Weiss et al. 2011) and in free-living conditions (Frenkel-Toledo et al. 2005; Schaafsma et al. 2003). Recent work suggests that transitions in movement recorded by an accelerometer (e.g. sit to stand) can discriminate between young and older adults, and fallers versus non-fallers (Iluz et al. 2015; Weiss et al. 2013). This work provides the foundation for using accelerometers to predict mobility impairment risk individually or in conjunction with traditional clinic-based and medically oriented markers.
There is little work done to understand the connection between activity patterns and mobility in older adults. Hip-worn accelerometers provide a unique opportunity to extract data patterns related to not only activity, but also gaps in activity time (i.e. bouts of sedentary time). Regarding the latter, sedentary behavior is an independent predictor of several chronic diseases (Ensrud et al. 2014), physical frailty (Cawthon et al. 2015; Song et al. 2015) and future decline in physical function (Langenberg et al. 2015). However, despite this knowledge, most of the existing work has focused on laboratory-based measures of mobility in patients with overt gait abnormalities (e.g., Parkinson’s Disease) (Weiss et al. 2011). Capturing free-living accelerometry data over an extended duration might reveal subtle signatures of current and/or future limitation. This information can provide an accelerometry profile that indicates future mobility problems.
In this paper, we aim to extend previous findings in the laboratory by examining accelerometer data to predicting mobility impairments. The overarching process involved extracting, selecting, and evaluating features from the actigraphy signal from a large source of multi-dimensional actigraphy data along from older adults enrolled in the Lifestyle Interventions and Independence for Elders (LIFE) study. In this study, free-living data for each participant was collected from a hip-worn accelerometer for a period of approximately one week. Our primary goal was to identify actigraphy features that are connected to a phenotype of mobility of impairment of slow walking speed — a strong predictor of mobility disability in older adults (Perera et al. 2015; Studenski et al. 2011). We then extended this evaluation to determine whether the features also predicted other mobility outcomes— specifically the inability to walk 400 meters. This approach is a novel extension from its original use, which was to monitor adherence and compliance to the physical activity intervention in the LIFE study.
2. Materials and methods
2.1. Participants
The LIFE study was a multicenter, single-blinded, parallel randomized trial (N=1635) conducted at 8 field centers across the U.S (Fielding et al. 2011). Participants were enrolled between February 2010 and December 2011 and who participated in the intervention for an average of 2.6 years. The inclusion criteria for the LIFE study were: (1) age 70–89 years, (2) summary score <10 on the Short Physical Performance Battery (SPPB) (Guralnik et al. 1995), (3) sedentary lifestyle, (4) ability to complete a 400m walk test, (5) ability to safely participate in the intervention as determined by medical history, physical exam and resting ECG, and (6) no major cognitive impairment (Modified Mini-Mental State Examination [3MSE] 1.5 standard deviations below education- and race-specific norms). Institutional review boards approved the study protocol at all sites. Written informed consent was obtained from all study participants. The study was monitored by a data and safety monitoring board appointed by the National Institute on Aging. The trial is registered at ClinicalsTrials.gov with the identifier NCT01072500.
2.2. Accelerometry
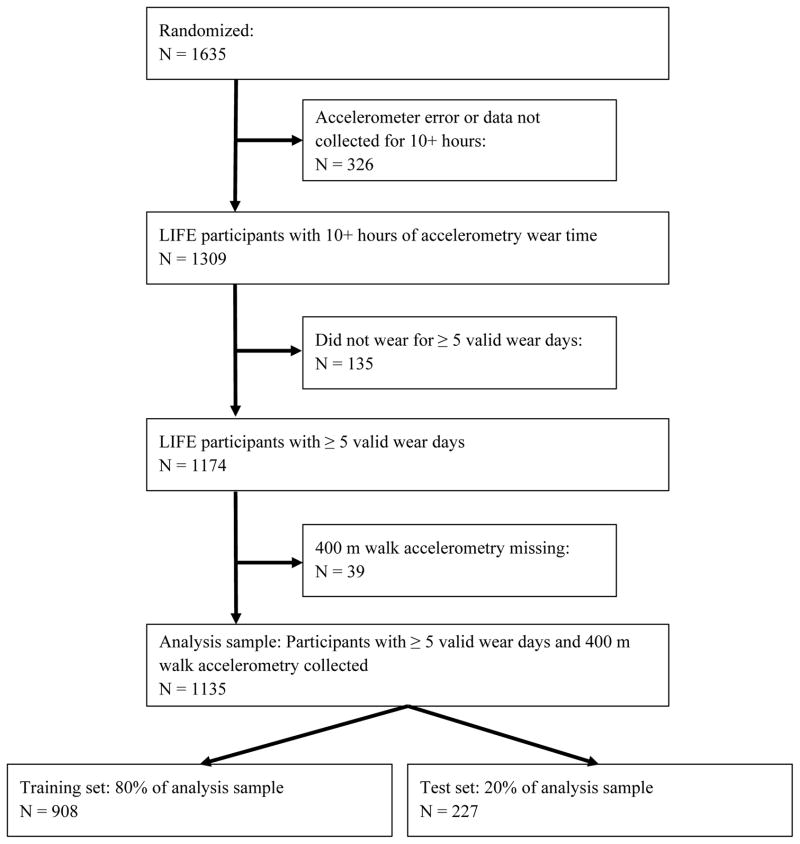
For this study, the analysis was performed on the baseline accelerometry data that were collected from a hip worn Actigraph GT3X. The device was worn on the right hip, which is considered an ideal location for capturing ambulatory activity and information about kinematics of gait (Preece et al. 2009). The Tri-axial accelerometer sampled movements in three directions (horizontal, perpendicular and vertical) and activity count data (calculated for 1 second epochs) were collected for approximately one week on each participant. Raw acceleration data was not collected due to limits in length of data collection, which was a maximum of a few hours for this version of hardware. The sample was constrained to participants having ≥5 valid days where a valid day was defined as having ≥10 hours of wear time. We followed standard guidelines for detecting time intervals when a participant was not wearing the monitor (i.e., non-wear) by calculating non-wear time using an amplitude threshold of zero for a period of a consecutive 90-min time window with an allowance of a 2-min interval of nonzero counts (Choi et al. 2011). There were 1135 participants who met all the necessary criteria to be included in the analysis. Figure 1 illustrates a flow diagram with the eligible participants available after applying the constraints. Version 2.1 of the LIFE accelerometry dataset was used for all computations.
Figure 1.
Eligible participant flow diagram for accelerometry analyses.
2.3. Walking speed, major mobility disability (MMD) and other measures
The walk speed was obtained from the baseline 400 m walk test that corresponded to participant’s baseline accelerometry data (Fielding et al. 2011; Pahor et al. 2014). Briefly, participants were asked to walk 400 m at their usual pace, without over-exerting, on a 20 m course for 10 laps (40 m per lap). Participants were allowed to stop for up to 1 minute for fatigue or other clinical symptoms. All participants were able to walk 400 m at baseline, but participants walking slower than <0.80 m/sec were defined as having mobility impairments (Perera et al. 2015; Studenski et al. 2011.) Participants were then re-evaluated every 6 months to measure the future occurrence of MMD— defined as the inability to complete the 400 m walk within 15 min without sitting and without the help of another person or walker. Use of a cane was acceptable. When MMD could not be objectively measured because of the inability of the participant to come to the clinic and also absence of a suitable walking course at the participant’s home, institution or hospital, an alternative adjudication of the outcome was based on objective inability to walk 4 m in <10 sec, or self-, proxy-, or medical record-reported inability to walk across a room. If participants met these alternative criteria, they would be classified as not being able to complete the 400 m walk within 15 minutes.
The main baseline measures included self-reported demographic characteristics of race and ethnicity reported according to National Institutes of Health (NIH) requirements, anthropometrics, medical and hospitalization history, cognitive function assessed with the Modified Mini-Mental Status Exam (Evelyn and Chui 1987), and the short physical performance battery (SPPB). These measures are described in detail elsewhere (Fielding et al. 2011; Marsh et al. 2013; Pahor et al. 2014).
2.4. Analysis design: feature extraction, selection and evaluation
The overall analytic design involved a four-pronged approach of extracting, selecting, evaluating and assessing the predictive value of actigraphy features. Feature extraction is a comprehensive approach to derive time-domain features in the temporal tri-axial accelerometry data. For the second step, machine learning methods were applied to select the features and evaluate the sensitivity and specificity to detect a low usual walking speed < 0.80 m/sec — a marker of mobility function (Perera et al. 2015; Studenski et al. 2011). Lastly, selected features were evaluated for their ability to predict the occurrence of MMD across a period of 24-months. More analytic details of these procedures are described in the subsequent sections.
In the first step, the activity count data were converted into feature vectors, where each vector (data point) represented a participant. Features were based on the time domain and were chosen from knowledge in the literature, visualization of the data and expert opinion from the coauthors (Ellis et al. 2014a; Ellis et al. 2014b; Ellis et al. 2014c; Kate et al. 2016). Frequency domain features were not calculated because the resolution did not satisfy the minimum rate at which a human movement signal (0.5 – 2.5 Hz) can be sampled without introducing errors, which is twice the highest frequency present in the signal (i.e. nyquist frequency). Within each daily identified wear time, bouts of activity were identified as time intervals, which had ≥100 activity counts per minute and <30 consecutive ≤2 activity counts per second. Gaps in activity where a participant was unlikely to be ambulatory were defined as <100 counts per minute. A total of 67 features that characterized the time-domain signal were extracted using custom code in Matlab®. Each feature was normalized to have a mean of zero and standard deviation of one. The features are described in detail in the supplementary table.
The next step was to select the major features associated with 400 m walking speed (assessed in meters/sec). Since many of the features were derived from correlated axes and vector magnitude, a variance inflation factor with correlation coefficient threshold of 0.9 was applied to reduce the number of features that possessed a high level of collinearity. We then conducted univariate Pearson correlations between each selected feature and 400 m walking speed. Next, assuming sample Xi has n different attributes Xi = {ai1, ai2, …, ain}, we chose those k features (k < n) which are sufficient to best explain the target Yi (walk speed). One straightforward solution would be examining every subset of k features, and select the best combination. However, there are possible subsets, which is impractical for traditional statistical approaches. We applied several well-known machine learning algorithms for feature selection: sequential feature selection (Pudil et al. 1994), LASSO (Tibshirani 1996), ridge regression (Hoerl and Kennard 1970), and elastic net (Zou and Hastie 2005). The general goal was to identify related features, as well as their linear combination ( ) such that they produced the target (walk speed) to its actual value (Yi). The methods minimize the sum of squared errors according to the following minimization parameters (assuming we have M samples):
Each method has strengths and limitations and has slightly different characteristics to minimize the sum of squared errors (RMSE). For regression-based methods, LASSO places a penalty on the number of features used as regressors, ridge regression adds a penalty to the summation of coefficients that are assigned to the features, elastic net applies a penalty to both the number of features used as regressors and the coefficients simultaneously. Sequential feature section works differently as it interactively adds features until the objective function, in this case RMSE, is minimized. We applied a 5-fold cross validation, such that four portions of the training set were used to learn a model and one test portion was used for evaluation purposes. Figure 1 includes information about the training and test datasets.
The predicted walk speed values of each machine learning technique were evaluated for its fit using root mean square error (RMSE) and R-squared values on the test sample. Additionally, the test sample was divided into those with walking speed <0.8 m/s as positive for mobility impairment and ≥0.8 m/s as negative for mobility impairment. Accuracy, sensitivity and specificity were calculated for each method. In order to gain an understanding about the importance of each feature, we ranked them based on their importance score. Importance score for each feature was computed as the summation of their assigned coefficients by linear regression weighted by the accuracy of the selected subset; i.e. If = Σs∈{SFS,LASSO,Ridge,Elastic} Cf,s × Accs, where If is the importance score for feature f, Cf,s is the coefficient assigned by linear regression and Accs is the accuracy for the selected subset. Features were sorted based on their importance score ranking (from highest to lowest) and incrementally added to the predictive model. For the sake of clarity consider the following example: method A selects features {f0 and f1} and method B favors {f0 and f2} – the features are sorted based on their coefficients. The features f1 and f2 are correlated (e.g., correlation > 0.5). If Cf1,A * AccA > Cf2,B * AccB then the final model will be {f0 and f1}; otherwise f2 would be included. This systematic stepwise process guarantees that the final model will include the features that were unanimously selected and include features contributing to better accuracy will be favored over their correlated counterparts.”
The next step in the analysis was to establish concurrent validity— ability to predict other outcomes— of the features. To do this, the features were used to predict the occurrence of a MMD outcome. For this approach nested Cox proportional hazards regression models were fit to evaluate the top five highest ranked actigraphy features for predicting the first occurrence of MMD. The approach fits nested models by sequentially adding blocks of variables and then comparing the relative fit of each model, one block at a time. A likelihood-ratio test along with the Harell’s Concordance Statistic (C-statistic) which measures the probability of concordance between the predicted and observed survival — a value of 0.50 equates to random predictions and a value of 1.0 equates to a perfectly discriminating model was used to evaluate the addition of each block. The blocks were designed to evaluate whether actigraphy features have added value to predicting MMD while also considering established predictors of disability in general. The blocks included: 1) age, gender and intervention arm (physical activity or health education), 2) body mass and height, 3) 4-meter walking speed at baseline assessed using the SPPB test, 4) comorbidity count (sum of myocardial infarction, diabetes, diagnosed high blood pressure, history of cancer, stroke, cardiac heart failure, chronic pulmonary disease, and depression diagnosis) and 5) the top five actigraphy features. For comparison purposes, continuously distributed covariates were normalized to have a mean of zero and standard deviation of one. For Cox models, failure time was measured from the time of randomization; follow-up was censored at the last successfully completed 400 m walk test. For participants who did not have any outcome assessments, we assigned one hour of follow-up time, since we knew that they completed the 400m walk at baseline. Results were considered statistically significant at p ≤ 0.05.
3. Results
The main baseline characteristics of the overall analytic sample are as follows: average age 78.7 years, women 66.5%, average BMI 30.3 kg/m2, and average SPPB score 7.5 (indicating a low functioning population and at risk of mobility incidents). Women had slower gait speed than men (mean [SD], 0.82 [0.15] m/sec vs 0.88 [0.17] m/sec; P<0.001) but similar body mass indices (mean [SD], 30.4 [6.3] vs 30.0 [5.5]; P=0.34). Table 1 depicts demographics, behavioral factors, medical conditions, and actigraphy metrics for participants dichotomized by gait speed defined as <0.80 and ≥0.80 m/sec. Older adults with slower walking speed were more likely to be women and generally less healthy—lower SPPB scores, higher BMI, less self-reported time spent in walking and weight training activities, lower 3MSE scores, and higher rates of heart attack and hypertension.
Table 1.
Qualitative characteristics for the participants by their gait speed
| Characteristic | Baseline 400 meter walk speed < 0.8 m/s N = 451 |
Baseline 400 meter walk speed ≥ 0.8 m/s N = 684 |
P Value |
|---|---|---|---|
| Age (years) | 79.6 ± 5.4 | 78.1 ± 5.1 | < 0.01 |
| Women | 334 (74.1) | 444 (61.5) | < 0.01 |
| Ethnicity/race | |||
| Hispanic | 17 (3.8) | 26 (3.8) | 0.99 |
| Caucasian | 355 (78.7) | 545 (79.7) | 0.75 |
| African American | 79 (17.5) | 113 (16.5) | 0.72 |
| SPPB score | 6.7 ± 1.6 | 8.0 ± 1.3 | < 0.01 |
| SPPB score <8 | 286 (64.4) | 189 (27.6) | < 0.01 |
| 400 m walking speed (m/sec) | 0.68 ± 0.09 | 0.94 ± 0.10 | < 0.01 |
| Body mass index (kg/m2) | 31.5 ± 6.9 | 29.5 ± 5.3 | < 0.01 |
| Self-reported minutes per week in walking/weight training activities | 14.0 ± 29.5 | 20.0 ± 35.7 | 0.02 |
| 3MSE score (0–100 scale) | 91.6 ± 5.6 | 92.4 ± 5.0 | 0.02 |
| Conditions a | |||
| Hypertension | 346 (76.7) | 460 (67.2) | < 0.01 |
| Diabetes | 131 (29.0) | 178 (26.0) | 0.29 |
| Heart attack or myocardial infraction | 46 (10.2) | 41 (6.0) | 0.01 |
| Stroke | 33 (7.3) | 47 (6.9) | 0.87 |
| Cancer | 111 (24.6) | 140 (20.5) | 0.12 |
| Chronic pulmonary disease | 75 (16.6) | 103 (15.0) | 0.52 |
Results are in a subset of individuals (451 in < 0.8 m/s gait speed and 684 in ≥ 0.8 m/s gait speed) with valid accelerometry data. Data are means and standard deviations or n (%);
SPPB = short physical performance battery.
Some values may slightly differ from those previously published data because of differences in analysis participants.
BMI, body mass index; 3MSE, Modified Mini-Mental Status Exam; SD, standard deviation; GS, gait speed.
Self-reported, physician diagnosed.
LIFE datasets version 2.1 were used for analyses
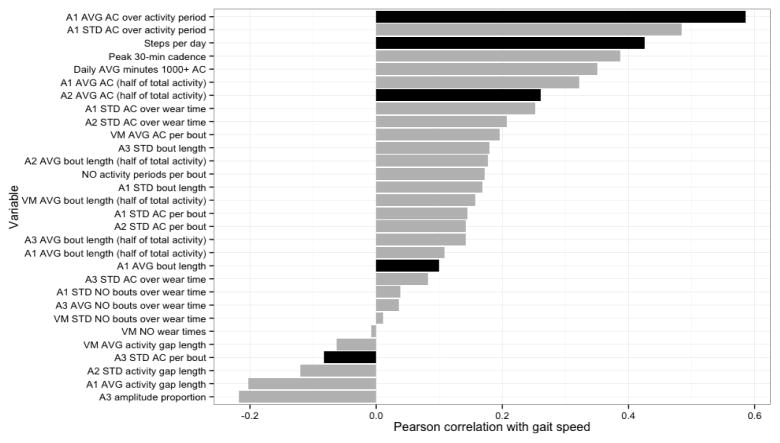
Sixty-seven features were extracted— 14 features for each axis and vector magnitude, amplitude proportion of each axis, one summary feature for vector magnitude, 2 step-related features, and 5 compound features (detailed description in the supplementary table). Application of the variance inflation factor reduced the number of features to 30 that are characterized in table 2. Univariate correlations between the 30 features and 400 meter walk speed are illustrated in figure 2. The average counts per second over activity periods had the highest positive correlation and the proportion of axis 3 counts/min to the total vector magnitude had the highest inverse correlation.
Table 2.
Descriptive characteristics of actigraphy wear times and actigraphy features without a high level of collinearity.
| Feature | Mean (SD) | Range (min and max) |
|---|---|---|
| Number of valid days worna | 8.4 (3.0) | 7 (5 – 21) |
| Average non-wear minutes per day | 559.7 (120.2) | 575.2 (0.9 – 786.2) |
| Average wear minutes per day | 845.8 (109.5) | 931.6 (631.6 – 1403.4) |
| Axis 3 Amplitude proportion | 0.73 (0.11) | 0.74 (0.26 – 0.96) |
| Axis 1 average number of active wear times (wear times) | 7.8 (3.0) | 7 (1 – 21) |
| Axis 1 standard deviation of number of bouts over wear time (bouts/wear time) | 17.2 (10.0) | 15.3 (0 – 105.7) |
| Axis 1 standard deviation of activity counts per bout (counts/bout) | 389.6 (90.2) | 379.9 (158.3 – 896.1) |
| Axis 1 average bout length (minutes) | 132.3 (26.9) | 128.3 (72.3 – 289.1) |
| Axis 1 standard deviation of bout length (minutes) | 143.4 (50.9) | 133.4 (48.9 – 356.7) |
| Axis 1 average of activity gap length (minutes) | 621.2 (272.2) | 565.2 (219.0 – 2772.0) |
| Axis 1 standard deviation of activity counts over wear times (counts/wear time) | 30015.2 (22371.4) | 24227.2 (0 – 172253.5) |
| Axis 1 average bout length where half of the total activity is accumulated (minutes) | 2.1 (0.5) | 2 (1 – 6) |
| Axis 1 average activity count where half of the total activity is accumulated (counts/minute) | 453.0 (110.4) | 442.7 (222.2 – 886.1) |
| Axis 1 average number of active wear times (wear times) | 7.8 (3.0) | 7 (1 – 21) |
| Axis 2 standard deviation of activity counts per bout (counts/bout) | 399.7 (85.8) | 391.6 (166.7 – 728.1) |
| Axis 2 standard deviation of activity gap length (minutes) | 980.9 (346.5) | 931.4 (305.1 – 2537.1) |
| Axis 2 standard deviation of activity counts over wear times (counts/wear time) | 40770.7 (26249.1) | 35183.0 (0 – 215984.6) |
| Axis 2 average bout length where half of the total activity is accumulated (minutes) | 3.0 (0.8) | 3 (1 – 6) |
| Axis 2 average activity count where half of the total activity is accumulated (counts/minutes) | 590.7 (135.0) | 586.4 (233.1 – 1126.1) |
| Axis 3 average number of bouts over wear time (bouts/wear time) | 77.8 (21.0) | 75.5 (30.3 – 279.3) |
| Axis 3 standard deviation of number of bouts over wear time (bouts/wear time) | 458.7 (110.1) | 447.6 (223.9 – 1011.2) |
| Axis 3 standard deviation of bout length (minutes) | 223.0 (76.8) | 208.6 (73.9 – 649.6) |
| Axis 3 standard deviation of activity counts over wear times (counts/wear time) | 56748.2 (35799.1) | 48860.7 (0 – 326040.0) |
| Axis 3 average bout length where half of the total activity is accumulated (minutes) | 3.1 (0.9) | 3 (1 – 7) |
| Axis 3 average number of bouts over wear time (bouts/wear time) | 77.8 (21.0) | 75.5 (30.3 – 279.3) |
| VM average number of wear times (wear times) | 7.8 | 7 (1 – 21) |
| VM standard deviation of number of bouts over wear time (bouts/wear time) | 17.4 (11.0) | 15.4 (0 – 122.9) |
| VM average activity counts per bout (counts/bout) | 1036.4 (208.2) | 1014.4 (469.7 – 2104.9) |
| VM average activity gap length (minutes) | 440.0 (164.0) | 413.5 (145.9 – 1368.6) |
| VM average bout length where half of the total activity is accumulated (minutes) | 3.7 (1.1) | 4 (2 – 9) |
| Number of activity periods found per bout (activity periods) | 0.1 (0.1) | 0 (0 – 0.4) |
| Axis 1 average activity counts over activity periods (counts/minute) | 1107 (456.6) | 1062.6 (163.8 – 3060.0) |
| Axis 1 standard deviation of activity counts over activity periods (count/minute) | 823.8 (327.6) | 795.6 (119.4 – 1612.8) |
| Average steps per day (steps) | 2744.9 (1366.5) | 2505.4 (274.6 – 10238.2) |
| Average daily number of minutes with 1000+ activity counts (minutes) | 16.5 (16.0) | 11.4 (0.4 – 148.2) |
| Average number of steps for the 30 minute cadence with maximum number of steps (steps/minute) | 18.9 (12.4) | 15.5 (1.7 – 94.8) |
Data is constrained to participants who wore the monitor for five valid wear days (>10 hours per day).
Figure 2.
Univariate correlation between features and walking speed (target variable). The minimal subset of features used for mobility assessment is bolded. AVG, average; STD, standard deviation; AC, activity counts; Ai, ith axis (e.g. A1= axis 1); VM, vector magnitude; NO, number of. The black bars were selected by machine learning techniques in Table 3 and the ranking algorithm described in the methods.
Table 3 shows the results of estimating accuracy between the actual and predicted values by each machine learning technique. Each machine learning technique performed similarly with Sequential Feature Selection having the least RMSE (0.07 m/sec) and LASSO with the best R-squared value (0.39). In terms of sensitivity and specificity, Elastic Net showed the highest sensitivity (71.3%) and Sequential Feature Selection had the highest specificity (83.6%). The five highest ranked features, according to their importance score, were: 1) average axis 1 counts per min during active periods, 2) standard deviation of axis 3 activity counts during bouts of activity, 3) number of steps per day, 4) average axis 2 activity counts where half of the total activity is accumulated, and 5) average duration of axis 1 bouts of activity. Applying the final list of top-5 features slightly improved the R-squared value (0.4107) and produced an accuracy of 78.8%, sensitivity of 72.4%, and specificity of 82.9% for classifying older adults with slow walking speed.
Table 3.
Results of fitting a linear model on subsets of features selected by different methods.
| Method | RMSE | R2 | # Miss | # Correct | % Correct | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Sequential Feature Selection | 0.070 | 0.374 | 51 | 176 | 77.53 | 0.68 | 0.84 |
| LASSO | 0.073 | 0.389 | 53 | 174 | 76.65 | 0.70 | 0.81 |
| Ridge Regression | 0.075 | 0.375 | 52 | 175 | 77.09 | 0.71 | 0.81 |
| Elastic Net | 0.072 | 0.388 | 51 | 176 | 77.53 | 0.71 | 0.81 |
| Top five featuresa | 0.072 | 0.410 | 47 | 180 | 79.30 | 0.74 | 0.83 |
Sensitivity, specificity and accuracy (# miss, # correct and % correct) calculated as classifying older adults with mobility impairment defined as walking speed <0.8 m/s.
Computed the summation of each assigned coefficients by weighted linear regression as described in the methods.
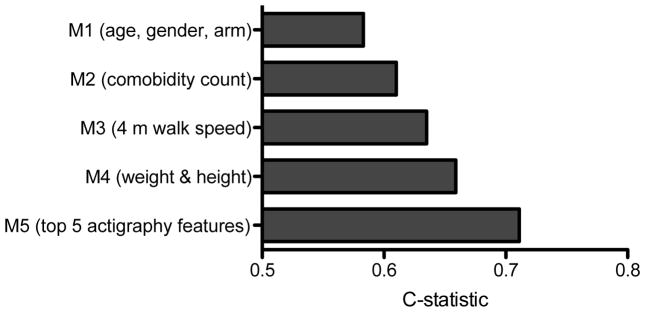
Over an average of 2.26 years (range: 0.01 – 3.6 years) of follow-up, 337 participants (131 per 1000 person-years) had a MMD event with an overall cumulative event rate of 29.7%. Each model in the sequence of Cox proportional hazard regression models demonstrated a significant improvement in fit (likelihood ratio test for addition of each block, p-value < 0.01). Figure 3 illustrates an increase in the C-statistic for each model and a final C-statistic of 0.70 after the five highest ranked actigraphy features were added to the model.
Figure 3.
Harell’s C-statistic where a value of 0.50 equates to a random chance of prediction was derived from post-estimations of Cox proportional hazards regression for predicting incident MMD (i.e., major mobility disability or the inability to walk 400 m). The results for sequential nested models: model 1 with covariates for age, gender and intervention arm, model 2 adds comorbidity count, model 3 adds 4-meter walking speed, model 4 adds body weight and height and model 5 adds the top five actigraphy features. All models are statistically significant and each sequential model represented a significant increase in prediction of MMD (p<0.05).
4. Discussion
The current study investigated a variety of actigraphy features derived on a large sample of older adults in free-living conditions. The main findings are that: 1) some of the most common machine learning techniques provide a similar predictive capacity for estimating walking speed and slow walking pace from actigraphy features; 2) despite a large number of derived actigraphy features, a smaller subset of only five appear to be able to capture the same information as the full 30 for estimating walking speed and slow walking speed; and 3) activity count amplitude, steps per day and some new actigraphy features, not commonly ascertained in the signal (e.g., variation in axis 3), improved the prediction of incident MMD in older adults. The results add to the existing literature regarding identification of important actigraphy features for estimating walking ability among community-dwelling older adults. They also contribute new knowledge about how actigraphy features might predict the occurrence of mobility disability that is specific to not only the amount and intensity of free-living physical activity, but also the variability in those movements in different anatomical orientations.
Several studies have sought to measure mobility in older adults using features constructed from accelerometer data. For example, (SankarPandi et al. 2014) extracted features such as the mean and standard deviation from accelerometer data collected during Time Up and Go (TUG) test. (Ezeugwu et al. 2015) evaluated the volume (e.g., sedentary and activity times) and pattern of activity and non-active (e.g., the gaps in activity) features (e.g., number of breaks in sedentary/activity time) to identify activity/inactivity behaviors in patients with multiple sclerosis. Additionally, there are a growing number of studies that exploit the raw signals to recognize specific physical activities (Ellis et al. 2014a; Ellis et al. 2014b; Ellis et al. 2014c) in free-living conditions. These results have also been extended to mobility impairments in patient populations such as Parkinson’s disease (Ellis et al. 2015). For example, Palmerini and colleagues demonstrated that frequency domain characteristics (high frequency power and frequency dispersion) from an accelerometer fixed to the lower back were associated with Parkinson’s disease severity and function (Palmerini et al. 2011). This study extends these concepts by deriving, selecting and evaluating actigraphy features in a large sample of older adults to predict the development of MMD without overt movement disorders.
Machine learning techniques are now commonly used to reduce the high dimensional actigraphy data (Garcia-Ceja et al. 2014; Gupta and Dallas 2014; Rothney et al. 2007; Staudenmayer et al. 2009). These techniques are useful because they are robust for sorting through a large number of features to identify individual and/or combinations that estimate a phenotype. Most research has focused on using these techniques to help recognize activity type and intensity. However, we took a different approach by using these techniques to estimate a clinically meaningful mobility phenotype of walking speed, given that it is a major predictor of overall health in older adults and has been consider a marker of vital status in older adults (Perera et al. 2015; Studenski et al. 2011). Interestingly, each analytic technique employed in this study yielded similar predictive capacity and generally the same predictive error for walking speed and classification error for mobility impairment. In that regard, the classification of mobility impaired older adults— or those exhibiting slow walk speeds— using these techniques was moderate with a sensitivity of ~0.70, a specificity of ~0.80 and a ~76% correct classification rate. These results suggest that categorizing older adults into mobility impairment categories is plausible with actigraphy data collected in free-living conditions. However, the classification accuracy was not perfect and estimates should be considered moderate for diagnosing slow walkers or those who might have mobility impairments.
We derived a method to rank the features that most contributed to the model fit and derived a parsimonious model. The following five features yielded an almost identical prediction error, sensitivity, specificity and classification error as the full set of features: average axis 1 counts per min during active periods, average length of axis 1 bout of activity, average axis 2 activity count where half of the total activity is accumulated, standard deviation axis 3 activity counts, and number of steps per day. Interestingly, these features were not universally correlated with walking speed in univariate analyses, which suggests that the combination of features as opposed to a specific feature is important to consider when choosing features and/or combinations of features.
The top five features appear to fall into fundamental categories that relate to traditional physical activity factors of movement amount and speed, pattern of accumulating physical activity, engagement in activity, and variability in movement. The average count amplitude in axis 2 (anterior-posterior plane) and variability of counts in axis 3 (medio-lateral plane) may also provide pseudo-biomechanical insights in free-living conditions that relate to gait characteristics. Axis 1, which captures accelerations in the vertical direction or the superior-inferior plan, is often associated with ambulatory activity intensity (e.g., speed) (Bassett et al. 2014; Freedson et al. 1998; Troiano et al. 2008; Troiano et al. 2014). In fact, a previous study by (Sherman 2003) demonstrated a significant relationship between activity counts generated by a hip-worn accelerometer and walking velocity on a treadmill test (coefficients range from 0.47 to 0.94). Our findings show that the average counts per min derived during activity periods (activity with >3 minutes length and >80% of nonzero values during a wear bout) was correlated to walking speed to a similar extent (coefficient= 0.59). Interestingly, this relationship was observed in both the superior-inferior (axis 1) and medio-lateral (axis 2) planes. What is different here is that actigraphy data were collected in free-living conditions and not synced to a test performed in the clinic as done in previous studies. Additionally, the average bout length for superior-inferior (axis 1) was one of the top five features in our data. This variable is highly (and negatively) correlated with the number of activity bouts, and can be conceptualized as the strength in activity engagements. In univariate comparisons, we found that walking speed was associated with both the average bout length and the average number of bouts per day (in opposite directions). Such finding suggests that mobility impaired individuals engage in more bouts, but these bouts were generally shorter than individuals with higher walking speeds. These findings are in line with Manns and coworkers who showed that older adults with mobility limitations had shorter bouts of activity (Manns et al. 2015). Moreover, the variability of counts in axis 3 was a key feature for predicting walking speed. This is consistent with the literature demonstrating that variability in gait is associated with mobility impairments and a predictor of mobility-related disabilities (Brach et al. 2007). Importantly, this feature demonstrated a low univariate correlation and thus the combination of variability along with a proxy of movement speed via information from the vertical axis appears to be critical. Lastly, despite the rigorous processing to identify unique actigraphy features, the number of steps per day— one of the simplest features that is often standard output— appeared as one of the most important features. There is a wealth of information noting the association between number of steps and mobility studied (Tudor-Locke et al. 2011; Tudor-Locke et al. 2012) and it is considered as one of the most important features (if not the only feature) to consider when investigating mobility quality (Davis et al. 2011). Our finding reinforces the features as critical component to predicting mobility in older adults. Despite its high correlation with the gait speed (~ 0.4), the accuracy of steps per day in discriminating slow walkers was no better than chance alone (49.4%, data not presented). Therefore, it is important to consider using actigraphy features in conjunction to provide an optimal fit for assessing mobility impairments.
A major addition of this work to the field is the transfer of knowledge from feature extraction and selection methods to predicting the occurrence of a MMD clinical endpoint. The results from the MMD analysis suggest that the top five actigraphy features significantly improved the prediction of MMD alongside variables consistently known for their association with disability in older adults. In fact, the model fit for predicting MMD was substantially improved after inclusion of the actigraphy features. As such, actigraphy data from free-living conditions may be a useful tool for generating a mobility disability risk profile in the future.
There are both strengths and limitations to the current work. The study included a comprehensive set of actigraphy features collected in a large sample of well-characterized older adults in free-living conditions. It also systematically evaluated these with clinically important endpoints. However, these strengths are balanced with some weaknesses that include not being able to evaluate the frequency domain features (e.g., dispersion) that are becoming more common with higher hardware resolution rates (Ellis et al. 2014b). Additionally, hip-worn actigraphy is not located at the center of mass and thus is not an ideal location to capture subtle impairments in ambulation (Weiss et al. 2015). Lastly, the sample of older adults in this study were moderate to low functioning and therefore not representative of the general population of older adults.
5. Conclusion
This study identified a subset of actigraphy features collected in free-living conditions that are moderately accurate in identifying older adults with slow walking speed— a proposed marker of vital status in this age group. These features capture domains related to movement pace and/or intensity, engagement in activity, patterns of activity, and the movement variability. When these actigraphy features are used in combination they significantly improve the prediction of mobility disability in older adults.
Supplementary Material
Acknowledgments
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (P30 AG028740), Wake Forest University (P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744).
Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
LIFE investigators are also partially supported by the following:
Dr. Todd Manini (University of Florida) is partially supported by NIH HL121023 and AG042525.
Dr. Thomas Gill (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging.
Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs.
Dr. Roger A. Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Dr. Bonnie Spring is partially supported by NIH DK097364, HL075451, U54EB020404, and AHA 14SFRN20740001
Dr. Catrine Tudor-Locke is partially supported by NIH 1R01AG049024, 1R21HD073807, and 1R21OH010785.
Appendix: Research Investigators for the LIFE Study
-
Administrative Coordinating Center, University of Florida, Gainesville, FL
Marco Pahor, MD – Principal Investigator of the LIFE Study
Jack M. Guralnik, MD, PhD – Co-Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD)
Christiaan Leeuwenburgh, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ching-ju Lu, MPH
-
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston Salem, NC
Michael E. Miller, PhD – DMAQC Principal Investigator
Mark A. Espeland, PhD – DMAQC Co-Investigator
Walter T. Ambrosius, PhD
William Applegate, MD
Daniel P. Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
Delilah Cook, CCRP
Curt D. Furberg, MD, PhD
Lea N. Harvin, BS
Leora Henkin, MPH, Med
John Hepler, MA
Fang-Chi Hsu, PhD
Laura Lovato, MS
Wesley Roberson, BSBA
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
W. Jack Rejeski, PhD
Jeffrey A. Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon L. Mihalko, PhD
Janine M. Jennings, PhD
-
National Institutes of Health, Bethesda, MD
Evan C. Hadley, MD (National Institute on Aging)
Sergei Romashkan, MD, PhD (National Institute on Aging)
Kushang V. Patel, PhD (National Institute on Aging)
National Heart, Lung and Blood Institute, Bethesda, MD
Denise Bonds, MD, MPH
-
Field Centers
Northwestern University, Chicago, IL
Mary M. McDermott, MD – Field Center Principal Investigator
Bonnie Spring, PhD – Field Center Co-Investigator
Joshua Hauser, MD – Field Center Co-Investigator
Diana Kerwin, MD – Field Center Co-Investigator
Kathryn Domanchuk, BS
Rex Graff, MS
Alvito Rego, MA
-
Pennington Biomedical Research Center, Baton Rouge, LA
Timothy S. Church, MD, PhD, MPH – Field Center Principal Investigator
Steven N. Blair, PED (University of South Carolina)
Valerie H. Myers, PhD
Ron Monce, PA-C
Nathan E. Britt, NP
Melissa Nauta Harris, BS
Ami Parks McGucken, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi K. Millet, MPA, BS
Catrine Tudor-Locke, PhD, FACSM
Ben P. Butitta, BS
Sheletta G. Donatto, MS, RD, LDN, CDE
Shannon H. Cocreham, BS
-
Stanford University, Palo Alto, CA
Abby C. King, PhD – Field Center Principal Investigator
Cynthia M. Castro, PhD
William L. Haskell, PhD
Randall S. Stafford, MD, PhD
Leslie A. Pruitt, PhD
Kathy Berra, MSN, NP-C, FAAN
Veronica Yank, MD
-
Tufts University, Boston, MA
Roger A. Fielding, PhD – Field Center Principal Investigator
Miriam E. Nelson, PhD – Field Center Co-Investigator
Sara C. Folta, PhD – Field Center Co-Investigator
Edward M. Phillips, MD
Christine K. Liu, MD
Erica C. McDavitt, MS
Kieran F. Reid, PhD, MPH
Won S. Kim, BS
Vince E. Beard, BS
-
University of Florida, Gainesville, FL
Todd M. Manini, PhD – Field Center Principal Investigator
Marco Pahor, MD – Field Center Co-Investigator
Stephen D. Anton, PhD
Susan Nayfield, MD
Thomas W. Buford, PhD
Michael Marsiske, PhD
Bhanuprasad D. Sandesara, MD
Jeffrey D. Knaggs, BS
Megan S. Lorow, BS
William C. Marena, MT, CCRC
Irina Korytov, MD
Holly L. Morris, MSN, RN, CCRC (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Margo Fitch, PT (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Floris F. Singletary, MS, CCC-SLP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jackie Causer, BSH, RN (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Katie A. Radcliff, MA (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
-
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH – Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH – Field Center Co-Investigator
Bret H. Goodpaster, PhD
Nancy W. Glynn, PhD
Oscar Lopez, MD
Neelesh K. Nadkarni, MD, PhD
Kathy Williams, RN, BSEd, MHSA
Mark A. Newman, PhD
George Grove, MS
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA (deceased)
Diane G. Ives, MPH
-
Wake Forest University, Winston Salem, NC
Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator
Anthony P. Marsh, PhD – Field Center Co-Investigator
Tina E. Brinkley, PhD
Jamehl S. Demons, MD
Kaycee M. Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
-
Yale University, New Haven, CT
Thomas M. Gill, MD – Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM – Field Center Co-Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, MPH (VA Connecticut Healthcare System)
Nathalie de Rekeneire, MD, MS
Joanne M. McGloin, MDiv, MS, MBA
Karen C. Wu, RN
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Lynne P. Iannone, MS, CCRP
Raeleen Mautner, PhD
Theresa Sweeney Barnett, MS, APRN
Sean N. Halpin, MA
Matthew J. Brennan, MA
Julie A. Bugaj, MS
Maria A. Zenoni, MS
Bridget M. Mignosa, AS
-
Cognition Coordinating Center, Wake Forest University, Winston Salem, NC
Jeff Williamson, MD, MHS – Center Principal Investigator
Kaycee M Sink, MD, MAS – Center Co-Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Mark Espeland, PhD
Janine Jennings, PhD
-
Electrocardiogram Reading Center, University of Florida, Gainesville, FL
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
Anita Szady, MD
-
Spirometry Reading Center, Yale University, New Haven, CT
Geoffrey L. Chupp, MD
Gail M. Flynn, RCP, CRFT
Thomas M. Gill, MD
John L. Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
-
Cost Effectiveness Analysis Center
Erik J. Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System)
Robert M. Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health)
References
- Aminian K, Najafi B. Capturing human motion using body-fixed sensors: outdoor measurement and clinical applications. Computer Animation and Virtual Worlds. 2004;15(2):79–94. [Google Scholar]
- Bassett DR, Troiano RP, McClain JJ, Wolff DL. Accelerometer-Based Physical Activity: Total Volume per Day and Standardized Measures. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000468. [DOI] [PubMed] [Google Scholar]
- Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(9):983–988. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela J, Pastorino M, Arredondo MT, Pansera M, Pastor-Sanz L, Villagra F, Pastor MA, Gonzalez AP. Gait assessment in Parkinson’s disease patients through a network of wearable accelerometers in unsupervised environments. In Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE; 2011. pp. 2233–2236. [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Blackwell TL, Cauley JA, Ensrud KE, Dam TT, Harrison SL, Peters KW, Mackey DC. Objective assessment of activity, energy expenditure, and functional limitations in older men: the osteoporotic fractures in men study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015;68(12):1518–1524. doi: 10.1093/gerona/glt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Medicine and science in sports and exercise. 2011;43(2):357. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MG, Fox KR, Hilsdon M, Sharp DJ, Coulson JC, Thompson JL. Objectively measured physical activity in a diverse sample of older urban UK adults. Med Sci Sports Exerc. 2011;43(4):647–654. doi: 10.1249/MSS.0b013e3181f36196. [DOI] [PubMed] [Google Scholar]
- Ellis K, Godbole S, Kerr J, Lanckriet G. Multi-Sensor physical activity recognition in free-living. In Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing: Adjunct Publication; ACM; pp. 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K, Godbole S, Marshall S, Lanckriet G, Staudenmayer J, Kerr J. Identifying active travel behaviors in challenging environments using GPS, accelerometers, and machine learning algorithms. Frontiers in public health. 2014b:2. doi: 10.3389/fpubh.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K, Kerr J, Godbole S, Lanckriet G, Wing D, Marshall S. A random forest classifier for the prediction of energy expenditure and type of physical activity from wrist and hip accelerometers. Physiological measurement. 2014c;35(11):2191. doi: 10.1088/0967-3334/35/11/2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Ng YS, Zhu S, Tan DM, Anderson B, Schlaug G, Wang Y. A Validated Smartphone-Based Assessment of Gait and Gait Variability in Parkinson’s Disease. PloS one. 2015;10(10):e0141694. doi: 10.1371/journal.pone.0141694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud KE, Blackwell TL, Cauley JA, Dam TTL, Cawthon PM, Schousboe JT, Barrett-Connor E, Stone KL, Bauer DC, Shikany JM, Mackey DC. Objective Measures of Activity Level and Mortality in Older Men. Journal of the American Geriatrics Society. 2014;62(11):2079–2087. doi: 10.1111/jgs.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evelyn LT, Chui CH. The modified mini-mental state (3MS) examination. The Journal of clinical psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- Ezeugwu V, Klaren RE, Hubbard EA, Manns PT, Motl RW. Mobility disability and the pattern of accelerometer-derived sedentary and physical activity behaviors in people with multiple sclerosis. Preventive Medicine Reports. 2015;2:241–246. doi: 10.1016/j.pmedr.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Rejeski WJ, Blair S, Church T, Espeland MA, Gill TM, Guralnik JM, Hsu FC, Katula J, King AC, Kritchevsky SB. The lifestyle interventions and independence for elders study: design and methods. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011:glr123. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine and science in sports and exercise. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Journal of NeuroEngineering and Rehabilitation. Journal of neuroengineering and rehabilitation. 2005;2(23):0003–2. doi: 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ceja E, Brena RF, Carrasco-Jimenez JC, Garrido L. Long-Term Activity Recognition from Wristwatch Accelerometer Data. Sensors. 2014;14(12):22500–22524. doi: 10.3390/s141222500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Dallas T. Feature selection and activity recognition system using a single triaxial accelerometer. Biomedical Engineering, IEEE Transactions on. 2014;61(6):1780–1786. doi: 10.1109/TBME.2014.2307069. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New England Journal of Medicine. 1995;332(9):556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerl AE, Kennard RW. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics. 1970;12(1):55–67. [Google Scholar]
- Iluz T, Weiss A, Gazit E, Tankus A, Brozgol M, Dorfman M, Mirelman A, Giladi N, Hausdorff JM. Can a Body-Fixed Sensor Reduce Heisenberg’s Uncertainty When It Comes to the Evaluation of Mobility? Effects of Aging and Fall Risk on Transitions in Daily Living. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015:glv049. doi: 10.1093/gerona/glv049. [DOI] [PubMed] [Google Scholar]
- Kate RJ, Swartz AM, Welch WA, Strath SJ. Comparative evaluation of features and techniques for identifying activity type and estimating energy cost from accelerometer data. Physiological measurement. 2016;37(3):360. doi: 10.1088/0967-3334/37/3/360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapisz JR, Weiss GM, Moore SA. Activity recognition using cell phone accelerometers. ACM SigKDD Explorations Newsletter. 2011;12(2):74–82. [Google Scholar]
- Lakka TA, Laaksonen DE, Lakka HM, Männikkö NIKO, Niskanen LK, Rauramaa RAINER, Salonen JT. Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Medicine and science in sports and exercise. 2003;35(8):1279–1286. doi: 10.1249/01.MSS.0000079076.74931.9A. [DOI] [PubMed] [Google Scholar]
- Langenberg DR, Papandony MC, Gibson PR. Sleep and physical activity measured by accelerometry in Crohn’s disease. Alimentary pharmacology & therapeutics. 2015;41(10):991–1004. doi: 10.1111/apt.13160. [DOI] [PubMed] [Google Scholar]
- Lemoyne R, Coroian C, Mastroianni T, Grundfest W. Accelerometers for quantification of gait and movement disorders: A perspective review. Journal of Mechanics in Medicine and Biology. 2008;8(2):137–152. [Google Scholar]
- Manns P, Ezeugwu V, Armijo-Olivo S, Vallance J, Healy GN. Accelerometer-Derived Pattern of Sedentary and Physical Activity Time in Persons with Mobility Disability: National Health and Nutrition Examination Survey 2003 to 2006. Journal of the American Geriatrics Society. 2015;63(7):1314–1323. doi: 10.1111/jgs.13490. [DOI] [PubMed] [Google Scholar]
- Marsh AP, Lovato LC, Glynn NW, Kennedy K, Castro C, Domanchuk K, McDavitt E, Rodate R, Marsiske M, McGloin J, Groessl EJ. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68(12):1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini L, Rocchi L, Mellone S, Valzania F, Chiari L. Feature selection for accelerometer-based posture analysis in Parkinson’s disease. Information Technology in Biomedicine, IEEE Transactions on. 2011;15(3):481–490. doi: 10.1109/TITB.2011.2107916. [DOI] [PubMed] [Google Scholar]
- Perera S, Patel KV, Rosano C, Rubin SM, Satterfield S, Harris T, Ensrud K, Orwoll E, Lee CG, Chandler JM, Newman AB. Gait speed predicts incident disability: a pooled analysis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015:glv126. doi: 10.1093/gerona/glv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece SJ, Goulermas JY, Kenney LP, Howard D. A comparison of feature extraction methods for the classification of dynamic activities from accelerometer data. Biomedical Engineering, IEEE Transactions on. 2009;56(3):871–879. doi: 10.1109/TBME.2008.2006190. [DOI] [PubMed] [Google Scholar]
- Pruitt LA, Glynn NW, King AC, Guralnik JM, Aiken EK, Miller G, Haskell WL. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. J Aging Phys Act. 2008;16(4):416–434. doi: 10.1123/japa.16.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudil P, Novoviov J, Kittler J. Floating search methodsin feature selection. Pattern Recognition Letters. 1994;15(11):1119–1125. [Google Scholar]
- Rothney MP, Neumann M, Béziat A, Chen KY. An artificial neural network model of energy expenditure using nonintegrated acceleration signals. Journal of applied physiology. 2007;103(4):1419–1427. doi: 10.1152/japplphysiol.00429.2007. [DOI] [PubMed] [Google Scholar]
- SankarPandi SK, Dlay S, Woo WL, Catt M. Predicting disability levels of community dwelling older individuals using single wrist mounted accelerometer. In Biomedical and Health Informatics (BHI), 2014 IEEE-EMBS International Conference on; IEEE; pp. 720–723. [Google Scholar]
- Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. Journal of the neurological sciences. 2003;212(1):47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Sherman WM. Ability of different physical activity monitors to detect movement during treadmill walking. Int J Sports Med. 2003;24:43–50. doi: 10.1055/s-2003-37196. [DOI] [PubMed] [Google Scholar]
- Song J, Lindquist LA, Chang RW, Semanik PA, Ehrlich-Jones LS, Lee J, Sohn MW, Dunlop DD. Sedentary Behavior as a Risk Factor for Physical Frailty Independent of Moderate Activity: Results From the Osteoarthritis Initiative. American Journal of Public Health. 2015;(0):e1–e7. doi: 10.2105/AJPH.2014.302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenmayer J, Pober D, Crouter S, Bassett D, Freedson P. An artificial neural network to estimate physical activity energy expenditure and identify physical activity type from an accelerometer. Journal of applied physiology. 2009;107(4):1300–1307. doi: 10.1152/japplphysiol.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. The Journal of the American Medical Association. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society: Series B (Methodological) 1996:267–288. [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and science in sports and exercise. 2008;40(1):181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. British journal of sports medicine. 2014;48(13):1019–1023. doi: 10.1136/bjsports-2014-093546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De bourdeaudhuij I, Ewald B, Gardner AW, Hatano Y, Lutes LD, Matsudo SM. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8(1):80. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Thyfault JP, Spence JC. A step-defined sedentary lifestyle index:<5000 steps/day. Applied pgysiology, nutrition, and metabolism. 2012;38(2):100–114. doi: 10.1139/apnm-2012-0235. [DOI] [PubMed] [Google Scholar]
- Weiss A, Herman T, Giladi N, Hausdorff JM. Association between Community Ambulation Walking Patterns and Cognitive Function in Patients with Parkinson’s Disease: Further Insights into Motor-Cognitive Links. Parkinson’s Disease. 2015 doi: 10.1155/2015/547065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Mirelman A, Buchman AS, Bennett DA, Hausdorff JM. Using a body-fixed sensor to identify subclinical gait difficulties in older adults with IADL disability: maximizing the output of the timed up and go. PloS one. 2013;8(7) doi: 10.1371/journal.pone.0068885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Sharifi S, Plotnik M, van Vugt JP, Giladi N, Hausdorff JM. Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabilitation and neural repair. 2011;25(9):810–818. doi: 10.1177/1545968311424869. [DOI] [PubMed] [Google Scholar]
- Zijlstra W, Aminian K. Mobility assessment in older people: new possibilities and challenges. European Journal of Ageing. 2007;4(1):3–12. doi: 10.1007/s10433-007-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2005;67(2):301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.