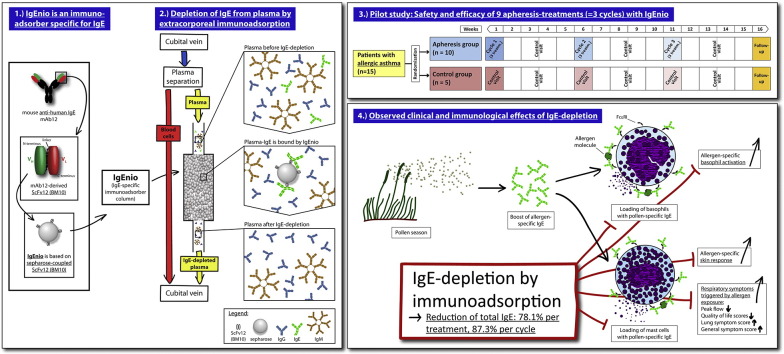
Abstract
Background
Prevention of IgE-binding to cellular IgE-receptors by anti-IgE (Omalizumab) is clinically effective in allergic asthma, but limited by IgE threshold-levels. To overcome this limitation, we developed a single-use IgE immunoadsorber column (IgEnio). IgEnio is based on a recombinant, IgE-specific antibody fragment and can be used for the specific extracorporeal desorption of IgE.
Objective
To study safety and efficacy of IgEnio regarding the selective depletion of IgE in a randomized, open-label, controlled pilot trial in patients with allergic asthma and to investigate if IgEnio can bind IgE-Omalizumab immune complexes.
Methods
Fifteen subjects were enrolled and randomly assigned to the treatment group (n = 10) or to the control group (n = 5). Immunoadsorption was done by veno-venous approach, processing the twofold calculated plasma volume during each treatment. A minimum average IgE-depletion of 50% after the last cycle in the intention-to-treat population was defined as primary endpoint. Safety of the treatment was studied as secondary endpoint. In addition, possible changes in allergen-specific sensitivity were investigated, as well as clinical effects by peak flow measurement and symptom-recording. The depletion of IgE-Omalizumab immune complexes was studied in vitro.
The study was registered at clinicaltrials.gov (NCT02096237) and conducted from December 2013 to July 2014.
Results
IgE immunoadsorption with IgEnio selectively depleted 86.2% (± 5.1% SD) of IgE until the end of the last cycle (p < 0.0001). Removal of pollen allergen-specific IgE was associated with a reduction of allergen-specific basophil-sensitivity and prevented increases of allergen-specific skin-sensitivity and clinical symptoms during pollen seasons. IgEnio also depleted IgE-Omalizumab immune complexes in vitro.
The therapy under investigation was safe and well-tolerated. During a total of 81 aphereses, 2 severe adverse events (SAE) were recorded, one of which, an episode of acute dyspnea, possibly was related to the treatment and resolved after administration of antihistamines and corticosteroids.
Conclusions
This pilot study indicates that IgE immunoadsorption with IgEnio may be used to treat patients with pollen-induced allergic asthma. Furthermore, the treatment could render allergic patients with highly elevated IgE-levels eligible for the administration of Omalizumab and facilitate the desorption of IgE-Omalizumab complexes.
This study was funded by Fresenius Medical Care Deutschland GmbH, Bad Homburg, Germany.
Abbreviations: AUC, area under the curve; AV, visit of apheresis group; BAT, basophil activation-testing; CV, visit of control group; IC, informed consent; IgE, immunoglobulin E; pt./pts., patient/s; SD, standard deviation; VAS, visual analogue scale
Keywords: Allergy, Asthma, Immunoadsorption, IgEnio, IgE
Graphical Abstract
Highlights
-
•
An IgE-specific immunoadsorber, IgEnio, was developed.
-
•
IgEnio selectively depleted IgE from subjects with allergic asthma.
-
•
IgEnio treatment was safe and well tolerated.
-
•
IgE-depletion prevented increase of pollen-induced symptoms.
-
•
In treated subjects, decreased skin- and basophil-reactivities were recorded.
IgE-associated allergy affects more than 25% of the population. Symptoms of allergy are caused by activation of inflammatory cells by IgE-allergen immune complexes. We developed an immunoadsorber, IgEnio, for the selective, effective and safe depletion of IgE from patients suffering from allergic asthma by immunoadsorption. IgE immunoadsorption with IgEnio may be useful for the treatment of allergen-induced asthma and perhaps other manifestations of IgE-associated allergies.
1. Introduction
IgE (immunoglobulin E)-associated allergies are a major health threat in industrialized countries affecting more than 25% of the population (Pawankar et al., 2012). Asthma represents one of the most severe manifestations of allergy because of its chronic course, strong impact on quality of life and severe, life-threatening symptoms (Holgate, 2012). Sensitization to airborne allergens from indoor allergen sources such as house dust mite, pets, mold and cockroach is a causative factor for asthma (Reed et al., 1985, Pollart et al., 1989, Konradsen et al., 2015). Furthermore, severe exacerbations are frequently observed after seasonal exposure to allergens from birch, grass, cypress and ragweed pollen (Pollart et al., 1988, Suphioglu et al., 1992, Schäppi et al., 1997, Midoro-Horiuti et al., 1992).
In allergic asthma, symptoms are caused by allergen-induced cross-linking of IgE antibodies on the surface of mast cells and basophils and the subsequent release of inflammatory mediators (Holgate, 2012). IgE may also play a major role in T cell activation through IgE-facilitated allergen presentation (Mudde et al., 1990, van der Heijden et al., 1993). T cell-derived cytokines such as IL-5 (Interleukin 5) and IL-13 contribute to mucus secretion and eosinophilic infiltration, respectively, both of which are involved in airway remodeling (Holgate, 2012). Since IgE antibodies play a central role in allergic asthma, several therapeutic strategies targeting IgE or the underlying Th2 (T helper 2 cell) immune response have evolved or are currently in clinical evaluation (Holgate, 2014). These strategies target cytokines which are involved in Th2-driven allergic asthma (Wenzel et al., 2013, Bel et al., 2014, Ortega et al., 2014, Gauvreau et al., 2014) or focus directly on IgE antibodies (O'Byrne and Tworek, 2015, Molimard et al., 2010, Humbert et al., 2014). Omalizumab, a therapeutic anti-IgE antibody preventing IgE from binding to its high-affinity receptor (FcεRI), is effective for the treatment of severe allergic asthma. According to clinical studies, it reduces exacerbations and the need for steroids (Molimard et al., 2010). One major drawback of injected Omalizumab is that its applicability is limited by a weight-dependent maximum level of total IgE up to which it can be administered, i.e., 1500 U/ml within the EU (EMA, 2015) and 700 U/ml in the US (FDA, 2015), respectively. This precludes many polysensitized patients with severe symptoms from anti-IgE treatment. In the present study, we report results from a clinical trial where an IgE-immunoadsorber, termed IgEnio, was used to treat subjects suffering from allergic asthma. Efficacy of IgE-depletion and safety of the treatment were studied as primary and secondary endpoints, respectively. In addition, several immunological and clinical parameters were assessed to investigate possible effects of the treatment on IgE-driven mechanisms.
2. Materials and Methods
2.1. Description of the Medical Device IgEnio
IgEnio (Fresenius Medical Care, Bad Homburg, Germany) is a single-use, IgE-specific immunoadsorber column based on a single-chain variable fragment, ScFv12 (Lupinek et al., 2009) (BM10, Biomay, Vienna, Austria), for the selective removal of IgE from plasma of allergic patients by extracorporeal immunoadsorption. ScFv12 is specific for IgE, does not cross-link IgE on mast cells and basophils (Lupinek et al., 2009) and is produced in E. coli under GMP (good manufacturing practice) conditions.
2.2. Study Design
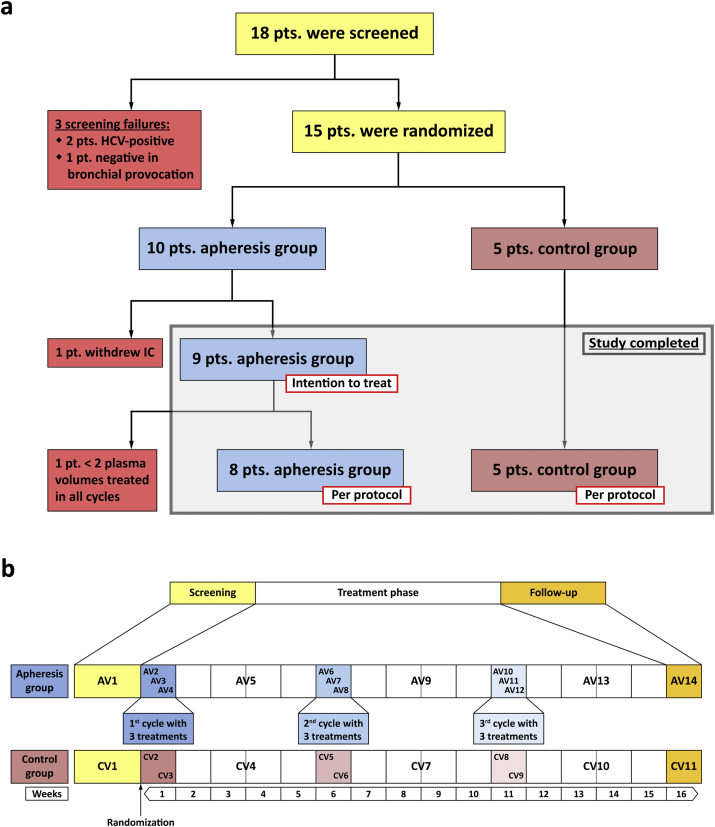
The study was designed in accordance with the declaration of Helsinki as a prospective, randomized, controlled, open-label trial and was performed in one study-center at the Medical University of Vienna, Austria. The trial was approved by Austrian health authorities (AGES), by the ethics committee of the Medical University of Vienna and was registered at clinicaltrials.gov (NCT02096237) (FMC, 2014).
Men and women, aged 18–50 years, who were suffering from allergy-driven asthma and with a minimum total IgE-level of 300 U/ml were eligible for participation. Diagnosis of allergic asthma was established by patient's history, measurement of allergen-specific IgE, skin prick testing (SPT) and by bronchial provocation with methacholine. Asthma-severity was graded according to guidelines of the German Medical Association (Bundesärztekammer, 2009) that are based on GINA-guidelines (GINA, 2007), the British Thoracic Society guidelines on the management of asthma (BTS, 2010) and guidelines for the diagnosis and management of asthma of the National Heart, Lung, and Blood Institute (NHLBI, 2007). Subjects suffering from non-allergic asthma, receiving Omalizumab-therapy or having received allergen-specific immunotherapy in the last 6 months were excluded from participation (FMC, 2014). Fifty years were selected as upper age level to reduce co-morbidities which would have precluded immunoadsorption.
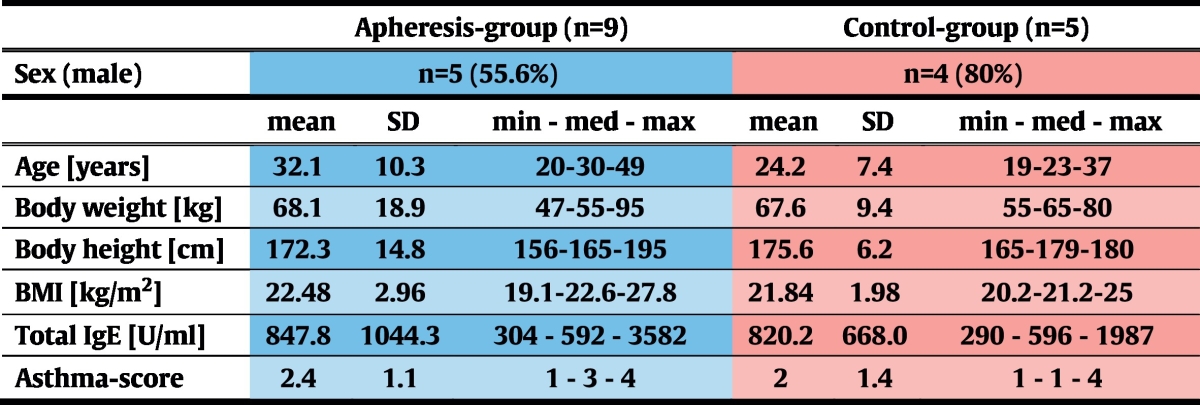
After enrollment of 15 eligible subjects, participants were ranked according to total-IgE levels and, in groups of three, randomly assigned to the apheresis (n = 10) or to the control group (n = 5; Fig. 1a) at a 2:1 ratio. This procedure facilitated achievement of comparable median total IgE-levels in the two groups.
Fig. 1.
Enrollment, randomization and study design. (a) After screening (yellow; AV1, CV1 in b), subjects were allocated to the apheresis (blue) or the control group (pink). (b) Treatment-visits (AV2, AV3, etc.) are indicated by blue boxes, corresponding visits of the control group (CV2, CV3, etc.) by pink boxes. Intervals between treatment weeks, including control visits (AV5, CV4, etc.) are shown in white. The study was completed after a follow-up visit in week 16 (orange; AV14 and CV11). Weeks after randomization are shown at the bottom. pt./pts. – patient/s; IC – informed consent; AV – visit apheresis group; CV – visit control group.
Treatment cycles consisted of 3 immunoadsorptions within one week and were followed by intervals of 4 weeks without intervention. Each patient was subjected to 3 cycles, i.e., 9 aphereses in total (Fig. 1b). Control visits were scheduled two weeks after the last treatment of each cycle, the final visit was planned for the 5th week after the last cycle. Subjects allocated to the control group were seen accordingly, but with 2 visits only in those weeks that corresponded to treatment weeks. Total study-duration was approximately 16 weeks for each patient.
The primary endpoint of the trial was defined as ≥ 50% reduction of total IgE after the last, i.e., the 9th immunoadsorption with IgEnio under the treatment scheme described above, when related to the pre-value of the first treatment. As secondary endpoint, safety of the treatment was investigated by recording adverse events and serious adverse events in accordance with ISO 14155 and MedDRA (http://www.meddra.org), and by analysis of blood and urine samples (Interlab GmbH Central Lab Services, Munich, Germany) (GCP, 2011). In order to detect possible effects of IgE-desorption on IgE-mediated mechanisms, additional immunological and clinical parameters were recorded (Table 1).
Table 1.
Clinical and immunological parameters.
| Primary variable: reduction of total IgE by ≥ 50% |
| Total IgE (ImmunoCAP) |
| Secondary variables: safety parameters |
| Adverse events (AE), serious adverse events (SAE) |
| Coagulation parameters: aPTT, Fibrinogen, Factor VIII, ionized calcium, Thrombin-Antithrombin III complex (TAT) |
| Complement-activation: C3a, C5a |
| Electrolytes (serum): Na, K, total Ca, PO4, Cl |
| Blood gas analysis: acid-base status, O2-saturation |
| Hematology: white blood cells, erythrocytes (number, MCV, MCH, MCHC), hemoglobin, hematocrit, platelets |
| Liver and kidney parameters: AST, ALT, γ-GT, creatinine, bilirubin, uric acid, BUN |
| Urine analysis: pH, leukocytes, erythrocytes, bilirubin, urobilinogen, ketone bodies, nitrite, glucose |
| Blood lipids: LDL, HDL, triglycerides |
| Bone metabolism: alkaline phosphatase |
| Hemolysis: lactate dehydrogenase |
| Protein loss: albumin |
| Inflammation/Infection: CRP (C-reactive protein) |
| Heart and muscle injury: CK (creatine kinase) |
| Additional clinical parameters: |
| Lung function parameters: PEF, FEV1 |
| Asthma control questionnaire |
| Patient's diary: symptoms, medication |
| Skin prick test |
| Body temperature |
| ECG |
| During treatment: |
| Treatment time |
| Blood flow/plasma flow |
| Processed plasma volume |
| Heart rate |
| Blood pressure (every 60 min) |
| Additional immunological parameters: |
| IgE and IgG reactivity profiles (ISAC, Thermo Fisher Scientific) |
| Specific IgE-levels (ImmunoCAP, Thermo Fisher Scientific) |
| Allergen-induced basophil activation (CD203c upregulation) |
| CD23-expression on B cells in peripheral blood |
| IgE positive cells among PBMCs (peripheral blood mononuclear cells) |
| FcεRI-expression on PBMCs |
| Allergen-induced T cell activation and cytokine secretion |
At every treatment visit, blood was collected immediately before and after apheresis. From control patients, one blood sample was obtained at corresponding visits (Fig. 1b). At screening, control- and follow-up visits, all patients were subjected to clinical examinations including lung function assessment, titrated skin prick testing, urine- and blood-collection. For the complete study duration, patients recorded results from daily peak flow measurements (Vitalograph peak-flow-meter, North Buckinghamshire, UK), symptom severity (general well-being graded by visual analogue scale [VAS]; eye-, nose- and lung-specific symptoms: severe, moderate, mild or no symptoms) and eventual adjustments of previously prescribed medication in patient diaries. At the beginning and at the end of the trial, patients completed a validated asthma control questionnaire (Juniper et al., 1999).
The complete study protocol is provided in the online repository.
2.3. Apheresis Treatment
Every week, 2 subjects were treated simultaneously by immunoadsorption. Blood was drawn via an 18-gauge needle from a peripheral vein at a flow rate of 50–80 ml/min. Anticoagulation during treatment was maintained by continuous citrate dosage (ACD-A, anticoagulant citrate dextrose, formula A; Fresenius Kabi, Bad Homburg, Germany) at a volume/citrate ratio of 1:25, and sodium heparin (1000–1500 IU/h, Heparin Immuno, Immuno AG, Vienna, Austria). Plasma was separated from blood cells by centrifugation (COM.TEC, Fresenius Kabi, Bad Homburg, Germany). Mean blood and plasma flow rates were 55.2 ml/min (± 6.1 ml/min SD) and 29.9 ml/min (± 0.5 ml/min SD), respectively, requiring an average time of 3.5 h (± 0.9 h SD) to treat the twofold plasma volume of the respective patient as calculated using the formula according to Sprenger et al. (1987). Mean deviation from plasma volume planned to be treated was − 10.7% (± 10.1% SD) for all 81 treatments. Separated plasma was conducted by a COM.TEC plasma pump from the centrifugal separation chamber directly to the IgE-adsorber where adsorption of IgE occurred. The processed plasma was thereafter passed through a particle filter as second safety barrier against accidental particle infusion, and reunified with the blood cells in the bubble catcher of the cell separator's return line. From there, it was returned to the patient by a second peripheral venous access at the opposite arm as used for blood collection. At the end of treatment residual blood in the blood lines and the adsorber was displaced by 0.9% saline and re-infused to the patient.
Since this clinical trial was conducted as a pre-CE study, apheresis treatments were performed using clinical samples of IgEnio.
2.4. Measurement of Total and Allergen-specific IgE
All serum samples were tested by ImmunoCAP (Thermo Fisher/Phadia AB, Uppsala, Sweden) for levels of total IgE and for IgE specific to defined seasonal (birch, ash, timothy grass, mugwort, Alternaria) and perennial allergen sources (Aspergillus, house dust mite, cat, dog). Results are reported in U/ml for total IgE, and in UA/ml for allergen-specific IgE (Bazaral and Hamburger, 1972). Relative IgE-depletion rates were calculated for each treatment day and cycle, respectively, as described below.
2.5. Measurement and Calculation of Changes in Concentrations of Serum IgG, IgA, IgM and Albumin
Levels of total IgG, IgA and IgM were measured in samples obtained before and immediately after apheresis treatments on a BNII nephelometer using N Antisera to human IgG, IgA and IgM (Siemens Healthcare Diagnostics, Vienna, Austria). Relative changes of antibody-concentrations were calculated for each treatment by relating post- to the respective pre-treatment values. In addition, mean changes were calculated for every apheresis cycle. Relative changes in serum albumin (Interlab GmbH Central Lab Services) were calculated accordingly and compared to results obtained for IgG, IgA and IgM in order to assess if decreases of antibody levels were due to interaction with the immunoadsorber or to serum dilution caused by infusion of electrolyte solution during apheresis.
2.6. Titrated Skin Prick Testing
At screening, control and follow-up visits, all patients were subjected to titrated skin prick testing (SPT) for one or two allergen sources, depending on individual sensitization profiles. Patients were asked to pause intake of antihistamines at least 48 h before skin prick testing. In case this was not possible due to severity of symptoms, SPT was omitted (Heinzerling et al., 2013).
Extracts from dog dander, house dust mite, birch pollen and Alternaria alternata were purchased from Allergopharma (Reinbek, Germany), cat dander and grass pollen extracts from Stallergenes (Antony, France). Dilutions of extracts were freshly prepared on the day of skin prick testing in sterile physiological NaCl-solution (Fresenius Kabi Austria GmbH, Graz, Austria). Extracts were diluted from 1:2 to 1:128 at twofold steps and pricked in duplicates on the patients' forearms, in parallel to undiluted extracts, NaCl solution and histamine-control. After 20 min, wheals were marked by felt pen and transferred to SPT-documentation forms using adhesive tape. Wheal areas were quantified by planimetry using ImageJ software, version 1.48 (Rasband, 1997–2014). The sum of mean wheal areas from duplicates was calculated for all wheals induced by dilutions of the same extract in the respective patient. Relative changes of total wheal areas were calculated using results from the screening visit as baseline.
2.7. Basophil Activation Experiments
Lyophilized extracts from grass pollen mix, cat and dog dander were purchased from Stallergenes (Antony, France), lyophilized birch pollen extract from Allergopharma (Reinbek, Germany). Extracts were reconstituted in sterile physiological NaCl solution (Fresenius Kabi Austria GmbH). Raw material for the preparation of an extract from Dermatophagoides pteronyssinus was obtained from Allergon (Allergon AB, Ängelholm, Sweden) and an aqueous extract in phosphate buffered saline (PBS) was prepared (Banerjee et al., 2015). Aliquots of all extracts were stored in glass vials at − 20 °C. For each basophil activation test, series of tenfold dilutions in physiological NaCl or PBS, respectively, ranging from 1 μg/ml to 10− 6 μg/ml were freshly prepared.
Triplicates of 45 μl from heparinized blood samples that were collected during screening, control and final visits were incubated with 5 μl of extract dilutions. Every patient was tested for basophil reactivity with extracts from the same allergen sources that were also used for SPT in this patient. As controls, anti-IgE antibody clone E124.2.8 (Beckman Coulter, Fullerton, CA, USA, RRID:AB_1575992), PBS and isotype-matched antibodies (phycoerythrin (PE) or fluorescein isothiocyanate (FITC)-labeled mouse IgG1/κ isotype control, clone MOPC-21, BD Biosciences, San Jose, CA, USA, RRID: AB_396091 and RRID: AB_396090, respectively) were used (Hauswirth et al., 2002). In addition, blood aliquots were incubated with 2.5 μl of PE-labeled CD203c mAb clone 97A6 (Beckman Coulter, RRID:AB_141295) and 2.5 μl of FITC-labeled CD63 mAb clone CLBGran/12 (Beckman Coulter, Art.-nr. IM1165U) for 15 min at 37 °C. Thereafter, samples were subjected to erythrocyte lysis with 1 ml of FACS Lysing Solution (BD Biosciences). Then, cells were washed, resuspended in PBS, and analyzed by means of 2-colour flow cytometry on a FACScan (BD Biosciences). Basophils were detected on the basis of forward side-scatter characteristics and of CD203c-expression and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Allergen-induced upregulation of CD203c and CD63 was calculated from mean fluorescence intensities (MFIs) obtained from stimulated (MFIstim) and unstimulated (MFIcontrol) cells and expressed as stimulation index (MFIstim/MFIcontrol). Areas under the curve (AUC) for stimulation indices of all dilution steps tested were calculated. Using results from the screening visit as baseline, relative changes in basophil reactivities were determined.
2.8. In Vitro Depletion of IgE From Sera After Addition of Omalizumab
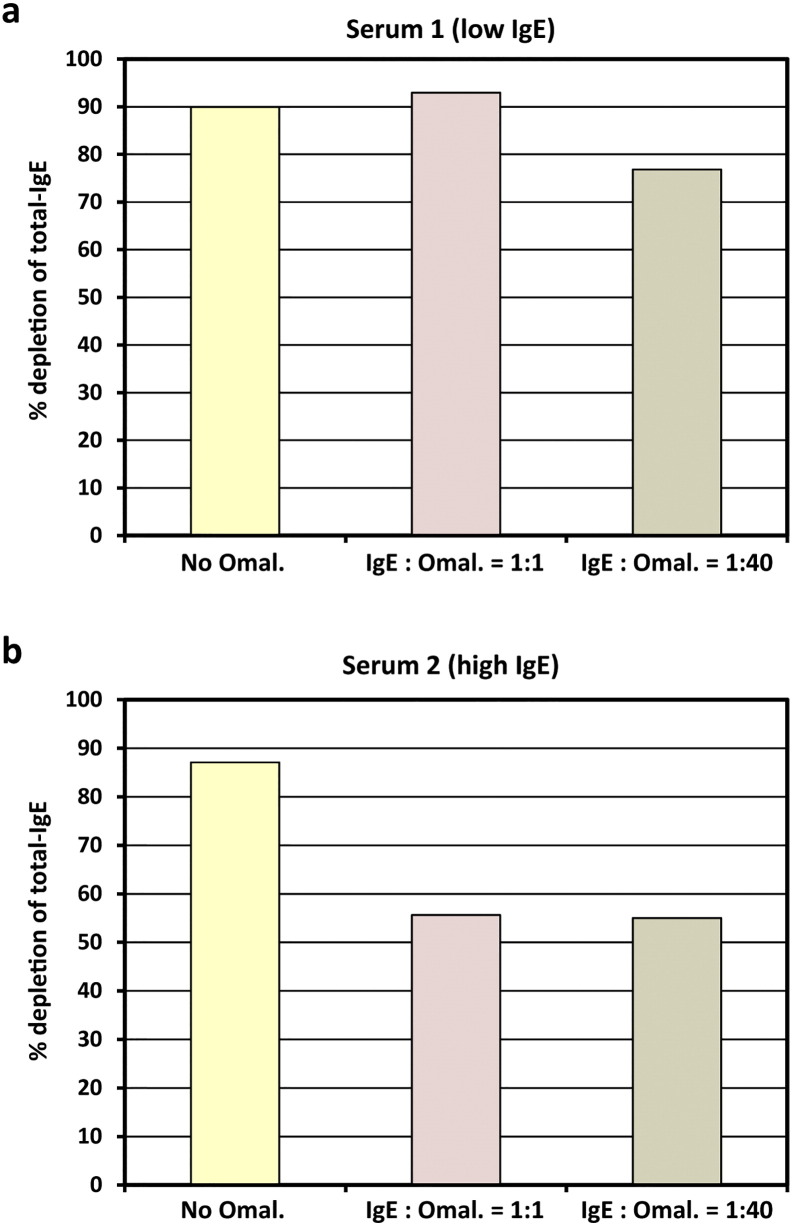
Sera from two different allergic donors with moderately and highly elevated total IgE-levels (i.e., serum 1: 263 U/ml; serum 2: 1427 U/ml), respectively, were collected. Omalizumab (Novartis, Basel, Switzerland) was added to aliquots of each sample at an IgE-to-Omalizumab ratio of 1:1 or 1:40, and incubated at room temperature for 1 h on a rocker. As reference, aliquots of both sera without Omalizumab were analyzed.
Five milliliter aliquots of each serum sample were applied to columns containing 2 ml of IgEnio adsorber material, flow-through was collected in 1 ml aliquots. Total IgE-levels were measured by ImmunoCAP (Thermo Fisher) before application to the column and in the last aliquot of each flow-through. For each sample the percentage of IgE-depletion after single passage through the column was calculated.
2.9. Additional Immunological Parameters
In blood samples collected at screening, control and follow-up visits, expression of IgE-receptors (FcεRI and CD23) on basophils and B cells, respectively, and allergen-induced T cell activation were studied. Details are provided in the online repository.
2.10. Statistical Methods
Sample size calculation was based on a one-sided t-test with a one-sided significance level of α = 0.025. Assuming a mean IgE reduction rate of 70% and a standard deviation of 17%, a sample size of n = 8 provided 80% statistical power to prove that the margin of 50% (primary endpoint) was significantly surpassed. To account for an expected drop-out rate of up to 20%, a total of 10 patients were included in the apheresis group, resulting in a final study population of n = 15, including 5 control patients.
All data collected in CRFs and patient diaries were entered into a ClinTrial database using double data entry. After database lock, complete data were exported to SAS V9.2 for statistical analysis. Prick test data were transmitted to the sponsor (Fresenius Medical Care, Bad Homburg, Germany) via Microsoft Excel and imported to ClinTrial database, as were transport files from the external laboratory (Interlab GmbH Central Lab Services, Munich, Germany). For generation of random codes SAS was used. Sample size estimation was performed with nQuery Advisor 5.0.
2.10.1. Primary Analysis
Primary analysis was based on the intention-to-treat (ITT) population. Additional analysis of IgE data of per-protocol (PP) patients was performed to assess validity of results (data not shown). Safety analyses were performed on the safety population.
For primary statistical analysis, the null hypothesis (H0), i.e., IgE reduction after 3 cycles of apheresis (in total, 9 treatments) is < 50%, was tested against the alternative hypothesis (H1), i.e., IgE reduction after 3 cycles of apheresis (in total, 9 treatments) is ≥ 50%. Statistical analysis was based on IgE assessments before treatment 1 of the first cycle, AV2pre (IgEpre; AV – apheresis visit), and after treatment 3 of the last cycle, AV12post (IgEpost). The percentage of IgE reduction (RR – reduction rate) was calculated as
A one-sided, one-sample t-test was calculated on all IgE reduction rates in the apheresis group and H0 was rejected if p < 0.025. Since primary analysis was based on a single statistical test no adjustment for multiplicity was necessary.
2.10.2. Secondary Analysis
For secondary analysis of changes in total IgE-levels, reduction rates were calculated as denoted above: For each treatment (pre- versus post-value of the same treatment) and for each cycle (pre-value of the first treatment to post-value of the third treatment of the same cycle). In case of single missing IgE data (pre- or post-treatment), the complete treatment was excluded from statistical analysis. Confidence intervals were calculated for reduction rates, incorporating the repeated measures design where applicable.
For the control group, relative changes of total IgE-levels were calculated for those weeks that corresponded to treatment weeks of the apheresis group (Fig. 1b). Comparisons between apheresis and control group were calculated employing linear mixed models.
For clinical outcome data, including prick test and lung function test, descriptive summary tables at study start and study end, as well as for differences between start and end of the trial were generated for the apheresis and the control group.
For the Asthma Quality of Life Questionnaire, data analysis was performed according to validated recommendations (Juniper et al., 1999). Statistical analysis was performed on inter-group differences as well as intra-group changes of the overall score. Patient diary data were analyzed accordingly.
Statistical comparisons were performed according to the measurement level of each parameter (t-test, Wilcoxon test, Fisher's Exact test). All p-values calculated in the context of secondary analyses were two-sided and considered purely descriptive and exploratory.
3. Results
3.1. Study Population
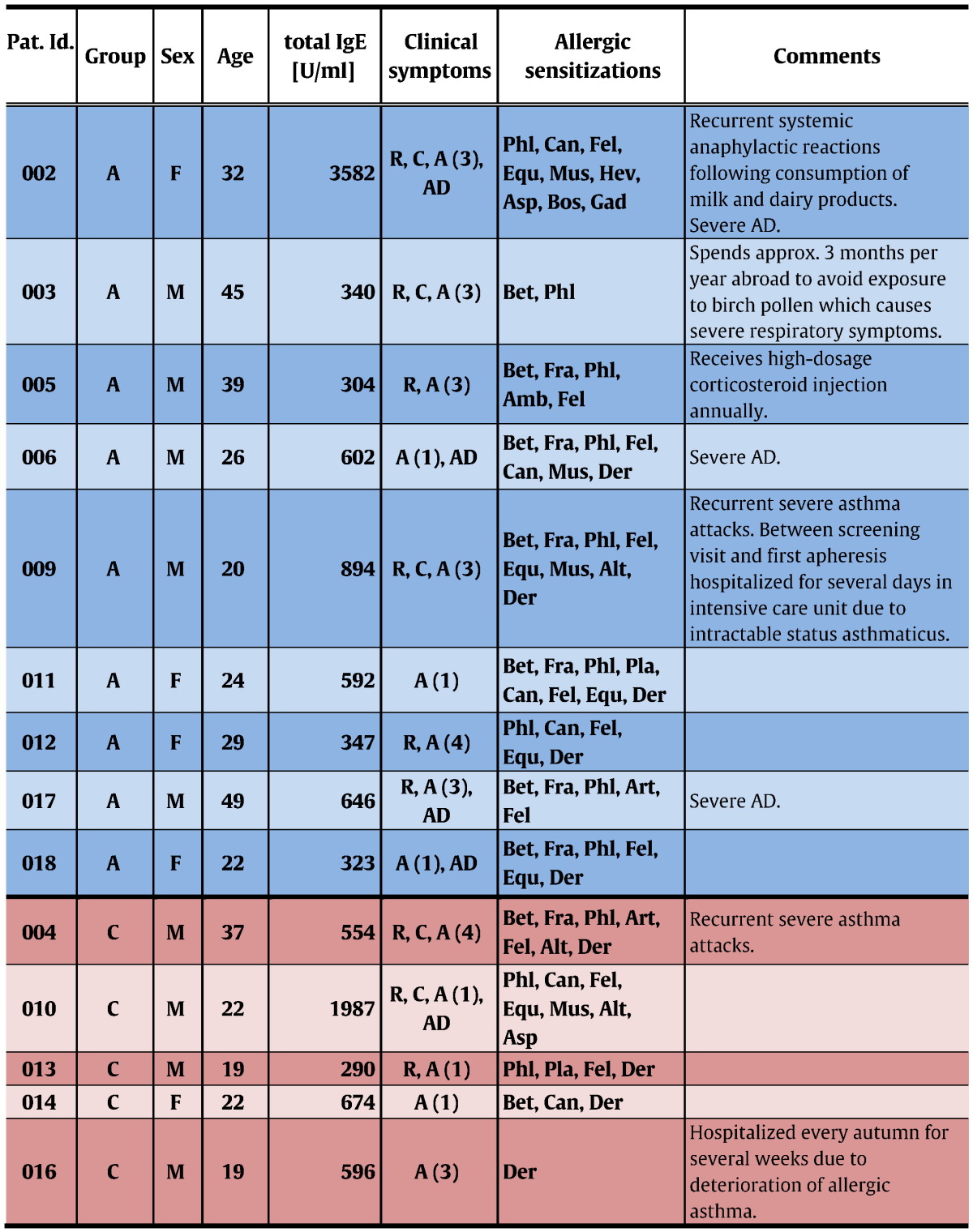
Patients were enrolled from December 2013 to February 2014. Follow-up visits were completed in July 2014. Among 18 patients who had given informed consent at the screening visit, 2 were excluded from participation due to positive serological results to HCV and one because of absence of bronchial hyperresponsiveness in methacholine provocation testing (Fig. 1a). After enrollment of 15 eligible subjects was completed, participants were ranked according to their total IgE-levels. Starting with those patients with the highest total IgE values, randomization to the apheresis (n = 10) or to the control group (n = 5) was done in groups of 3 subjects each at a 2:1 ratio. After randomization and before the first immunoadsorption treatment was started, one patient of the treatment group withdrew informed consent for personal reasons. All remaining 14 individuals completed the trial (Fig. 1a, Table 2).
Table 2.
Characterization of study subjects.
Pat. Id.: patient-identification; Groups: A – apheresis (blue boxes), C – control (pink boxes); Sex: M – male, F – female; Age: age when informed consent was given; Clinical symptoms: R – allergic rhinitis, C – allergic conjunctivitis, A (grade of severity) – allergic asthma, AD – atopic dermatitis; Allergic sensitizations: Bet – birch, Fra – ash tree, Phl – grass, Amb – ragweed, Art – mugwort, Pla – plantain, Can – dog, Fel – cat, Equ – horse, Mus – mouse, Der – house dust mite, Asp – Aspergillus, Alt – Alternaria, Hev – latex, Bos – cow's milk, Gad – codfish.
Due to this randomization algorithm, both groups were comparable in terms of total IgE-levels. In the apheresis group, median asthma-severity was higher than in the control group, but this difference was statistically not significant (p = 0.62; Table 3).
Table 3.
Characterization of study groups.
SD – standard deviation; min – minimum; med – median; max – maximum.
The highest total IgE level at the screening visit (AV1/CV1) was 3582 U/ml (Table 2). No subjects suffering from allergic asthma with higher IgE-levels could be recruited in our study. This is in accordance with results obtained for patients with allergic asthma in different countries where IgE-levels in such populations were usually lower than 10,000 U/ml (Gergen et al., 2009, Davila et al., 2015, Manise et al., 2016, Tay et al., 2016).
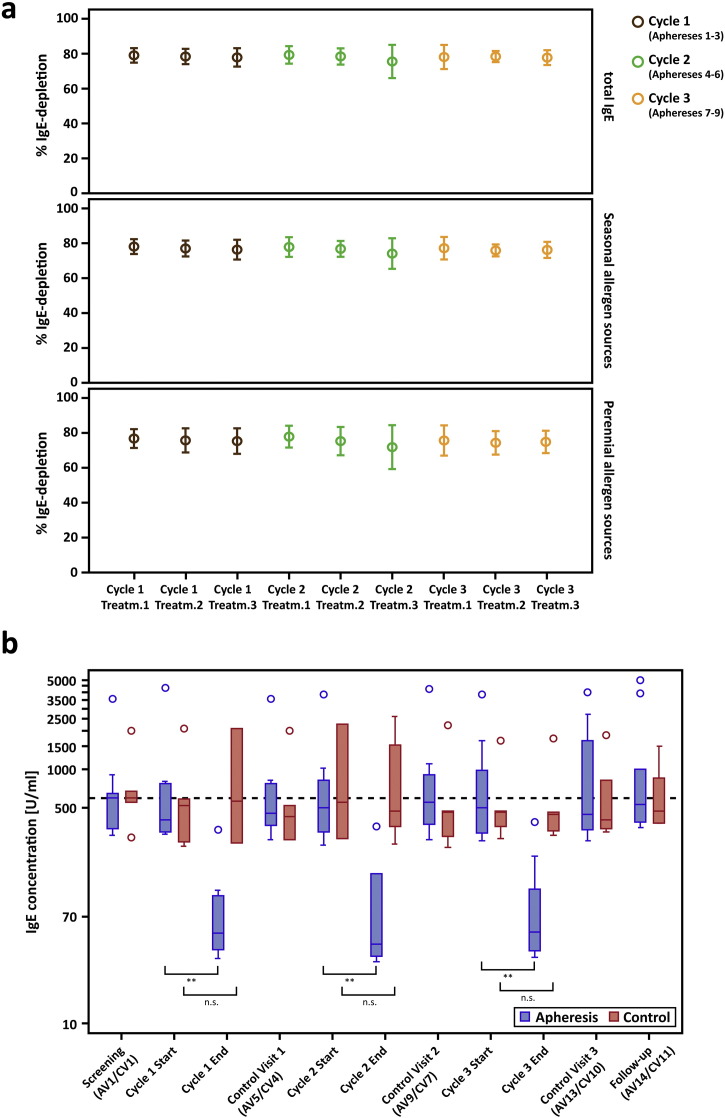
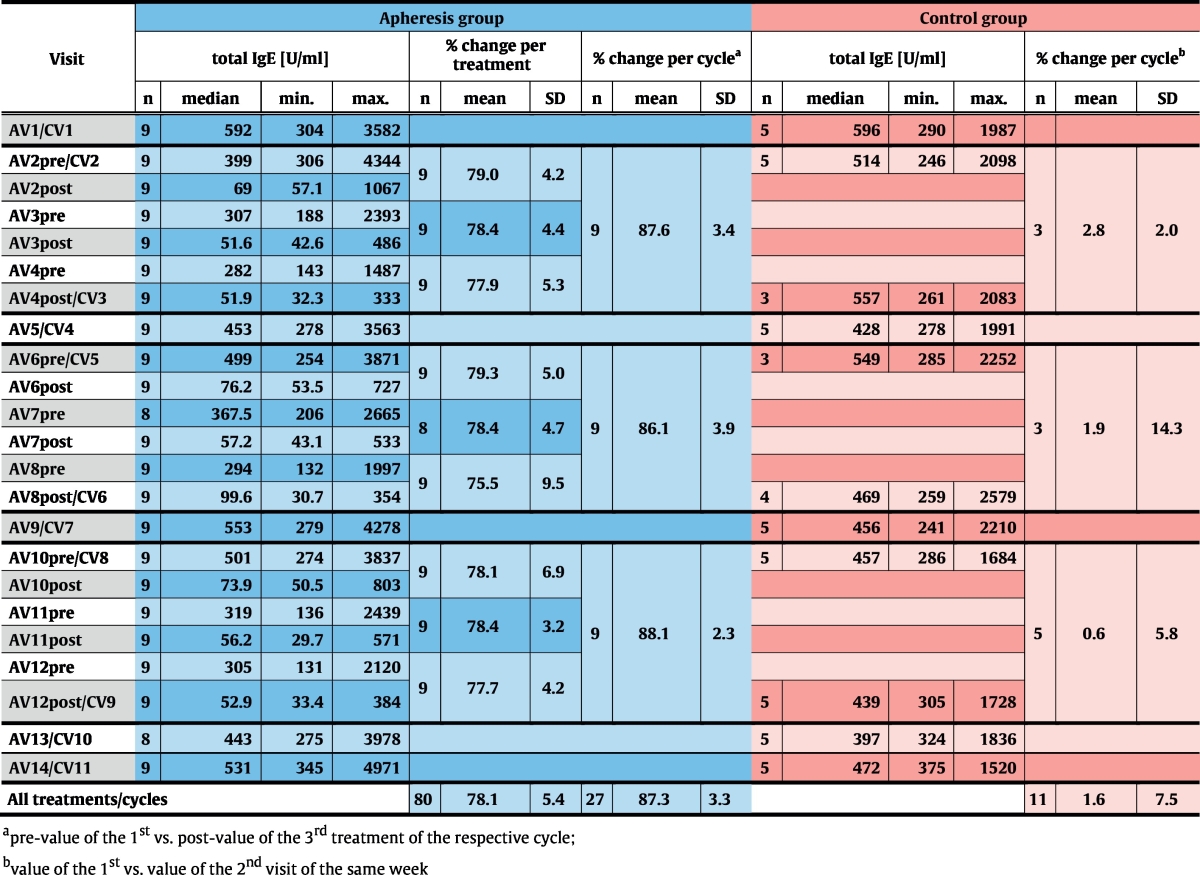
3.2. Reproducible and Selective Depletion of 87.3% of total IgE per Cycle of Immunoadsorption with IgEnio
Mean IgE-depletion rates were 78.1% (± 5.4% SD, p < 0.0001) per treatment and 87.3% (± 3.3% SD, p < 0.0001) per cycle (Fig. 2a, Table 4). Despite a wide range of individual pre-treatment IgE-levels (AV2pre: 306–4344 U/ml), IgE-depletion rates were similar in all patients (Table 4 and Table E1, online repository) and highly reproducible (observed differences between mean desorption rates of all 9 treatment days: p = 0.95). Comparing pre-treatment values of the very first apheresis (AV2pre; AV – apheresis visit) with post-treatment values of the last visit of cycle 3 (AV12post), the mean IgE-desorption rate was 86.2% (± 5.1% SD) in the intention-to-treat group. Thus, the primary endpoint of the study was achieved in all apheresis patients (p < 0.0001).
Fig. 2.
Relative IgE-depletion and absolute IgE-levels. (a) Mean (± SD) depletion rates (y-axes: % IgE-reduction) of total IgE (top chart), IgE to seasonal (center) and perennial allergen sources (bottom) are shown for each treatment visit (x-axes) of the apheresis group. (b) Absolute total IgE-levels (y-axis: U/ml) are shown as box-and-whisker plots for the screening visit, the first (Start) and the last visit (End) of each treatment cycle, for control- and follow-up visits (x-axis). Blue boxes show results for the treatment, red boxes for the control group, outliers are indicated by circles. The dashed line corresponds to median total IgE-levels for both groups at the screening visit. Significant differences between start and end of the respective cycle are indicated (**p ≤ 0.01).
Table 4.
Absolute levels and relative reduction of total IgE in the intention-to-treat population. Median, minimum (min.) and maximum (max.) total IgE-levels [U/ml] are shown for each visit for the apheresis (blue) and the control group (pink); pre – levels measured before apheresis; post – levels measured after apheresis; AV – visits of the apheresis group; CV – visits of the control group. Mean (± SD) relative reductions of total IgE-levels are shown for each treatment visit for the apheresis group. For both groups, mean (± SD) values of relative changes are shown for each treatment cycle or for corresponding weeks of the control group, respectively.
Reduction rates were almost identical for total IgE and IgE specific for seasonal and perennial allergen sources (Fig. 2a, Table E1, online repository). Significant reduction of absolute total IgE-levels between the first and the last visit of each cycle were observed in the apheresis group (p = 0.004) but not in the control group at corresponding time points. At each control visit, i.e., 2 weeks after treatment cycles, IgE-levels had returned to baseline, indicating that IgE-depletion does not influence IgE-production (Fig. 2b, Table 4, Table E1, online repository).
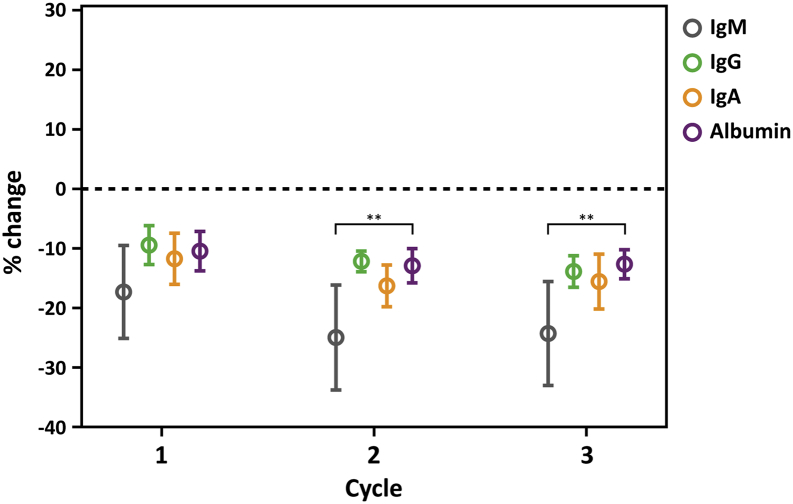
Specificity of the treatment for IgE was demonstrated by comparing changes in IgG, IgA and IgM with changes in serum albumin (Fig. 3, Table 5).
Fig. 3.
Isotype specificity of IgEnio. Relative changes (y-axis: % changes to pre-treatment values) in serum IgM (gray), IgG (green), IgA (orange) and albumin (purple) are shown for each treatment cycle (x-axis) for the apheresis group. Error bars represent mean relative changes ± standard deviations. Significant differences to changes in serum albumin are indicated (**p ≤ 0.01).
Table 5.
Mean relative changes (± SD) of serum IgM, IgG, IgA and albumin-levels are shown for each treatment day and -cycle. AV – visits of the apheresis group.
3.3. Immunoadsorption with IgEnio is Safe and Well-tolerated
With the exception of 1 patient of the treatment group who withdrew informed consent for personal reasons before the first immunoadsorption treatment, all subjects completed the trial. All 9 patients of the intention-to-treat group were subjected to 3 complete cycles of 3 aphereses each. All but one subject were treated per protocol (Fig. 1a). Due to low body weight of this patient (subject 009; Table 2) < 80% of the planned plasma volume were desorbed during each apheresis.
Almost all adverse events (AE) judged by the investigators as treatment-related were clinically irrelevant deviations of laboratory parameters which are frequently observed after immunoadsorption, and resolved without any treatment within 1–2 days (not shown). Allergy-related AEs were mild skin-reactions and general disorders (Table 6).
Table 6.
Mild allergy-related adverse events that occurred after the screening phase.
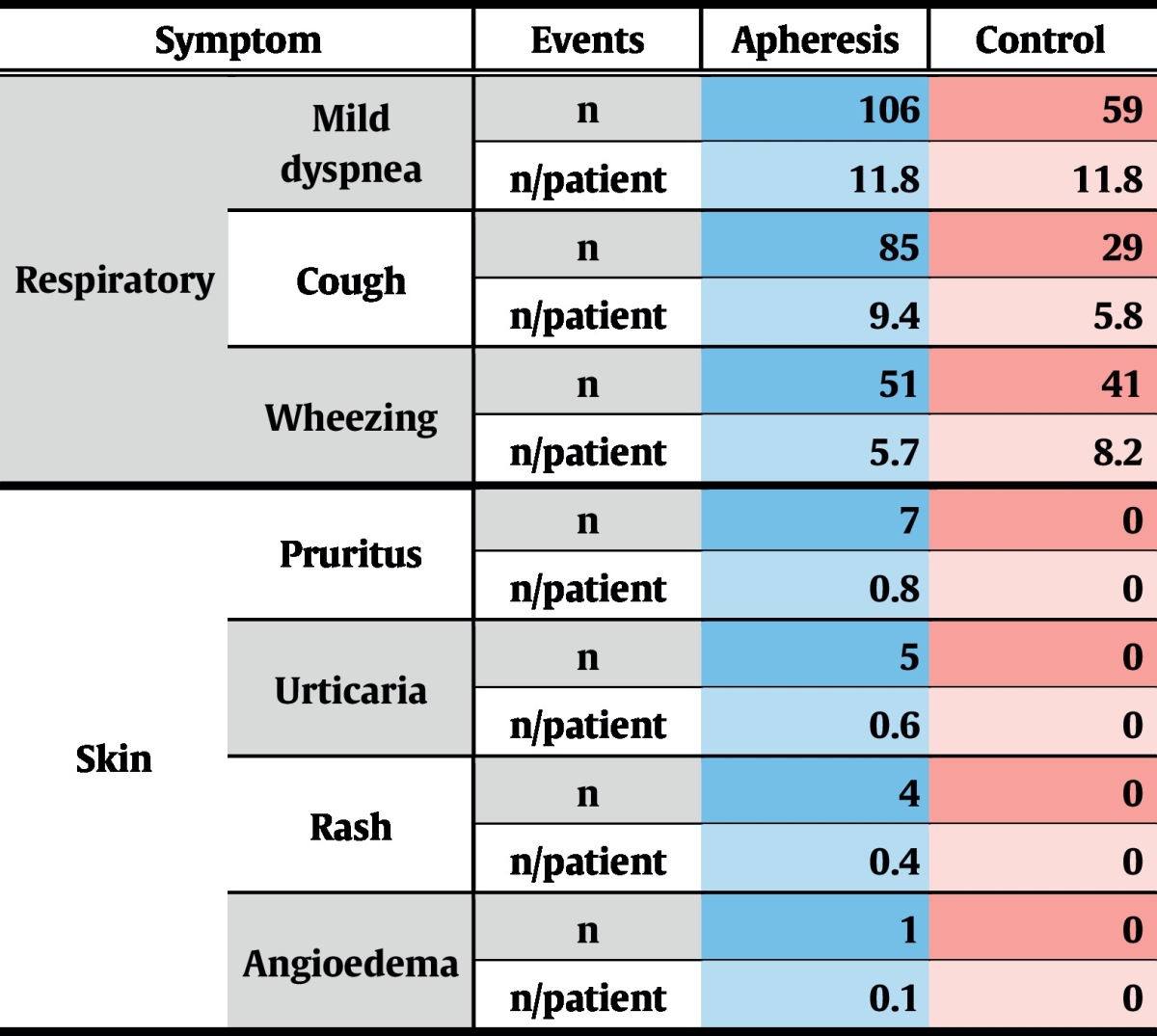
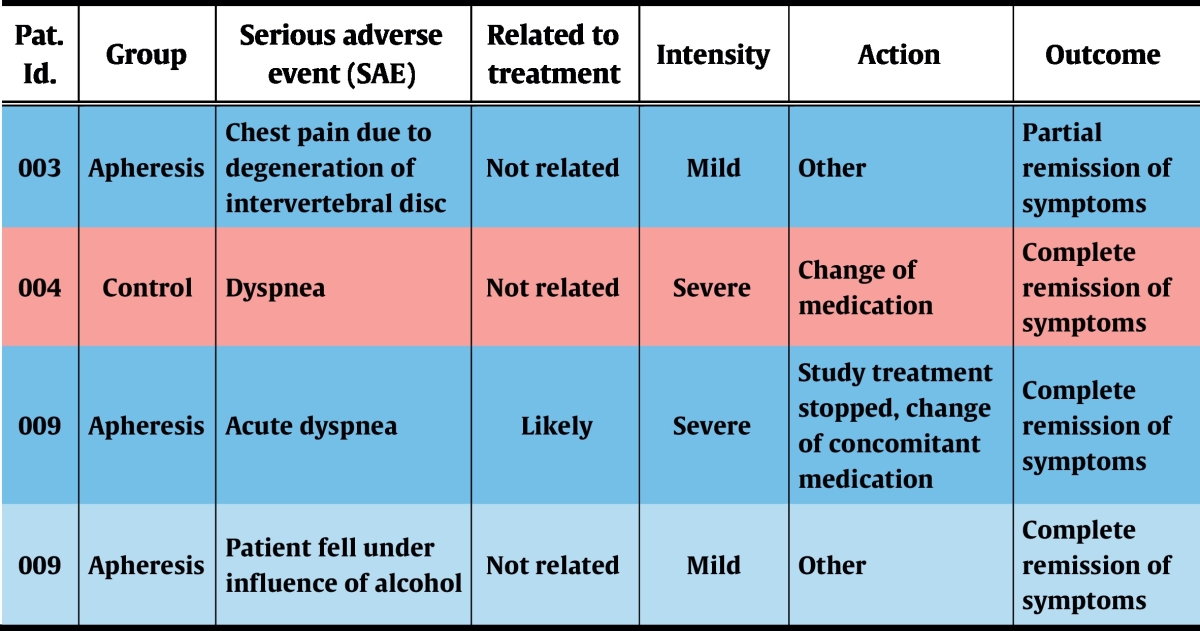
During the complete study period, 7 severe adverse events (SAE) were recorded, 4 of which occurred during the treatment phase. Of these, 1 SAE was reported in the control group and 3 in the treatment group (Table 7). Only 2 SAEs emerged during apheresis treatments and were thus rated as treatment-associated: one patient reported chest pain that started while being treated, which turned out to be due to chronic degenerative changes of the spinal column. The second patient developed acute dyspnoea, starting a few minutes before completion of immunoadsorption. This acute condition resolved quickly under symptomatic medication and the patient could be discharged on the same day. This patient already had exhibited severe asthma attacks during the screening phase and was even hospitalized once due to an intractable status asthmaticus.
Table 7.
Serious adverse events that occurred during the treatment phase.
3.4. Immunoadsorption with IgEnio Reduces Sensitivity to Seasonal Pollen Allergens
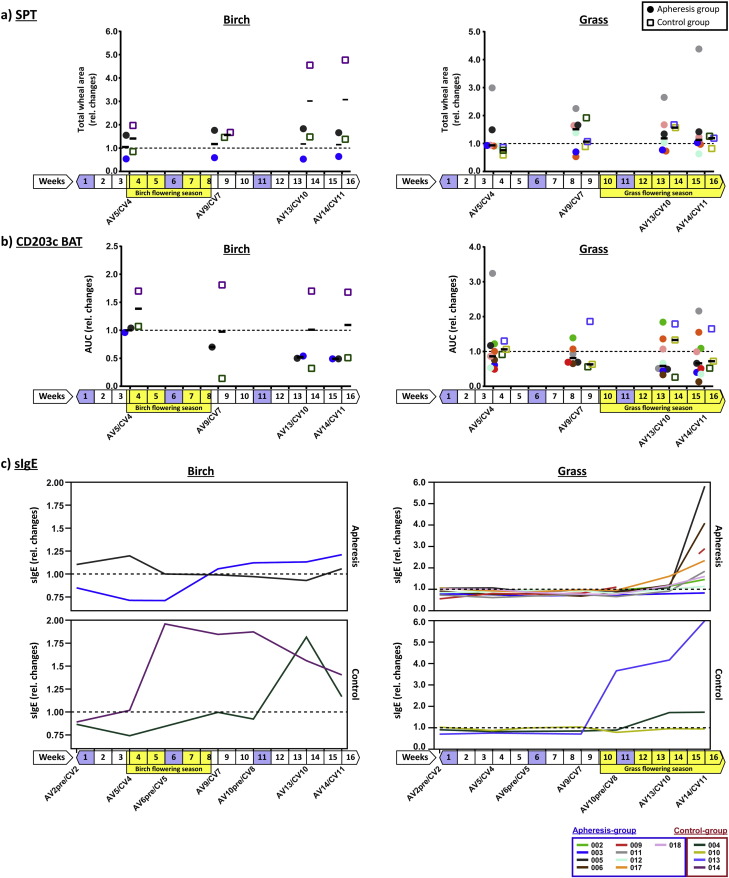
During the study, patients were seasonally exposed to birch and grass pollen which is known to boost allergen-specific IgE-levels (Niederberger et al., 2007). We therefore analyzed birch- and grass pollen allergen-specific IgE-levels, as well as skin- and basophil-reactivity in birch and grass pollen allergic subjects among our study participants. Patient 014 from the control group showed a strong boost of birch-specific IgE (Fig. 4c, left chart, purple curve) which was associated with an almost twofold increase in birch-specific basophil-reactivity. A more than fourfold increase in birch-specific skin-reactivity occurred with a delay of several weeks in this patient (Fig. 4a and b, left charts, purple squares). Birch pollen-specific IgE-levels, as well as basophil- and skin-reactivity of control patient 004 (dark green) showed no relevant changes during the birch season, while basophil-reactivity declined and no relevant change of skin-sensitivity to birch pollen was found. The strong increase in birch-specific IgE after week 10, i.e., outside the birch season, was due to polyclonal activation during a protracted respiratory infection, resulting in an increase of all IgE-specificities tested (Fig. E1, online repository). Unlike control patient 014, patients treated by apheresis (003, 005) showed no relevant increase of birch pollen-specific IgE-levels during the pollen season, there was a gradual decline in basophil-sensitivity and no increase in skin-sensitivity occurred in either patient (Fig. 4, left).
Fig. 4.
Changes in allergen-specific skin- and basophil-reactivity and in sIgE-levels. Relative changes (y-axes: fold changes) of (a) total wheal-areas from skin prick testing (SPT) and (b) of basophil-sensitivity (CD203c BAT – basophil activation test measuring CD203c upregulation; AUC – area under the curve) are shown as scatter plots for control and follow-up visits (x-axes) for birch (left) and grass pollen allergens (right). Results from individual subjects are colour-coded and displayed for the apheresis (filled circles) and for the control group (open boxes) with the respective median levels indicated by horizontal black lines for each group and visit. (c) Relative changes of sIgE-levels to birch (left) and grass (right) are displayed for the same subjects from (a) and (b) (top-charts: apheresis group, bottom-charts: control group). Weeks of study duration are shown underneath the x-axes with birch- and grass seasons highlighted by yellow boxes and treatment weeks in purple. Dashed horizontal lines indicate baseline levels. AV – visit apheresis group; CV – visit control group.
For the nine grass pollen allergic patients treated by apheresis, with the exception of patient 017, increases of grass pollen-specific IgE-levels were detected only at the follow-up visit (AV14) approximately four weeks after the last apheresis (Fig. 4c, right). This is in contrast to the control group, where two out of three patients showed a boost already at control visit 8 (CV8, patient 013) or CV10 (patient 004), respectively, soon after start of the grass pollen season. Accordingly, we found that grass pollen-specific basophil-sensitivity in the apheresis group declined until AV13 and skin-sensitivity did not increase (Fig. 4a and b, right). Control patient 013, who had shown a more than 5-fold increase of grass pollen-specific IgE-levels also increased in basophil- and skin-sensitivity.
In contrast to seasonal allergen-sources, no significant differences were observed for perennial allergen sources (e.g., house dust mite) regarding allergen-specific IgE-levels, basophil- or skin-sensitivity (Fig. E2, online repository).
No effect of apheresis treatment on allergen-specific T cell proliferation was found (data not shown).
3.5. Treatment with IgEnio is Associated with Improved Lung Function and Reduced Lung Symptoms
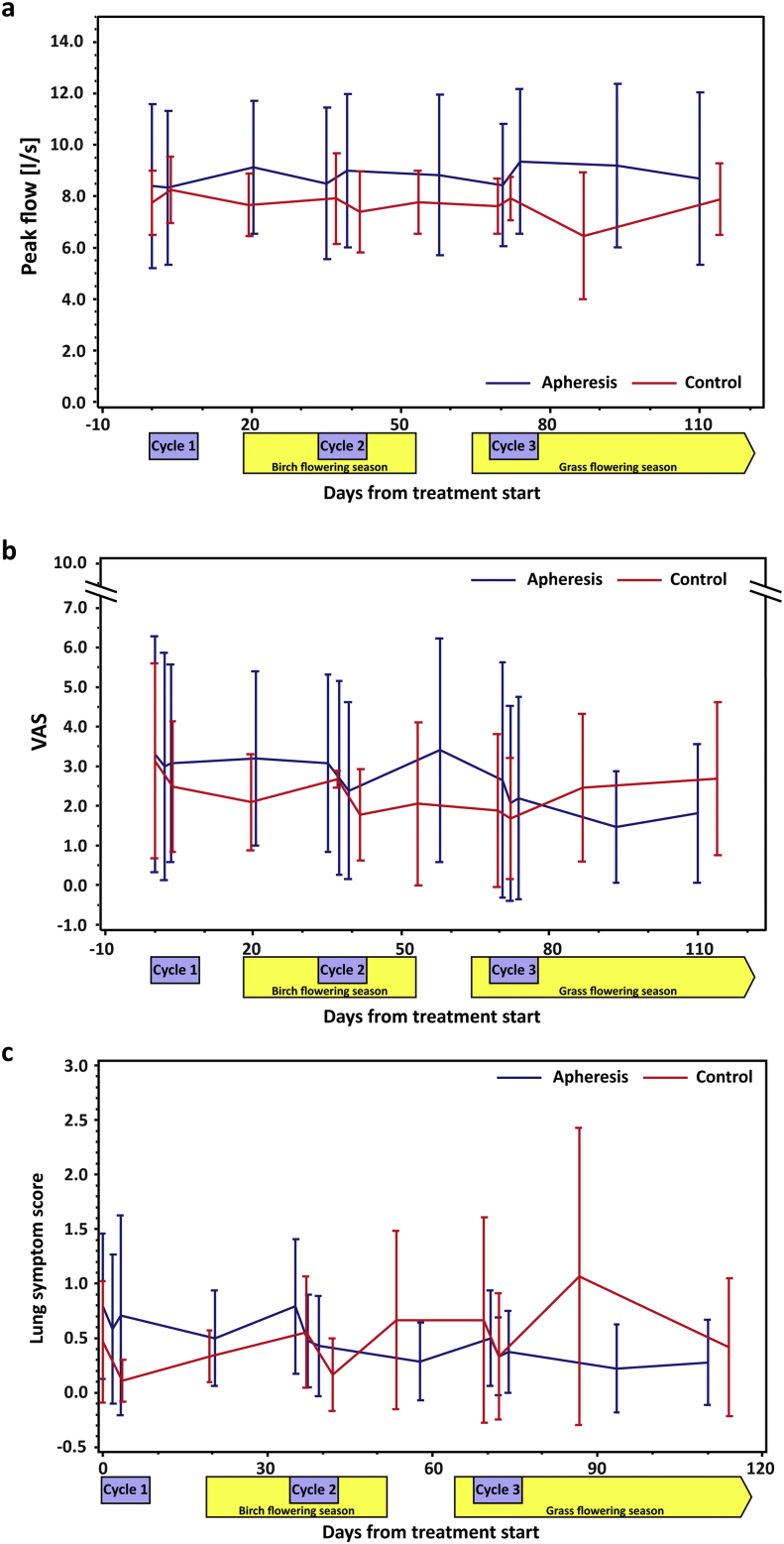
Starting from similar baseline levels, a steady improvement in mean peak flow was observed in the apheresis group whereas the control group had relatively unchanged levels up to day 70 when, coinciding with the onset of the grass pollen season, a drop of peak flow levels was measured (Fig. 5a). In accordance with these data, apheresis patients showed continuous improvement regarding overall (VAS) and lung-specific symptoms while control subjects deteriorated in both parameters (Fig. 5b and c). No differences were found between the two groups for eye- and nose-related symptoms (not shown). Due to the small number of patients, results were not statistically significant.
Fig. 5.
Changes in peak flow and symptom scores. (a) Peak flow results (y-axis: l/s), (b) overall severity of allergy-related symptoms (y-axis: VAS – visual analogue scale) and (c) severity of lung symptoms (y-axis: lung symptom score), based on daily assessment by the patients, are shown for the complete study period (x-axes: days from treatment start). Blue curves represent results from the apheresis group and red curves from the control group. Error bars show mean values, ± SD. Treatment weeks are highlighted by purple boxes, birch and grass pollen seasons by yellow boxes.
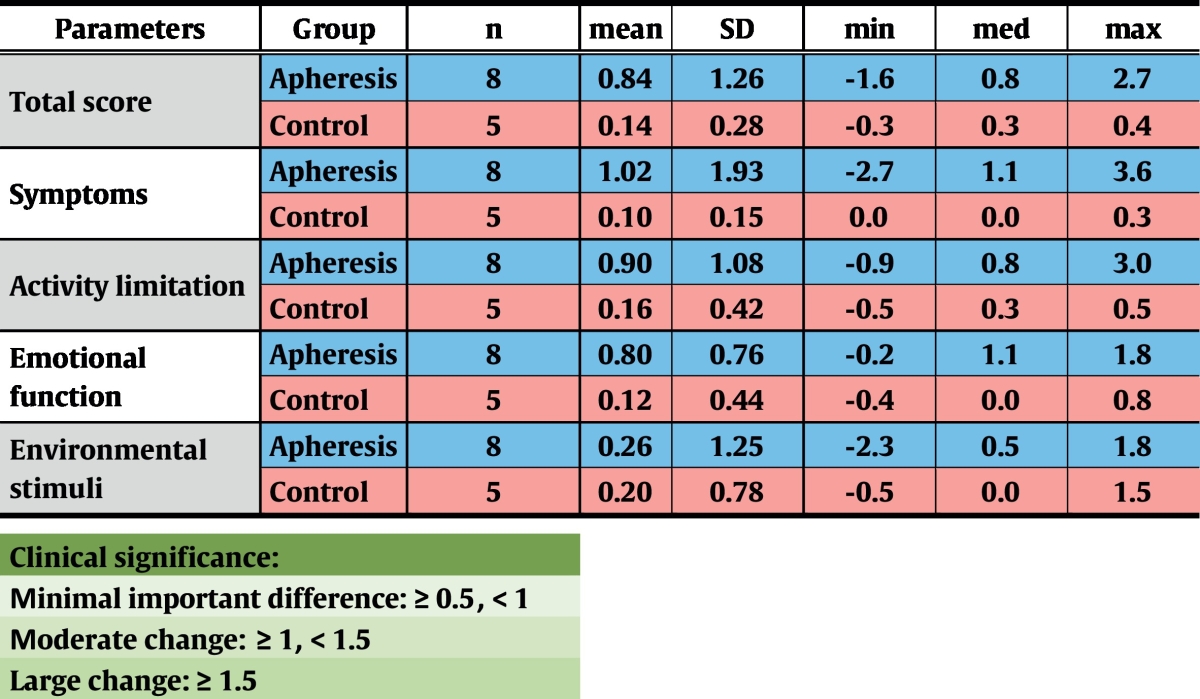
Results from an Asthma Quality of Life Questionnaire (Juniper et al., 1999) were obtained from all but one patient (Table 8), showing a moderate change in symptoms in the apheresis group at the follow-up visit when compared to results from treatment start (n.s.). For activity limitation (n.s.), emotional function (p = 0.02) and total score (n.s.), minimal clinically important changes were observed. By contrast, no shifts in any parameter were recorded in the control group.
Table 8.
Results from a validated Asthma Quality of Life Questionnaire. In every category, several questions had to be answered by selection of a value between 1 (complete impairment) and 7 (no impairment). Differences between results obtained at the end and at the beginning of the trial are shown. SD – standard deviation; min – minimum; med – median; max – maximum.
3.6. IgEnio Depletes IgE In Vitro in Presence of Omalizumab
It is known from previous studies (Jardieu and Fick, 1999) that in patients who are subjected to Omalizumab treatment, considerable concentrations of IgE/Omalizumab immune complexes accumulate in the blood. In a serum sample with moderately elevated total IgE-level (serum 1, 263 U/ml), addition of an equal amount of Omalizumab as related to the IgE-level did not interfere with IgE-depletion by IgEnio adsorber material in vitro (Fig. 6a and Table 9). Even in presence of a 40-fold excess of Omalizumab, in vitro depletion rates were only slightly lower than in the serum sample alone (76.8% versus 90% depletion). When the same experiment was performed using a serum sample with a higher total IgE-level, i.e., 1427 U/ml (serum 2), reduction of depletion rates from 87.1% in samples without Omalizumab to 55% was demonstrated, regardless if an equimolar amount or a 40-fold excess of Omalizumab were added (Fig. 6b and Table 9).
Fig. 6.
In vitro IgE-depletion from serum samples after addition of Omalizumab. Two serum samples with total IgE-levels < 300 U/ml (a, low IgE) or > 1400 U/ml (b, high IgE), without (yellow bars) and with different IgE-to-Omalizumab ratios (x-axes: 1:1 – pink bars; 1:40 – brown bars) were applied to IgEnio adsorber material. Relative depletion rates of total-IgE are shown (y-axes).
Table 9.
In vitro depletion of IgE from sera after addition of Omalizumab.
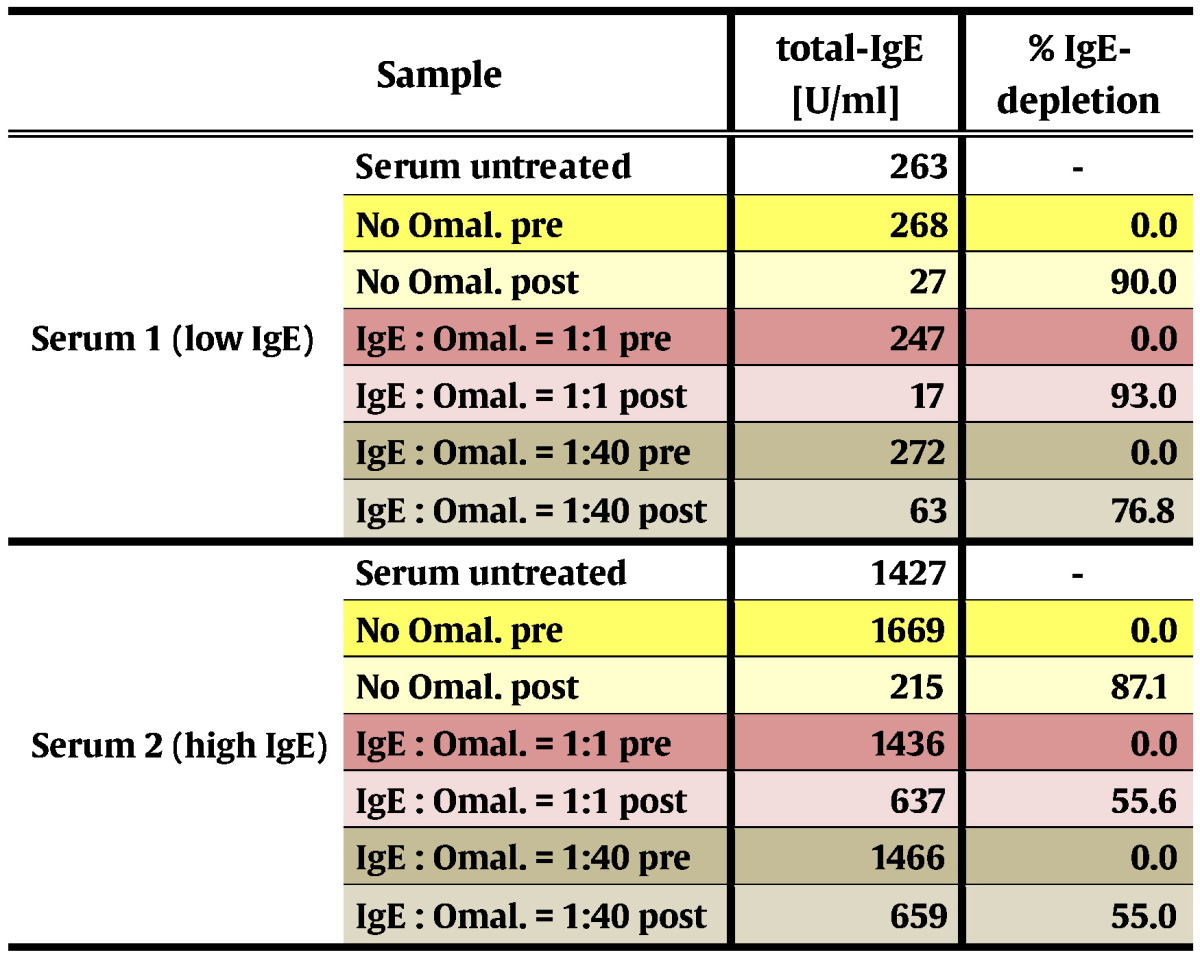
Omal. – Omalizumab; pre – samples obtained before application to IgEnio; post – samples obtained after application to IgEnio.
4. Discussion
Our study shows that IgE-desorption with IgEnio leads to selective and highly reproducible IgE-reduction rates of 87.3% per cycle in patients with a broad range of pre-treatment IgE-levels (306–4344 U/ml). This is in line with results from previous in vitro tests and indicates that subjects with even higher total IgE-levels can be treated with IgEnio. No relevant changes of IgE-levels were noted in the control group.
Since desorption with the single-use columns was done by peripheral venous access, treatment with IgEnio was convenient, safe and well-tolerated. In total, 81 treatments were performed by veno-venous access in highly sensitive patients suffering from allergic asthma and other allergic co-morbidities such as allergic rhinitis, atopic dermatitis and life-threatening food allergies. Most adverse events were subclinical deviations of laboratory parameters that resolved without any treatment. Mild respiratory AEs such as dyspnea, cough or wheezing occurred at similar frequencies in both groups. Skin reactions, such as pruritus, urticaria, rash and one case of angioedema were observed in the apheresis group, but rapidly resolved under antihistamines and steroids, respectively. Likewise, the only SAE likely related to the treatment, i.e., an asthma attack at the end of the first apheresis in one patient, resolved under medication and the patient was discharged on the same day. The remaining 8 treatments were well tolerated by this patient.
Even though aphereses required 3.5 h on average, compliance of patients was high, as indicated by the absence of drop-outs during the treatment phase.
Although the study was designed to investigate efficacy, specificity and safety of IgE-desorption by IgEnio, data on immunological and clinical effects were also collected. The trial was conducted from end of February until mid-July 2014, covering birch and grass flowering seasons. Usually, strong increases of allergen-specific IgE, basophil- and skin-sensitivity are observed in birch and grass pollen allergic patients after seasonal exposure (Niederberger et al., 2007). Interestingly, pollen allergen-specific basophil-sensitivity declined in the apheresis group and skin-sensitivity did not increase after the pollen season. This may be explained by the fact that, due to removal of the newly synthesized IgE, cells were not loaded with the latter. Effects of IgE immunoadsorption on basophil-sensitivity appeared earlier than those on skin-sensitivity which may be due to the shorter life-span of basophils as compared to mast cells. Immunological effects observed were paralleled by clinical effects: improvement in peak flow was detected in the treated group already after the first cycle of immunoadsorption that was not reversed even during the peak of the birch pollen season (Fig. 5a). Likewise, steady improvement in lung symptoms was recorded for the apheresis group, in contrast to worsening in the control group after day 40, when the highest birch pollen counts were reported (Fig. 5c). After the onset of the grass pollen season, the 3rd cycle of immunoadsorption was performed and thus, all patients of the apheresis group had been subjected to two cycles already before being exposed to grass pollen. Shortly after start of the grass season, around day 70, the control group deteriorated in general and lung-associated symptoms and in peak flow measurements, whereas the treated group remained stable or even showed improvement (Fig. 5a–c).
A limitation of our study was that, due to the uneven distribution of allergic co-morbidities (i.e., rhinitis, dermatitis) in the study groups and due to the low number of study participants, effects of the treatment on co-morbidities could not be investigated. The effects of treatment with IgEnio on clinical endpoints will be assessed in follow-up studies. Likewise, effects of long-term treatment will be analyzed in future studies.
Based on these results, two treatment scenarios emerge: first, we suggest IgE immunoadsorption with IgEnio in patients with pollen-induced asthma attacks, initiated shortly before and continued during the pollen season. This treatment can be prescribed regardless of total IgE-levels due to the high binding-capacity of IgEnio. Since treatment with IgEnio was well tolerated, we think that it also can be applied in patients suffering from severe and uncontrolled asthma. Accordingly, the second clinical application suggested by our data concerns polysensitized allergic patients who are not eligible for Omalizumab treatment due to IgE-levels beyond the current limits of the drug (1500 U/ml in the EU (EMA, 2015) and 700 U/ml in the US (FDA, 2015)). Among our study population, 3 treated subjects had pre-treatment levels exceeding these limits (Table E1, online repository, patient 002: 4344 U/ml, patient 006: 797 U/ml, patient 017: 764 U/ml). In such patients, IgE-levels could be reduced by immunoadsorption in a first step, followed by administration of Omalizumab. An equivalent strategy already was successfully applied in a child suffering from severe asthma (Kerzel et al., 2011), in patients with severe atopic dermatitis (Zink et al., 2016) and in a case of life-threatening food allergy (Dahdah et al., 2015). However, reduction of total IgE-levels in these patients was achieved either by plasma exchange (Kerzel et al., 2011), a very cumbersome and unspecific treatment, by using an adsorber non-specifically removing antibodies of all isotypes (Zink et al., 2016) or by an IgE-specific adsorber that also reduced IgG, IgM and IgA by approximately 40% (Dahdah et al., 2015). In contrast, IgE-depletion with IgEnio would facilitate Omalizumab administration after fewer desorptions and without alteration of other plasma components than IgE which would allow to use IgEnio also in children.
In addition, results from in vitro experiments demonstrated that IgEnio also depletes IgE in presence of Omalizumab (Fig. 6, Table 9). This observation indicates that IgEnio may also be used in Omalizumab-treated patients with extremely elevated IgE-levels in order to remove potentially harmful immune complexes. Other treatment scenarios to be studied include application in polysensitized patients, in atopic dermatitis (Kasperkiewicz et al., 2011, Kasperkiewicz et al., 2014, Reich et al., 2016) and in the context of allergen-specific immunotherapy.
In summary, we provide evidence that IgE immunoadsorption with IgEnio is a treatment strategy for patients with severe pollen-induced asthma attacks and for allergic patients with high serum total IgE-levels. Additional clinical investigations with a higher number of participants are planned to confirm these results and to further improve treatment efficacy.
Funding Sources
Sponsor of this study was Fresenius Medical Care Deutschland GmbH (Bad Homburg, Germany and in part by grants F4605, F4611 and F4613 of the Austrian Science Fund (FWF)), providing funding for planning and conduction of the trial and for immunological and in vitro safety analyses. Employees of the sponsor contributed to development of the medical device, study design and data analysis (see Author contributions section).
Conflicts of Interest
Christian Lupinek's salary during the conduction of the trial was partly funded by Fresenius Medical Care (Bad Homburg, Germany).
Rudolf Valenta has received research grants from Biomay AG (Vienna, Austria), Thermo Fisher (Uppsala, Sweden) and Fresenius Medical Care (Bad Homburg, Germany) and serves as consultant for these companies.
René Cervenka, Thomas Plaichner, Hans Peter Leinenbach, Adelheid Gauly and Ingrid Uhlenbusch-Koerwer are employees of Fresenius Medical Care (producer of IgEnio and sponsor of the study).
Volker Schoder and Justyna Kozik-Jaromin were employees of Fresenius Medical Care (producer of IgEnio and sponsor of the study) when the trial was planned and conducted.
Gottfried Stegfellner, Hans Huber and Rainer Henning are employees of Biomay AG (producer of BM10, the ligand of IgEnio).
No conflicts of interest related to the present article: Kurt Derfler, Silvia Lee, Thomas Prikoszovich, Oliver Movadat, Eva Wollmann, Ventzislav Petkov, Carolin Cornelius, Milena Weber, Renate Fröschl, Regina Selb, Katharina Blatt, Dubravka Smiljkovic, Thomas Perkmann, Verena Niederberger, Peter Valent.
Author Contributions
Christian Lupinek, Rudolf Valenta: study design, data analysis and -interpretation, paper writing, figures.
Kurt Derfler, Silvia Lee, Thomas Prikoszovich, Oliver Movadat, Eva Wollmann, Ventzislav Petkov: recruitment, treatment and clinical examination of patients, critical reading of the paper.
Carolin Cornelius, Milena Weber, Renate Fröschl, Regina Selb, Katharina Blatt, Dubravka Smiljkovic, Thomas Perkmann, Verena Niederberger, Peter Valent: immunological and serological in vitro testing, critical reading of the paper.
Volker Schoder: sample size calculation, planning and execution of statistical data analysis.
René Cervenka, Thomas Plaichner, Hans Peter Leinenbach: development (coupling of the ligand, sterilization) and pre-clinical testing of IgEnio, production of columns for the clinical trial, critical reading of the paper.
Gottfried Stegfellner, Hans Huber, Rainer Henning: process development and production of BM10, i.e., the ligand for IgEnio.
Justyna Kozik-Jaromin: safety and SAE reporting during the trial, critical reading of the paper.
Adelheid Gauly, Ingrid Uhlenbusch-Koerwer: study design, coordination of the study team, critical reading of the paper.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.02.007.
Appendix A. Supplementary Data
Supplementary methods and results.
Study protocol.
References
- Banerjee S., Resch Y., Chen K.-W., Swoboda I., Focke-Tejkl M., Blatt K., Novak N., Wickman M., van Hage M., Ferrara R., Mari A., Purohit A., Pauli G., Sibanda E.N., Ndlovu P., Thomas W.R., Krzyzanek V., Tacke S., Malkus U., Valent P., Valenta R., Vrtala S. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J. Invest. Dermatol. 2015;135:102–109. doi: 10.1038/jid.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Hamburger R.N. Standardization and stability of immunoglobulin E (IgE) J. Allergy Clin. Immunol. 1972;49:189–191. doi: 10.1016/0091-6749(72)90113-3. [DOI] [PubMed] [Google Scholar]
- Bel E.H., Wenzel S.E., Thompson P.J., Prazma C.M., Keene O.N., Yancey S.W., Ortega H.G., Pavord I.D., Investigators S., Gibson P., Sajkov D., Thompson P., Laviolette M., Lemiere C., Nair P., Bystron J., Chlumsky J., Kindlova A., Kolek V., Pauk N., Aubier M., Bourdin A., De Blay F., Donazzolo Y., Magnan A., Ballenberger S., Beck E., Hoffmann M., Korn S., Kornmann O., Ludwig-Sengpiel A., Rolke M., Schroeder-Babo W., Hernandez-Colin D.D., Bel E.H.D., ten Brinke A., Mroz R., Pulka G., Howarth P., Mortimer K., Pavord I., Spencer D., Castro M., Chupp G., Katial R., Meltzer S., Wenzel S. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- BTS . British Thoracic Society, Scottish Intercollegiate Guidelines Network (SIGN); 2010. British Guideline on the Management of Asthma. SIGN Guideline 101. 2008.http://www.sign.ac.uk/pdf/sign101.pdf [Online] Available from: [Google Scholar]
- Bundesärztekammer . Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF); 2009. Nationale Versorgungsleitlinie Asthma–Langfassung.http://www.versorgungsleitlinien.de/themen/asthma [Online] Available from: [Google Scholar]
- Dahdah L., Ceccarelli S., Amendola S., Campagnano P., Cancrini C., Mazzina O., Fiocchi A. IgE immunoadsorption knocks down the risk of food-related anaphylaxis. Pediatrics. 2015;136:e1617–e1620. doi: 10.1542/peds.2015-1757. [DOI] [PubMed] [Google Scholar]
- Davila I., Valero A., Entrenas L.M., Valveny N., Herraez L., Group, S. S Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J. Investig. Allergol. Clin. Immunol. 2015;25:120–127. [PubMed] [Google Scholar]
- EMA . European Medicines Agency; 2015. Xolair (Omalizumab)http://www.ema.europa.eu [Online] Available from: [Google Scholar]
- FDA . Food and Drug Administration; 2015. Xolair Prescribing Information.www.accessdata.fda.gov/drugsatfda_docs/label/2015/103976s5224lbl.pdf [Online] Available from: [Google Scholar]
- FMC . Fresenius Medical Care Deutschland GmbH; 2014. Extracorporeal SPecific IgE Removal From the Plasma of Allergic Asthma Patients (ESPIRA-study)https://clinicaltrials.gov [Online] Available from: [Google Scholar]
- Gauvreau G.M., O'Byrne P.M., Boulet L.-P., Wang Y., Cockcroft D., Bigler J., FitzGerald J.M., Boedigheimer M., Davis B.E., Dias C., Gorski K.S., Smith L., Bautista E., Comeau M.R., Leigh R., Parnes J.R. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- GCP . ISO; 2011. Clinical Investigation of Medical Devices for Human Subjects - Good Clinical Practice.http://www.iso.org [Online] Available from: [Google Scholar]
- Gergen P.J., Arbes S.J., Jr., Calatroni A., Mitchell H.E., Zeldin D.C. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 2009;124:447–453. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINA . Global Initiative for Asthma (GINA); 2007. The Global Strategy for Asthma Management and Prevention.http://www.ginasthma.com/download.asp?intId=309 [Online] Available from: [Google Scholar]
- Hauswirth A.W., Natter S., Ghannadan M., Majlesi Y., Schernthaner G.H., Sperr W.R., Buhring H.J., Valenta R., Valent P. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J. Allergy Clin. Immunol. 2002;110:102–109. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- Heinzerling L., Mari A., Bergmann K.C., Bresciani M., Burbach G., Darsow U., Durham S., Fokkens W., Gjomarkaj M., Haahtela T., Bom A.T., Wohrl S., Maibach H., Lockey R. The skin prick test - European standards. Clin. Transl. Allergy. 2013;3:3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- Holgate S.T. New strategies with anti-IgE in allergic diseases. World Allergy Organ. J. 2014;7:17. doi: 10.1186/1939-4551-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M., Busse W., Hanania N.A., Lowe P.J., Canvin J., Erpenbeck V.J., Holgate S. Omalizumab in asthma: an update on recent developments. J. Allergy Clin. Immunol. Pract. 2014;2(525–36):e1. doi: 10.1016/j.jaip.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Jardieu P.M., Fick R.B., Jr. IgE inhibition as a therapy for allergic disease. Int. Arch. Allergy Immunol. 1999;118:112–115. doi: 10.1159/000024043. [DOI] [PubMed] [Google Scholar]
- Juniper E.F., O'Byrne P.M., Guyatt G.H., Ferrie P.J., King D.R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M., Schmidt E., Frambach Y., Rose C., Meier M., Nitschke M., Falk T.M., Reich K., Ludwig R.J., Zillikens D. Improvement of treatment-refractory atopic dermatitis by immunoadsorption: a pilot study. J. Allergy Clin. Immunol. 2011;127 doi: 10.1016/j.jaci.2010.07.042. 267–70, 270 e1–6. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M., Sufke S., Schmidt E., Zillikens D. IgE-specific immunoadsorption for treatment of recalcitrant atopic dermatitis. JAMA Dermatol. 2014;150:1350–1351. doi: 10.1001/jamadermatol.2014.2082. [DOI] [PubMed] [Google Scholar]
- Kerzel S., Zemlin M., Rogosch T., Ollert M., Renz H., Klaus G., Maier R.F. Plasmapheresis prior to omalizumab administration in a 15-year-old boy with severe asthma and very high IgE levels: sustained effect over 2 years. Klin. Padiatr. 2011;223:356–359. doi: 10.1055/s-0031-1287824. [DOI] [PubMed] [Google Scholar]
- Konradsen J.R., Fujisawa T., van Hage M., Hedlin G., Hilger C., Kleine-Tebbe J., Matsui E.C., Roberts G., Ronmark E., Platts-Mills T.A.E. Allergy to furry animals: new insights, diagnostic approaches, and challenges. J. Allergy Clin. Immunol. 2015;135:616–625. doi: 10.1016/j.jaci.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Lupinek C., Roux K.H., Laffer S., Rauter I., Reginald K., Kneidinger M., Blatt K., Ball T., Pree I., Jahn-Schmid B., Allam J.P., Novak N., Drescher A., Kricek F., Valent P., Englund H., Valenta R. Trimolecular complex formation of IgE, FcεRI, and a recombinant nonanaphylactic single-chain antibody fragment with high affinity for IgE. J. Immunol. 2009;182:4817–4829. doi: 10.4049/jimmunol.0800726. [DOI] [PubMed] [Google Scholar]
- Manise M., Bakayoko B., Schleich F., Corhay J.L., Louis R. IgE mediated sensitisation to aeroallergens in an asthmatic cohort: relationship with inflammatory phenotypes and disease severity. Int. J. Clin. Pract. 2016;70:596–605. doi: 10.1111/ijcp.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midoro-Horiuti T., Nouno S., Seino Y. Skin tests of pollen grains of taxodiaceae and cupressaceae in children with bronchial asthma. Acta Paediatr. Jpn. 1992;34:501–504. doi: 10.1111/j.1442-200x.1992.tb00996.x. [DOI] [PubMed] [Google Scholar]
- Molimard M., Buhl R., Niven R., Le Gros V., Thielen A., Thirlwell J., Maykut R., Peachey G. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir. Med. 2010;104:1381–1385. doi: 10.1016/j.rmed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Mudde G.C., Van Reijsen F.C., Boland G.J., de Gast G.C., Bruijnzeel P.L., Bruijnzeel-Koomen C.A. Allergen presentation by epidermal Langerhans' cells from patients with atopic dermatitis is mediated by IgE. Immunology. 1990;69:335–341. [PMC free article] [PubMed] [Google Scholar]
- NHLBI . National Heart, Lung and Blood Institute (NHLBI); 2007. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007.http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf [Online] Available from: [Google Scholar]
- Niederberger V., Ring J., Rakoski J., Jager S., Spitzauer S., Valent P., Horak F., Kundi M., Valenta R. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int. Arch. Allergy Immunol. 2007;142:133–144. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- O'Byrne P.M., Tworek D. Timing is everything: targeting IgE to reduce asthma exacerbation risk. J. Allergy Clin. Immunol. 2015;136:1486–1487. doi: 10.1016/j.jaci.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Ortega H.G., Liu M.C., Pavord I.D., Brusselle G.G., FitzGerald J.M., Chetta A., Humbert M., Katz L.E., Keene O.N., Yancey S.W., Chanez P., Investigators M., Ardusso L., De Salvo M., Raso E., Rey L., Rodriguez A., Scherbovsky P.S., Wehbe L., Bardin P., Gibson P., Robinson P., Sajkov D., Thompson P., Brusselle G., Dupont L., Louis R., Michel O., Bergeron C., Bhutani M., FitzGerald J.M., Houle P.-A., Killian K., Killorn P., Laviolette M., Leigh R., Lemiere C., Martin J., Pek B., Sharma S., Bisbal C., Patricio M., Saavedra R., Silva Orellana R., Vargas S., Bourdin A., Chanez P., Deschildre A., Devouassoux G., Humbert M., Paganin F., Verdier S., Wallaert B., Ballenberger S., Beck E., Ehlers M., Foerster K., Hofmann S., Illies G., Keller C., Korn S., Kornmann O., Rolke M., Schroeder-Babo W., Zielen S., Baglioni S., Canonica G.W., Chetta A., Foschino Barbaro M., Idotta G., Mereu C., Paggiaro P., Sofia M., Agnese S.A., Fukushima Y., Haida M., Harada T., Hataji O., Hozawa S., Kaneko N., Kato M., Kikuchi K., Kinoshita M., Kishaba T., Matsumoto M., Matsuoka H., Mori M., Nakatani Y., Odajima H., Oki K., Saito T., Ekino H., Shimoda T., Tohyama K., Tsuburai T., Tsuda T., Yamazaki Y., Choi B.W., Jung K.-S., Kim M.-K. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- Pawankar R., Canonica G.W., Holgate S.T., Lockey R.F. Allergic diseases and asthma: a major global health concern. Curr. Opin. Allergy Clin. Immunol. 2012;12:39–41. doi: 10.1097/ACI.0b013e32834ec13b. [DOI] [PubMed] [Google Scholar]
- Pollart S.M., Chapman M.D., Fiocco G.P., Rose G., Platts-Mills T.A. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J. Allergy Clin. Immunol. 1989;83:875–882. doi: 10.1016/0091-6749(89)90100-0. [DOI] [PubMed] [Google Scholar]
- Pollart S.M., Reid M.J., Fling J.A., Chapman M.D., Platts-Mills T.A. Epidemiology of emergency room asthma in northern California: association with IgE antibody to ryegrass pollen. J. Allergy Clin. Immunol. 1988;82:224–230. doi: 10.1016/0091-6749(88)91003-2. [DOI] [PubMed] [Google Scholar]
- Rasband W.S. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2014. ImageJ. [Google Scholar]
- Reed C.E., Swanson M.C., Agarwal M.K., Yunginger J.W. Allergens that cause asthma. Identification and quantification. Chest. 1985;87:40S–44S. [PubMed] [Google Scholar]
- Reich K., Deinzer J., Fiege A.K., von Gruben V., Sack A.L., Thraen A., Weisenseel P., Breuer K., Jackle S., Meier M. Panimmunoglobulin and IgE-selective extracorporeal immunoadsorption in patients with severe atopic dermatitis. J. Allergy Clin. Immunol. 2016;137(1882–1884):e6. doi: 10.1016/j.jaci.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Schäppi G.F., Suphioglu C., Taylor P.E., Knox R.B. Concentrations of the major birch tree allergen Bet v 1 in pollen and respirable fine particles in the atmosphere. J. Allergy Clin. Immunol. 1997;100:656–661. doi: 10.1016/s0091-6749(97)70170-2. [DOI] [PubMed] [Google Scholar]
- Sprenger K.B., Huber K., Kratz W., Henze E. Nomograms for the prediction of patient's plasma volume in plasma exchange therapy from height, weight, and hematocrit. J. Clin. Apher. 1987;3:185–190. doi: 10.1002/jca.2920030313. [DOI] [PubMed] [Google Scholar]
- Suphioglu C., Singh M.B., Taylor P., Bellomo R., Holmes P., Puy R., Knox R.B. Mechanism of grass-pollen-induced asthma. Lancet. 1992;339:569–572. doi: 10.1016/0140-6736(92)90864-y. [DOI] [PubMed] [Google Scholar]
- Tay T.R., Bosco J., Aumann H., O'Hehir R., Hew M. Elevated total serum immunoglobulin E (> 1000 IU/mL): implications? Intern. Med. J. 2016;46:846–849. doi: 10.1111/imj.13073. [DOI] [PubMed] [Google Scholar]
- van der Heijden F.L., Joost van Neerven R.J., van Katwijk M., Bos J.D., Kapsenberg M.L. Serum-IgE-facilitated allergen presentation in atopic disease. J. Immunol. 1993;150:3643–3650. [PubMed] [Google Scholar]
- Wenzel S., Ford L., Pearlman D., Spector S., Sher L., Skobieranda F., Wang L., Kirkesseli S., Rocklin R., Bock B., Hamilton J., Ming J.E., Radin A., Stahl N., Yancopoulos G.D., Graham N., Pirozzi G. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- Zink A., Gensbaur A., Zirbs M., Seifert F., Suarez I.L., Mourantchanian V., Weidinger S., Mempel M., Ring J., Ollert M. Targeting IgE in severe atopic dermatitis with a combination of immunoadsorption and omalizumab. Acta Derm. Venereol. 2016;96:72–76. doi: 10.2340/00015555-2165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods and results.
Study protocol.