There is a wealth of evidence from both epidemiological studies (Hales and Barker, 2001) and animal models (Tarry-Adkins and Ozanne, 2016) that environmental exposures during early mammalian development may alter developmental trajectories in a manner that results in altered disease susceptibility and stress responsiveness in later life. This phenomenology is termed ‘developmental programming.’ Developmental programming is of key interest because it has the potential to expand phenotypic diversity within a population in a manner that is not entirely accounted for by the underlying genetic diversity. Furthermore, there are many indicators that such factors may contribute to increased risk of human cardio-metabolic disease. Yet, despite convincing evidence at the phenotypic and epidemiological level, the molecular underpinnings of this phenomenon have remained enigmatic.
The last decade has seen the emergence of genome-wide association studies (GWAS) aimed at addressing the genetic foundation of common human traits and multifactorial disease. Whilst GWAS have been highly successful in delineating many common genetic variants that are associated with particular traits, a surprising outcome is that, collectively, the common variants associated with a given trait only explain a relatively small proportion of its heritability. This intriguing conundrum of ‘missing heritability’ has led to much speculation as to its origin (Trerotola et al., 2015). This article will discuss recent work that suggests that developmental programming may act at genomic regions not profiled within the context of GWAS to contribute to phenotypic diversity through epigenetic mechanisms. Such regions of the genome are overlooked in the context of large-scale genomic studies as current sequencing and computational technologies are unable to resolve their repetitive sequence architecture to sufficient accuracy.
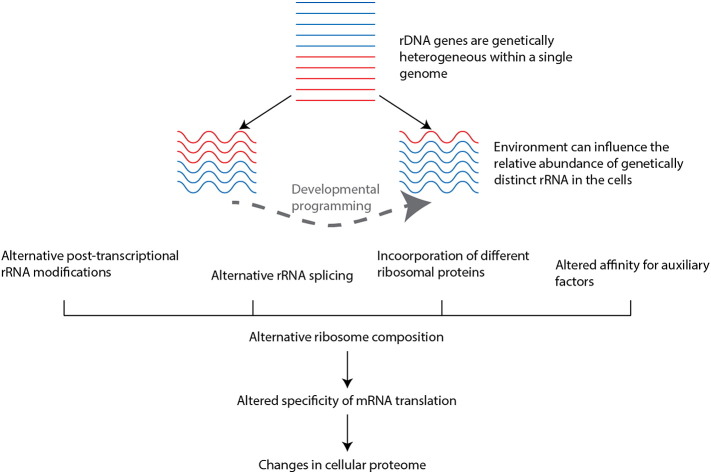
Ribosomal DNA (rDNA) is such a part of the genome. It encodes the RNA components of ribosomes; the cellular machines responsible for making proteins. rDNA exists in 100s of copies/genome and is organised into large repetitive arrays clustered on a number of chromosomes (Dev et al., 1977). Ribosomes have often been assumed to lack functional specificity in their role in protein manufacture. This, together with the technical challenges cited above, has meant that the genetic variation within this part of the genome has remained under-explored. However, more recent work has revealed surprising functional specificity in the role of ribosomes in regulating genomic output via control of selection of which proteins are preferentially produced (Shi and Barna, 2015). These studies have focussed on the incorporation of alternative protein components of ribosomes. However, it is conceivable that genetic variation amongst rDNA copies within the cell could contribute to greater diversity in the repertoire of functional ribosomes by contributing alternative forms of the rRNA components (Fig. 1).
Fig. 1.
Schematic for how environmental influences on rDNA epigenetic regulation may potentially influence the translational machinery. Genetically distinct copies of rDNA are represented as either red or blue.
Furthermore, a recent study has also implicated rDNA as a part of the genome that is responsive to early life dietary exposures in a mouse model of developmental programming. Intriguingly, early life nutrition was found to influence the epigenetic state (i.e. selectively silence) a specific, genetically distinct subset of rDNA within the genome of a given individual. Once established, this effect was permanent throughout the life-course, and altered which genetically distinct rRNA copies are available to form ribosomes (Holland et al., 2016). The implications of these findings for subsequent effects on ribosome composition and protein translation are subject to ongoing investigation. Yet, collectively, this recent work raises the curious possibility that environment and hitherto under-explored genetic variation can act in the context of developmental programming to alter functional genomic output and give rise to phenotypic variation that does not follow expected inheritance patterns.
Such genomic responses may be critical for balancing ‘robustness’-the ability of an organism to maintain a stable phenotypic state in the face of environmental or genetic perturbations; versus ‘plasticity’-the ability of an organism to alter physiology, morphology and development (Lachowiec et al., 2016). How such mechanisms contribute to individual phenotype and disease prevalence across populations in the face of changing environmental pressures should reveal some exciting results in the years to come.
Author Disclosure
The author declares no competing influences.
Acknowledgements
Michelle L. Holland is supported by a Research Council UK Academic Fellowship.
References
- Dev V.G., Tantravahi R., Miller D.A., Miller O.J. Nucleolus organizers in Mus musculus subspecies and in the RAG mouse cell line. Genetics. 1977;86(2 Pt. 1):389–398. [PMC free article] [PubMed] [Google Scholar]
- Hales C.N., Barker D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Holland M.L., Lowe R., Caton P.W., Gemma C., Carbajosa G., Danson A.F. Early-life nutrition modulates the epigenetic state of specific rDNA genetic variants in mice. Science. 2016;353(6298):495–498. doi: 10.1126/science.aaf7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowiec J., Queitsch C., Kliebenstein D.J. Molecular mechanisms governing differential robustness of development and environmental responses in plants. Ann. Bot. 2016;117(5):795–809. doi: 10.1093/aob/mcv151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Barna M. Translating the genome in time and space: specialized ribosomes, RNA regulons, and RNA-binding proteins. Annu. Rev. Cell Dev. Biol. 2015;31:31–54. doi: 10.1146/annurev-cellbio-100814-125346. [DOI] [PubMed] [Google Scholar]
- Tarry-Adkins J.L., Ozanne S.E. Nutrition in early life and age-associated diseases. Ageing Res. Rev. 2016 doi: 10.1016/j.arr.2016.08.003. (pii: S1568-1637(16)30179-9) [DOI] [PubMed] [Google Scholar]
- Trerotola M., Relli V., Simeone P., Alberti S. Epigenetic inheritance and the missing heritability. Hum. Genomics. 2015;9:17. doi: 10.1186/s40246-015-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]