Abstract
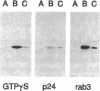
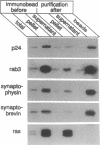
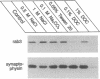
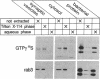
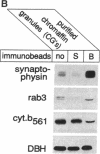
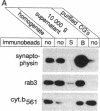
rab3, a low molecular weight GTP-binding protein, is primarily expressed in brain, where it is present in soluble and membrane-bound forms. Membrane-bound rab3 in brain is exclusively localized on synaptic vesicles, the secretory organelles of the synapse that store and release neurotransmitters. rab3 is also expressed in endocrine tissues such as the adrenal medulla, where it is found together with other synaptic vesicle proteins on microvesicles distinct from chromaffin granules. The tight binding of rab3 to membranes correlates with hydrophobic modifications that are different in the membrane-bound and soluble forms of rab3. The results demonstrate the exclusive targeting of a small GTP-binding protein to secretory vesicles of a subset of the regulated pathway of secretion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989 Feb;8(2):379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burger P. M., Mehl E., Cameron P. L., Maycox P. R., Baumert M., Lottspeich F., De Camilli P., Jahn R. Synaptic vesicles immunoisolated from rat cerebral cortex contain high levels of glutamate. Neuron. 1989 Dec;3(6):715–720. doi: 10.1016/0896-6273(89)90240-7. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. A., Cameron P. L., Stukenbrok H., Jahn R., De Camilli P., Südhof T. C. Synaptophysin is targeted to similar microvesicles in CHO and PC12 cells. EMBO J. 1989 Oct;8(10):2863–2872. doi: 10.1002/j.1460-2075.1989.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. A., Jahn R., Südhof T. C. Transmembrane topography and evolutionary conservation of synaptophysin. J Biol Chem. 1989 Jan 15;264(2):1268–1273. [PubMed] [Google Scholar]
- Lapetina E. G., Reep B. R. Specific binding of [alpha-32P]GTP to cytosolic and membrane-bound proteins of human platelets correlates with the activation of phospholipase C. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2261–2265. doi: 10.1073/pnas.84.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe A. W., Madeddu L., Kelly R. B. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biol. 1988 Jan;106(1):51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaule P., Axel R. A novel ras-related gene family. Cell. 1985 May;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kondo J., Hishida T., Teranishi Y., Takai Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11071–11074. [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Nagy A., Baker R. R., Morris S. J., Whittaker V. P. The preparation and characterization of synaptic vesicles of high purity. Brain Res. 1976 Jun 11;109(2):285–309. doi: 10.1016/0006-8993(76)90531-x. [DOI] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B., Chardin P., Touchot N., Zahraoui A., Tavitian A. Expression of the ras-related ralA, rho12 and rab genes in adult mouse tissues. Oncogene. 1988 Aug;3(2):231–234. [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Slaughter C. A., Südhof T. C. The structure of cytochrome b561, a secretory vesicle-specific electron transport protein. EMBO J. 1988 Sep;7(9):2697–2703. doi: 10.1002/j.1460-2075.1988.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sano K., Kikuchi A., Matsui Y., Teranishi Y., Takai Y. Tissue-specific expression of a novel GTP-binding protein (smg p25A) mRNA and its increase by nerve growth factor and cyclic AMP in rat pheochromocytoma PC-12 cells. Biochem Biophys Res Commun. 1989 Jan 31;158(2):377–385. doi: 10.1016/s0006-291x(89)80058-0. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Puzicha M., Gallwitz D. Study of a temperature-sensitive mutant of the ras-related YPT1 gene product in yeast suggests a role in the regulation of intracellular calcium. Cell. 1988 May 20;53(4):635–647. doi: 10.1016/0092-8674(88)90579-x. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Czernik A. J., Kao H. T., Takei K., Johnston P. A., Horiuchi A., Kanazir S. D., Wagner M. A., Perin M. S., De Camilli P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989 Sep 29;245(4925):1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. Synaptic vesicles. Curr Opin Cell Biol. 1989 Aug;1(4):655–659. doi: 10.1016/0955-0674(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Walworth N. C., Goud B., Kabcenell A. K., Novick P. J. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 1989 Jun;8(6):1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]