Abstract
Epidemiological data on dengue in Africa are still scarce. We investigated imported dengue infection among travelers with a high proportion of subjects from Africa over a 9-year period. From January 2005 to December 2013, blood samples from travelers with clinical suspicion of dengue were analyzed. Dengue was diagnosed using serological, antigen detection, and molecular methods. Subjects were classified according to birthplace (Europeans versus non-Europeans) and last country visited. Overall, 10,307 serum samples corresponding to 8,295 patients were studied; 62% were European travelers, most of them from Spain, and 35.9% were non-Europeans, the majority of whom were born in Africa (mainly Equatorial Guinea) and Latin America (mainly Bolivia, Ecuador, and Colombia). A total of 492 cases of dengue were identified, the highest number of cases corresponding to subjects who had traveled from Africa (N = 189), followed by Latin America (N = 174) and Asia (N = 113). The rate of cases for Africa (4.5%) was inferior to Asia (9%) and Latin America (6.1%). Three peaks of dengue were found (2007, 2010, and 2013) which correlated with African cases. A total of 2,157 of past dengue infections were diagnosed. Non-Europeans who had traveled from Africa had the highest rate of past infection (67.8%), compared with non-Europeans traveling from Latin America (38.7%) or Asia (35%). Dengue infection in certain regions of Africa is underreported and the burden of the disease may have a magnitude similar to endemic countries in Latin America. It is necessary to consider dengue in the differential diagnosis of other febrile diseases in Africa.
Introduction
Dengue is the most prevalent arthropod-borne viral disease worldwide and has become a major international public health problem. More than 3.5 billion people, 40–60% of the world's population, live in tropical and subtropical regions at risk for dengue virus (DENV) infection.1,2 DENVs comprise four phylogenetically and antigenically distinct serotypes (DENV 1–4) belonging to the genus Flavivirus, family Flaviridae.3 There has been a dramatic rise in the number of dengue cases worldwide with an estimated 30-fold increase in the incidence of dengue infections over the last 50 years, and the actual global infection burden may be triple the original World Health Organization estimate.1,4
While the impact of dengue is well known in Asia-Pacific and the Americas, Africa has the poorest record of data. Surveillance is often deficient and new information would help to better define the hidden burden in this continent.1,5 A source of data about the epidemiological status of Africa is the information obtained from the recent wave of migration from this continent. In the last decade, Europe and in particular Spain has received a massive number of immigrants. Between 2000 and 2009 Spain's foreign-born population more than quadrupled, rising from under 1.5 million to over 6.5 million, making Spain the second largest recipient of immigrants in absolute terms, following the United States.6 Because of Spain's geographic location and cultural ties, a considerable proportion of these immigrants arrived from Latin America and Africa. In Europe, most cases of dengue are imported and related to travel to endemic regions, whereas autochthonous cases are scarce and geographically limited.7
With all this in mind, we aimed to analyze the epidemiological features of dengue infection among subjects who had traveled from Africa in comparison with those traveling from other dengue-endemic American and Asia-Pacific countries over a 9-year period.
Patients and Methods
Patients.
A retrospective study was carried out at Hospital Carlos III, a leading Center for Tropical Medicine in the Community of Madrid, Spain. From January 2005 to December 2013, we analyzed blood samples from subjects with clinical suspicion of dengue who were seen in the Tropical Medicine Department. Dengue was diagnosed using serological and molecular methods. The following information was retrieved retrospectively: age, gender, birthplace, and last country visited (country of exposure). According to birthplace, patients were classified as Europeans (subjects born in Spain or another European country) or non-Europeans (subjects born outside Europe). Countries were grouped into regions using the United Nations classification.8 Patients who had traveled to more than one country were considered as patients with multiple exposure.
Serology.
Anti-dengue immunoglobulin M (IgM) antibodies were detected using an IgM-capture ELISA (Panbio® Dengue IgM Capture ELISA; Alere Inc., Waltham, MA). For anti-dengue IgG antibodies, an indirect ELISA (Panbio® Dengue IgG Indirect ELISA; Alere Inc.) was used. Both tests were performed and interpreted according to the manufacturer's instructions. Paired sera were requested but convalescent serum was not always available.
Antigen detection.
From May 2010, detection of dengue NS1 antigen by enzyme immunoassay was introduced in the laboratory routine. Only patients with suspicion of a very early onset of the disease were studied for NS1 antigen. Samples were tested using the Platelia™ Dengue NS1 Ag (Bio-Rad Laboratories, Marnes-la-Coquette, France) according to the manufacturer's instructions.
Molecular diagnosis and serotyping.
Real-time reverse transcription polymerase chain reaction (RT-PCR) for DENV was performed retrospectively. Patients who were positive for dengue NS1 antigen or who presented anti-dengue IgM antibodies were tested if samples were available. Diagnosis of pan-DENV was made using a real-time RT-PCR.9 Positive samples were serotyped using a specific multiplex real-time RT-PCR described elsewhere.10
Diagnostic criteria.
Cases of dengue were defined by IgM or IgG seroconversion or a 4-fold IgG titer increase in paired sera; if only a single sample was available, cases were considered when IgM, NS1 antigen, and/or RNA virus were detected in serum. Patients who only showed anti-dengue IgG antibodies were defined as past dengue infections. Those subjects who presented all negative results for DENV were considered as nondengue-infected patients.11
Malaria.
Malaria diagnosis was made by conventional microscopy using thin and thick blood smears stained with Field's stain according to standard methods.12
Statistical analyses.
We used χ2 or linear-trend χ2 tests when appropriate for comparison of proportions. A P value of < 0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS, version 19 software (IBM, New York, NY).
Results
Study population.
A total of 10,307 serum samples corresponding to 8,295 patients were analyzed. More than a half of patients were women (N = 4,604, 55.5%). The mean age of the subjects at the first consultation was 37.5 years (standard deviation: 12.5; range: 5 months–88 years). Age was not available in 13 subjects. According to birthplace, about 62% were Europeans, most of them from Spain. In this group, only one subject was born in a European country with risk of dengue (Albania); 35.9% of subjects were non-Europeans, the majority of them born in Africa and Latin America. Most African patients were from Equatorial Guinea, a former colony of Spain, whereas the majority of Latin American patients were from Bolivia, Ecuador, or Colombia. The country of birth was not specified in 21 patients born in Latin America and seven born in Africa. In 2% (N = 164) of subjects, the birthplace was unknown.
Regarding the region of exposure, Europeans had traveled mainly to sub-Saharan Africa, Latin America, and the Caribbean. Among 2,976 non-European subjects, the travel destination matches with their country of birth in almost all travelers (97%). Table 1 (Supplemental Figure 1) shows the distribution of European and non-European patients according to region of exposure. Over the 9-year study period, the number of European patients seeking medical care due to clinical suspicion of dengue remained relatively constant, except for a peak in 2010. In contrast, non-European population grew until 2007 and since then has gradually declined, mainly due to African subjects.
Table 1.
Distribution of population study according to birthplace and region of exposure
| Region of exposure* | Total | Birthplace | ||
|---|---|---|---|---|
| Europeans | Non-Europeans | Unknown | ||
| N | n (%) | n (%) | n (%) | |
| Africa | 3,874 | 2,312 (59.7) | 1,557 (40.2) | 5 (0.1) |
| Northern Africa | 138 | 129 (93.5) | 7 (5.1) | 2 (1.4) |
| Sub-Saharan Africa | 3,736 | 2,183 (58.4) | 1,550 (41.5) | 3 (0.1) |
| Eastern Africa | 691 | 641 (92.8) | 50 (7.2) | 0 (0) |
| Ethiopia | 121 | 105 (86.8) | 16 (13.2) | 0 (0) |
| Kenya | 119 | 106 (89.1) | 13 (10.9) | 0 (0) |
| Mozambique | 107 | 99 (92.5) | 8 (7.5) | 0 (0) |
| Tanzania | 159 | 154 (96.9) | 5 (3.1) | 0 (0) |
| Middle Africa | 2,055 | 811 (39.5) | 1,244 (60.5) | 0 (0) |
| Angola | 130 | 102 (78.5) | 28 (21.5) | 0 (0) |
| Cameroon | 188 | 140 (74.5) | 48 (25.5) | 0 (0) |
| Equatorial Guinea | 1,570 | 435 (27.7) | 1,135 (72.3) | 0 (0) |
| Southern Africa | 83 | 82 (98.8) | 1 (1.2) | 0 (0) |
| Western Africa | 899 | 646 (71.9) | 250 (27.8) | 3 (0.3) |
| Mali | 106 | 73 (68.9) | 33 (31.1) | 0 (0) |
| Nigeria | 136 | 52 (38.2) | 84 (61.8) | 0 (0) |
| Senegal | 279 | 234 (83.9) | 45 (16.1) | 0 (0) |
| Americas | 2,638 | 1,216 (46.1) | 1,327 (50.3) | 95 (3.6) |
| Latin America and the Caribbean | 2,633 | 1,211 (46) | 1,327 (50.4) | 95 (3.6) |
| Caribbean | 312 | 207 (66.3) | 82 (26.3) | 23 (7.4) |
| Dominican Republic | 138 | 75 (54.3) | 52 (37.7) | 11 (8) |
| Central America | 520 | 400 (76.9) | 82 (15.8) | 38 (7.3) |
| Mexico | 164 | 129 (78.7) | 20 (12.2) | 15 (9.1) |
| South America | 1,785 | 603 (33.8) | 1,150 (64.4) | 32 (1.8) |
| Bolivia | 686 | 89 (13) | 597 (87) | 0 (0) |
| Brazil | 185 | 138 (74.6) | 47 (25.4) | 0 (0) |
| Colombia | 187 | 58 (31) | 126 (67.4) | 3 (1.6) |
| Ecuador | 282 | 77 (27.3) | 203 (72) | 2 (0.7) |
| Peru | 247 | 147 (59.5) | 85 (34.4) | 15 (6.1) |
| Northern America | 5 | 5 (100) | 0 (0) | 0 (0) |
| Asia | 1,082 | 1,039 (96) | 40 (3.7) | 3 (0.3) |
| Eastern Asia | 52 | 51 (98.1) | 1 (1.9) | 0 (0) |
| Central Asia | 3 | 3 (100) | 0 (0) | 0 (0) |
| Southern Asia | 597 | 570 (95.5) | 26 (4.4) | 1 (0.1) |
| India | 529 | 508 (96) | 21 (3.8) | 0 (0) |
| Southeastern Asia | 421 | 406 (96.4) | 13 (3.1) | 2 (0.5) |
| Thailand | 127 | 124 (97.6) | 3 (2.4) | 0 (0) |
| Western Asia | 9 | 9 (100) | 0 (0) | 0 (0) |
| Oceania | 13 | 13 (100) | 0 (0) | 0 (0) |
| Unknown | 382 | 291 (76.2) | 30 (7.9) | 61 (16) |
| Multiple exposure | 306 | 284 (92.8) | 22 (7.2) | 0 (0) |
| Total | 8,295 | 5,155 (62.1) | 2,976 (35.9) | 164 (2) |
Only countries with more than 100 patients studied are shown.
Cases.
Of 8,295 patients included, a proportion of them were seen several times over the 9-year study period, the number of suspected episodes of dengue infections studied amounting to 9,247. Overall, 492 (5.3%) cases of dengue were identified. Cases were diagnosed by serology (N = 411), NS1 antigen (N = 71), and/or molecular methods (N = 35). In 13 cases, serology was negative and diagnosis was made only by NS1 antigen and/or PCR detection.
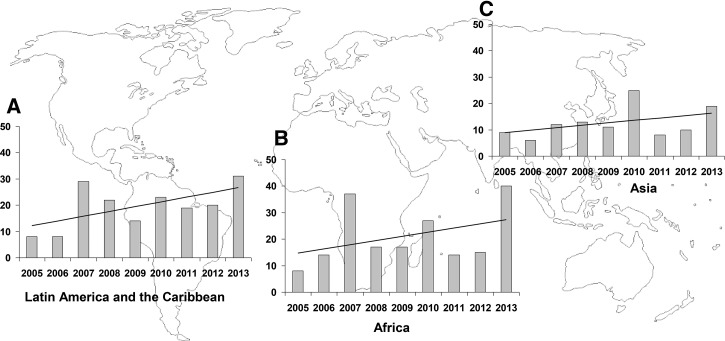
The highest number of cases was detected in subjects who had returned from Africa (N = 189), the majority from the sub-Saharan region (N = 185): Equatorial Guinea (N = 87) and Senegal (N = 14) in particular. It is worth noting that, except for southern Africa, the rate of distribution of cases throughout sub-Saharan Africa was similar. In the Americas, 174 cases were diagnosed, notably in Bolivia (N = 26), Brazil (N = 19), and Dominican Republic (N = 15). Finally, 113 cases were detected in patients who had traveled from Asia, mainly from India (N = 52) and Thailand (N = 21). Figure 1 shows the distribution of cases of dengue by year and continent. Detail information about countries in which cases of dengue were detected is shown in Supplemental Table 1.
Figure 1.
Number of cases of dengue diagnosed in (A) Latin America and the Caribbean, (B) Africa, and (C) Asia. The x axis represents years and the y axis the number of cases. The linear trend of cases over the 9-year study period is shown by each continent.
Distribution of dengue cases was similar between male (5.4%) and female (5.3%) travelers. With regard to age, more than 75% of dengue cases corresponded to subjects aged between 20 and 40. In terms of relative frequency, the rate of cases was similar among the different age groups (see Supplemental Figure 2). There was no difference in the rate of cases between European and non-European patients, although when we stratified by areas, African continent and eastern Africa region showed a slightly higher rate in the non-European population versus the European one (P < 0.05) (Table 2). Although Africa was the continent where most cases were diagnosed, its rate of cases (4.5%) was lower than that of Asia (9%) and Latin America (6.1%).
Table 2.
Cases and past infections of dengue according to birthplace and region of exposure
| Region of exposure* | Cases | Past infections | ||||
|---|---|---|---|---|---|---|
| Total | Europeans | Non-Europeans | Total | Europeans | Non-Europeans | |
| N (%)† | n (%)† | n (%)† | N (%)† | n (%)† | n (%)† | |
| Africa | 173 (4.5) | 90 (3.9) | 83 (5.3)‡ | 1,311 (33.8) | 254 (11) | 1,056 (67.8)¶ |
| Northern Africa | 4 (2.9) | 4 (3.1) | 0 (0) | 8 (5.8) | 6 (4.7) | 2 (28.5) |
| Sub-Saharan Africa | 169 (4.5) | 86 (3.9) | 83 (5.4) | 1,303 (34.9) | 248 (11.4) | 1,054 (68)¶ |
| Eastern Africa | 23 (3.3) | 19 (3) | 4 (8)§ | 53 (7.7) | 45 (7) | 8 (16) |
| Ethiopia | 4 (3.3) | 1 (1) | 3 (1.9) | 10 (8.3) | 8 (7.6) | 2 (12.5) |
| Kenya | 8 (6.7) | 7 (6.6) | 1 (7.7) | 10 (8.4) | 8 (7.5) | 2 (15.3) |
| Mozambique | 1 (0.9) | 1 (1) | 0 (0) | 12 (11.2) | 11 (11.1) | 1 (12.5) |
| Tanzania | 3 (1.9) | 3 (1.9) | 0 (0) | 12 (7.5) | 10 (6.5) | 2 (40) |
| Middle Africa | 107 (5.2) | 44 (5.4) | 63 (5.1) | 1,043 (50.7) | 146 (18) | 897 (72.1)¶ |
| Angola | 6 (4.6) | 5 (4.9) | 1 (3.5) | 44 (33.8) | 19 (18.6) | 25 (89.2)¶ |
| Cameroon | 9 (4.8) | 6 (4.3) | 3 (6.2) | 43 (22.9) | 16 (11.4) | 27 (56.2)¶ |
| Equatorial Guinea | 84 (5.4) | 27 (6.2) | 57 (5) | 923 (58.8) | 93 (21.3) | 830 (73.1)¶ |
| Western Africa | 37 (4.1) | 22 (3.4) | 15 (6) | 195 (21.7) | 48 (7.4) | 146 (58.4)¶ |
| Mali | 5 (4.7) | 3 (4.1) | 2 (6.1) | 20 (18.9) | 2 (2.7) | 18 (54.5) |
| Nigeria | 7 (5.1) | 3 (5.8) | 4 (4.8) | 71 (52.2) | 4 (7.7) | 67 (79.8)¶ |
| Senegal | 11 (3.9) | 8 (3.4) | 3 (6.7) | 34 (12.2) | 15 (6.4) | 19 (42.2)¶ |
| Americas | 161 (6.1) | 75 (6.2) | 77 (5.8) | 598 (22.7) | 69 (5.6) | 513 (38.7)¶ |
| Latin America and the Caribbean | 161 (6.1) | 75 (6.2) | 77 (5.8) | 598 (22.7) | 69 (5.6) | 513 (38.7)¶ |
| Caribbean | 21 (6.7) | 10 (4.8) | 9 (10.9) | 61 (20) | 11 (5.3) | 45 (54.9)¶ |
| Dominican Republic | 13 (9.4) | 5 (6.7) | 6 (11.5) | 41 (29.7) | 2 (2.6) | 35 (67.3)¶ |
| Central America | 45 (8.7) | 31 (7.8) | 10 (12.2) | 42 (8.8) | 19 (4.8) | 16 (19.5)¶ |
| Mexico | 10 (6.1) | 7 (5.4) | 2 (10) | 8 (4.9) | 4 (3.1) | 1 (5) |
| South America | 94 (5.3) | 34 (5.6) | 57 (5) | 487 (27.3) | 39 (6.5) | 444 (38.6)¶ |
| Bolivia | 26 (3.8) | 1 (1.1) | 25 (4.2) | 249 (36.3) | 8 (9) | 241 (40.4)¶ |
| Brazil | 19 (10.3) | 12 (8.7) | 7 (14.9) | 27 (14.6) | 12 (8.7) | 15 (31.9)¶ |
| Colombia | 13 (6.7) | 7 (12.1) | 6 (4.8) | 71 (38) | 4 (6.9) | 67 (53.2)¶ |
| Ecuador | 10 (3.5) | 3 (3.9) | 7 (3.4) | 82 (29.1) | 5 (6.5) | 76 (37.4)¶ |
| Peru | 6 (2.4) | 3 (2) | 2 (2.4) | 21 (8.5) | 8 (5.4) | 11 (12.9) |
| Asia | 97 (9) | 94 (9) | 2 (5) | 98 (9.1) | 84 (8.1) | 14 (35)¶ |
| Southern Asia | 54 (9) | 53 (9.3) | 1 (3.8) | 53 (8.9) | 47 (8.2) | 6 (23.1)¶ |
| India | 49 (9.3) | 49 (9.6) | 0 (0) | 48 (9.1) | 43 (8.5) | 5 (23.8)§ |
| Southeastern Asia | 42 (10) | 41 (10.1) | 0 (0) | 42 (10) | 34 (8.4) | 8 (61.5)¶ |
| Thailand | 20 (15.7) | 19 (15.3) | 0 (0) | 8 (6.3) | 7 (5.6) | 1 (33.3) |
| Unknown | 14 (3.7) | 10 (3.4) | 1 (3.3) | 51 (13.4) | 24 (8.2) | 10 (33.3)¶ |
| Multiple exposure | 45 (14.7) | 40 (14.1) | 5 (22.7) | 99 (32.4) | 80 (28.2) | 19 (86.4)¶ |
| Total | 492 (5.9) | 311 (6) | 168 (5.6) | 2,157 (26) | 511 (9.9) | 1,612 (54.2)¶ |
Only areas and countries with more than 100 subjects studied are shown.
Rates were calculated using as denominator the figures of Table 1.
Significant differences between Europeans versus non-Europeans in each group (cases and past infections): P < 0.05; P < 0.01; P < 0.001, respectively.
Yearly distribution and seasonality.
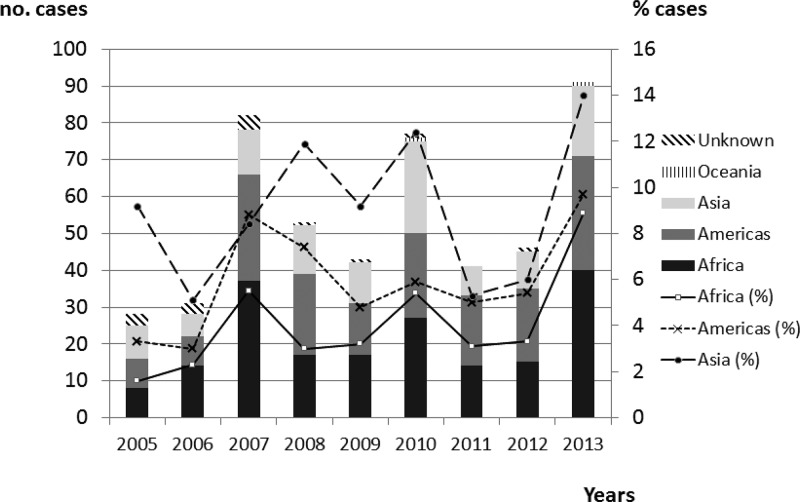
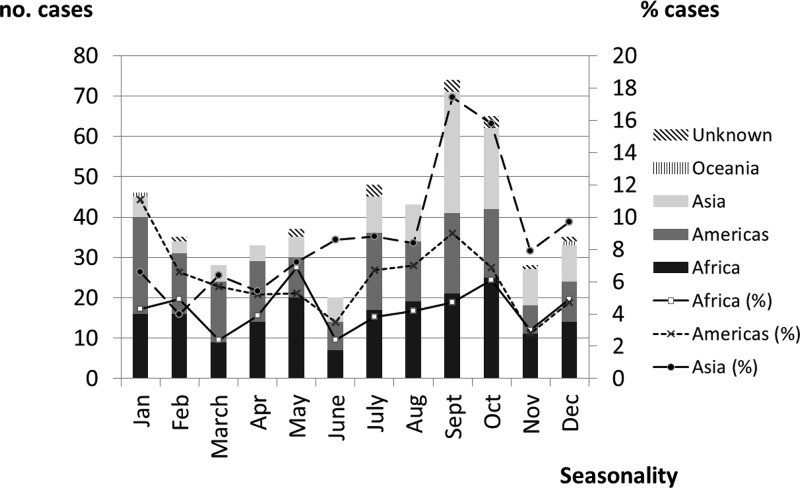
We found three peaks of diagnosed dengue cases corresponding to the years 2007 (N = 82), 2010 (N = 77), and 2013 (N = 91). Figure 2 shows the distribution (and rate) of cases according to birthplace and geographical exposure over a 9-year period. It is worth noting that Africa had three peaks, in 2007 (N = 37, 5.5%), 2010 (N = 27, 5.4%), and 2013 (N = 40, 8.9%), respectively. Although more than half of the cases were detected in middle Africa (mainly in Equatorial Guinea), the cases detected in western Africa only show a peak in 2010 (N = 12, 27.9%). By seasonality, the months of September and October showed the highest number of cases, largely due to cases detected in travelers coming from Asia. No seasonal pattern was observed in patients coming from Africa (Figure 3 ).
Figure 2.
Distribution of cases and rate (%) by year and geographical dengue exposure.
Figure 3.
Distribution of cases and rate (%) by seasonality and geographical dengue exposure over the 9-year study period.
Serotyping.
A total of 223 patients were studied for PCR, of whom 35 (15.7%) were positive. In 34 of them, PCR-specific species could be performed with the following distribution of serotypes: 15 DENV-1, 8 DENV-2, 10 DENV-3, and one patient with coinfection with DENV-1 and DENV-2. No cases of DENV-4 were found. Table 3 shows the distribution of serotypes according to geographic exposure.
Table 3.
Serotypes of dengue according to region of exposure
| Region of exposure | Serotype | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| N (year) | N (year) | N (year) | |
| Africa | |||
| Angola | 3 (2013) | ||
| Cameroon | 1 (2005) | ||
| Equatorial Guinea | 1 (2012) | ||
| Kenya | 1 (2012) | 1 (2013) | 1 (2006) |
| Asia | |||
| Bhutan | 1 (2009) | ||
| India | 2 (2010) | 1 (2008) | 1 (2005) |
| Indonesia | 2 (2010, 2013) | ||
| Myanmar | 1 (2005) | ||
| Sri Lanka | 1 (2005) | ||
| Thailand | 1 (2013) | 1 (2008) | 4 (2009, 2012, 2013†) |
| Latin America | |||
| Brazil | 1 (2011) | ||
| Colombia | 2 (2009, 2013) | ||
| Dominican Republic | 1 (2010) | ||
| Panama | 1 (2009) | ||
| Paraguay | 1 (2011*) | 2 (2011*, 2012) | |
| Venezuela | 2 (2007, 2013) | ||
| Unknown | 2 (2006, 2007) | ||
| Total | 16 | 9 | 10 |
One case with coinfection with serotype 1 and 2.
Two cases.
Past dengue infections.
Overall, 2,157 of past dengue infections were diagnosed. As expected, there was a significant increase in past infections by age (P < 0.00001) (Supplemental Figure 2), and they were more frequent in non-European subjects than in European ones (54.2% versus 9.9%, respectively; P < 0.00001). Table 2 shows the distribution of past dengue infections according to birthplace and travel destination. Among non-European patients, those who had traveled from Africa had the highest rate of past infection (67.8%), compared with those coming from Latin America and the Caribbean (38.7%) or Asia (35%). Although non-European subjects who had traveled from Africa were slightly older compared with those coming from the Americas or Asia, when we stratified by age group, we obtained similar rates of past infection across continents (data not shown).
Malaria.
Investigation for malaria was performed in 7,231 (78.2%) of suspected dengue episodes, of which 106 (1.5%) were positive (99 Plasmodium falciparum, four Plasmodium vivax, two Plasmodium ovale, and one Plasmodium malariae). Most cases of malaria were detected in subjects who had traveled from sub-Saharan Africa (N = 92, 86.8%) and, to a much lesser degree, from Latin America and the Caribbean (N = 10, 9.4%). More malaria cases were diagnosed among non-European patients than among their European counterparts (N = 57, 53.8% versus N = 48, 45.2%, P < 0.001). In the study population, there was a significant decrease in the number of subjects with malaria over time, with a similar distribution of cases by seasonality (data not shown). Only seven cases of dengue/malaria coinfection were detected: all but one were subjects who had traveled to Africa.
Discussion
A recent report has suggested that the worldwide impact of dengue infection is far greater than previously thought.1 This may be especially dramatic for Africa. The true burden of dengue is uncertain because surveillance is still deficient and infection may be masked by the many febrile diseases in this continent often being overlooked. Although several outbreaks have been reported in this continent and the number of epidemiological studies has increased in recent years, studies are still scarce.13,14 Therefore, there is a need to collect epidemiological data on dengue infection in Africa to uncover the probably large hidden African dengue burden and its extension within this continent.15 In our study, we found the highest rate of diagnosed cases in patients who had visited Asia (9%), followed by those traveling from Latin America and the Caribbean (6.1%) and, very close behind, from Africa (4.5%). These data tally with the findings of Bhatt and others, who estimated that Africa's dengue burden is lower than that of Asia but similar to that of America.1 We found that most cases from Africa had traveled from sub-Saharan countries within the tropical belt; consequently, only a minority of African dengue cases was diagnosed outside this area. These data are in agreement with previous reports that showed that although infection is widespread throughout the continent, the risk of dengue does not seem to be evenly distributed, predominating in tropical and subtropical regions, whereas the regions of northern and southern Africa are less affected.1,2
In our research, we divided the study population between European and non-European patients according to birthplace. The epidemiological context of the study suggests that non-European travelers probably represent recent migrants or settled immigrants who visit friends and relatives in their native countries. Therefore, the risk of acquiring dengue infection, which is related to the length of stay,16 may be higher in this last group. Analysis of these travelers who come from or visit their home countries could provide a clearer picture of the true impact of dengue in these countries. We did not find any differences in the rate of cases of dengue infection between European and non-European travelers, except for subjects who have traveled from Africa. However, the seroprevalence of past dengue infections in most regions of traveling was significantly higher in non-European travelers than in European ones (Table 2).
A significant proportion of non-European subjects who had traveled to Africa are from Equatorial Guinea (about 40%) and, to a lesser extent, Senegal and Nigeria. Epidemiological studies in these countries are scarce. Dengue infection had been reported in Equatorial Guinea previously,13 but to our knowledge no studies have been conducted in this country. In Nigeria, a recent report carried out on 310 febrile patients showed a 67.7% seroprevalence of dengue using plaque reduction neutralization testing to rule out cross-reactivity.17 This figure is slightly lower than that found in our study (73.6%). There are no data about seroprevalence of dengue in Senegal, although outbreaks have been reported there.18
In our study, we noted that the rate of past dengue infections found among non-Europeans who had traveled from Africa was almost twice as high as that detected in non-Europeans coming from Asia or the Americas. We found no significant age or gender-related difference among these subjects that could explain this paradox. A plausible explanation is that African immigrants may previously have been exposed to other circulating flaviviruses and alphaviruses in their countries of origin that can cause cross-reactivity in the IgG serological tests for dengue. In addition, exposure to parasites may produce polyclonal B-cell activation responsible for the high rate of false-positive immunoassays results reported in African patients.19 Vairo and others have estimated that although sensitivity of IgG enzyme immunoassay test for dengue is nearly 100%, specificity could be substantially diminished in African populations.20 Taken all together, these data suggest that prevalence studies using anti-dengue IgG antibodies in the African continent may be overestimating dengue infection and should be interpreted cautiously.
We observed three main peaks of cases in 2007, 2010, and 2013, in keeping with a recent report.21 In subjects traveling from Africa, the peaks in 2007 and 2013 were due mainly to those traveling from Equatorial Guinea. These data suggest this country probably experienced successive dengue epidemics during the study period. The increase in cases from Latin America, in particular Venezuela and Dominican Republic (2007) and Brazil (2013), and from Asia, notably Thailand (2008 and 2013) and India (2010), is probably related to ongoing outbreaks in these countries at that time.22,23
Another risk factor for acquiring dengue is the season of travel. We found that most cases had been detected after the summer (September or October) and correspond mainly to cases from Asia (namely, India and Thailand). Several reports have found a similar seasonal pattern in these countries related to the rainy season (the monsoon), which extends from May/June to September/October.24,25 Our data did not show a seasonality distribution in Africa, even when the study was restricted to Equatorial Guinea. To our knowledge, only one study has analyzed the seasonality of dengue in Africa; the authors did not find a seasonal pattern, although the number of cases included was very small.26
All four DENV serotypes are found worldwide, including in Africa. Our study included few serotyped samples, and for this reason we did not find any subjects with DENV-4, which is the least prevalent globally.3 In sub-Saharan Africa, we found serotypes 1, 2, and 3. Interestingly, all three cases of DENV-1 were detected in travelers coming from Angola in 2013, in which year an outbreak of DENV-1 was reported there.27 Another case of DENV-1 was detected in Equatorial Guinea, but there were no reports about serotypes circulating in this country. The remaining serotypes found in sub-Saharan African countries have been reported previously.3
The study aimed to investigate epidemiological dengue infection features in ill travelers with clinical suspicion of dengue. Therefore, although malaria infection data were collected, they are not representative of the disease's impact on this population and must be interpreted cautiously. Nevertheless, we found that most malaria cases were diagnosed in sub-Saharan travelers, whereas the proportion of malaria cases among subjects coming from Asia and the Americas was very low. This is to some extent in agreement with previous studies that maintain that dengue is now more frequent than malaria as a cause of febrile disease in travelers returning from southeast Asia and from America–Caribbean, whereas malaria predominates in sub-Saharan Africa.28 In addition, our data support the claim that coinfection malaria/dengue is not a rare event and may have clinical implications.29
Our study has several limitations: First, no antibody-neutralizing test was performed to rule out cross-reaction with other circulating flaviviruses or with yellow fever vaccination. This is particularly relevant when using IgG dengue antibody for seroprevalence determination in non-European subjects, mainly African travelers, and may cause the rate to be overestimated. Second, we have established the presence of anti-DENV IgM antibodies in a single specimen as a criterion to define a confirmed case of dengue, therefore we cannot rule out the possibility of a false-positive result. Third, NS1 antigen and molecular methods were not used routinely for dengue diagnosis; however, few cases were detected using only these methods and they do not interfere with the conclusion on comparing the rate of cases between continents. Finally, our study focused exclusively on ill travelers in whom dengue was clinically suspected; therefore we cannot draw conclusions about the actual incidence of the disease.
In summary, our work suggests that dengue infection in certain regions of Africa has a magnitude approaching that of other endemic countries in Latin America and the Caribbean. These data suggest that dengue infection is underdiagnosed in Africa and may be misdiagnosed and therefore confused with other diseases, mainly malaria. Implementation of measures aimed at ensuring correct diagnoses and appropriate treatment should be a priority.
Supplementary Material
Supplemental table and figures.
ACKNOWLEDGMENTS
We are indebted to Peter Bonney for his assistance in the final preparation of the manuscript in English.
Footnotes
Authors' addresses: Carlos Toro, Mercedes Subirats, and Margarita Baquero, Service of Microbiology and Parasitology, Carlos III Hospital, Madrid, Spain, E-mails: carlostororueda@hotmail.com, mercedessubirats@gmail.com, and margaritabaqueromochales@yahoo.es. Patricia Trevisi, Beatriz López-Quintana, and Nuria Iglesias, Department of Microbiology, Investigación Hospital La Paz, Madrid, Spain, E-mails: crickettrevisi@hotmail.com, bealq@hotmail.com, and nurinu@telefonica.net. Aránzazu Amor, National Centre of Tropical Medicine, Carlos III Institute of Health, Madrid, Spain, E-mail: aranchazu@gmail.com. Concepción Ladrón de Guevara, Mar Lago, Marta Arsuaga, Fernando de la Calle-Prieto, and Sabino Puente, Tropical Medicine Unit, Infectious Diseases Department, Carlos III Hospital, Madrid, Spain, E-mails: mconcepcion.ladron@salud.madrid.org, mariamar.lago@salud.madrid.org, martaarsuaga@gmail.com, fercalleprieto@gmail.com, and sabinopuentepuente@gmail.com. Dolores Herrero, Service of Internal Medicine, Quironsalud University Hospital, Madrid, Spain, E-mail: mdolhermen@yahoo.es. Margarita Rubio, School of Biomedical Sciences, Universidad Europea de Madrid, Madrid, Spain, E-mail: margarita.rubio@universidadeuropea.es.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, Bhatt S, Katzelnick L, Howes RE, Battle KE, Simmons CP, Hay SI. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 2014;22:138–146. doi: 10.1016/j.tim.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Global Strategy for Dengue Prevention and Control, 2012–2020. Geneva, Switzerland: World Health Organization; 2012. WHO Report. [Google Scholar]
- 5.Messina JP, Brady OJ, Pigott DM, Brownstein JS, Hoen AG, Hay SI. A global compendium of human dengue virus occurrence. Sci Data. 2014;1:140004. doi: 10.1038/sdata.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization for Economic Cooperation and Development (OECD) International Migration Outlook 2013: Spain. Paris, France: OECD Publishing; 2013. [Google Scholar]
- 7.Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect Dis. 2014;14:1271–1280. doi: 10.1016/S1473-3099(14)70834-5. [DOI] [PubMed] [Google Scholar]
- 8.United Nations Statistic Division 2016. http://unstats.un.org/unsd/methods/m49/m49regin.htm Available at. Accessed December 29, 2016.
- 9.Gurukumar KR, Priyadarshini D, Patil JA, Bhagat A, Singh A, Shah PS, Cecilia D. Development of real time PCR for detection and quantitation of dengue viruses. Virol J. 2009;6:10. doi: 10.1186/1743-422X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito M, Takasaki T, Yamada K, Nerome R, Tajima S, Kurane I. Development and evaluation of fluorogenic TaqMan reverse transcriptase PCR assays for detection of dengue virus types 1 to 4. J Clin Microbiol. 2004;42:5935–5937. doi: 10.1128/JCM.42.12.5935-5937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, Enria DA, Farrar J, Gubler DJ, Guzman MG, Halstead SB, Hunsperger E, Kliks S, Margolis HS, Nathanson CM, Nguyen VC, Rizzo N, Vázquez S, Yoksan S. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol. 2010;8((Suppl 12)):S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 12.Cheesbrough M. Medical Laboratory Manual for Tropical Countries. 2nd edition. Vol. I. Cambridge, United Kingdom: University Press; 1987. [Google Scholar]
- 13.Were F. The dengue situation in Africa. Paediatr Int Child Health. 2012;32((Suppl 1)):18–21. doi: 10.1179/2046904712Z.00000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17:1349–1354. doi: 10.3201/eid1708.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaenisch T, Junghanss T, Wills B, Brady OJ, Eckerle I, Farlow A, Hay SI, McCall PJ, Messina JP, Ofula V, Sall AA, Sakuntabhai A, Velayudhan R, Wint GR, Zeller H, Margolis HS, Sankoh O, Dengue in Africa Study Group Dengue expansion in Africa—not recognized or not happening? Emerg Infect Dis. 2014;20:e140487. doi: 10.3201/eid2010.140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massad E, Wilder-Smith A. Risk estimates of dengue in travelers to dengue endemic areas using mathematical models. J Travel Med. 2009;16:191–193. doi: 10.1111/j.1708-8305.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 17.Baba M, Logue CH, Oderinde B, Abdulmaleek H, Williams J, Lewis J, Laws TR, Hewson R, Marcello A, D' Agaro P. Evidence of arbovirus co-infection in suspected febrile malaria and typhoid patients in Nigeria. J Infect Dev Ctries. 2013;7:51–59. doi: 10.3855/jidc.2411. [DOI] [PubMed] [Google Scholar]
- 18.Faye O, Ba Y, Faye O, Talla C, Diallo D, Chen R, Mondo M, Ba R, Macondo E, Siby T, Weaver SC, Diallo M, Sall AA. Urban epidemic of dengue virus serotype 3 infection, Senegal, 2009. Emerg Infect Dis. 2014;20:456–459. doi: 10.3201/eid2003.121885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klarkowski D, O'Brien DP, Shanks L, Singh KP. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti Infect Ther. 2014;12:49–62. doi: 10.1586/14787210.2014.866516. [DOI] [PubMed] [Google Scholar]
- 20.Vairo F, Nicastri E, Yussuf SM, Cannas A, Meschi S, Mahmoud MA, Mohamed AH, Maiko PM, De Nardo P, Bevilacqua N, Castilletti C, Di Caro A, Racalbuto V, Ippolito G. IgG against dengue virus in healthy blood donors, Zanzibar, Tanzania. Emerg Infect Dis. 2014;20:465–468. doi: 10.3201/eid2003.130150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verschueren J, Cnops L, van Esbroeck M. Twelve years of dengue surveillance in Belgian travellers and significant increases in the number of cases in 2010 and 2013. Clin Microbiol Infect. 2015;21:867–872. doi: 10.1016/j.cmi.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Pan American Health Organization (PAHO) Number of Reported Cases of Dengue and Severe Dengue (SD) in the Americas by Country. 2016. http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=3274&Itemid=40734&lang=en Available at. Accessed December 29, 2016.
- 23.Bureau of Epidemiology, MoPH, Thailand Natural Disease Surveillance (Report 506): Dengue Fever. 2016. http://www.boe.moph.go.th/boedb/surdata/506wk/y58/en/d66_2658_en.pdf Available at. Accessed December 29, 2016.
- 24.Limkittikul K, Brett J, L'Azou M. Epidemiological trends of dengue disease in Thailand (2000–2011): a systematic literature review. PLoS Negl Trop Dis. 2014;8:e3241. doi: 10.1371/journal.pntd.0003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatnagar S, Lal V, Gupta SD, Gupta OP. Forecasting incidence of dengue in Rajasthan, using time series analyses. Indian J Public Health. 2012;56:281–285. doi: 10.4103/0019-557X.106415. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz E, Weld LH, Wilder-Smith A, von Sonnenburg F, Keystone JS, Kain KC, Torresi J, Freedman DO, GeoSentinel Surveillance Network. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg Infect Dis. 2008;14:1081–1088. doi: 10.3201/eid1407.071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sessions OM, Khan K, Hou Y, Meltzer E, Quam M, Schwartz E, Gubler DJ, Wilder-Smith A. Exploring the origin and potential for spread of the 2013 dengue outbreak in Luanda, Angola. Glob Health Action. 2013;6:21822. doi: 10.3402/gha.v6i0.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Travel-associated dengue surveillance: United States, 2006–2008. Morb Mortal Wkly Rep. 2010;59:715–719. [PubMed] [Google Scholar]
- 29.Epelboin L, Hanf M, Dussart P, Ouar-Epelboin S, Djossou F, Nacher M, Carme B. Is dengue and malaria co-infection more severe than single infections? A retrospective matched-pair study in French Guiana. Malar J. 2012;11:142. doi: 10.1186/1475-2875-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table and figures.