Abstract
Children living in homes with livestock may have both an increased risk of enteric infections and improved access to food, and therefore improved nutritional status. Few studies, however, have characterized these relationships in tandem. This study investigated the association between child health and household ownership of livestock. A cross-sectional study was performed using data from Demographic and Health Surveys conducted in 30 sub-Saharan African countries with 215,971 rural children under 5 years of age from 2005 to 2015. Logistic regression was performed for each country to estimate the relationship between a log2 increase in the number of livestock owned by the household and three child-health outcomes: 2-week prevalence of diarrhea, stunting, and all-cause mortality. Results for each country were combined using meta-analyses. Most countries (22 of 30) displayed an odds ratio (OR) less than 1 for child stunting associated with livestock (pooled OR = 0.97; 95% confidence interval [CI] = 0.95, 0.99). The results for diarrhea were more even with 14 countries displaying ORs greater than 1 and 10 displaying ORs less than 1. Most countries (22 of 30) displayed an OR greater than 1 for child mortality (pooled OR = 1.04; 95% CI = 1.02, 1.06). All meta-analyses displayed significant heterogeneity by country. Our analysis is consistent with the theory that livestock may have a dual role as protective against stunting, an indicator of chronic malnutrition, and a risk factor for all-cause mortality in children, which may be linked to acute infections. The heterogeneity by country, however, indicates more data are needed on specific household livestock management practices.
Introduction
Undernutrition is estimated to be an underlying cause for 3.1 million annual deaths in children under 5 years of age, occurring mainly in low- and middle-income countries (LMICs).1 Undernutrition has also been shown to detrimentally affect children's physical and cognitive development and make them more susceptible to infectious diseases, such as diarrhea.2 Diarrheal disease is a contributor to chronic ailments associated with undernutrition. Enteric infections may also lead to a subclinical condition of the gut called environmental enteric dysfunction, resulting in impaired nutrient absorption and altered immune function that can compromise growth and cognitive development in children over time.3 Diarrheal disease also contributes to acute disease burden and is the second leading cause of mortality in children under 5 years of age globally.2,4
A substantial amount of attention has been focused on poor water, sanitation, and hygiene practices (WASH), which are recognized risk factors for the majority of diarrheal deaths in LMICs.5 WASH interventions are generally focused on breaking the human fecal–oral route of disease. One underrecognized source of fecal contamination, however, is animal feces, which are common contaminants in the environment of children living in poor communities of LMICs.6 Exposures to animal feces will likely increase as small-scale animal agriculture—promoted for rural development—expands.7 A systematic review of human diarrhea infections and livestock ownership suggested evidence for animals as risk factors for diarrhea, especially when specific animal and pathogen combinations were studied.8 The greatest risk observed in the review was for poultry. Campylobacter, associated with chicken feces, was the main enteric pathogen observed in infected children.8–13 There remains, however, an important gap in our ability to estimate the risk of specific animal species, animal holding practices, and zoonotic pathogenic species contributing to the transmission of zoonotic enteric infections to children.14 Understanding the role that small-scale livestock production plays in contributing to diarrhea and other zoonotic infectious diseases is critical.
In addition to zoonotic infectious diseases, such as diarrhea, there is increasing evidence that fecal contamination associated with human and animal feces may be an important risk factor for environmental enteric dysfunction, a subclinical condition of the gastrointestinal tract that can detrimentally affect child nutrition and growth.15 Researchers have found geophagy to be a common behavior among young children and soil has often been found to be contaminated with animal feces.16 Researchers in India identified high levels of animal fecal markers in households and community water sources.17 Research has also linked geophagy and chicken ownership to environmental enteric dysfunction and child stunting.3,18
Despite concerns about zoonotic exposures, livestock plays an essential role as sources of income and nutrition for households. Consumption of animal source foods, including eggs, milk, milk products, meat, poultry, and fish, have been shown to be protective against stunting and undernutrition.19–21 In a study of 1,500 infants and 1,658 toddlers, most of whom were breastfed, researchers found that consumption of meat was associated with a reduced likelihood of stunting (odds ratio [OR] = 0.64; 95% confidence interval [CI] = 0.46–0.90).22 A cross-sectional study of 183 Kenyan children under the age of 5, showed a positive association between female-owned livestock and children's weight-for-age z scores.23 Further, in a review of interventions promoting animal production, researchers identified 14 studies that assessed the impacts of livestock ownership on dietary intake and household income—all studies showed a positive effect. Four of the 14 studies included nutritional status as an outcome measure and all identified positive effects.24
In this study, we used publicly available data to explore small-scale livestock ownership as both a risk factor and a protective factor for two acute child health outcomes, 2-week prevalence of diarrhea and all-cause child mortality, and a chronic outcome, stunting. We focus on children under 5 years of age in sub-Saharan Africa.
Materials and Methods
Data.
Data for the analysis came from Demographic and Health Surveys (DHS) conducted in countries across sub-Saharan Africa between 2005 and 2015. We chose to focus on sub-Saharan Africa, due to the high burden of morbidity and mortality in children.25 The DHS are large nationally representative cross-sectional surveys that use the national census bureau to first stratify the country by geographic regions and then by urban/rural. From each stratum, DHS draws clusters from a census and randomly samples households from that cluster. The DHS survey is thus designed to produce representative estimates of the entire country, for urban/rural areas separately and for the geographical regions.26 The target population for the survey is women age 15–49 and children under 5 years of age. Livestock ownership was much less prevalent among urban households and we expected a different dynamic between livestock and child health, so only children in rural households were included in the study.
Variables.
Anthropometric measurements on children were restricted to children born up to 5 years before the survey and alive at the time of the survey. Stunting was defined as having a height-for-age z score (HAZ) below or equal to −2 standard deviations (SDs), as measured against the World Health Organization's (WHO) international growth standards.27,28 We used stunting as an indicator of long-term health impacts on child health.29,30 For the diarrhea analyses, mothers were asked about the occurrence of diarrhea in the past 2 weeks. For the analysis of all-cause child mortality, we included all children born to mothers in the previous 5 years.
When comparing children with and without anthropometry data as well as children that were living and not living, we found that children without anthropometry data and children that were not living were significantly different from their counterparts across many of the countries with regard to age, maternal education, number of children in the household, and household wealth. Children without anthropometry data and children that were not living were on average younger, had mothers with less education, had fewer children in the household, and were less wealthy. To account for potential selection bias, we used a form of imputation suggested by Langkampt and others, where we redistributed the sample weights from missing samples to nonmissing samples based on covariate subgroups.31 We first created subgroups based on all possible combinations of the differing covariates mentioned earlier, resulting in 300 possible subgroups. For each subgroup, we summed the weights from missing children in the stunting and diarrhea analyses, that is, children without anthropometry data and/or children that were not living, and then redistributed them equally to children that were in the sample.
Household ownership of livestock was defined as the number of the following animals owned: chickens, cows, goat, sheep, or pigs. Although most countries reported ownership of pigs as a country-specific variable, this animal species was not included in surveys from eight countries. An additional exposure of interest, based on research by Zambrano and others, was the presence of chickens and the number of chickens owned by the household.8 The study results for chicken ownership are provided in the Supplemental Materials.
Covariates for the model were selected based on their potential to be confounding factors or strong predictors identified through existing literature. Covariates included mother's education, mother's age, improved water supply, safe treatment of water, improved sanitation, practice of open defecation, child's age, child's sex, number of members in household per sleeping room, number of children under 5 years of age in the household, and asset-based wealth. Breastfeeding was considered as a covariate but we found that it had a minor effect on our outcome variables. Water supply and sanitation facilities were recoded as improved and unimproved according to United Nations Children's Fund/WHO Joint Monitoring Program for Water Supply and Sanitation definitions.32
To include a proxy for household wealth as a covariate, principal components analysis (PCA) was performed on rural households' assets for each country.33,34 Household asset variables included electricity, radio, television, fridge, bicycle, motorbike, car, floor materials, wall materials, roof materials, stove type, watch, cart, boat, land, mobile phone, separate kitchen, and any other country-specific assets. As most variables were already binary, all household assets were converted to binary variables and a tetrachoric correlation for binary variables was used to produce appropriate weights.35 Using the first principal component as a wealth score, all households were categorized into quintiles of wealth.
Statistical analysis.
Multivariable logistic regression was performed to estimate ORs. Animal ownership was log2 transformed, because we hypothesized a logarithmic relationship between the number of animals owned (i.e., dose) and child health outcomes (i.e., response). We chose log2 for ease of interpretation, so a one unit increase in log2 corresponds to a 2-fold increase in livestock ownership. ORs were interpreted as the outcome associated with a doubling in the number of animals owned. A recently published study using DHS data from Ethiopia, Kenya, and Uganda was used to inform this analysis.36 All statistical analyses were conducted separately by country using the survey package in Stata 12 (StataCorp LP, College Station, TX) to specify the rural subpopulation, weights, strata, and clustering of primary sampling units. We performed the analysis at the child level as clustering at the primary sampling unit was sufficient to account for potential correlation between children within households. A meta-analysis with random effects using the Mantel–Haenszel method combined the final ORs to create a summary OR and demonstrate potential heterogeneity between countries. We reported the I2 measure, which is the percentage of total variation across the effect estimates that is due to heterogeneity rather than chance, and a P value from the test for heterogeneity.37
Ethics statement.
DHS data collection activities were approved by the ICF International (Calverton, MD) institutional review board as well as the country-level entity that approves research on human subjects.38
Results
Descriptive statistics using sample weights and the unweighted household sample sizes for all countries are reported in Table 1. Household ownership of animals, as well as chickens only, was heavily skewed to the right, thus means and medians are provided. Across all countries, the total unweighted sample size for rural children was 215,971 after removing 832 children missing data on certain covariates. We also calculated the total number of rural households affected by livestock ownership as the following for each country:
Table 1.
Descriptive characteristics for rural HHs in sub-Saharan Africa based on DHS data including number of HHs with livestock (calculated using the total population size according to the World Bank)
| Survey year | HHs in sample (N) | Livestock ownership (%) | HHs with livestock '000 | Animals* per HH (mean [SD]) | Animals* per HH (med. [IQR]) | Chickens per HH (mean [SD]) | Chickens per HH (med. [IQR]) | |
|---|---|---|---|---|---|---|---|---|
| Benin | 2011–2012 | 5,084 | 40 | 460 | 9.9 (22.6) | 0 (0–11) | 5.8 (13.3) | 0 (0–6) |
| Burkina Faso | 2010 | 6,345 | 93 | 2,070 | 41.2 (42.7) | 29 (14–53) | 12.8 (14.2) | 10 (3–20) |
| Burundi | 2010 | 3,924 | 62 | 1,250 | 3.4 (5.0) | 2 (0–5) | 1.0 (2.5) | 0 (0–1) |
| Cameroon | 2011 | 3,648 | 70 | 1,460 | 11.0 (17.4) | 5 (0–14) | 5.5 (9.5) | 2 (0–8) |
| Comoros | 2012 | 1,223 | 50 | 50 | 4.2 (7.1) | 0 (0–6) | 1.6 (3.7) | 0 (0–1) |
| Congo | 2011 | 4,205 | 44 | 180 | 5.5 (13.8) | 0 (0–8) | 4.6 (11.5) | 0 (0–7) |
| Congo DR | 2013–2014 | 7,320 | 56 | 5,250 | 3.9 (6.7) | 1 (0–6) | 2.8 (4.9) | 0 (0–4) |
| Cote d'Ivoire | 2011–2012 | 2,850 | 47 | 1,030 | 7.8 (15.8) | 0 (0–10) | 5.6 (12.6) | 0 (0–6) |
| Ethiopia | 2011 | 6,043 | 92 | 13,940 | 8.6 (8.6) | 6 (3–11) | 3.0 (4.2) | 2 (0–4) |
| Gabon | 2012 | 1,271 | 37 | 30 | 5.0 (16.5) | 0 (0–7) | 4.6 (15.4) | 0 (0–6) |
| Ghana | 2008 | 1,291 | 64 | 2,190 | 11.3 (17.7) | 5 (0–16) | 7.5 (12.2) | 3 (0–10) |
| Guinea | 2012 | 2,796 | 69 | 900 | 9.9 (14.3) | 5 (0–13) | 4.3 (6.9) | 0 (0–6) |
| Kenya | 2008–2009 | 2,854 | 83 | 6,090 | 11.6 (19.2) | 6 (2–14) | 5.1 (7.3) | 3 (0–7) |
| Lesotho | 2009 | 2,355 | 66 | 250 | 11.9 (25.8) | 4 (0–12) | 2.3 (5.3) | 0 (0–3) |
| Liberia | 2013 | 3,065 | 53 | 200 | 5.6 (11.8) | 1 (0–8) | 4.7 (10.1) | 0 (0–6) |
| Madagascar | 2008–2009 | 6,535 | 75 | 3,130 | 8.8 (12.6) | 5 (1–12) | 5.6 (8.0) | 3 (0–8) |
| Malawi | 2010 | 11,837 | 67 | 2,010 | 7.7 (11.6) | 4 (0–11) | 5.9 (9.4) | 2 (0–8) |
| Mali | 2012–2013 | 4,375 | 75 | 1,640 | 31.6 (43.0) | 16 (0–40) | 6.7 (12.5) | 0 (0–10) |
| Mozambique | 2011 | 4,628 | 66 | 2,860 | 7.9 (12.0) | 4 (0–11) | 5.7 (8.3) | 3 (0–8) |
| Namibia | 2013 | 1,785 | 75 | 180 | 29.3 (43.2) | 16 (0–40) | 9.0 (11.7) | 6 (0–13) |
| Niger | 2012 | 4,992 | 80 | 2,100 | 8.8 (11.7) | 5 (1–12) | 3.0 (5.4) | 0 (0–4) |
| Nigeria | 2013 | 11,327 | 73 | 15,200 | 15.6 (23.5) | 8 (0–21) | 7.6 (12.3) | 3 (0–10) |
| Rwanda | 2010–2011 | 5,280 | 58 | 1,350 | 2.1 (3.3) | 1 (0–3) | 0.7 (2.0) | 0 (0–0) |
| Senegal | 2010–2011 | 3,539 | 81 | 590 | 23.1 (40.3) | 11 (2–27) | 6.6 (11.3) | 3 (0–10) |
| Sierra Leone | 2013 | 4,829 | 63 | 450 | 6.8 (11.6) | 3 (0–10) | 5.2 (7.5) | 2 (0–8) |
| Swaziland | 2006–2007 | 1,292 | 79 | 130 | 19.4 (20.2) | 13 (3–28) | 11.3 (11.7) | 9 (1–17) |
| Togo | 2013–2014 | 3,014 | 76 | 580 | 17.8 (25.2) | 10 (1–25) | 12.1 (17.4) | 6 (0–20) |
| Uganda | 2011 | 3,515 | 72 | 4,430 | 7.0 (10.1) | 4 (0–9) | 3.7 (6.3) | 1 (0–5) |
| Zambia | 2007 | 2,484 | 76 | 1,550 | 10.5 (14.3) | 6 (1–14) | 7.2 (9.5) | 4 (0–10) |
| Zimbabwe | 2010–2011 | 2,860 | 79 | 1,770 | 12.6 (15.1) | 8 (2–18) | 8.3 (10.6) | 5 (0–12) |
DHS = Demographic and Health Survey; HH = household; IQR = interquartile range; med. = median; SD = standard deviation.
Animals include chickens, cows, goat, sheep, or pigs per HH.
Population size data were obtained from the World Bank.39 The total number of rural households that own livestock across all 30 countries is approximately 73 million. In all countries combined, the unweighted prevalence of livestock ownership, as defined previously, was 71% with a mean number of all animals owned at 13.7 (SD = 25.3) and median at 5 (interquartile range [IQR] = 0–16). The unweighted mean and median number of poultry owned overall was 6 (SD = 10.6) and 2 (IQR = 0–8), respectively. In the weighted statistics, 22 of 30 of the countries had ownership levels between 60% and 90%. Gabon had the lowest level of household livestock ownership at 37% and Burkina Faso the highest at 93%. The number of livestock and chickens was also greatest in Burkina Faso. The weighted mean wealth score, representing the quintile (1–5) of wealth categorized by PCA, ranged from 2.8 to 3.0 in all countries.
The unweighted sample sizes for the mortality, diarrhea, and stunting analyses were 215,971, 195,784, and 108,286, respectively (Table 2). Although the sample sizes differed, the statistics in Table 1 were very similar across samples. The sample sizes for the stunting analyses were much smaller for two possible reasons: 1) DHS randomly samples children for anthropometry in some countries and 2) anthropometry data can often be missing for children for various reasons. When we compared our results to those without the imputation, we found they were quite similar, suggesting little impact of selection bias by availability of anthropometry or child's vital status.
Table 2.
Unweighted sample sizes of analyses and weighted statistics of child health indicators
| Stunting sample (N) | Stunting (%) | HAZ (mean [SD]) | Diarrhea sample (N) | Diarrhea (%) | Mortality sample (N) | Mortality (%) | |
|---|---|---|---|---|---|---|---|
| Benin | 4,828 | 43.9 | −1.5 (2.2) | 7,897 | 6.4 | 8,470 | 5.7 |
| Burkina Faso | 5,064 | 36.1 | −1.4 (1.5) | 10,539 | 15.0 | 11,741 | 9.6 |
| Burundi | 2,831 | 58.2 | −2.2 (1.2) | 5,878 | 26.0 | 6,348 | 7.2 |
| Cameroon | 2,925 | 38.7 | −1.4 (1.6) | 6,085 | 24.4 | 6,995 | 10.0 |
| Comoros | 1,587 | 29.8 | −1.2 (1.8) | 1,914 | 17.3 | 2,059 | 4.7 |
| Congo | 3,333 | 28.8 | −1.2 (1.7) | 6,461 | 15.2 | 6,911 | 4.8 |
| Congo DR | 5,677 | 45.1 | −1.7 (1.7) | 11,922 | 16.5 | 13,148 | 7.8 |
| Cote d'Ivoire | 2,109 | 32.6 | −1.3 (1.4) | 4,537 | 18.0 | 5,145 | 8.9 |
| Ethiopia | 8,038 | 43.8 | −1.6 (1.7) | 8,847 | 14.4 | 9,633 | 7.1 |
| Gabon | 1,304 | 27.0 | −1.1 (1.9) | 2,066 | 18.2 | 2,335 | 6.3 |
| Ghana | 1,582 | 29.9 | −1.1 (1.7) | 1,830 | 21.6 | 1,985 | 6.3 |
| Guinea | 2,209 | 33.2 | −1.2 (1.7) | 4,430 | 16.9 | 4,987 | 9.8 |
| Kenya | 3,910 | 35.5 | −1.4 (1.5) | 4,265 | 17.0 | 4,605 | 6.4 |
| Lesotho | 1,398 | 36.9 | −1.4 (1.6) | 2,927 | 12.1 | 3,327 | 10.8 |
| Liberia | 2,165 | 30.3 | −1.3 (1.6) | 4,641 | 24.9 | 5,176 | 7.0 |
| Madagascar | 3,964 | 49.3 | −1.8 (1.8) | 9,445 | 8.2 | 10,195 | 5.7 |
| Malawi | 4,116 | 46.3 | −1.7 (1.6) | 16,460 | 18.1 | 18,013 | 8.4 |
| Mali | 3,250 | 39.4 | −1.5 (1.7) | 7,124 | 8.7 | 7,801 | 7.8 |
| Mozambique | 6,367 | 44.6 | −1.7 (1.6) | 6,861 | 11.0 | 7,494 | 7.6 |
| Namibia | 908 | 21.1 | −0.9 (1.3) | 2,439 | 21.3 | 2,736 | 4.8 |
| Niger | 3,720 | 44.7 | −1.7 (1.5) | 8,809 | 14.4 | 9,766 | 8.6 |
| Nigeria | 15,890 | 42.3 | −1.6 (2.0) | 18,609 | 11.3 | 20,999 | 10.4 |
| Rwanda | 3,514 | 44.3 | −1.8 (1.3) | 7,250 | 13.4 | 7,721 | 5.9 |
| Senegal | 2,495 | 30.3 | −1.2 (1.5) | 7,943 | 19.6 | 8,681 | 6.0 |
| Sierra Leone | 2,889 | 39.2 | −1.4 (1.7) | 7,073 | 11.3 | 8,217 | 11.3 |
| Swaziland | 1,605 | 26.8 | −1.1 (1.4) | 1,837 | 15.8 | 2,084 | 9.2 |
| Togo | 2,303 | 30.7 | −1.3 (1.4) | 4,591 | 17.9 | 4,975 | 7.0 |
| Uganda | 1,639 | 35.7 | −1.5 (1.4) | 5,630 | 24.7 | 6,192 | 7.0 |
| Zambia | 3,481 | 45.7 | −1.7 (1.7) | 3,868 | 15.3 | 4,280 | 8.5 |
| Zimbabwe | 3,185 | 31.8 | −1.3 (1.4) | 3,606 | 12.9 | 3,952 | 6.9 |
HAZ = height-for-age z score; SD = standard deviation.
Stunting ranged from 21.1% in Namibia to 58.2% in Burundi (Table 2). Most of the countries (24 of 30) had between 30% and 60% of their children undernourished. In all countries but Namibia, the mean HAZ score was below −1.0, so many children were 1 SD under the height for their age according to WHO standards. The 2-week prevalence of diarrhea was the lowest at 6.4% in Benin and the highest at 26.0% in Burundi, where the stunting prevalence is also the greatest. Mortality was the greatest in Sierra Leone, where under-five mortality was 11.3%. Mortality was least common in Comoros at 4.7%.
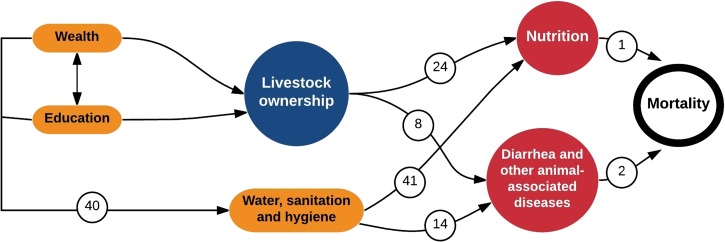
We developed a conceptual diagram, based on evidence that identifies the potential causal pathways considered in this study (Figure 1 ). The diagram highlights relationships hypothesized and documented in the literature: 1) household wealth and education have been associated with WASH conditions,40 2) WASH has been found to impact child diarrhea and child growth,14,41 and 3) nutrition and diarrhea have strong links to child mortality.1,2
Figure 1.
Conceptual diagram that includes covariates (wealth, education, water, sanitation, and hygiene), exposure variable of interest (livestock ownership), and health outcomes. Relationships noted may be positive or negative. Each number refers to a reference.
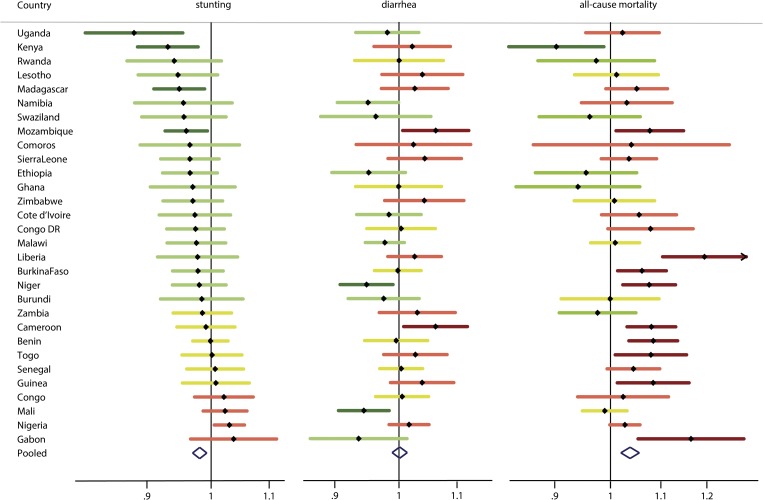
The results from the adjusted logistic regressions for each child health indicator associated with a log2 increase in the number of livestock owned are displayed in Figure 2 , with the actual numbers in Table 3. In Figure 2, the ORs are visualized by the direction of the OR (protective, null, or harmful) to illustrate overall patterns and concordance or discordance across indicators within each country. For the figure, we categorized ORs within the range of 0.99–1.01 as null, those below 0.99 as protective and those above 1.01 as harmful. The unadjusted livestock results are reported in Supplemental Figures 1–3. The logistic regression results for chickens were very similar to the overall livestock results and are reported in the Supplemental Figures 4–6.
Figure 2.
Adjusted odds ratios associated with log2 increase in animal ownership for each child health indicator across countries in sub-Saharan Africa categorized as significantly protective (light grey with asterisk/dark green), protective (light grey/green), null (grey/yellow), harmful (black/red), and significantly harmful (black with asterisk/dark red). Adjusted for mother's education and age, improved water supply, safe treatment of water, improved sanitation, open defecation, child's age and sex, household members per sleeping room, children in the household, and asset wealth.
Table 3.
ORs, CIs, and meta-analysis weights for the association of a log2 increase in animal ownership with each child health indicator
| Country | Stunting | Diarrhea | All-cause mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Weight (%) | OR | 95% CI | Weight (%) | OR | 95% CI | Weight (%) | |
| Uganda | 0.88 | 0.81, 0.96 | 1.72 | 0.98 | 0.93, 1.03 | 3.46 | 1.02 | 0.95, 1.10 | 3.34 |
| Kenya | 0.93 | 0.89, 0.98 | 3.42 | 1.02 | 0.96, 1.09 | 2.70 | 0.90 | 0.83, 0.99 | 2.46 |
| Rwanda | 0.94 | 0.87, 1.02 | 1.81 | 1.00 | 0.93, 1.08 | 2.18 | 0.97 | 0.87, 1.09 | 1.85 |
| Lesotho | 0.95 | 0.89, 1.01 | 2.36 | 1.04 | 0.97, 1.11 | 2.50 | 1.01 | 0.93, 1.10 | 2.88 |
| Madagascar | 0.95 | 0.91, 0.99 | 4.20 | 1.03 | 0.97, 1.08 | 3.22 | 1.05 | 0.99, 1.11 | 3.95 |
| Namibia | 0.96 | 0.88, 1.04 | 1.72 | 0.95 | 0.90, 1.00 | 3.58 | 1.03 | 0.95, 1.12 | 2.62 |
| Swaziland | 0.96 | 0.89, 1.03 | 2.16 | 0.96 | 0.88, 1.05 | 1.55 | 0.96 | 0.87, 1.06 | 2.27 |
| Mozambique | 0.96 | 0.93, 0.99 | 5.02 | 1.06 | 1.01, 1.12 | 3.27 | 1.08 | 1.01, 1.15 | 3.63 |
| Comoros | 0.97 | 0.89, 1.05 | 1.67 | 1.02 | 0.93, 1.13 | 1.46 | 1.04 | 0.86, 1.25 | 0.79 |
| Sierra Leone | 0.97 | 0.92, 1.01 | 3.57 | 1.04 | 0.98, 1.11 | 2.85 | 1.04 | 0.98, 1.09 | 4.32 |
| Ethiopia | 0.97 | 0.92, 1.01 | 3.86 | 0.95 | 0.90, 1.01 | 2.84 | 0.95 | 0.87, 1.05 | 2.26 |
| Ghana | 0.97 | 0.91, 1.04 | 2.14 | 1.00 | 0.93, 1.07 | 2.28 | 0.94 | 0.84, 1.06 | 1.70 |
| Zimbabwe | 0.97 | 0.92, 1.02 | 3.49 | 1.04 | 0.98, 1.11 | 2.57 | 1.01 | 0.93, 1.09 | 3.02 |
| Cote d'Ivoire | 0.97 | 0.92, 1.03 | 2.76 | 0.98 | 0.93, 1.04 | 3.37 | 1.06 | 0.98, 1.13 | 3.27 |
| Congo DR | 0.97 | 0.93, 1.02 | 3.64 | 1.00 | 0.95, 1.06 | 3.12 | 1.08 | 1.00, 1.17 | 2.82 |
| Malawi | 0.98 | 0.93, 1.02 | 3.56 | 0.98 | 0.95, 1.01 | 5.49 | 1.01 | 0.96, 1.06 | 4.71 |
| Liberia | 0.98 | 0.92, 1.05 | 2.34 | 1.03 | 0.98, 1.07 | 4.15 | 1.19 | 1.10, 1.29 | 2.89 |
| Burkina Faso | 0.98 | 0.94, 1.02 | 4.21 | 1.00 | 0.96, 1.04 | 4.74 | 1.06 | 1.01, 1.11 | 4.73 |
| Niger | 0.98 | 0.94, 1.03 | 3.95 | 0.95 | 0.91, 0.99 | 4.21 | 1.08 | 1.02, 1.13 | 4.48 |
| Burundi | 0.98 | 0.92, 1.05 | 2.24 | 0.98 | 0.92, 1.03 | 2.98 | 1.00 | 0.91, 1.10 | 2.37 |
| Zambia | 0.99 | 0.94, 1.03 | 3.58 | 1.03 | 0.97, 1.10 | 2.72 | 0.98 | 0.91, 1.05 | 3.19 |
| Cameroon | 0.99 | 0.95, 1.04 | 3.61 | 1.06 | 1.01, 1.12 | 3.42 | 1.08 | 1.03, 1.13 | 4.73 |
| Benin | 1.00 | 0.97, 1.03 | 5.81 | 1.00 | 0.95, 1.05 | 3.46 | 1.08 | 1.04, 1.14 | 4.73 |
| Togo | 1.00 | 0.95, 1.05 | 3.47 | 1.03 | 0.98, 1.08 | 3.44 | 1.08 | 1.01, 1.16 | 3.44 |
| Senegal | 1.01 | 0.96, 1.06 | 3.70 | 1.00 | 0.97, 1.04 | 5.10 | 1.04 | 0.99, 1.10 | 4.48 |
| Guinea | 1.01 | 0.95, 1.07 | 3.01 | 1.04 | 0.99, 1.09 | 3.44 | 1.08 | 1.01, 1.16 | 3.42 |
| Congo | 1.02 | 0.97, 1.07 | 3.57 | 1.01 | 0.96, 1.05 | 4.20 | 1.02 | 0.94, 1.12 | 2.61 |
| Mali | 1.02 | 0.99, 1.06 | 4.87 | 0.94 | 0.91, 0.98 | 4.39 | 0.99 | 0.95, 1.03 | 4.99 |
| Nigeria | 1.03 | 1.01, 1.06 | 6.43 | 1.02 | 0.98, 1.05 | 5.40 | 1.03 | 1.00, 1.06 | 5.93 |
| Gabon | 1.04 | 0.97, 1.11 | 2.10 | 0.94 | 0.86, 1.01 | 1.91 | 1.16 | 1.05, 1.29 | 2.13 |
| Pooled OR | 0.98 | 0.97, 0.99 | 1.00 | 0.99, 1.01 | 1.04 | 1.02, 1.06 | |||
CI = confidence interval; OR = odds ratio. Adjusted for mother's education and age, improved water supply, safe treatment of water, improved sanitation, open defecation, child's age and sex, household members per sleeping room, children in the household, and asset wealth.
Animal ownership and stunting.
The ORs for stunting combined using a meta-analysis had an I2 of 83.3% (P < 0.001) in the unadjusted models (Supplemental Figure 1) and 39.8% (P = 0.014) after adjustment for the covariates described previously. After adjustment, many of the ORs were attenuated. The pooled OR indicated a slight protective effect for a log2 increase in the number of animals owned on stunting (OR = 0.98, 95% CI = 0.97, 0.99). Uganda displayed the most extreme adjusted OR < 1 (OR = 0.88, 95% CI = 0.81, 0.96), illustrating a protective effect of livestock ownership. Approximately, two-thirds of the countries (22 of 30 countries) suggested a protective effect with ORs < 1, though only four of those countries had CIs below 1. Six countries showed livestock ownership to be a risk for stunting with ORs > 1, however, only one country, Nigeria, had a CI above 1 (OR = 1.03, 95% CI = 1.01, 1.06). For the 22 countries with an OR > 1, the pooled OR was 0.96 (95% CI = 0.95, 0.97).
Animal ownership and diarrhea.
For diarrhea, we also observed significant heterogeneity across countries (I2 = 40.9%, P = 0.011) in the adjusted models, but we did not see the same pattern in the ORs as we observed for stunting. Instead, we saw a somewhat even distribution with 10 of the countries demonstrating ORs < 1, six ORs null, and 14 ORs > 1. The most suggestive ORs were in Mali (OR = 0.94, 95% CI = 0.91, 0.98), Niger (OR = 0.95, 95% CI = 0.91, 0.99), Cameroon (OR = 1.06, 95% CI = 1.01, 1.12), and Mozambique (OR = 1.06, 95% CI = 1.01, 1.12). The pooled OR was null (OR = 1.00, 95% CI = 0.99, 1.01). Overall, the results did not suggest a clear pattern of child diarrhea risk for households that own livestock. The pooled estimate for those 10 countries classified as exhibiting a protective effect was OR = 0.96 (95% CI = 0.95, 0.98), and the pooled estimate for those 14 countries classified as exhibiting a risk was OR = 1.03 (95% CI = 1.02, 1.05).
Animal ownership and child mortality.
The adjusted analyses for all-cause child mortality showed a pattern opposite to that of the stunting analyses. The overall I2 for the adjusted analyses was 53.2% (P < 0.001). Approximately, two-thirds of the countries (22 of 30) displayed ORs > 1, of which nine had CIs above 1. The pooled OR also suggested livestock as a risk factor (OR = 1.04, 95% CI = 1.02, 1.06). In contrast, only one country, Kenya, displayed a strong protective effect of livestock toward mortality (OR = 0.90, 95% CI = 0.83, 0.99). The most extreme OR indicating livestock as a risk factor was in Liberia (OR = 1.19, 95% CI = 1.10, 1.29), suggesting a 19% increased odds of mortality associated with a doubling of livestock ownership. The pooled estimate of the 22 countries exhibiting livestock as a risk was OR = 1.06 (95% CI = 1.05, 1.08).
Discussion
We analyzed small-scale livestock ownership as both a risk factor and a protective factor for child health outcomes including stunting, 2-week prevalence of diarrhea, and mortality in children less than 5 years of age in sub-Saharan Africa. Livestock ownership has been shown both to be associated with an increased risk of infection as well as health benefits through improved nutrition and socioeconomic status. Our multi-country analysis of livestock ownership and child morbidity and mortality agrees with this dual impact. Our analyses suggested a protective effect of livestock on the chronic condition of stunting in 22 of the 30 countries, a mixed effect on diarrhea (both associated with acute infection and chronic malnutrition), and a harmful effect on all-cause child mortality (potentially associated with acute infections) in 22 of the 30 countries, with all analyses displaying significant heterogeneity across countries.
On the one hand, livestock ownership may result in consumption of more nutrient dense food by children, and thus explain our finding that livestock, as well as poultry ownership, on average was associated with less stunting in children. This finding is also in line with past research that identified a protective effect of household livestock ownership on child stunting prevalence in three east African countries.36 It is possible that improved childhood nutrition improves immune function and offsets risk associated with exposure to enteric pathogens in animal feces.42 Current evidence, however, suggests that animals are a risk factor for enteric infections and diarrhea.8 Our results for diarrhea only somewhat reflect this evidence, with approximately a third of the countries suggesting an increased risk and a third suggesting a protective effect. Nonetheless, the finding that animal ownership had a positive association with child mortality may reflect this evidence. There is also the potential that other zoonotic infectious diseases (i.e., nonenteric infections) were responsible for the increased all-cause child mortality.
The strength of this analysis was its use of large, nationally representative data sets from 30 countries. DHS is a key source for measuring child mortality and undernutrition across LMICs. In the DHS, a complete birth and death history is collected for each eligible woman's children, including date of birth and, when applicable, age at death of each child. Further, the DHS has extensive training for enumerators and uses standardized measurement tools that include a core set of questions with pretesting to ensure that data are standardized and comparable across diverse settings. Spot checks and validation of completed surveys are regularly conducted as part of the DHS, but the quality of measurement also differs by country. A recent methodological report by DHS highlighted differences in quality of anthropometric measurements by country, which may explain some of the heterogeneity by country.28
The significant heterogeneity by country may also indicate country-specific dynamics in livestock practices that are not captured by DHS. Using the ownership of livestock variable, we could not ascertain whether the animals owned were kept near the household. For example, it may be that households own livestock but keep them at a location distant from where children are raised or that livestock were corralled rather than allowed to roam. The lack of detailed information on livestock management may lead to residual confounding that this analysis could not address. Also, it may be that livestock ownership under certain contexts is positively or negatively associated with poverty, which may have not been fully captured in our proxy measure for wealth. In this scenario, poverty, not animal ownership, could be the main risk factor for mortality. A study in Madagascar estimated that the burden of diseases among poultry exacerbated the economic impacts on poor households, leading to a 10–15% monthly income loss.43 Although this could suggest wealth as a causal intermediate between livestock and child health, in our study, wealth was an asset-based index that did not incorporate livestock. Also, if this were true, however, we should have observed a similar relationship for stunting and diarrhea given that they have both been shown to have positive associations with poverty.
This analysis has other limitations. Results come from cross-sectional surveys, so longitudinal studies could strengthen our understanding of the relationship between livestock ownership and child health. The mortality analyses included any children that had died in the past 5 years of any cause, so the animal exposures identified in the surveys may not have reflected the child's actual exposure before their death. This situation could also apply to stunting given that it is a less acute health outcome and livestock exposures in the past may not be accurately reported by the household's livestock ownership at the time of the survey. A substantial change in livestock ownership by households, however, is also unlikely. Further, all-cause mortality is a fairly crude measure and includes a variety of causes that are unlikely to be influenced by livestock ownership. Selection bias for living children may be a concern for the stunting and diarrhea analyses; however, we examined this using a form of imputation and found little to no differences, which minimized selection bias from our observed variables. Finally, an important discussion in the context of the many analyses conducted in this article is multiple testing. We chose not to display P values for each country to focus more on the trends observed in each meta-analysis. We also advise against heavy reliance on the pooled estimates, especially given the significant heterogeneity observed across countries. Further, the DHS data were not designed to conduct an in-depth analysis of livestock ownership and health outcomes, so future studies should focus on what is happening within countries to improve our mechanistic understanding of the risks and protective effects of livestock.
Livestock ownership is highly prevalent in rural sub-Saharan Africa and other regions of the world. Furthermore, many development organizations provide livestock to households for poverty alleviation. The results of this article highlight the dual role of livestock and underscore the need to understand what aspects of livestock management are harmful or beneficial. Given the economic importance of livestock, it is surprising that few studies have emphasized both the protective and harmful effects on child health. More effort should be made to ascertain the mechanisms between livestock management practices and child health, and thereby better understand the country-level heterogeneity observed in the DHS data. This mechanistic understanding can provide direction for points of intervention in livestock management and related sanitary practices to mitigate risks and accentuate benefits.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Alan Hubbard, Maureen Lahiff, John Colford, James Fuller, and the Demographic and Health Survey staff for their support on the analysis. We would also like to acknowledge United States Agency for International Development, all partner organizations, and participants involved in the surveys.
Footnotes
Financial support: This work was partially supported by Fogarty International Center of the National Institutes of Health (K01 TW 009484). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Authors' addresses: Maneet Kaur, Division of HIV, Infectious Diseases, and Global Medicine, University of California, San Francisco, CA, E-mail: maneet.kaur@ucsf.edu. Jay P. Graham, Public Health Institute, Oakland, CA, E-mail: jay.graham@phi.org. Joseph N. S. Eisenberg, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, E-mail: jnse@umich.edu.
References
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 2.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet Lond Engl. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George CM, Oldja L, Biswas SK, Perin J, Lee GO, Ahmed S, Haque R, Sack RB, Parvin T, Azmi IJ, Bhuyian SI, Talukder KA, Faruque AG. Fecal markers of environmental enteropathy are associated with animal exposure and caregiver hygiene in Bangladesh. Am J Trop Med Hyg. 2015;93:269–275. doi: 10.4269/ajtmh.14-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, Guerrant RL, Bhutta Z, Mason C, Kang G, Kabir M, Amour C, Bessong P, Turab A, Seidman J, Olortegui MP, Quetz J, Lang D, Gratz J, Miller M, Gottlieb M. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink G, Günther I, Hill K. The effect of water and sanitation on child health: evidence from the demographic and health surveys 1986–2007. Int J Epidemiol. 2011;40:1196–1204. doi: 10.1093/ije/dyr102. [DOI] [PubMed] [Google Scholar]
- 6.Vasco K, Graham JP, Trueba G. Detection of zoonotic enteropathogens in children and domestic animals in a semi-rural community in Ecuador. Appl Environ Microbiol. 2016;82:4218–4224. doi: 10.1128/AEM.00795-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conan A, Goutard FL, Sorn S, Vong S. Biosecurity measures for backyard poultry in developing countries: a systematic review. BMC Vet Res. 2012;8:240. doi: 10.1186/1746-6148-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambrano LD, Levy K, Menezes NP, Freeman MC. Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2014;108:313–325. doi: 10.1093/trstmh/tru056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Tras WF, Holt HR, Tayel AA, El-Kady NN. Campylobacter infections in children exposed to infected backyard poultry in Egypt. Epidemiol Infect. 2015;143:308–315. doi: 10.1017/S095026881400096X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grados O, Bravo N, Black RE, Butzler JP. Paediatric Campylobacter diarrhoea from household exposure to live chickens in Lima, Peru. Bull World Health Organ. 1988;66:369–374. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee G, Pan W, Peñataro Yori P, Paredes Olortegui M, Tilley D, Gregory M, Oberhelman R, Burga R, Chavez CB, Kosek M. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis. 2013;7:e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marquis GS, Ventura G, Gilman RH, Porras E, Miranda E, Carbajal L, Pentafiel M. Fecal contamination of shanty town toddlers in households with non-corralled poultry, Lima, Peru. Am J Public Health. 1990;80:146–149. doi: 10.2105/ajph.80.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberhelman RA, Gilman RH, Sheen P, Cordova J, Taylor DN, Zimic M, Meza R, Perez J, LeBron C, Cabrera L, Rodgers FG, Woodward DL, Price LJ. Campylobacter transmission in a Peruvian shantytown: a longitudinal study using strain typing of Campylobacter isolates from chickens and humans in household clusters. J Infect Dis. 2003;187:260–269. doi: 10.1086/367676. [DOI] [PubMed] [Google Scholar]
- 14.Curtis V, Schmidt W, Luby S, Florez R, Touré O, Biran A. Hygiene: new hopes, new horizons. Lancet Infect Dis. 2011;11:312–321. doi: 10.1016/S1473-3099(10)70224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed S, Ali A, Duggan C. Environmental enteric dysfunction in children: a review. J Pediatr Gastroenterol Nutr. 2016;63:6–14. doi: 10.1097/MPG.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngure FM, Humphrey JH, Mbuya MNN, Majo F, Mutasa K, Govha M, Mazarura E, Chasekwa B, Prendergast AJ, Curtis V, Boor KJ, Stoltzfus RJ. Formative research on hygiene behaviors and geophagy among infants and young children and implications of exposure to fecal bacteria. Am J Trop Med Hyg. 2013;89:709–716. doi: 10.4269/ajtmh.12-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schriewer A, Odagiri M, Wuertz S, Misra PR, Panigrahi P, Clasen T, Jenkins MW. Human and animal fecal contamination of community water sources, stored drinking water and hands in rural India measured with validated microbial source tracking assays. Am J Trop Med Hyg. 2015;93:509–516. doi: 10.4269/ajtmh.14-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George CM, Oldja L, Biswas S, Perin J, Lee GO, Kosek M, Sack RB, Ahmed S, Haque R, Parvin T, Azmi IJ, Bhuyian SI, Talukder KA, Mohammad S, Faruque AG. Geophagy is associated with environmental enteropathy and stunting in children in rural Bangladesh. Am J Trop Med Hyg. 2015;92:1117–1124. doi: 10.4269/ajtmh.14-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darapheak C, Takano T, Kizuki M, Nakamura K, Seino K. Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int Arch Med. 2013;6:29. doi: 10.1186/1755-7682-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dror DK, Allen LH. The importance of milk and other animal-source foods for children in low-income countries. Food Nutr Bull. 2011;32:227–243. doi: 10.1177/156482651103200307. [DOI] [PubMed] [Google Scholar]
- 21.Iannotti L, Lesorogol C. Animal milk sustains micronutrient nutrition and child anthropometry among pastoralists in Samburu, Kenya. Am J Phys Anthropol. 2014;155:66–76. doi: 10.1002/ajpa.22547. [DOI] [PubMed] [Google Scholar]
- 22.Krebs NF, Mazariegos M, Tshefu A, Bose C, Sami N, Chomba E, Carlo W, Goco N, Kindem M, Wright LL, Hambidge KM, Complementary Feeding Study Group Meat consumption is associated with less stunting among toddlers in four diverse low-income settings. Food Nutr Bull. 2011;32:185–191. doi: 10.1177/156482651103200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin M, Iannotti LL. Livestock production, animal source food intake, and young child growth: the role of gender for ensuring nutrition impacts. Soc Sci Med. 1982;2014:16–21. doi: 10.1016/j.socscimed.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Leroy JL, Frongillo EA. Can interventions to promote animal production ameliorate undernutrition? J Nutr. 2007;137:2311–2316. doi: 10.1093/jn/137.10.2311. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE, Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet Lond Engl. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 26.ICF International . Demographic and Health Survey Sampling and Household Listing Manual. MEASURE DHS. Calverton, MD: ICF International; 2012. [Google Scholar]
- 27.WHO Multicentre Growth Reference Study Group Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr. 1992;450((Suppl. 2006)):38–46. doi: 10.1111/j.1651-2227.2006.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 28.Assaf S, Kothari MT, Pollum T. An Assessment of the Quality of DHS Anthropometric Data, 2005–2014. Rockville, MD: ICF International; 2015. https://dhsprogram.com/pubs/pdf/MR16/MR16.pdf Available at. [Google Scholar]
- 29.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7:5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, International Child Development Steering Group Developmental potential in the first 5 years for children in developing countries. Lancet Lond Engl. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langkamp DL, Lehman A, Lemeshow S. Techniques for handling missing data in secondary analyses of large surveys. Acad Pediatr. 2010;10:205–210. doi: 10.1016/j.acap.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO/UNICEF Joint Monitoring Program for Water Supply and Sanitation . Progress on Drinking Water and Sanitation: 2014 Update. Geneva, Switzerland: World Health Organization; 2014. http://www.wssinfo.org/fileadmin/user_upload/resources/JMP_report_2014_webEng.pdf Available at. Accessed December 9, 2014. [Google Scholar]
- 33.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India*. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 34.Rutstein SO, Johnson K. DHS Wealth Index. Calverton, MD: ORC Macro; 2004. http://www.dhsprogram.com/pubs/pdf/CR6/CR6.pdf Available at. [Google Scholar]
- 35.Howe LD, Galobardes B, Matijasevich A, Gordon D, Johnston D, Onwujekwe O, Patel R, Webb EA, Lawlor DA, Hargreaves JR. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41:871–886. doi: 10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosites EM, Rabinowitz PM, Thumbi SM, Montgomery JM, Palmer GH, May S, Rowhani-Rahbar A, Neuhouser ML, Walson JL. The relationship between livestock ownership and child stunting in three countries in eastern Africa using national survey data. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DHS Program Protecting the Privacy of DHS Survey Respondents. http://dhsprogram.com/What-We-Do/Protecting-the-Privacy-of-DHS-Survey-Respondents.cfm Available at. Accessed January 10, 2016.
- 39.The World Bank Population, Total. 2016. http://data.worldbank.org/indicator/SP.POP.TOTL Available at. Accessed April 11, 2016.
- 40.Sara S, Graham J. Ending open defecation in rural Tanzania: which factors facilitate latrine adoption? Int J Environ Res Public Health. 2014;11:9854–9870. doi: 10.3390/ijerph110909854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, Cavill S, Allen E, Uauy R. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev. 2013:CD009382. doi: 10.1002/14651858.CD009382.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rytter MJH, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS One. 2014;9:e105017. doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rist CL, Ngonghala CN, Garchitorena A, Brook CE, Ramananjato R, Miller AC, Randrianarivelojosia M, Wright PC, Gillespie TR, Bonds MH. Modeling the burden of poultry disease on the rural poor in Madagascar. One Health. 2015;1:60–65. doi: 10.1016/j.onehlt.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.