Abstract
Neuronally differentiated PC12 cells secrete decreasing amounts of [3H]norepinephrine when repetitively stimulated by depolarizing concentrations of potassium ion. The decreasing response shows attributes that have been classically ascribed to response habituation, a behavior commonly observed in nervous systems but found here in a homogeneous cell type. Alteration of the habituation pattern was caused by activators of the protein kinase C pathway and of voltage-gated calcium channels.
Full text
PDF
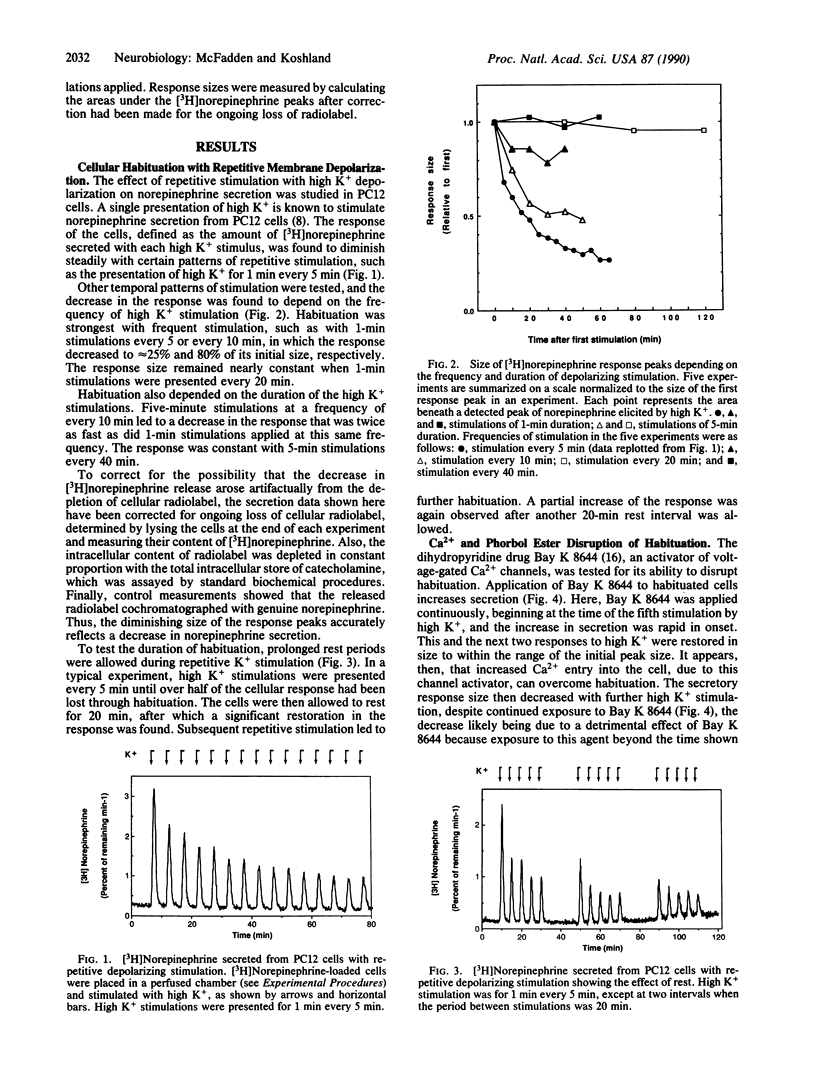


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Sahley C. L. Invertebrate learning and memory: from behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
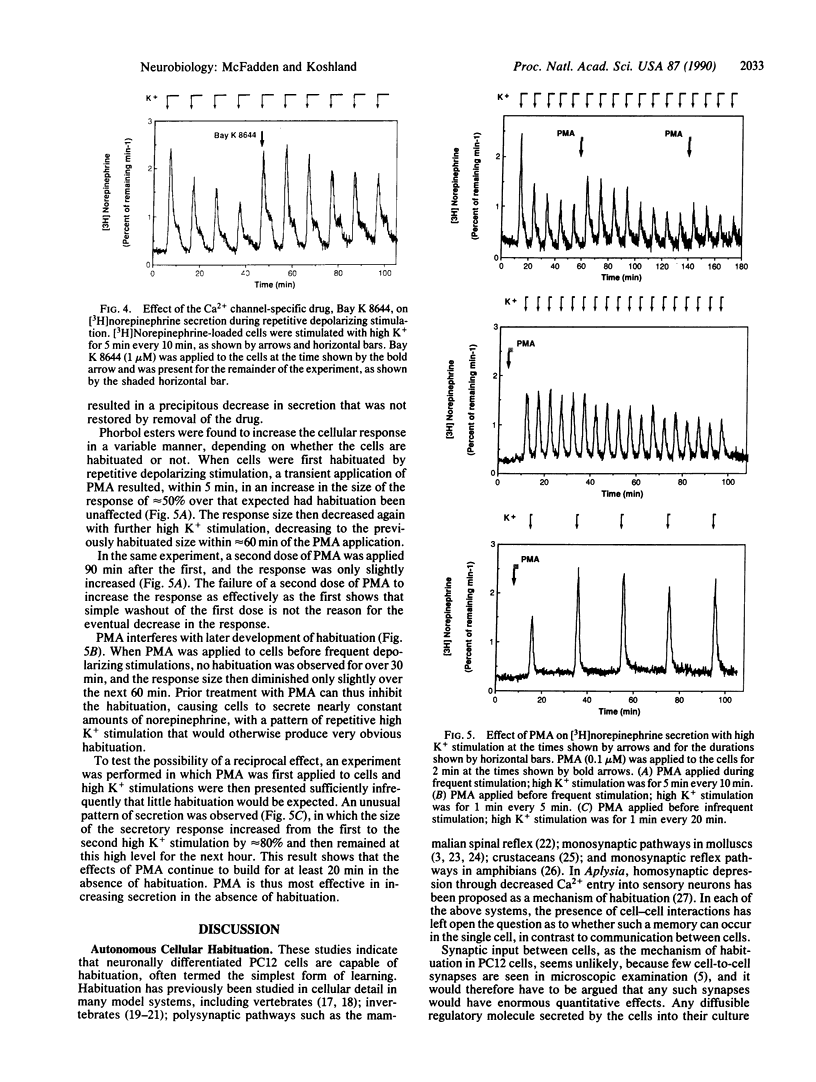
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I., Kandel E. R. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Davis M., Wagner A. R. Habituation of startle response under incremental sequence of stimulus intensities. J Comp Physiol Psychol. 1969 Apr;67(4):486–492. doi: 10.1037/h0027308. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Tischler A. S., Greene L. A. Nerve growth factor-induced increase in electrical excitability and acetylcholine sensitivity of a rat pheochromocytoma cell line. Nature. 1977 Aug 11;268(5620):501–504. doi: 10.1038/268501a0. [DOI] [PubMed] [Google Scholar]
- Farel P. B. Dual processes control response habituation across a single synapse. Brain Res. 1974 Jun 7;72(2):323–327. doi: 10.1016/0006-8993(74)90874-9. [DOI] [PubMed] [Google Scholar]
- Farel P. B., Glanzman D. L., Thompson R. F. Habituation of a monosynaptic response in vertebrate central nervous system: lateral column-motoneuron pathway in isolated frog spinal cord. J Neurophysiol. 1973 Nov;36(6):1117–1130. doi: 10.1152/jn.1973.36.6.1117. [DOI] [PubMed] [Google Scholar]
- Farley J., Alkon D. L. Cellular mechanisms of learning, memory, and information storage. Annu Rev Psychol. 1985;36:419–494. doi: 10.1146/annurev.ps.36.020185.002223. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res. 1977 Jul 1;129(2):247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M., Kao L. S., Curran D. J., Westhead E. W. Flow-injection analysis of catecholamine secretion from bovine adrenal medulla cells on microbeads. Anal Biochem. 1985 Jan;144(1):218–227. doi: 10.1016/0003-2697(85)90109-5. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Toselli M., Swandulla D., Lux H. D. A diacylglycerol analogue reduces neuronal calcium currents independently of protein kinase C activation. Nature. 1989 Mar 23;338(6213):340–342. doi: 10.1038/338340a0. [DOI] [PubMed] [Google Scholar]
- Jaken S., Tashjian A. H., Jr, Blumberg P. M. Characterization of phorbol ester receptors and their down-modulation in GH4C1 rat pituitary cells. Cancer Res. 1981 Jun;41(6):2175–2181. [PubMed] [Google Scholar]
- Klein M., Shapiro E., Kandel E. R. Synaptic plasticity and the modulation of the Ca2+ current. J Exp Biol. 1980 Dec;89:117–157. doi: 10.1242/jeb.89.1.117. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Kongsamut S., Miller R. J. Nerve growth factor modulates the drug sensitivity of neurotransmitter release from PC-12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2243–2247. doi: 10.1073/pnas.83.7.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I., Castellucci V., Pinsker H., Kandel E. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1743–1745. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- Matthies H. J., Palfrey H. C., Hirning L. D., Miller R. J. Down regulation of protein kinase C in neuronal cells: effects on neurotransmitter release. J Neurosci. 1987 Apr;7(4):1198–1206. doi: 10.1523/JNEUROSCI.07-04-01198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Huttner W. B., Tsien R. Y., Pozzan T. Free cytoplasmic Ca2+ and neurotransmitter release: studies on PC12 cells and synaptosomes exposed to alpha-latrotoxin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):620–624. doi: 10.1073/pnas.81.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Kauer J. A., Malenka R. C. The current excitement in long-term potentiation. Neuron. 1988 Apr;1(2):97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Pocotte S. L., Frye R. A., Senter R. A., TerBush D. R., Lee S. A., Holz R. W. Effects of phorbol ester on catecholamine secretion and protein phosphorylation in adrenal medullary cell cultures. Proc Natl Acad Sci U S A. 1985 Feb;82(3):930–934. doi: 10.1073/pnas.82.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Gatti G., Dozio N., Vicentini L. M., Meldolesi J. Ca2+-dependent and -independent release of neurotransmitters from PC12 cells: a role for protein kinase C activation? J Cell Biol. 1984 Aug;99(2):628–638. doi: 10.1083/jcb.99.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S. G., Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986 Jan;83(1):184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Knight D. E. Stimulus-secretion coupling: a perspective highlighting the contributions of Peter Baker. J Exp Biol. 1988 Sep;139:1–30. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Spencer W. A., Thompson R. F., Neilson D. R., Jr Response decrement of the flexion reflex in the acute spinal cat and transient restoration by strong stimuli. J Neurophysiol. 1966 Mar;29(2):221–239. doi: 10.1152/jn.1966.29.2.221. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ogura A. Dihydropyridines as potent calcium channel blockers in neuronal cells. FEBS Lett. 1983 Feb 21;152(2):191–194. doi: 10.1016/0014-5793(83)80377-9. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tsukui H., Hatanaka H. Neuronal differentiation of Ca2+ channel by nerve growth factor. Brain Res. 1985 Aug 26;341(2):381–384. doi: 10.1016/0006-8993(85)91079-0. [DOI] [PubMed] [Google Scholar]
- Thompson R. F., Spencer W. A. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966 Jan;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thompson R. F. The neurobiology of learning and memory. Science. 1986 Aug 29;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Wagner J. A. Structure of catecholamine secretory vesicles from PC12 cells. J Neurochem. 1985 Oct;45(4):1244–1253. doi: 10.1111/j.1471-4159.1985.tb05549.x. [DOI] [PubMed] [Google Scholar]
- Wood D. C. Habituation in Stentor: a response-dependent process. J Neurosci. 1988 Jul;8(7):2248–2253. doi: 10.1523/JNEUROSCI.08-07-02248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972 Sep;35(5):621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]