Abstract
Treatment of metastatic colorectal cancer (CRC) has continuously improved over the last decade. However, disease monitoring remains underdeveloped and mostly dependent on imaging e.g. RECIST 1.1 criteria. The genetic landscape of individual cancers and subsequently occurring treatment-induced evolution remain neglected in current surveillance strategies. Novel biomarkers demand minimally invasive and repetitive tracking of the cancer mutagenome for therapy stratification and to make prognostic predictions. Carcinoembryonic antigen (CEA), a routinely used tumor marker for CRC, does not meet these goals and thus prevents its use as a reliable monitoring tool. A tumor-derived fraction of circulating cell-free DNA (cfDNA), isolated from blood samples, may bypass the limitations of currently available biomarkers and could be a tool for noninvasive disease monitoring. Here, total cfDNA levels differentiated a cohort of metastatic CRC patients from healthy controls. Furthermore, we correlated cfDNA during chemotherapy of 27 stage IV patients with clinical parameters to establish its prognostic and predictive value. Indeed, cfDNA levels in chemotherapy naive patients correlate with the tumor burden and CEA values at diagnosis and increase upon disease progression during 1st and 2nd line treatment. Moreover, we confirm the possibility of cfDNA-based genotyping of KRAS to early detect the emergence of resistance during chemotherapy. These data indicate that repetitive quantitative and mutational analysis of cfDNA might complement current treatment standards but may have also limited value in some patients.
Introduction
Despite intense efforts in the prevention and screening, colorectal cancer (CRC) remains an important contributor to cancer morbidity and mortality worldwide. In Germany, CRC is the second most common cancer among men and women. Various treatment options are available depending mostly on disease stage. In recent years, there has been a substantial improvement of survival in the metastatic situation due to novel therapeutic agents such as anti-EGFR antibodies in the absence of RAS mutations. However, valid prognostic and/or predictive biomarkers or noninvasive measures to study response or failure of a given therapeutic regimen are still missing.
The image-based Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) considers changes in the longest axial tumor diameters assessed by cross section imaging and remains the gold standard to define treatment response [1]. RECIST 1.1 evaluation has several limitations and size measurements not necessarily reflect the true tumor burden [2, 3]. Furthermore, spatial heterogeneity of tumor lesions has been suggested to correlate with tumor biology and treatment resistance but is not reflected by RECIST 1.1 criteria. In addition, sole RECIST-based tumor response in metastatic CRC fails to predict survival to an individual patient [4].
Besides its impact on histological diagnosis, the molecular characterization of biopsies and resected tumor specimens enabled a prediction of disease progression and response to therapies in CRC [5]. However, there are also limitations in studying a single snapshot of a tumor due to both intertumoral and intratumoral heterogeneity and treatment associated tumor evolution. Hence, a single biopsy is likely to underestimate the complexity of the tumors genomic landscape [6]. This highlights the difficulty to determine an optimal and mutation tailored treatment process based on a single or even repetitive tumor tissue biopsy.
Blood based biomarkers for treatment stratification and therapy monitoring would be desirable tools. Analysis of carcinoembryonic antigen (CEA) is routinely used as a CRC-specific tumor marker. Even though it is elevated in multiple other malignancies and benign conditions and performs with limited sensitivity and specificity [7–9]. New minimal or noninvasive approaches to observe tumor dynamics and tumor genetics have evolved but are not yet clinically validated. One of these approaches is the analysis of circulating cell-free DNA (cfDNA) isolated from a patient´s blood sample. In the past 10 years, cfDNA has been a subject of intense research in different clinical scenarios, such as muscle training, end-stage renal failure, prenatal assessment of the unborn, stroke, surgery and trauma including myocardial infarction [10]. In clinical oncology cfDNA has been suggested as a new surrogate marker for therapy response, disease progression or early relapse [11–20].
Healthy subjects exhibit only low levels of circulating nucleic acids in their blood stream due to physiological tissue renewal. This balance can be disturbed even upon benign tumorigenesis as recently shown by our group for patients with cystic pancreatic tumors, having significantly higher cfDNA levels compared to healthy individuals. Moreover, cfDNA has the potential to discriminate patients with benign and malignant pancreatic diseases [21] and thus confirms data from previous reports. Therefore, cfDNA could be a promising tool for repeated noninvasive tumor assessment in oncology [22]. Moreover, studies have revealed a significant correlation between disease stage and the presence of tumor-associated genetic aberrations in the blood of patients with resectable [13, 23] or metastatic CRC and other early- and late-stage human malignancies [24, 25]. Tumor burden also correlates with the quantity of released cfDNA [26]. Similarly, cfDNA dynamics may predict therapy response or failure, or progression free survival (PFS) in metastatic CRC patients [27, 28]. Larger clinical trials to verify these observations are few and hampered by the limited amount of plasma samples for analysis after baseline [27]. Hence, the prognostic or predictive impact of cfDNA dynamics during treatment in metastatic CRC patients remains to be clarified.
Besides quantification, cfDNA analysis also provides the opportunity for targeted genotyping to detect the emergence of therapy resistance. The latter is considered as the most promising application of cfDNA in clinical oncology as shown in pioneering work of Diaz LA Jr et al. [11] and Misale et al. [12]. In addition, the non-invasive detection of emerging KRAS mutations in cfDNA from peripheral blood can help to measure resistance to EGFR blockade [29, 30]. Similarly, high levels of KRAS mutant alleles in the plasma indicate a poor outcome for patients when treated with cetuximab [19]. Bettegowda et al were able to show, that circulating tumor DNA is a broadly applicable, sensitive, and specific biomarker that can be used for a variety of clinical and research purposes in patients with multiple different types of cancer [24]. Murtaza et al. reported on sequencing of cancer exomes in serial plasma samples to track the genomic evolution of metastatic cancers during treatment. These proof-of-principle studies showed that cfDNA-based genotyping could complement current invasive biopsy approaches to identify mutations associated with acquired drug resistance in advanced cancers [31].
In this study, we hypothesized that quantitative cfDNA analysis complemented with targeted genotyping for KRAS under palliative chemotherapy may fulfill the requirements for implementation of cfDNA as a noninvasive monitoring tool in metastatic CRC patients during subsequent lines of palliative chemotherapy.
Patients and methods
Institutional review board
Prior to start of the study a positive vote from the institutional review board of Ulm University was obtained (Ethics Committee of Ulm University, Ulm, Germany, Approval numbers: 317/12, 230/14, 128/15). Participation to the study was voluntary. All patients, prior to inclusion, signed a written informed consent.
Patient characteristics and study design
Twenty-seven patients with histologically confirmed metastatic CRC (UICC stage IV) were enrolled into a treatment surveillance cohort. All of these patients received palliative chemotherapy: 15 patients were analyzed during 1st line, another 13 patients during 2nd line, 3 different patients during 3rd line and one patient during 4th line treatment. Of note, not the same patients were analyzed during all mentioned lines of treatment. The clinical data of theses 27 patients are shown in Table 1, detailed patient characteristics and information about chemotherapeutic regimens are provided in S1 Table.
Table 1. Patient characteristics.
Characteristics and clinical parameters of metastatic colorectal cancer patients (surveillance cohort).
| Characteristic | N (%) | Characteristic | N (%) |
|---|---|---|---|
| Patients | 27 | Resected primary tumor | |
| Age (years) | 65.4±1.8 | Yes | 23 (85) |
| Gender | No | 4 (15) | |
| Male | 23 (85) | Metastatic sites | |
| Female | 4 (15) | 1 | 5 (19) |
| Therapy lines | 32 | 2 | 12 (44) |
| 1st line | 15 (47) | >2 | 10 (37) |
| 2nd line | 13 (41) | KRAS Status | |
| 3rd line | 3 (9) | Wild-type | 16 (59) |
| 4th line | 1 (3) | Mutated | 11 (41) |
| Median PFS (months) | Chemotherapy regimen* | ||
| 1st line | 5.0±2.75 | Oxaliplatin-based | 11 (34) |
| 2nd line | 2.5±0.75 | Irinotecan-based | 16 (50) |
| 3rd line | 2.0±0.5 | Other | 5 (16) |
| 4th line | 2.75 | antiEGFR | 8 (25) |
| Primary tumor site | antiVEGF | 17 (53) | |
| Right-sided colon | 8 (30) | ||
| Left-sided colon | 9 (33) | ||
| Rectum | 10 (37) | ||
* Of total 32 therapy lines; detailed characteristics available in S1 Table.
Blood samples for cfDNA analyses were taken prospectively at predefined time points (“baseline”: at least 7 days prior therapy initiation; “upon treatment”: 3.6±0.15 weeks after treatment initiation; “progression”: at radiological confirmed disease progression, ±7 days after the respective CT scan) and analyzed retrospectively. CfDNA was quantified fluorometrically. The KRAS status of the tumor was assessed routinely in FFPE tumor tissues at baseline (at initial diagnosis) by pyro-sequencing. Furthermore, we used droplet digital PCR (ddPCR) for KRAS genotyping of cfDNA at the respective time points. For the analysis of mutational concordance between tissue DNA and cfDNA we analyzed additional 23 therapy naïve patients with histologically confirmed metastatic CRC (S3 Table) in addition to 17 selected patients of the treatment surveillance cohort. All of these 40 tissue and blood samples were taken prior to start of 1st line treatment. CT-scans were done at baseline and tumor burden was determined according to the guidelines laid out by RECIST 1.1 for measurable lesions. For restaging CT scans were performed at a mean of 2.35±0.14 months after therapy initiation and target lesions were again evaluated according to RECIST 1.1 criteria. CEA measurements were performed in parallel to cfDNA analyses at baseline, staging and restaging time points. Additionally, cfDNA was compared to a previously reported [21] cohort of 38 healthy controls (S2 Table) without any chronic, malignant or inflammatory diseases.
Plasma collection
7.5ml of whole venous blood were collected in EDTA tubes (Sarstedt, Nümbrecht, Germany) by peripheral blood draw. The tubes were kept at 4°C until separation, which was carried out within one hour after collection. Whole blood was centrifuged for 10 minutes (820 x g at 4°C) and the plasma fraction was transferred in cold 2ml tubes (Eppendorf RNA/DNA LoBind micro-centrifuge tubes, Eppendorf, Hamburg, Germany). These were subsequently centrifuged again for 10 minutes (20,000 x g at 4°C) and pure plasma was recovered in fresh 2ml tubes for immediate storage at -80°C until cfDNA extraction.
Extraction of circulating cell-free DNA
Circulating cell-free DNA was extracted from plasma using the QIAamp Circulating Nucleic Acid Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. For each patient we used 2ml of plasma for cfDNA extraction and recovered cfDNA in 50μl of elution buffer. DNA was stored at 4°C until further use.
Quantification of cell-free DNA
The total amount of cfDNA was determined by fluorometric measurement using Qubit 2.0 Fluorometer (ThermoFisher Scientific, Waltham, Massachusetts, USA). We used 2μl of DNA eluate gained through the QIAamp Circulating Nucleic Acid Kit and measured the concentration using the Qubit dsDNA HS Assay Kit (ThermoFisher Scientific, Waltham, Massachusetts, USA) according to the manufacturer’s instructions.
Droplet digital PCR (ddPCR) analyses
Isolated cfDNA was amplified using ddPCR™ Supermix for Probes (Bio-Rad®, Hercules, California, USA) and the respective PrimePCR™ ddPCR™ Mutation Assay for KRAS p.A146T, KRAS p.A59T, KRAS p.Q61H, KRAS p.Q61L and the ddPCR™ KRAS Screening Multiplex Kit (Bio-Rad®, Hercules, California, USA) which covers the 7 most frequent KRAS mutations in colorectal cancer (KRAS p.G12A/G12C/G12D/G12R/G12S/G12V/G13D). 8μl/9μl of isolated cfDNA were used in each reaction and mixed with 2μl/1μl of primers/probes and 10μl of Supermix. The reaction mix was then vortexed and immediately transferred into a DG8™ Cartridge together with 70μl of Droplet Generation Oil for Probes for droplet generation in a QX200™ Droplet Generator (all: Bio-Rad®, Hercules, California, USA). Droplets were carefully transferred into a 96-well plate, which was sealed with PX1™ PCR Plate Sealer for subsequent amplification in a T100™ Thermal Cycler according to the manufacturer’s instructions (all: Bio-Rad®, Hercules, California, USA). Droplets were analyzed in QX200™ Droplet Reader (Bio-Rad®, Hercules, California, USA) for fluorescent measurement of FAM and HEX probes. We used reference DNA by Horizon® (SW48, human, CRC stage IV) as positive (50% WT, 50% Mutant) and negative (100% WT) controls and H20 as no-template-control. Thresholding was done based on positive and negative controls for each assay. False-positive-rates (FPR) were determined for each assay individually using wild-type reference DNA (Horizon®) in appropriate concentrations. Samples were called positive based on Poisson distribution when reaching 99% confidence level for being positive. DdPCR data were analyzed by QuantaSoft analysis software (version 1.7.4) according to the manufacturer’s instructions (Bio-Rad®, Hercules, California, USA).
Assessment of KRAS-Status in tumor tissue at baseline
For the identification of KRAS mutations, H&E-stained slides from FFPE tumor samples were reviewed by a pathologist and tumor tissue was selected for analysis. Corresponding tissue from two unstained, 5-μm-thick tissue sections was removed by micro-dissection and the tissue was lysed by incubation in TEN buffer (1mM EDTA, 10mM TRIS-HCl, 0.1M NaCl, pH 8,0) including Proteinase K (20mg/ml) over night at 62°C. The resulting lysates were used for PCR reactions to amplify the regions encoding codons 12/13 in exon 2, 59 to 61 in exon 3, and 117 and 146 in exon 4 of the KRAS gene. For this 3μl of the lysate were added to 47μl of the PCR reaction including 25μl PCR Master Mix S (Peqlab), 0,5μl of the specific PCR primer mix (25pmol of each primer) and 21,5μl Millipore H2O. The cycling conditions were: 95°C 5 min; 2x[95°C 30sec; 62°C 30sec; 72°C 30sec]; 2x[95°C 30sec; 60°C 30sec; 72°C 30sec]; 2x[95°C 30sec; 58°C 30sec; 72°C 30sec]; 35x[95°C 30sec; 58°C 30sec; 72°C 30sec]; 72°C 10min. A fraction of the PCR reaction was separated on a 1% agarose gel to ensure the successful amplification of the KRAS regions. The mutation status at the different KRAS codons was determined by pyro-sequencing using the PyroMark Q24 sequencer (QIAGEN, Hilden, Germany). Pyro-sequencing was performed according the instructions of the manufacturer using the pyro-sequencing reagents from QIAGEN (Hilden, Germany). The sequences of all primers are available upon request.
Statistical analyses
Results for continuous variables are presented as median ± median absolute deviation (MAD) or mean ± standard error of the mean (SEM) unless stated otherwise. Treatment groups were compared with the Student’s t-test or the Mann-Whitney U test. Comparison of categorical variables was generated by Fisher’s exact test. Survival curves were compared with the Mantel-Cox log-rank test. Correlation analyses were performed by Pearson or Spearman correlation analysis, p-values < 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism version 7 (GraphPad Software, La Jolla, California, USA).
Results
Baseline cfDNA and CEA values
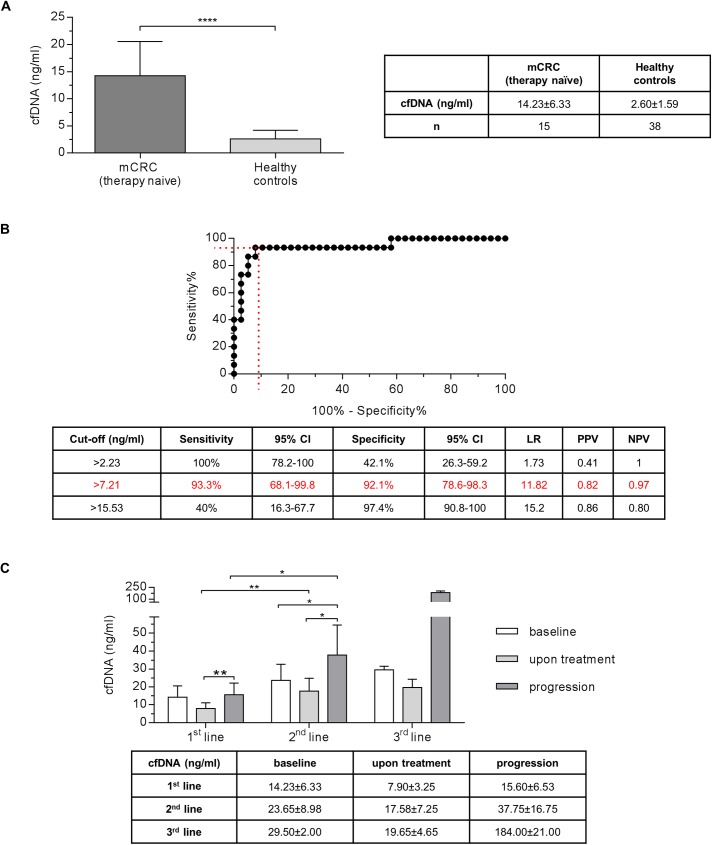
First, we examined the level of cfDNA in therapy naïve UICC stage IV metastatic CRC patients prior to systemic treatment. The median baseline cfDNA value was 14.23±6.33ng/ml. There was no significant difference between the cfDNA concentrations of right- and left-sided primary CRCs (15.76±9.15ng/ml vs.14.23±6.23ng/ml, p = 0.9495, data not shown). However, there was a significant difference in cfDNA content between patients with metastatic CRC and healthy individuals with a median value of 2.60±1.59ng/ml (p<0.0001, Fig 1A). Test performance analyzed by Receiver operating characteristic (AUC = 0.94, Fig 1B) showed reasonable sensitivity (93.3%) and specificity (92.1%) when using a threshold of 7.21 ng cfDNA per ml of plasma to discriminate the two cohorts. Total cfDNA levels were independent of age (data not shown) or sex (therapy naïve mCRC cohort p = 0.400; healthy controls p = 0.5297, data not shown). CEA was elevated [≥ 2.5μg/l] at baseline in 86.7% of the cases with a median baseline level of 19.80±17.9μg/l (data not shown).
Fig 1. Diagnostic power of cfDNA in metastatic colorectal cancer patients and its changes under palliative treatment.
(A) Therapy naïve patients with metastatic colorectal cancer (CRC) show significantly higher cfDNA concentration than healthy controls (Mann-Whitney, p<0.0001). (B) Receiver operating characteristic (ROC) of cfDNA level as a test parameter to discriminate patients with metastatic CRC from healthy controls. (C) CfDNA levels over the time course of therapy in 1st, 2nd and 3rd line treatment. The amount of cfDNA increases at time-point of progression in 1st and 2nd line (Mann-Whitney, p<0.01, p<0.05, respectively). CI = confidence interval, LR = likelihood ratio, PPV = positive predictive value, NPV = negative predictive value. Level of significance: * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001.
cfDNA levels during palliative 1st and 2nd line chemotherapy
Next, we examined cfDNA levels and their changes during 1st and 2nd line chemotherapy. CfDNA concentration was determined prior to start of chemotherapy in each therapy line (1st or 2nd line treatment), after 3.96±0.28 weeks of treatment (termed “upon treatment” time point) and upon radiologically confirmed disease progression (termed “progression”).
Under 1st line palliative chemotherapy reduced cfDNA levels were observed after 4 weeks of chemotherapy dropping from 14.23±6.23ng/ml to 7.9±3.25ng/ml (p = 0.0515). At the time of disease progression, cfDNA levels were significantly increased in the 1st line setting to 15.6±6.53ng/ml compared to the “upon treatment” time point (p<0.01) (Fig 1C). During 2nd line treatment, we observed increased cfDNA levels compared to the 1st line situation. Though this observation was not statistical significance at baseline (p = 0.2005) but upon treatment (p<0.01) and at progression (p<0.05) a significant difference could be observed (Fig 1C). Similarly, there was no significant difference (p = 0.798) between cfDNA levels at baseline of 2nd line (23.65±8.98ng/ml) and upon treatment of 2nd line (7.58±7.25ng/ml). However, upon disease progression median cfDNA value significantly increased to 37.75±16.75ng/ml (vs. “baseline”: p<0.05; and vs. “upon treatment”: p<0.05, Fig 1C). CfDNA dynamic during 3rd line chemotherapy was overall higher with a similar pattern compared to the respective states prior 1st and 2nd line treatment. Intriguingly, a substantial increase in cfDNA levels was observed “at progression” in 3rd line but again not significant (p = 0.2000, Fig 1C).
Correlation of cfDNA and tumor burden (RECIST 1.1)
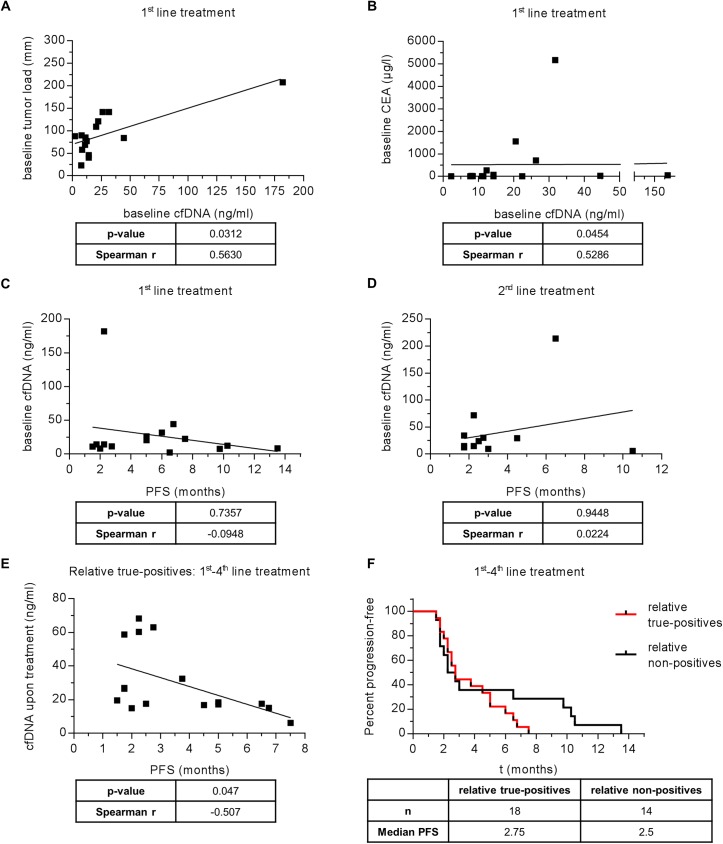
Tumor burden of all individuals in our study was determined according to the guidelines laid out by RECIST 1.1 for measurable lesions. Baseline cfDNA levels in therapy naïve patients significantly correlated with the respective tumor burden (p<0.05, r = 0.563). (Fig 2A). However, such a correlation could not be observed in subsequent therapy lines (p = 0.5746, r = 0.1706, data not shown). Interestingly, cfDNA levels did not correlate with tumor burden according to RECIST 1.1 criteria at disease progression under 1st line (p = 0.3538, data not shown) and 2nd line (p = 0.2769, data not shown) treatment.
Fig 2. Correlation of respective cfDNA and CEA values with clinical parameters in therapy naïve and pretreated patients.
Correlation of baseline cfDNA levels in therapy naïve metastatic CRC patients with (A) baseline tumor load (RECIST 1.1) and (B) baseline CEA level (C) with progression-free survival (PFS). (D) Correlation of baseline cfDNA levels in pretreated metastatic CRC patients with progression-free survival (PFS). (E) + (F): patients defined as relative true-positives concerning cfDNA level at baseline (>95% of highest control). (E) Correlation of cfDNA level upon treatment with PFS of relative true-positive group. (F) Kaplan-Meier-plot showing PFS of relative true-positives vs relative non-positives.
Predictive value of cfDNA for progression-free survival (PFS)
To determine whether these findings would correlate with PFS we analyzed all data obtained in the 1st and 2nd line setting. No significant influence on PFS for cfDNA levels both at baseline (Fig 2C) and upon treatment (S1A Fig) in the 1st line and 2nd line setting could be observed (Fig 2D, S1C Fig). We then normalized cfDNA baseline values of our treatment surveillance cohort to 95% of the highest cfDNA level in our healthy control cohort to identify relative true-positives. By excluding the relative non-positives from the analysis, a significant correlation between the cfDNA levels upon treatment and PFS can be demonstrated (Fig 2E, p = 0.047, r = -0.507). The respective cfDNA levels at baseline did not correlate with PFS (p = 0.600, data not shown). Similarly, the “relative true-positives” does not significantly differ from the “relative non-positives” group in the Kaplan Mayer analysis to assess PFS (Fig 2F, p = 0.2584).
Correlation of cfDNA and CEA levels
Corresponding cfDNA levels and CEA measurements were available from all patients at corresponding time points. There was a significant, positive correlation between baseline cfDNA levels and CEA values in therapy naïve patients (p = 0.0454, r = 0.5286, Fig 2B), while no correlation was observed at later time points during 1st line (”upon treatment”: p = 0.3310;”progression”: p = 0.9132, data not shown) or in the 2nd line setting (“baseline”: p = 0.6960, “upon treatment”: p = 0.4511, progression: p = 0.2316, data not shown). Interestingly, there was also no correlation between CEA baseline levels and PFS in the 1st line (p = 0.7602, data not shown) or the 2nd line setting (p = 0.4035, data not shown). In contrast, CEA levels upon treatment correlated with PFS in the 1st (p = 0.0280, r = -0.6410, S1B Fig) but not during 2nd line treatment (P = 0.6638, S1D Fig).
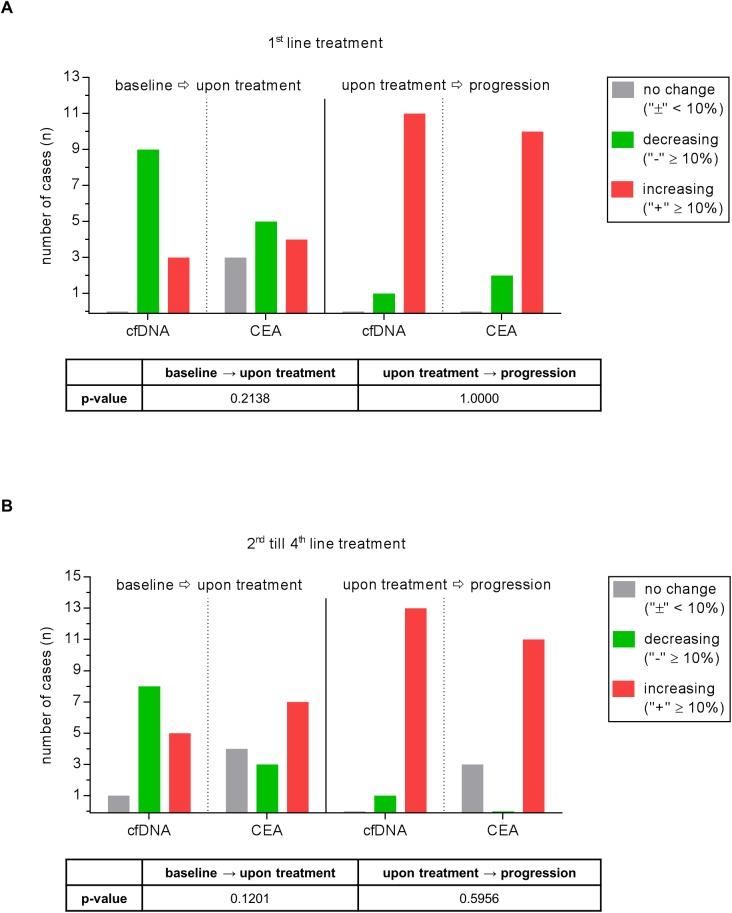
Next, we investigated the power of cfDNA and CEA to mirror disease course. A 10% change in the CEA or cfDNA value across various time points was determined as a clinically meaningful difference [28]. In the 1st line situation cfDNA decreased in 75% of cases under therapy while CEA decreased in only 56%. At the time of disease progression there was an increase in cfDNA levels in 92% of cases and in CEA levels in 83% (Fig 3A). This was even more pronounced in advanced treatment lines (2nd - 4th line). Here we observed decreasing cfDNA levels under therapy in 57% of cases versus 21% for CEA and increasing cfDNA levels towards disease progression in 93% versus 79% for CEA (Fig 3B).
Fig 3. Comparison of the power of cfDNA and CEA course to reflect the course of disease.
Values are depicted for both therapy naïve patients (A) and patients during further therapy lines (B). A change in cfDNA amount of ≥10% is considered as “increase” /”decrease”. A change in value <10% is defined as “no change”.
KRAS genotyping of tumor tissue and cfDNA in therapy naïve patients
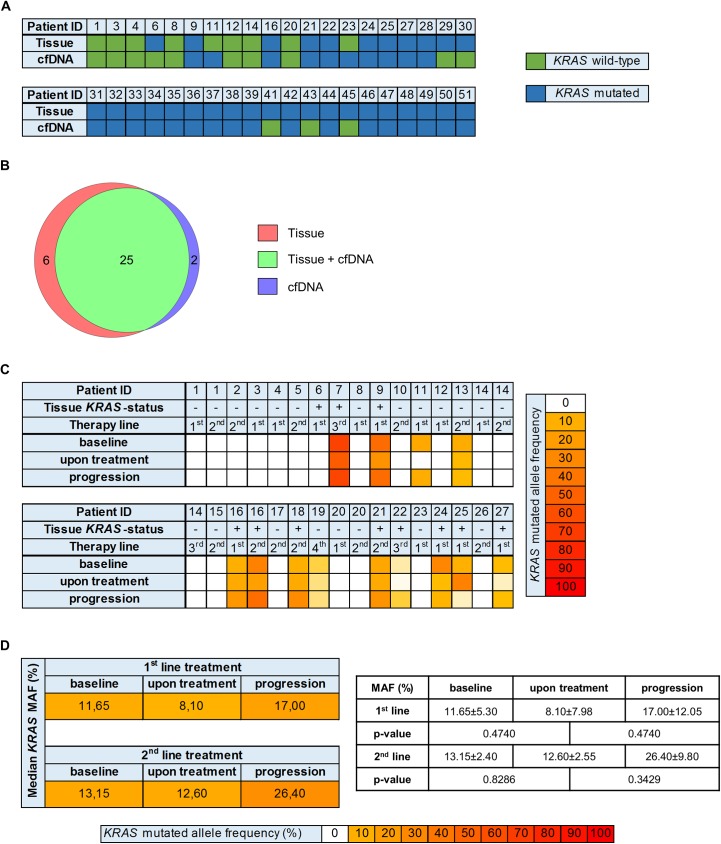
First, we applied pyro-sequencing technologies of hot-spot regions in the KRAS gene at initial diagnosis of metastatic CRC. Specifically, we assessed the KRAS status in exon 2 (codons 12/13), exon 3 (codon 59 and 61) and exon 4 (codon 117 and 146) and identified 77.5% (31 out of 40) to carry any KRAS mutation within these hotspots. These data were complemented using digital droplet PCR techniques (ddPCR) from cfDNA of the respective patients. Such analysis identified 2 additional patients to be KRAS mutated based on cfDNA genotyping suggesting heterogeneity. Vice versa, cfDNA analysis missed 6 patients with KRAS mutations. Details are shown in Fig 4A and 4B, S3 and S4 Tables. To specify these numbers, we calculated the overall per patient concordance of KRAS mutational status between cfDNA and tumor tissue with 80% in therapy naïve patients (Fig 4B).
Fig 4. KRAS genotyping of cfDNA.
(A) KRAS mutational status per patient for tissue and cfDNA analysis at time-point of initial diagnosis (n = 40, all: therapy naïve). (B) Venn diagram showing that 25 KRAS mutations, detected in tumor tissue could also be found in cfDNA, 6 were only present in tumor tissue and 2 only in cfDNA. (C) KRAS mutated allele frequencies in cfDNA under treatment per patient and therapy line. (D) Median KRAS mutated allele frequencies (MAF) in 1st and 2nd line treatment group at the respective time-points.
Fig 4C shows KRAS mutated allele frequencies (MAF) for the treatment surveillance cohort during treatment. 6 of the 15 therapy naïve patients (40.0%) showed KRAS mutations. The median MAF of the patients with mutated KRAS decreased from baseline (11.65±5.30%) to the “upon treatment” time point (8.10±7.98%, p = 0.4740) and increased again at progression (17.00±12.05%, p = 0.4740) in the 1st line treatment group (Fig 4D).
KRAS genotyping of tumor tissue and cfDNA in advanced therapy lines
In pretreated patients, 4/13 (30.8%) patients under 2nd line treatment and 2/3 (66.7%) patients under 3rd line treatment and 1/1 (100%) patient under 4th line treatment exhibited mutated KRAS in cfDNA at baseline (Fig 4C). The median MAF for KRAS of the patients under 2nd line treatment decreased from baseline (13.15±2.40%) to the “upon treatment” time point (12.60±2.55%, p = 0.8286) and increased again at progression (26.40±9.80%, p = 0.3429) (Fig 4D).
KRAS genotyping of cfDNA during therapy
It has been suggested that amongst other causes KRAS mutations emerge during anti-EGFR treatment and mediate acquired resistance. Therefore, we analyzed the respective parameters in some of our patients (S2 Fig).
In one patient (S2A Fig), the primary tumor tissue was KRAS exon 2–4 wild type, similarly cfDNA analysis confirmed the KRAS wild type status at baseline before initiating of 1st line therapy. In this patient, the drop in cfDNA correlated with a sustained response during treatment. There was also a correlation of radiologically confirmed disease progression with a rise in cfDNA. The CEA value of this patient also dropped during treatment, but failed to increase upon progression. However, a KRAS Exon 2 mutation was detectable at the time point of radiologically confirmed disease progression.
In another patient (S2B Fig), where the tumor also was determined as KRAS exon 2–4 wild type, we detected a KRAS Exon 3 mutation by cfDNA analysis already at baseline (prior 1st line therapy), namely Q61H. Since tissue analysis was the standard of KRAS assessment at that time the patient received a combination of FOLFIRI plus panitumumab. Interestingly, the KRAS mutation was not any more detectable one month after therapy initiation, possibly due to the treatment with cytotoxic agents (irinotecan-based chemotherapy). However, the same mutation was detectable again in cfDNA about 5 months later, more than 2 months prior to radiologically confirmed disease progression. The results of this patient indicate the potential usefulness of cfDNA-based KRAS screening during treatment for early detection of disease progression in contrast to sole cfDNA quantification.
Another patient (S2C Fig) received an anti-EGFR antibody as single agent as 4th line treatment. The tumor tissue was defined as KRAS exon 2–4 wild type prior to 1st line therapy and no further tissue analyses were done according to standard clinical practice. However, we detected a KRAS exon 3 mutation (Q61H) over the whole period of 4th line treatment in our retrospective analysis. Consequently, there was disease progression already at the first staging after 2 months of treatment. As expected from the data described above, disease progression was accompanied by an increase in total cfDNA value.
Discussion
Data from this study and from other groups [32] demonstrate that cfDNA can be faithfully isolated and analyzed in patient samples. First described in 1948, cfDNA has been analyzed for many years to test its potential as a noninvasive biomarker in cancer patients [13, 31, 33–37]. Patients with malignant tumors carry significantly more cfDNA compared to those with benign or premalignant lesions or healthy individuals, for example in pulmonary or gastrointestinal diseases [21, 38–42]. In this study, we confirm this concept in patients with metastatic CRC compared to healthy individuals. Accordingly, cfDNA may be considered as a useful and unique biomarker in malignancy. However, the role of cfDNA as a noninvasive monitoring tool over the continuum of care is still largely elusive and only limited data are available, in particular in advanced therapy lines [27, 28, 30, 43].
For dynamic cfDNA analysis we focused on three time points that appear clinically relevant: baseline, upon treatment and at disease progression as determined by imaging. In this study, cfDNA concentration and tumor burden as determined according to the guidelines laid out by RECIST 1.1 for measurable lesions at baseline prior to 1st line treatment positively correlated in therapy naïve patients (Fig 2A) as well as cfDNA and CEA (Fig 2B), which is in line with previously reported data [28]. Therefore, cfDNA quantification in therapy naïve patients allows to determine the tumor load in an easy noninvasive procedure. But comparisons between the various studies are difficult to make, not at least due to a lack of standardized procedures and technologies for isolation and quantification of cfDNA, like BEAMing, several quantitative real-time PCR protocols [27], ddPCR technologies [21, 30] or digital genomic assays, for example Safe-SeqS [28], resulting in a limited generalizability. Interestingly, cfDNA levels in our study did not correlate with tumor burden according to RECIST 1.1 criteria at disease progression under 1st line and 2nd treatment (S1E and S1F Fig). This may be caused by the fact, that RECIST 1.1 dependent disease progression is defined by a predefined increase in diameter but also by the occurrence of new metastases. When new metastases define disease progression this is not reflected by the diameter. In those cases, cfDNA level may increase due to high cellular turnover although the RECIST 1.1 diameter is not increasing. Nevertheless, the observed correlations between tumor load (RECIST 1.1), CEA and baseline cfDNA in therapy naïve patients could not be confirmed under therapy and in later therapy lines. However, an increase in cfDNA correlates well with disease progression either in 1st or in 2nd line treatment and cfDNA levels rise during the therapeutic course of metastatic CRC.
Moreover, it has not been conclusive, if cfDNA values at baseline and cfDNA changes under therapy are prognostic. In our study, total cfDNA values both prior 1st line treatment and 2nd line treatment did not show a significant correlation to PFS in the respective therapy line. Furthermore, cfDNA fold change in all therapy lines did not have a significant correlation with PFS. To further examine the prognostic value of cfDNA we normalized cfDNA baseline values of our treatment surveillance cohort to 95% of the highest cfDNA level in our healthy control cohort and thereby identified the relative true-positives, which showed a significant correlation between the cfDNA levels upon treatment and PFS (Fig 2E, p = 0.047, r = -0.507), in line with previously published reports [27, 28]. Similarly, CEA values upon treatment significantly correlated with PFS in the 1st line situation (S1B Fig, p = 0.0280, r = -0.641). Accordingly, CEA seemed to outperform cfDNA in the prediction of a highly relevant endpoint (PFS) in the 1st line setting. This fact may render the use of a more complicated and expensive biomarker rather redundant and needs further clarification. An assumed multivariate model would likely show limited to no value of total cfDNA quantification as a prognostic marker. Moreover, it is important to state, that the reliance of a dichotomized rather than continuous biomarker, which could help to prove the variability in calls is relevant. This is especially important to gain assumptions from longitudinal changes between the respective time points of cfDNA measurement (baseline, therapy and disease progression).
Nevertheless, our data suggest that quantitative cfDNA analysis in metastatic CRC shows some important strengths, like estimation of tumor load in therapy naïve patients but also some weaknesses. In particular, the lack of standardized procedures in cfDNA isolation, quantification and genomic analysis, and the lack of validated cut-offs for cfDNA changes, makes it difficult to assess the correlation of such changes with PFS or tumor response across clinical trials. The value of total cfDNA quantification and its impact as a predictive and prognostic tool is also limited due to several individual variations in the cellular turnover under treatment with subsequent cfDNA release. Several mechanisms of tumor evolution under treatment also affect the total amount of cfDNA release, which limits the individual comparison of total cfDNA assessment and the derivation of prognostic and/or predictive information. For sure, also the low number of patients in our study limits its meaningfulness.
Besides the quantitative assessment of cfDNA during the course of several chemotherapeutic regimens we focused on the molecular characterization of cfDNA by targeted genotyping of KRAS using droplet digital PCR (ddPCR), an established technology for this application [21, 30]. KRAS genotyping of cfDNA is not considered as a standard in metastatic CRC patients undergoing treatment with anti-EGFR antibodies. Primarily, it should be clarified how representative KRAS genotyping in cfDNA is in comparison to tumor tissue assessment, which is the gold standard in deciding regarding anti-EGFR treatment. In the retrospective analysis of our cohort, tissue KRAS mutational status at baseline could be confirmed in cfDNA in 80% of the therapy naïve patients (Fig 4A and 4B), highlighting the potential of this promising tool for noninvasive molecular assessment and underlining other concordance analyses of cfDNA and tumor tissue [13, 31]. The high concordance status provides the prerequisite for molecular tumor characterization on cfDNA level, which was fulfilled in our study cohort. Of interest, in 2 patients tumor tissue genotyping for KRAS revealed a wild type situation, while the respective baseline cfDNA harbored KRAS mutations. In contrast, 6 patients were confirmed as KRAS mutated in tumor tissue, while the corresponding cfDNA genotyping for KRAS showed a wild type situation. To understand the possible path forward, if they are false positives or true positives likely an outcome study is needed. The fact that different methodologies were used to assess the presence of KRAS mutations in cfDNA (ddPCR) and in the respective tumor tissue (pyro-sequencing) could potentially account for some discordance in our study. Moreover, while total cfDNA quantification is not suitable to study both inter- and intratumoral heterogeneity, cfDNA targeted genotyping, as it was performed for KRAS within our study, is indeed a means to overcome reports about single biopsy bias.
Many studies focused on KRAS assessment of cfDNA during the last years and demonstrated the occurrence and recurrence of mutated KRAS alleles under anti-EGFR treatment [11, 12, 19, 27–32, 37, 43]. However, in the clinical scenario it remains as yet unclear how to deal with these results. On the one hand, what to do, if inconsistent results are occurring between tissue and cfDNA at baseline and on the other hand how to proceed when (K)RAS mutations occur under EGFR-blockade. As shown by our case study (S2A–S2C Fig), KRAS mutated clones can indeed disappear under a combination of chemotherapy plus anti-EGFR antibody and become again detectable upon disease progression. Therefore, in some cases obviously combination chemotherapy is also capable of suppressing KRAS mutated clones in CRC.
Our data indicate that combined cfDNA quantification and targeted genotyping of cfDNA in metastatic CRC patients can provide some useful information for treatment monitoring and noninvasive early detection of a disease progression under a given therapeutic regimen which underlines previously reported data [11, 12, 19, 27–32, 37, 43]. Still several, in particular technical, obstacles have to be overcome. Therefore, cfDNA as a novel means of assessing tumor progression was already being integrated into many clinical trials to validate its significance and to enable technical standardization and to potentially use it for treatment stratification. Nevertheless, robust data are not yet available.
Conclusion
In summary, the most important findings of our study are that quantitative cfDNA measurement and targeted cfDNA genotyping for KRAS by ddPCR provides an easy to perform and promising approach for noninvasive treatment monitoring, in particular for disease progression but not treatment response in patients with metastatic CRC. Furthermore, baseline cfDNA quantification prior 1st line treatment positively correlates with tumor burden. This finding only applies to therapy naïve patients and could complement determination of the real tumor burden with a potential impact on the choice of the therapeutic regimen. Our data of this univariate analysis show, that serial cfDNA measurements provide the possibility for dynamic disease monitoring and can complement chemical laboratory (CEA) and radiological (RECIST 1.1) data for treatment monitoring in metastatic CRC patients. Upon both 1st and 2nd line treatment a cfDNA increase indicates a disease progression underlining its value as a monitoring tool. cfDNA true positives significantly correlated with progression free survival, highlighting the impact of cfDNA as a prognostic tool in mCRC. Moreover, cfDNA KRAS status is highly concordant with tissue results in therapy naïve patients. Our case reports indicate the usefulness but also the limitations of KRAS mutational screening prior therapy initiation, under therapy and prior to later therapy lines when using EGFR blocking strategies.
Supporting information
Correlation of progression-free survival (PFS) under 1st line treatment with (A) cfDNA level upon treatment (B) and CEA level upon treatment. Correlation of progression-free survival (PFS) under second line treatment with (C) cfDNA level upon treatment and (D) CEA level upon treatment. Correlation of cfDNA level and tumor load at progression in 1st line (E) and 2nd line treatment (F).
(TIF)
Case reports: Tracking of cfDNA, CEA, mutated KRAS allele levels over the time course of treatment of patient (A) 11, (B) 19 and (C) 23. Selected CT-scans and corresponding RECIST 1.1 classification are depicted below the respective graphs. BL = baseline, SD = stable disease, PR = partial remission, PD = progressive disease.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors wish to thank Mrs. Magdalena Bienek-Ziolkowski and Mrs. Rosina Sing (Biobank, Department of Internal Medicine I, Ulm University) for excellent technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific, no third party funding for this work.
References
- 1.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 2.Sharma MR, Maitland ML, Ratain MJ. RECIST: no longer the sharpest tool in the oncology clinical trials toolbox—point. Cancer Res. 2012;72(20):5145–9; discussion 50. 10.1158/0008-5472.CAN-12-0058 [DOI] [PubMed] [Google Scholar]
- 3.Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326–34. 10.1200/JCO.2008.16.3212 [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Hedrick EE, Mass RD, Sarkar S, Suzuki S, Ramanathan RK, et al. Response-independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol. 2008;26(2):183–9. 10.1200/JCO.2007.13.8099 [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. PubMed Central PMCID: PMCPMC3401966. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366(10):883–92. PubMed Central PMCID: PMCPMC4878653. 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bast RC Jr., Ravdin P, Hayes DF, Bates S, Fritsche H Jr., Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(6):1865–78. 10.1200/JCO.2001.19.6.1865 [DOI] [PubMed] [Google Scholar]
- 8.Sorbye H, Dahl O. Carcinoembryonic antigen surge in metastatic colorectal cancer patients responding to oxaliplatin combination chemotherapy: implications for tumor marker monitoring and guidelines. J Clin Oncol. 2003;21(23):4466–7. 10.1200/JCO.2003.99.200 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23(4):338–51. [DOI] [PubMed] [Google Scholar]
- 10.Diaz LA Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–86. PubMed Central PMCID: PMCPMC4820760. 10.1200/JCO.2012.45.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz LA Jr., Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537–40. PubMed Central PMCID: PMCPMC3436069. 10.1038/nature11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532–6. PubMed Central PMCID: PMCPMC3927413. 10.1038/nature11156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–90. PubMed Central PMCID: PMCPMC2820391. 10.1038/nm.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4(136):136ra68 10.1126/scitranslmed.3003726 [DOI] [PubMed] [Google Scholar]
- 15.Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091–6. 10.1200/JCO.2009.21.9170 [DOI] [PubMed] [Google Scholar]
- 16.Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013;31(8):1105–11. PubMed Central PMCID: PMCPMC4209068. 10.1200/JCO.2012.44.5353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez D, Fearfield L, Nathan P, Taniere P, Wallace A, Brown E, et al. BRAF mutation testing algorithm for vemurafenib treatment in melanoma: recommendations from an expert panel. Br J Dermatol. 2013;168(4):700–7. 10.1111/bjd.12248 [DOI] [PubMed] [Google Scholar]
- 18.Simon R, Roychowdhury S. Implementing personalized cancer genomics in clinical trials. Nat Rev Drug Discov. 2013;12(5):358–69. 10.1038/nrd3979 [DOI] [PubMed] [Google Scholar]
- 19.Spindler KL, Pallisgaard N, Vogelius I, Jakobsen A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin Cancer Res. 2012;18(4):1177–85. 10.1158/1078-0432.CCR-11-0564 [DOI] [PubMed] [Google Scholar]
- 20.Spindler KL, Sorensen MM, Pallisgaard N, Andersen RF, Havelund BM, Ploen J, et al. Phase II trial of temsirolimus alone and in combination with irinotecan for KRAS mutant metastatic colorectal cancer: outcome and results of KRAS mutational analysis in plasma. Acta Oncol. 2013;52(5):963–70. 10.3109/0284186X.2013.776175 [DOI] [PubMed] [Google Scholar]
- 21.Berger AW, Schwerdel D, Costa IG, Hackert T, Strobel O, Lam S, et al. Detection of Hot-Spot Mutations in Circulating Cell-Free DNA From Patients With Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology. 2016;151(2):267–70. 10.1053/j.gastro.2016.04.034 [DOI] [PubMed] [Google Scholar]
- 22.Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer—a survey. Biochim Biophys Acta. 2007;1775(1):181–232. 10.1016/j.bbcan.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Frattini M, Gallino G, Signoroni S, Balestra D, Lusa L, Battaglia L, et al. Quantitative and qualitative characterization of plasma DNA identifies primary and recurrent colorectal cancer. Cancer Lett. 2008;263(2):170–81. 10.1016/j.canlet.2008.03.021 [DOI] [PubMed] [Google Scholar]
- 24.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24 PubMed Central PMCID: PMCPMC4017867. 10.1126/scitranslmed.3007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92 10.1126/scitranslmed.aaf6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thierry AR, Mouliere F, Gongora C, Ollier J, Robert B, Ychou M, et al. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res. 2010;38(18):6159–75. PubMed Central PMCID: PMCPMC2952865. 10.1093/nar/gkq421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabernero J, Lenz HJ, Siena S, Sobrero A, Falcone A, Ychou M, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16(8):937–48. 10.1016/S1470-2045(15)00138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26(8):1715–22. PubMed Central PMCID: PMCPMC4511218. 10.1093/annonc/mdv177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arena S, Siravegna G, Mussolin B, Kearns JD, Wolf BB, Misale S, et al. MM-151 overcomes acquired resistance to cetuximab and panitumumab in colorectal cancers harboring EGFR extracellular domain mutations. Sci Transl Med. 2016;8(324):324ra14 10.1126/scitranslmed.aad5640 [DOI] [PubMed] [Google Scholar]
- 30.Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):795–801. PubMed Central PMCID: PMCPMC4868598. 10.1038/nm.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108–12. 10.1038/nature12065 [DOI] [PubMed] [Google Scholar]
- 32.Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014;20(4):430–5. 10.1038/nm.3511 [DOI] [PubMed] [Google Scholar]
- 33.Mandel P, Metais P. [Not Available]. C R Seances Soc Biol Fil. 1948;142(3–4):241–3. [PubMed] [Google Scholar]
- 34.Koffler D, Agnello V, Winchester R, Kunkel HG. The occurrence of single-stranded DNA in the serum of patients with systemic lupus erythematosus and other diseases. J Clin Invest. 1973;52(1):198–204. PubMed Central PMCID: PMCPMC302243. 10.1172/JCI107165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gormally E, Caboux E, Vineis P, Hainaut P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: practical aspects and biological significance. Mutat Res. 2007;635(2–3):105–17. 10.1016/j.mrrev.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Jung K, Fleischhacker M, Rabien A. Cell-free DNA in the blood as a solid tumor biomarker—a critical appraisal of the literature. Clin Chim Acta. 2010;411(21–22):1611–24. 10.1016/j.cca.2010.07.032 [DOI] [PubMed] [Google Scholar]
- 37.Spindler KL, Pallisgaard N, Andersen RF, Brandslund I, Jakobsen A. Circulating free DNA as biomarker and source for mutation detection in metastatic colorectal cancer. PLoS One. 2015;10(4):e0108247 PubMed Central PMCID: PMCPMC4395277. 10.1371/journal.pone.0108247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–61. PubMed Central PMCID: PMCPMC3266542. 10.1016/j.cell.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146(3):647–51. PubMed Central PMCID: PMCPMC4514438. 10.1053/j.gastro.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szpechcinski A, Chorostowska-Wynimko J, Struniawski R, Kupis W, Rudzinski P, Langfort R, et al. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer. 2015;113(3):476–83. PubMed Central PMCID: PMCPMC4522634. 10.1038/bjc.2015.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szpechcinski A, Rudzinski P, Kupis W, Langfort R, Orlowski T, Chorostowska-Wynimko J. Plasma cell-free DNA levels and integrity in patients with chest radiological findings: NSCLC versus benign lung nodules. Cancer Lett. 2016;374(2):202–7. 10.1016/j.canlet.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 42.Toth K, Wasserkort R, Sipos F, Kalmar A, Wichmann B, Leiszter K, et al. Detection of methylated septin 9 in tissue and plasma of colorectal patients with neoplasia and the relationship to the amount of circulating cell-free DNA. PLoS One. 2014;9(12):e115415 PubMed Central PMCID: PMCPMC4272286. 10.1371/journal.pone.0115415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov. 2016;6(2):147–53. PubMed Central PMCID: PMCPMC4744519. 10.1158/2159-8290.CD-15-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of progression-free survival (PFS) under 1st line treatment with (A) cfDNA level upon treatment (B) and CEA level upon treatment. Correlation of progression-free survival (PFS) under second line treatment with (C) cfDNA level upon treatment and (D) CEA level upon treatment. Correlation of cfDNA level and tumor load at progression in 1st line (E) and 2nd line treatment (F).
(TIF)
Case reports: Tracking of cfDNA, CEA, mutated KRAS allele levels over the time course of treatment of patient (A) 11, (B) 19 and (C) 23. Selected CT-scans and corresponding RECIST 1.1 classification are depicted below the respective graphs. BL = baseline, SD = stable disease, PR = partial remission, PD = progressive disease.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.