Abstract
Platelets play critical roles in hemostasis and thrombosis. Emerging evidence indicates that they are versatile cells and also involved in many other physiological processes and disease states. Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is a life threatening bleeding disorder caused by fetal platelet destruction by maternal alloantibodies developed during pregnancy. Gene polymorphisms cause platelet surface protein incompatibilities between mother and fetus, and ultimately lead to maternal alloimmunization. FNAIT is the most common cause of intracranial hemorrhage in full-term infants and can also lead to intrauterine growth retardation and miscarriage. Proper diagnosis, prevention and treatment of FNAIT is challenging due to insufficient knowledge of the disease and a lack of routine screening as well as its frequent occurrence in first pregnancies. Given the ethical difficulties in performing basic research on human fetuses and neonates, animal models are essential to improve our understanding of the pathogenesis and treatment of FNAIT. The aim of this review is to provide an overview on platelets, hemostasis and thrombocytopenia with a focus on the advancements made in FNAIT by utilizing animal models.
Keywords: Fetal and neonatal alloimmune thrombocytopenia, Integrins and GPIb complex, Platelets and platelet receptors, Thrombosis and hemostasis and Animal models of human disease
Platelets are versatile cells – Critical roles in hemostasis and thrombosis
Platelets are small anucleated blood cells, derived from precursor megakaryocytes in bone marrow1, 2 that play a key role in vascular repair and hemostasis.2, 3, 4 Their major role is to accumulate at sites of damaged blood vessels and initiate clotting in order to prevent blood loss. Resting platelets in the blood circulation of mammals are smooth and discoid in shape. When the vessel wall becomes injured, collagen and other subendothelial matrix proteins are exposed and interact with receptors on the platelet surface, resulting in a series of intracellular signaling events and platelet activation.3, 4, 5 The activated platelets then change their shape and adhere to the site of injury. Recruitment of additional platelets to the damaged site leads to platelet aggregation and platelet plug formation (i.e. the first wave of hemostasis).4, 5
The second wave of hemostasis is mediated by the coagulation system, which can be activated by either extrinsic (tissue factor pathway) or intrinsic pathway following vessel damage. The vital product of coagulation cascades is thrombin, which converts fibrinogen to fibrin, leading blood coagulation that further assists the formation of a clot/hemostatic plug. Thrombin is also the most potent platelet agonist that induces platelet activation, granule release and platelet adhesion/aggregation. Thus, the second wave of hemostasis significantly enforces the first wave of hemostasis. On the other hand, following platelet activation, phosphatidylserine exposure occurs, which generates a negatively charged membrane surface that can host coagulation factors and markedly increase thrombin generation.6 These intensive interactions between the first and the second waves of hemostasis synergistically contribute to the formation of a hemostatic plug.3, 7, 8
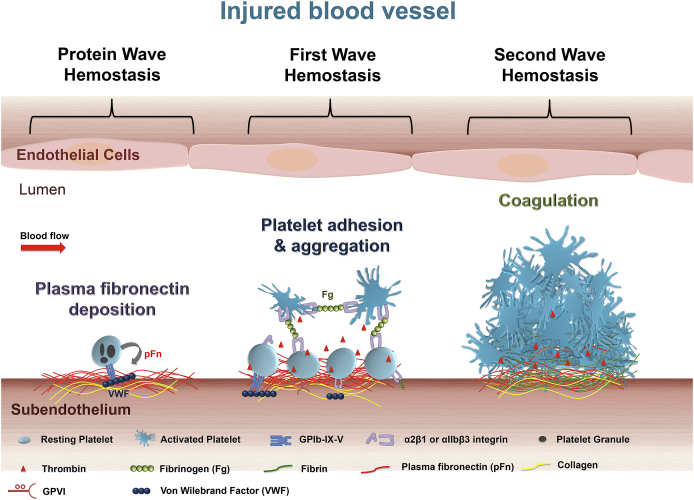
An interesting and recent discovery describes the deposition of plasma fibronectin9, 10 into the injured vessel wall and initiating a “protein wave of hemostasis”11 (Fig. 1). This “protein wave” hemostasis occurs even earlier than the classical “first wave of hemostasis”, although platelet recruitment and their locally released granule fibronectin (internalized from plasma fibronectin via αIIbβ3 integrin)12, 13, 14, 15 may be also involved. However, the importance of this new discovery in diseases including possible plasma fibronectin transfusion in controlling bleeding disorders, particularly those associated with anticoagulant therapies requires further investigation.11 It is notable that platelets contribute to all the aforementioned three waves of hemostasis, which may explain why a decreased platelet count in blood (i.e. thrombocytopenia)16 usually causes bleeding disorders, such as autoimmune thrombocytopenia (ITP) and fetal and neonatal alloimmune thrombocytopenia (FNAIT).
Figure 1.
Roles of platelets in thrombosis and hemostasis. After vascular injury, plasma fibronectin quickly deposits onto the injured vessel wall. Platelets may also release their internalized plasma fibronectin from their granules. These plasma and platelet sources of fibronectin likely synergistically contribute to the protein wave of hemostasis. Platelet adhesion and aggregation (i.e. the classical first wave of hemostasis) are then initiated via platelet receptors and their ligands. Activated platelets also provide a negatively charge surface and mediate cell-based thrombin generation, which contributes to blood coagulation that is initiated following tissue damage (i.e. the classical second wave of hemostasis). In a growing hemostatic plug/thrombus, the fibrin and fibronectin matrix is usually formed in the interface between the injured vessel wall and platelet plug.
Despite the vital role in hemostasis, platelet adhesion and aggregation at inappropriate sites may lead to thrombosis and vessel occlusion. In both men and women, heart attack and stroke are the leading cause of morbidity and mortality worldwide3, 17 and are caused by thrombosis in the coronary or cerebral arteries.7, 18 Thrombotic events may also occur in the placenta which may lead to miscarriage.19 In addition to their important roles in thrombosis and hemostasis, platelets have several other functions including acceleration of atherosclerosis,20, 21, 22 lymphatic vessel development,23, 24, 25 aiding in tumor growth,26 destruction of microorganisms27, 28 and infected cells29, 30 and modulation of inflammation and immune responses.31, 32, 33, 34 Thus, platelets are versatile cells that play critical roles in multiple human diseases.
Platelet αIIbβ3 integrin and GPIbα: The abundant functional receptors and common targeted antigens in immune mediated thrombocytopenia
There are many receptors expressed on platelets which maintain platelet physiological and pathological functions. The two major platelet surface receptors are glycoprotein (GP) IIbIIIa (αIIbβ3 integrin) and the GPIb-IX complex, which mediate platelet adhesion and aggregation.4, 35, 36 GPIbα, of the GPIb-IX complex, is a leucine rich repeat family protein, which is essential for platelet tethering and adhesion to the injured vessel wall primarily through binding to immobilized von Willebrand factor (VWF), particularly at high shear stress.3, 37, 38 The interaction between GPIbα and VWF can deliver a signal to platelets, which subsequently causes a conformational change in αIIbβ3 integrin on the platelet surface39 leading to fibrinogen (Fg) binding.40 Fg cross-links adjacent platelets, leading to platelet aggregation and platelet plug formation. Although it has been documented for more than a half century that Fg is required for platelet aggregation, interestingly, recent studies demonstrated that the formation of a hemostatic plug and thrombus formation can occur independently of VWF and Fg,12, 41 but not αIIbβ3 integrin.42 These findings suggest that other unidentified ligands of αIIbβ3 integrin exist which can also mediate platelet aggregation, and hemostatic plug/thrombus formation.5, 7, 42 This mechanism and its significance in thrombosis and hemostasis is currently under investigation.41, 42, 43, 44, 45
There are approximately 80,000 copies of αIIbβ3 integrin and 25,000 copies of GPIbα on the surface of resting human platelets.46, 47 It was previously documented that both GPIbα and the αIIb subunit of the αIIbβ3 integrin are exclusively expressed on platelets and their precursors, while the β3 subunit of the αIIbβ3 integrin is expressed on many other cell types such as endothelial and endothelial progenitor cells,48, 49 microglia,50 astrocytes,51 sperm cells52 and trophoblast cells of the placenta.53 The expression of GPIbα on endothelial cells (ECs) has been demonstrated under certain conditions,54, 55 however this remains controversial.56 Within the last decade αIIb has been shown to be expressed on early trophoblast cells of the placenta,57 mast cells,58 embryonic stem cells59 and hematopoietic stem cells (HSCs).60 Little is known about the role αIIb plays in stem cells, however it is thought to maintain HSC activity during embryonic development,61 although the exact function of αIIb in stem and mast cells remain unclear.
In addition to their important physiological and pathological roles in hemostasis and thrombosis, platelet αIIbβ3 integrin and GPIbα are the major targeted antigens in both auto- and alloimmune thrombocytopenias (e.g. ITP and FNAIT).62, 63 Although the immunogenecity and the exact mechanisms of immune response are still not well understood, their abundant expression and significant amounts of polymorphisms likely contribute to the pathogenesis of these immune-mediated thrombocytopenias.
Thrombocytopenia disorders
Thrombocytopenia refers to a decrease in the number of circulating platelets in the blood. In healthy adults, the normal range in circulating platelets is 150−400 × 109/L and a platelet count below this range may lead to severe bleeding, where in some cases may be life threatening.31 The three major categories of thrombocytopenia are immune-mediated, genetic deficiency-associated and malignancy-associated. Immune-mediated thrombocytopenia can be further sub-classified into autoimmune or alloimmune thrombocytopenia.33, 64
Autoimmune thrombocytopenia is caused by a loss of self-tolerance resulting in an abnormal immune response, targeting one's own platelets. Autoimmune thrombocytopenia is categorized into primary immune thrombocytopenia (ITP), drug-induced thrombocytopenia and infection associated thrombocytopenia.65, 66, 67 ITP is the most common with an incidence of 1–2.5 per 10,000 individuals68 and the degree of thrombocytopenia can range from mild to severe.62 It has been shown that both platelet destruction and impaired platelet production may contribute to low platelet counts, although the exact contributions by each remain unclear.69 To date, autoantibodies are considered to be the predominant effector in thrombocytopenia. In adult ITP patients, approximately 70% of detectable platelet autoantibodies are directed against αIIbβ3 integrin, and roughly 20%–40% have specificity for the GPIbα complex, or both.70 Interestingly, there is a subpopulation of ITP patients who are thrombocytopenic but have no detectable antibodies. One proposed explanation is that in this group of patients, ITP is mediated by CD8+ T cells (i.e. cytotoxic T-lymphocytes).71, 72, 73 On the other hand, it may be that the current antibody detection systems are not optimal and may be unable to detect some of the autoantibodies that recognize conformation-dependent epitopes. The conformations of these platelet antigens may be changed during the anticoagulant treatment and sample preparations, subsequently losing their binding sites for some antibodies.74 This may be particularly important for αIIbβ3 integrin, since divalent cations play important roles in maintaining integrin structure and function.75, 76, 77, 78, 79 Divalent cation chelators (e.g. sodium citrate, and EDTA) in the anti-coagulated blood may generate false negative results and decrease the autoantibody detection.
Platelet clearance mediated by anti-platelet autoantibodies typically result in platelet phagocytosis by Fcγ-receptor bearing cells of the reticuloendothelial system (RES) such as macrophages, and the majority of these opsonized platelets are cleared in the spleen. However some ITP patients, particularly those with anti-GPIbα antibodies who do not respond well to treatments,80, 81, 82, 83 may mediate platelet clearance through an Fc-independent pathway,84, 85 leading to alternative sites of platelet clearance.86 A recent study found that platelet desialylation87 may occur after antibody binding, particularly those platelets opsonized by anti-GPIbα antibodies.88 This mechanism leads to platelet clearance in the liver via Ashwell-Morell receptors on hepatocytes, which is fundamentally different from the classical Fc-FcγR-dependent macrophage phagocytosis in spleen.88 This discovery may be important not only in basic research, but also for diagnosis and treatment of refractory ITP (Nature Communications in revision).89, 90 It is currently unknown whether this novel Fc-independent platelet clearance pathway also occurs in fetuses and contributes to FNAIT.
ITP in pregnancy and other neonatal thrombocytopenias
Expecting mothers with pre-existing ITP have been less well studied and as such the effect of the disease on neonates is controversial.91, 92 Neonatal thrombocytopenia in mothers with ITP cannot be predicted by history or platelet count, however an affected older sibling is a reliable risk factor in subsequent pregnancies.93 Despite being rare and less of a clinical concern, some cases of neonates born to mothers with ITP have been associated with extensive hemorrhage and fetal death.94, 95 Neonatal thrombocytopenias which are not due to anti-platelet antibodies are generally associated with chronic fetal hypoxia, as seen in mothers with pregnancy-induced hypertension, diabetes or intrauterine growth restriction (IUGR).96 The mechanism of non-antibody mediated forms of neonatal thrombocytopenia is thought to be reduced megakaryopoiesis97 and cases are usually mild since neonate platelet count normally resolves within 10 days.98
Alloimmune thrombocytopenia is due to alloantibody-mediated platelet depletion and develops when an immune response is generated following exposure to allogenic platelets. This can occur after transfusion of platelets from allogenic donors, termed post-transfusion purpera (PTP) or during pregnancy following maternal exposure to paternal alloantigens on fetal platelets, termed fetal and neonatal alloimmune thrombocytopenia (FNAIT).99 FNAIT is similar to the more common condition hemolytic disease of the fetus and newborn (HDFN) where maternal alloantibodies target antigens on fetal red blood cells. In contrast to HDFN that occurs following antigen exposure in the previous pregnancy, FNAIT may develop in the first pregnancy in up to 50% of all cases,100, 101 making diagnosis and treatment more difficult.
Fetal and neonatal alloimmune thrombocytopenia
FNAIT is caused by fetal platelet destruction by maternal alloantibodies developed during pregnancy.102, 103 Harrington et al were the first to formally describe neonatal thrombocytopenia in 1953, where two infants were born with significantly decreased platelet counts, delivered from mothers without ITP.104 Although serological techniques were not available at the time, this was the first identification and report describing what we now know as FNAIT. Shulman et al105 first identified that maternal alloimmunization and antibodies targeting platelet antigens was the reason for platelet destruction in neonates with the disease. Today FNAIT is the most common cause of severe thrombocytopenia in live born neonates100 and accounts for up to 40% of all neonates admitted into the neonatal intensive care unit.106 FNAIT is now recognized as a critical complication in pregnancy with severe and adverse outcomes. Due to the life threatening nature of FNAIT, there are ethical concerns in performing basic research on human fetuses and neonates with the disease. The use of animal models in FNAIT research will surely help decode the complexity of this disease and may lead to treatment in humans.
FNAIT is also the most common cause of intracranial hemorrhage (ICH) in full-term infants,107 which is the most severe clinical complication of FNAIT and may lead to neurological impairments or death.95, 108, 109, 110 ICH occurs at a rate of 10%–20% in neonates born with FNAIT and has fatal consequences in approximately 5% of documented cases.102, 111 An ICH can also occur in utero as early as week 14 of pregnancy in fetuses affected by FNAIT,112 suggesting that early diagnosis and management are necessary to prevent morbidity and mortality. The recurrence rate of a subsequent pregnancy affected by FNAIT is close to 100% in antigen positive siblings and they usually have a similar or more severe form of thrombocytopenia.109, 113 Therefore, the only established biomarker for severity of the disease is if a previous sibling presents with ICH.113, 114 Interestingly, in some cases ICH is independent of the severity of thrombocytopenia,113, 115 indicating that other mechanisms aside from low platelet count likely contribute to bleeding in the brain as seen in some neonates.
Several large studies have shown an incidence of FNAIT between 1 and 1.5/1000 live births.106, 116, 117, 118 However, these reports do not include the rate of miscarriage associated with the disease, therefore FNAIT may be much more prevalent than previously thought. Although it has been reported by several groups,101, 119, 120 the rate of miscarriage in affected pregnant women has not been adequately studied. One explanation for this is that rare human platelet antigens (HPAs) may induce severe FNAIT leading to miscarriage, which could confound the severity and frequency of reported cases of FNAIT in humans.
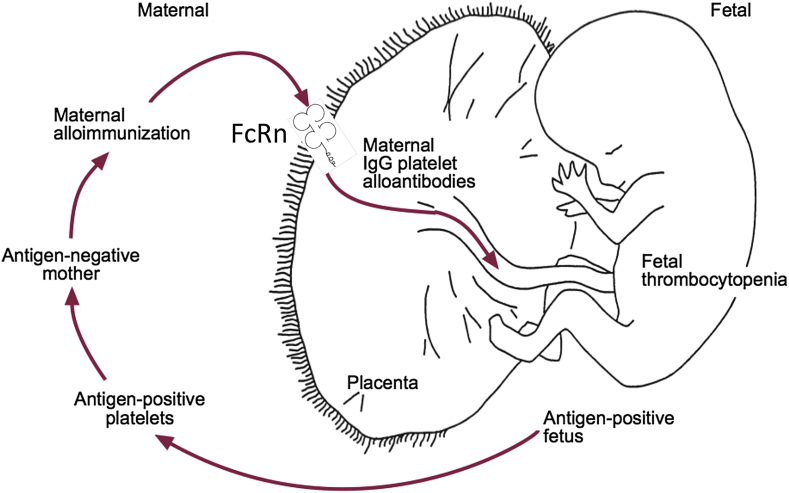
In healthy human fetuses, the platelet count reaches approximately 300 × 109/L by 30–35 weeks of gestation121 and thrombocytopenia in the fetus or neonate has been defined as a platelet count less than 150 × 109/L, however FNAIT can cause severe thrombocytopenia where platelet counts are often below 20 × 109/L.120 Alloantibodies generated in the mother are transported across the placenta via the neonatal Fc receptor (FcRn) during pregnancy and enter fetal circulation and opsonize platelets and mediates clearance122, 123 (Fig. 2). It is thought that platelet destruction in FNAIT is similar to that in ITP. Alloantibody bound platelets interact with Fc receptors on macrophages through the Fc fragment of IgG, resulting in clearance of the antibody-bound platelets by the RES system in the spleen.124 However, some ITP patients with antibodies targeting GPIbα may have Fc-independent platelet clearance,86 which may also be occurring in FNAIT patients. It is currently unclear whether there are significant differences between adult and fetal/neonatal platelet clearance mechanisms.
Figure 2.
Pathogenesis of fetal and neonatal alloimmune thrombocytopenia (FNAIT). This figure is adapted from Blanchette VS, Johnson J and Rand M, Baillieres Best Pract. Res Clin Haematol, 2000, 13(3): 365–90 and Chen P, Li C, Lang S, Zhu G, Reheman A, Spring CM, Freedman J and Ni H, Blood, 2010, 116 (18), 3660–3668.
Gene polymorphisms and platelet alloantigens in FNAIT
In 1990, the Platelet Serology Working Party of the International Society of Blood Transfusion (ISTB) established a nomenclature for platelet antigens.125 They classified platelet antigens as HPAs, where each platelet antigen is numbered based on date of discovery and the more common alleles are ordered in alphabetical pairs based on frequency – ‘a’ for the common allele and ‘b’ for the rare allele.126 All HPAs result in a single amino acid substitution except for HPA-14w where the antigen is a result of a one amino acid deletion.127 The database of all immunizing HPAs that have caused at least one case of FNAIT can be found at http://www.ebi.ac.uk/ipd/hpa/table2.html. To date there are 36 HPAs on six platelet surface proteins that have been discovered and may lead to FNAIT.128 Of these, 18 are located on integrin β3 subunit (including HPAs 1a, 1b, 1c, 4a, 4b, 6bw, 7b, 7c, 8bw, 10bw, 11bw, 14bw, 16bw, 17bw, 19bw, 21bw, 23bw and 26bw), 8 are located on the αIIb subunit (HPAs 3a, 3b, 9bw, 20bw, 22bw, 24bw, 27bw and 28bw), 5 on integrin α2 (HPAs 5a, 5b, 13bw, 18bw and 25bw), 2 on GPIbα (HPAs 2a and 2b), 1 on GPIbβ (HPA 12bw) and 2 on CD109 (HPAs 15a and 15b). 26 HPAs are caused by bi-allelic polymorphisms and two HPAs are due to tri-allelic polymorphisms.129
Despite the existence of multiple platelet antigens, only few are responsible for the majority of reported cases of FNAIT. Incompatibility of HPA-1 (a leucine–proline difference at residue 33 of the β3 integrin130) accounts for approximately 75%–85% of cases in Caucasians.131 Although FNAIT due to HPA-1a incompatibility is very rare in African and Asian populations,132, 133 irrespective of race, HPAs in the β3 integrin subunit account for the vast majority of reported cases.134, 135, 136 It is interesting that certain polymorphisms are found much more abundantly in certain populations than others. The HPA-4 polymorphism, also located on the subunit β3 integrin, resides on residue 143 and is more prevalent in the Asian population, as well as HPA-21bw.137 In addition, HPA-6bw is a more common polymorphism to the Finnish population that causes FNAIT.138 HPA-5 is located on integrin α2 subunit of the collagen receptor and is the second most common antigen causing FNAIT accounting for 10%–15% of all cases.139 The reported incidence of FNAIT due to HPA-2 on GPIbα is rare.140, 141 However, it is currently unknown whether prothrombotic events are induced by anti-GPIbα antibodies in the placenta,19 which may cause miscarriage and mask the reported incidence in humans. FNAIT caused by HPA-2 may also be rare due to the lower immunogenicity of this leucine repeat family protein that may stimulate a weaker immune response in women.86
Interestingly, 5 out of 8 HPAs on the αIIb subunit are located in very close proximity within the calf-2 domain, including the most common αIIb antigens HPA-3a and HPA-9bw. These HPAs only account for 2%–5% of all cases of FNAIT,106, 131 however they are typically involved in more severe cases of FNAIT.142, 143 HPA-3b and HPA-9b are in a linkage disequilibrium, explained by the fact that they are located only 19 base pairs apart.129 HPA-22bw resides on the β-propeller domain, close to the ligand-binding site, which may interfere with fibrinogen and other ligand binding and cause severe bleeding in affected neonates.144 Both polymorphisms themselves and alloantibody binding may affect hemostasis/thrombosis. HPA-13w on the α2 integrin and both HPA-1b and HPA-21bw on the β3 integrin subunit have also been identified as HPAs that interfere with receptor function.145, 146 However the extent to which various HPAs contribute to hemorrhagic or thrombotic risk remains to be determined.147
Glycoprotein IV (CD36) deficiency occurs in 3%–5% of individuals of African or Asian ancestry. It is a member of the class B scavenger receptor family of proteins expressed on platelets, red blood cells, endothelial cells, monocytes and macrophages.148 Mothers who lack CD36 are at risk of becoming immunized if exposed to the protein by transfusion or pregnancy.149 It is interesting that affected neonates have a severely decreased platelet count without solid evidence of other tissue damage despite CD36 being expressed on endothelial cells and other blood cells.148 Immunization against CD36 is termed ‘Nak’ isoantibody generation. Since CD36 is expressed on multiple cells, it does not have HPA nomenclature.150
Mechanisms of alloantibody generation in FNAIT
FNAIT is thought to be initiated by fetal platelet alloantigens that are inherited from the father but absent in the mother. The mechanism of exposure of the mother to fetal alloantigens is not well understood. Similar to HDFN, immunization may occur as a result of fetomaternal hemorrhage during parturition.116, 151 Since FNAIT often occurs during the first pregnancy, fetal platelets may “leak” into the maternal circulation and initiate an immune response. The smaller size and greater mobility of platelets compared to red blood cells may attribute to FNAIT development in the first pregnancy as compared to HDFN. It has been demonstrated that β3 integrin is expressed on human trophoblast cells of the placenta,57, 152 suggesting that an immune response may be generated independently of platelets but via a maternal immune response against β3 integrins on trophoblast cells. In addition, β3 integrin is also expressed on spermatozoa,52, 153 therefore suggesting that women may become immunized with preconceptional exposure.
When platelet antigens are exposed to the maternal circulation, they are processed by antigen presenting cells and subsequently presented to T helper lymphocytes similar to other foreign antigens.99, 153, 154, 155, 156, 157 T cells may then stimulate B cells to become activated and differentiate into plasma cells and secrete antigen-specific IgG antibodies. Macrophages themselves may also directly stimulate the differentiation of B cells to plasma cells.158 Interestingly, not all mothers with incompatible fetuses will develop FNAIT; prospective studies indicate only approximately 10% of women with an HPA-1a incompatible fetus will become immunized during pregnancy.116, 159, 160 The most plausible explanation for this may be the strong association between the human leucocyte antigen (HLA) class II DRB3*0101 and DRB4*0101 alleles and HPA-1a alloantibody generation.155 The DRB3 allele has also been demonstrated to have binding affinity for HPA-1a but not HPA-1b.154 This implies that specific maternal antigen presentation pathway and a specific genetic background may be involved in fetal antigen alloimmunization. In addition, co-existing viral or bacterial infections may also enhance the maternal immune response against fetal platelet antigens.156
Once antibodies against fetal platelet alloantigens are generated in the mother, they can enter fetal circulation via FcRn122 and cause platelet opsonization and clearance.123 It is thought that platelet destruction in FNAIT is similar to that in ITP. Alloantibody-bound platelets interact with Fc receptors on macrophages through the Fc fragment of IgG, resulting in clearance of the antibody-bound platelets by the RES system in the spleen.124 However, some ITP patients with antibodies targeting GPIbα may have Fc-independent platelet clearance,86 which may also be occurring in FNAIT patients. It is currently unclear whether there are significant differences between adult and fetal/neonatal platelet clearance mechanisms.
Therapies and management of FNAIT
Although there have been significant advancements in the diagnosis and management of FNAIT within the past decade, existing therapies are currently limited and prophylactic treatments are lacking. FNAIT is a life-threatening disease that may occur during the first pregnancy and antenatal management is challenging but necessary. Since there is currently no screening procedure in practice, diagnosis occurs after the delivery of a symptomatic child.161 Several treatments including intravenous immunoglobulin G (IVIG), corticosteroids and fetal and neonatal platelet transfusions have been used to manage FNIAT,162 however antenatal treatment has not been standardized.163 Although in utero platelet transfusions may be effective in some FNAIT patients, most centers have abandoned this technique due to its invasiveness and due to the high risk of fetal loss.161, 164 It is notable that recent studies in murine models suggested that neither platelets nor fibrin clots are essential for hemostasis in fetuses,165, 166 therefore in utero fetal platelet transfusion may be less important. However, platelet transfusion after birth has been demonstrated to be very beneficial for neonates.120, 167 IVIG has been used to treat FNAIT albeit with varying results.118, 168 IVIG is pooled from the plasma of healthy donors; it is expensive and in limited supply. There are also risks of transmitting blood born pathogens with administration of IVIG and it is often not well tolerated in pregnant women.169 Recent clinical studies suggest a combination of IVIG and corticosteroids is the most effective treatment, specifically when corticosteroids are administered during the last trimester.170
Postnatal therapeutic options depend on the severity of thrombocytopenia, since severe bleeding and ICH often occur in neonates with platelet counts less than 20 × 109/L.170 Platelet transfusions are the first line of therapy in neonates with low platelet counts. Fortunately, random donor platelets, even when incompatible, are frequently effective either as sole treatment of FNAIT167, 171 or when administered together with IVIG. This is of significance since matched platelets may not be available and obtaining washed maternal platelets may not be possible in certain cases. The challenge for effective therapy and management is not only due to FNAIT occurring in the first pregnancy, rather it is due to a shortage of knowledge, which requires research and animal models of this disease.
Animal models of FNAIT
Given the ethical difficulties in performing basic research on human fetuses and neonates, animal models are essential to improve our understanding of the pathogenesis and treatment of FNAIT. Previously we established the fist animal model of FNAIT, while other animal models have been generated in order to study immune thrombocytopenia.73, 172, 173, 174, 175, 176, 177 These include mouse models such as (NZW × BXSB) F1 mice173 that develop lupus nephritis, myocardial infarction, and thrombocytopenia; and an immunodeficient SCID mouse/human chimera model, in which human cord blood cells or splenocytes from ITP patients were implanted into SCID mice.174 Other animal models such as murine,178, 179 rat,175 dog,176 and baboon177 have been studied, which were generated by injection of anti-β3 integrin antibodies. In models of alloimmune thrombocytopenia, genetically or chemically modified anti-HPA-1a competitive antibodies (B2G1 and SZ21) have been demonstrated to be able to block anti-HPA-1a antibodies binding to HPA-1a-positive platelets in murine models, suggesting their therapeutic potential.180, 181 These models provide important information for gaining a better understanding of the antibody-mediated macrophage phagocytosis and the role of IVIG, which shed light on the mechanisms of platelet destruction in FNAIT. However, they provide little information to our understanding of the maternal immune responses causing FNAIT or how pathogenic antibodies affect the fetus throughout development.
Previous experiments from our laboratory have demonstrated β3 integrin deficient (β3−/−) mice generate antibodies when transfused with wild type (WT) platelets. After immunized β3−/− females were bred with WT males, thrombocytopenia and ICH were observed in the heterozygote pups. We also examined the role of FcRn in FNAIT via combined deficiencies of β3−/− and FcRn–/– mice.122 Furthermore, we established a model of FNAIT mediated by anti-GPIbα antibodies.19
Lessons learned from animal models of FNAIT
Prior to establishing a murine model of FNAIT, the process of the maternal immune response to fetal platelet antigens was largely unknown. Although in clinical cases, mothers usually generate antibodies against one specific site on the integrin; our animal models have the advantage in that they generate anti-platelet antibodies which are capable of targeting multiple epitopes on platelet receptors as seen in some cases.128 In addition, there are 18 alloantigens (approximately half of all HPAs) located throughout the β3 integrin subunit (from the N-terminus to the C-terminus of the extracellular domain), which are capable of eliciting an immune response. Therefore the study of the immune response against the entire β3 integrin subunit is of importance to the understanding of FNAIT that are mediated by different anti-HPA antibodies.
Our first model was established by transfusing female β3 deficient mice with WT platelets.153 These immunized female mice were then bred with WT male mice, to generate a heterozygous fetus. Antibodies from the maternal circulation cross the placenta via the FcRn and bind to fetal platelets, leading to bleeding symptoms.122 Some mothers underwent miscarriage and did not deliver pups, while other pups were delivered stillborn. The severity of fetal symptoms is correlated with the antibody titers in the maternal circulation. Both IgG1 and IgG2a antibodies were detected in the maternal serum, indicating that both T-helper 1 (Th1) and Th2-like immune responses exist. Therapeutic administration of IVIG to immunized mothers decreased antibody titers in both mothers and neonates, however, no anti-idiotype activity of IVIG was found in this model.
Alloantibodies may have other roles in the pathogenesis of FNAIT besides the destruction of fetal platelets. ICH has been observed in some patients whose platelet counts are within the normal range.115 Our lab demonstrated that anti-β3 antibodies generated in our model of FNAIT can cross react with αVβ3 integrin on angiogenic endothelial cells in vivo, leading to impaired angiogenesis.182 Neonates born to our model of anti-β3 mediated FNAIT present with ICH and decreased blood vessel density in both their brains and retinas. However neonates from the anti-GPIbα model of FNAIT did not show any evidence of bleeding in the brain or decreased vascular density. In vitro studies with anti-HPA-1a antibodies demonstrate inhibited proliferation and network formation of human umbilical vein endothelial cells (HUVECs). Interestingly, anti-β3 antibodies injected into αIIb–/– neonates induce ICH, suggesting that ICH may develop independently of thrombocytopenia. Therefore, ICH likely results from interference of αVβ3 integrin on ECs by antibodies in FNAIT, leading to impairments in blood vessel development in the brains of fetuses and neonates.182, 183
Miscarriage is a devastating outcome of FNAIT, however the incidence and mechanism of miscarriage has not been adequately studied. Therefore, an animal model of FNAIT is particularly useful in examining the pathophysiological mechanism of miscarriage. In contrast to the 20%–40% prevalence of anti-GPIbα antibodies in patients with immune thrombocytopenia,67 there are few reported cases of anti-GPIbα FNAIT.140 Our laboratory has demonstrated in an FNAIT animal model that anti-GPIbα antibodies cause spontaneous miscarriage,19 which may explain the low frequency of anti-GPIbα mediated FNAIT in humans. Miscarriage may also account for the low frequency of other rare antigens reported in FNAIT such as anti-αIIb mediated FNAIT, however more studies are needed to confirm this phenomenon.
Antenatal treatments such as IVIG and steroids have been used to treat FNAIT, however the mechanisms of these treatments are still not well understood and they are often accompanied by side effects. Therefore, a safer and more effective therapy remains to be developed. The FcRn has been shown to be important in transplacental transfer of IgG antibodies from mother to the fetus during pregnancy.122, 184 However, it has been speculated that other IgG associated proteins may also contribute for the transport of IgG across the placenta, specifically in inflammatory conditions. We have shown that in an animal model of FNAIT, fetuses lacking the FcRn prevented the transfer of pathogenic antibodies from the mothers.122 In addition, targeting the FcRn with anti-FcRn antibody or IVIG ameliorates FNAIT. Prior to our discovery, it was previously unknown whether FcRn independent IgG placental transport plays a significant role in FNAIT, and whether this pathway can be upregulated during inflammation or other pathologies that occur in the placenta during FNAIT. However, in our animal models, we clearly demonstrated that fetal but not maternal FcRn is required for all IgG placental transport and required for induction of FNAIT.122
While infection in the pathogenesis of ITP has been investigated,66 infection in FNAIT has not. In our mouse model of FNAIT, injections of lipopolysaccharide (LPS) (mimicking bacterial infection) or Poly I:C (mimicking viral infection) was performed together with immunization of WT platelet in GPIbα–/– and β3−/− mice. We found enhanced antibody generation, more severe bleeding and increased rates of miscarriage in these mice.156 This suggests that bacterial or viral infection may enhance the severity of FNAIT and also other alloimmune thrombocytopenias such as post-transfusion purpura (PTP). Control of infection may be an important strategy during pregnancy to attenuate FNAIT and PTP, and after pregnancy to prevent miscarriage in subsequent pregnancies.
Future treatments of FNAIT
Treating and preventing FNAIT is especially difficult because of the severity of bleeding symptoms and also because it may occur during the first pregnancy. However results from a retrospective study indicate that FNAIT occurring in the first pregnancy may be less common than expected185 and that FNAIT may be treated prophylactically similar to HDFN. Antibody-mediated immune suppression (AMIS) has successfully been used for prevention of RhD immunization for the past 4 decades.186 Results from a proof of concept study using our mouse model of FNAIT suggest that the same principle may be applied for the prevention of FNAIT. In our murine model of FNAIT, AMIS led to a 90% reduction in anti-platelet antibodies in maternal circulation, significantly elevated neonatal platelet counts and vastly improved pregnancy outcomes.187 The idea of a prophylactic approach in FNAIT is supported and deserves further studies in clinical trials.
Therapeutics targeting the FcRn may be considered as a useful therapy alternative to current treatments. The dose of anti-FcRn required to mediate therapeutic effects are much lower than that of IVIG (at least 200-fold less), suggesting that treatment with anti-FcRn antibody may be a more efficient therapy.122 Anti-FcRn therapy would also be advantageous, as it is not prepared from pooled human plasma (as is the case for IVIG), thereby decreasing the chance of patient exposure to blood born micropathogens as well as being a more cost effective therapy. Therefore, the use of anti-FcRn monoclonal antibody is promising as a future treatment for this disease and deserves further clinical consideration. In conclusion, further clinical studies on treatments are warranted for FNAIT. It is also of significance to investigate the possible differences of platelet function, the coagulation system, and RES system between fetus and adult.
Acknowledgments
The authors gratefully acknowledge Dr. Richard O. Hynes and Dr. Jerry Ware and Dr. Zaviero M. Ruggeri for providing the β3 integrin and GPIbα deficient mice. This work was supported by Canadian Institutes of Health Research (MOP 68986, MOP 119551, MOP 97918, and 119540), Heart and Stroke Foundation of Canada (Ontario), and Canadian Blood Services/Canadian Institutes of Health Research operating grant, Equipment Funds from St. Michael's Hospital, Canadian Blood Services and Canada Foundation for Innovation. B. Vadasz is a recipient of graduate fellowships from the Department of Laboratory Medicine and Pathobiology, University of Toronto and of the Queen Elizabeth II Ontario Graduate Scholarship. I. Yougbaré is a recipient of the Canadian Blood Services postdoctoral fellowship. D. Zdravic is a recipient of Canadian Blood Services graduate fellowship. J. Li is a recipient of Laboratory Medicine and Pathobiology Departmental Fellowship, University of Toronto. C. Li is a recipient of Laboratory Medicine and Pathobiology Departmental Fellowships and of the Connaught Scholarship, University of Toronto. We acknowledge Alexandra Marshall for her contribution to Figure 1.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Junt T., Schulze H., Chen Z. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Andrews M., Yang Y. Platelets in thrombosis and hemostasis: old topic with new mechanisms. Cardiovasc Hematol Disord Drug Targets. 2012;12:126–132. doi: 10.2174/1871529x11202020126. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri Z.M. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 4.Ni H., Freedman J. Platelets in hemostasis and thrombosis: role of integrins and their ligands. Transfus Apher Sci. 2003;28:257–264. doi: 10.1016/S1473-0502(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 5.Jackson S.P. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 6.Roberts H.R., Hoffman M., Monroe D.M. A cell-based model of thrombin generation. Semin Thromb Hemost. 2006;32(suppl 1):32–38. doi: 10.1055/s-2006-939552. [DOI] [PubMed] [Google Scholar]
- 7.Reheman A., Xu X., Reddy E.C., Ni H. Targeting activated platelets and fibrinolysis: hitting two birds with one stone. Circ Res. 2014;114:1070–1073. doi: 10.1161/CIRCRESAHA.114.303600. [DOI] [PubMed] [Google Scholar]
- 8.Clemetson K.J. Platelets and primary haemostasis. Thromb Res. 2012;129:220–224. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Ni H., Yuen P.S., Papalia J.M. Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc Natl Acad Sci U S A. 2003;100:2415–2419. doi: 10.1073/pnas.2628067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni H. Unveiling the new face of fibronectin in thrombosis and hemostasis. J Thromb Haemost. 2006;4:940–942. doi: 10.1111/j.1538-7836.2006.01899.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Reheman A., Spring C.M. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014;124:4281–4293. doi: 10.1172/JCI74630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni H., Denis C.V., Subbarao S. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni H., Papalia J.M., Degen J.L., Wagner D.D. Control of thrombus embolization and fibronectin internalization by integrin alpha IIb beta 3 engagement of the fibrinogen gamma chain. Blood. 2003;102:3609–3614. doi: 10.1182/blood-2003-03-0850. [DOI] [PubMed] [Google Scholar]
- 14.Xu X., Wu J., Zhai Z. A novel fibrinogen Bbeta chain frameshift mutation in a patient with severe congenital hypofibrinogenaemia. Thromb Haemost. 2006;95:931–935. doi: 10.1160/TH06-01-0020. [DOI] [PubMed] [Google Scholar]
- 15.Zhai Z., Wu J., Xu X. Fibrinogen controls human platelet fibronectin internalization and cell-surface retention. J Thromb Haemost. 2007;5:1740–1746. doi: 10.1111/j.1538-7836.2007.02625.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrington W.J., Minnich V., Hollingsworth J.W., Moore C.V. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med. 1951;38:1–10. [PubMed] [Google Scholar]
- 17.Heart attack and stroke: men vs. women. For both men and women, cardiovascular disease is the leading cause of death. But their risks and symptoms can differ. Harv Heart Lett: from Harvard Medical School. 2014;24:1–7. [PubMed] [Google Scholar]
- 18.Yang Y., Shi Z., Reheman A. Plant food delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis: novel protective roles against cardiovascular diseases. PloS One. 2012;7:e37323. doi: 10.1371/journal.pone.0037323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C., Piran S., Chen P. The maternal immune response to fetal platelet GPIbalpha causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti-FcRn therapies. J Clin Invest. 2011;121:4537–4547. doi: 10.1172/JCI57850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy A.J., Bijl N., Yvan-Charvet L. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med. 2013;19:586–594. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podrez E.A. Bad versus good cholesterol in the bone marrow. Nat Med. 2013;19:541–543. doi: 10.1038/nm.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni H. The platelet “sugar high” in diabetes. Blood. 2012;119:5949–5951. doi: 10.1182/blood-2012-04-420794. [DOI] [PubMed] [Google Scholar]
- 23.Hess P.R., Rawnsley D.R., Jakus Z. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest. 2014;124:273–284. doi: 10.1172/JCI70422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertozzi C.C., Schmaier A.A., Mericko P. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog B.H., Fu J., Wilson S.J. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coupland L.A., Parish C.R. Platelets, selectins, and the control of tumor metastasis. Semin Oncol. 2014;41:422–434. doi: 10.1053/j.seminoncol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Clark S.R., Ma A.C., Tavener S.A. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 28.Youssefian T., Drouin A., Masse J.M., Guichard J., Cramer E.M. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 29.McMorran B.J., Wieczorski L., Drysdale K.E. Platelet factor 4 and duffy antigen required for platelet killing of Plasmodium falciparum. Science. 2012;338:1348–1351. doi: 10.1126/science.1228892. [DOI] [PubMed] [Google Scholar]
- 30.Yeaman M.R. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12:426–437. doi: 10.1038/nrmicro3269. [DOI] [PubMed] [Google Scholar]
- 31.Semple J.W., Italiano J.E., Jr., Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A., Garlanda C. Platelet-macrophage partnership in innate immunity and inflammation. Nat Immunol. 2013;14:768–770. doi: 10.1038/ni.2666. [DOI] [PubMed] [Google Scholar]
- 33.Li C., Li J., Li Y. Crosstalk between platelets and the immune system: old systems with new discoveries. Adv Hematol. 2012;2012:384685. doi: 10.1155/2012/384685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H., Lang S., Zhai Z. Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression. Blood. 2009;114:425–436. doi: 10.1182/blood-2008-03-145821. [DOI] [PubMed] [Google Scholar]
- 35.Ruggeri Z.M. Mechanisms initiating platelet thrombus formation. Thromb Haemost. 1997;78:611–616. [PubMed] [Google Scholar]
- 36.Jackson S.P. Arterial thrombosis – insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 37.Lei X., Reheman A., Hou Y. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb Haemost. 2014;111:279–289. doi: 10.1160/TH13-06-0490. [DOI] [PubMed] [Google Scholar]
- 38.Ni H., Ramakrishnan V., Ruggeri Z.M., Papalia J.M., Phillips D.R., Wagner D.D. Increased thrombogenesis and embolus formation in mice lacking glycoprotein V. Blood. 2001;98:368–373. doi: 10.1182/blood.v98.2.368. [DOI] [PubMed] [Google Scholar]
- 39.Xiao T., Takagi J., Coller B.S., Wang J.H., Springer T.A. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shattil S.J., Hoxie J.A., Cunningham M., Brass L.F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 41.Reheman A., Yang H., Zhu G. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood. 2009;113:1809–1817. doi: 10.1182/blood-2008-04-148361. [DOI] [PubMed] [Google Scholar]
- 42.Yang H., Reheman A., Chen P. Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo. J Thromb Haemost. 2006;4:2230–2237. doi: 10.1111/j.1538-7836.2006.02116.x. [DOI] [PubMed] [Google Scholar]
- 43.Dunne E., Spring C.M., Reheman A. Cadherin 6 has a functional role in platelet aggregation and thrombus formation. Arterioscler Thromb Vasc Biol. 2012;32:1724–1731. doi: 10.1161/ATVBAHA.112.250464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reheman A., Tasneem S., Ni H., Hayward C.P. Mice with deleted multimerin 1 and alpha-synuclein genes have impaired platelet adhesion and impaired thrombus formation that is corrected by multimerin 1. Thromb Res. 2010;125:e177–183. doi: 10.1016/j.thromres.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Reheman A., Gross P., Yang H. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3:875–883. doi: 10.1111/j.1538-7836.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 46.Modderman P.W., Admiraal L.G., Sonnenberg A., von dem Borne A.E. Glycoproteins V and Ib-IX form a noncovalent complex in the platelet membrane. J Biol Chem. 1992;267:364–369. [PubMed] [Google Scholar]
- 47.Wagner C.L., Mascelli M.A., Neblock D.S., Weisman H.F., Coller B.S., Jordan R.E. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88:907–914. [PubMed] [Google Scholar]
- 48.Brooks P.C., Clark R.A., Cheresh D.A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 49.Brooks P.C., Montgomery A.M., Rosenfeld M. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 50.Welser-Alves J.V., Boroujerdi A., Tigges U., Milner R. Microglia use multiple mechanisms to mediate interactions with vitronectin; non-essential roles for the highly-expressed alphavbeta3 and alphavbeta5 integrins. J Neuroinflammation. 2011;8:157. doi: 10.1186/1742-2094-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermosilla T., Munoz D., Herrera-Molina R. Direct Thy-1/alphaVbeta3 integrin interaction mediates neuron to astrocyte communication. Biochim Biophys Acta. 2008;1783:1111–1120. doi: 10.1016/j.bbamcr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fusi F.M., Tamburini C., Mangili F., Montesano M., Ferrari A., Bronson R.A. The expression of alpha v, alpha 5, beta 1, and beta 3 integrin chains on ejaculated human spermatozoa varies with their functional state. Mol Hum Reprod. 1996;2:169–175. doi: 10.1093/molehr/2.3.169. [DOI] [PubMed] [Google Scholar]
- 53.Kumpel B.M., Sibley K., Jackson D.J., White G., Soothill P.W. Ultrastructural localization of glycoprotein IIIa (GPIIIa, beta 3 integrin) on placental syncytiotrophoblast microvilli: implications for platelet alloimmunization during pregnancy. Transfusion. 2008;48:2077–2086. doi: 10.1111/j.1537-2995.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 54.Konkle B.A., Shapiro S.S., Asch A.S., Nachman R.L. Cytokine-enhanced expression of glycoprotein Ib alpha in human endothelium. J Biol Chem. 1990;265:19833–19838. [PubMed] [Google Scholar]
- 55.Wu G., Essex D.W., Meloni F.J. Human endothelial cells in culture and in vivo express on their surface all four components of the glycoprotein Ib/IX/V complex. Blood. 1997;90:2660–2669. [PubMed] [Google Scholar]
- 56.Ware J. Molecular analyses of the platelet glycoprotein Ib-IX-V receptor. Thromb Haemost. 1998;79:466–478. [PubMed] [Google Scholar]
- 57.Snir A., Brenner B., Paz B., Lanir N. Presence of integrin alpha(IIb)beta 3 in early gestation human trophoblasts: possible involvement of fibrin as a matrix ligand. Thromb Res. 2010;125:253–256. doi: 10.1016/j.thromres.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Berlanga O., Emambokus N., Frampton J. GPIIb (CD41) integrin is expressed on mast cells and influences their adhesion properties. Exp Hematol. 2005;33:403–412. doi: 10.1016/j.exphem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J., Chen H., Li S. Fibroblastic potential of CD41+ cells in the mouse aorta-gonad-mesonephros region and yolk sac. Stem Cells Dev. 2012;21:2592–2605. doi: 10.1089/scd.2011.0572. [DOI] [PubMed] [Google Scholar]
- 60.Corbel C., Vaigot P., Salaun J. (alpha)IIb Integrin, a novel marker for hemopoietic progenitor cells. Int J Dev Biol. 2005;49:279–284. doi: 10.1387/ijdb.041936cc. [DOI] [PubMed] [Google Scholar]
- 61.Boisset J.C., Clapes T., Van Der Linden R., Dzierzak E., Robin C. Integrin alphaIIb (CD41) plays a role in the maintenance of hematopoietic stem cell activity in the mouse embryonic aorta. Biol Open. 2013;2:525–532. doi: 10.1242/bio.20133715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodeghiero F., Ruggeri M. ITP and international guidelines: what do we know, what do we need? Presse Med. 2014;43(4 Pt 2):e61–67. doi: 10.1016/j.lpm.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Bussel J.B., Husebekk A., Kaplan C. Pathophysiology and management of fetal and neonatal alloimmune thrombocytopenia (FNAIT) Blood. 2013 in press. [Google Scholar]
- 64.Lo E., Deane S. Diagnosis and classification of immune-mediated thrombocytopenia. Autoimmun Rev. 2014;13:577–583. doi: 10.1016/j.autrev.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 65.Liebman H. Other immune thrombocytopenias. Semin Hematol. 2007;44(4 suppl 5):S24–S34. doi: 10.1053/j.seminhematol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Cines D.B., Bussel J.B., Liebman H.A., Luning Prak E.T. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cines D.B., Blanchette V.S. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 68.Abrahamson P.E., Hall S.A., Feudjo-Tepie M., Mitrani-Gold F.S., Logie J. The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 2009;83:83–89. doi: 10.1111/j.1600-0609.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 69.Semple J.W., Provan D., Garvey M.B., Freedman J. Recent progress in understanding the pathogenesis of immune thrombocytopenia. Curr Opin Hematol. 2010;17:590–595. doi: 10.1097/MOH.0b013e32833eaef3. [DOI] [PubMed] [Google Scholar]
- 70.Beardsley D.S., Ertem M. Platelet autoantibodies in immune thrombocytopenic purpura. Transfus Sci. 1998;19:237–244. doi: 10.1016/s0955-3886(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 71.Olsson B., Andersson P.O., Jernas M. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123–1124. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 72.Sayeh E., Sterling K., Speck E., Freedman J., Semple J.W. IgG antiplatelet immunity is dependent on an early innate natural killer cell-derived interferon-gamma response that is regulated by CD8+ T cells. Blood. 2004;103:2705–2709. doi: 10.1182/blood-2003-10-3552. [DOI] [PubMed] [Google Scholar]
- 73.Chow L., Aslam R., Speck E.R. A murine model of severe immune thrombocytopenia is induced by antibody- and CD8+ T cell-mediated responses that are differentially sensitive to therapy. Blood. 2010;115:1247–1253. doi: 10.1182/blood-2009-09-244772. [DOI] [PubMed] [Google Scholar]
- 74.Allen D.L., Metcalfe P., Kaplan C. Sensitivity of assays for the detection of HPA-1a antibodies: results of an international workshop demonstrating the impact of cation chelation from integrin alphaIIbbeta3 on three widely used assays. Vox Sang. 2013;105:167–173. doi: 10.1111/vox.12043. [DOI] [PubMed] [Google Scholar]
- 75.Ni H., Li A., Simonsen N., Wilkins J.A. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J Biol Chem. 1998;273:7981–7987. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- 76.Ni H., Wilkins J.A. Localisation of a novel adhesion blocking epitope on the human beta 1 integrin chain. Cell Adhes Commun. 1998;5:257–271. doi: 10.3109/15419069809040296. [DOI] [PubMed] [Google Scholar]
- 77.Luo B.H., Carman C.V., Springer T.A. Structural basis of integrin regulation and signaling. Ann Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 79.Wilkins J.A., Li A., Ni H., Stupack D.G., Shen C. Control of beta1 integrin function. Localization of stimulatory epitopes. J Biol Chem. 1996;271:3046–3051. [PubMed] [Google Scholar]
- 80.Zeng Q., Zhu L., Tao L. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPIIbIIIa versus GPIbalpha antibodies. Am J Hematol. 2012;87:206–208. doi: 10.1002/ajh.22211. [DOI] [PubMed] [Google Scholar]
- 81.Peng J., Ma S.H., Liu J. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J Thromb Haemost. 2014;12:497–504. doi: 10.1111/jth.12524. [DOI] [PubMed] [Google Scholar]
- 82.Webster M.L., Sayeh E., Crow M. Relative efficacy of intravenous immunoglobulin G in ameliorating thrombocytopenia induced by antiplatelet GPIIbIIIa versus GPIbalpha antibodies. Blood. 2006;108:943–946. doi: 10.1182/blood-2005-06-009761. [DOI] [PubMed] [Google Scholar]
- 83.Chang M., Nakagawa P.A., Williams S.A. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–895. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 84.Nieswandt B., Bergmeier W., Rackebrandt K., Gessner J.E., Zirngibl H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96:2520–2527. [PubMed] [Google Scholar]
- 85.Webster M.L., Zhu G., Li Y., Ni H. Fc-independent phagocytosis: implications for intravenous IgG therapy in immune thrombocytopenia. Cardiovasc Hematol Disord Drug Targets. 2008;8:278–282. doi: 10.2174/187152908786786223. [DOI] [PubMed] [Google Scholar]
- 86.Li J., van der Wal D.E., Zhu L. Fc-independent phagocytosis: implications for IVIG and other therapies in immune-mediated thrombocytopenia. Cardiovasc Hematol Disord Drug Targets. 2013;13:50–58. doi: 10.2174/1871529x11313010006. [DOI] [PubMed] [Google Scholar]
- 87.Jansen A.J., Peng J., Zhao H.G., Hou M., Ni H. Sialidase inhibition to increase platelet counts: a new treatment option for thrombocytopenia. Am J Hematol. 2015;90(5):E94–E95. doi: 10.1002/ajh.23953. [DOI] [PubMed] [Google Scholar]
- 88.Li J, van der Wal DE, Zhu G, et al. Platelet desialylation: a novel mechanism of Fc-independent platelet clearance and a potential diagnostic biomarker and therapeutic target in immune thrombocytopenia. Paper presented at: 56th ASH Annual Meeting; December 4–6, 2014; San Francisco, CA.
- 89.Li J., Callum J.L., Lin Y., Zhou Y., Zhu G., Ni H. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica. 2014;99:e61–63. doi: 10.3324/haematol.2013.102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shao L., Wu Y., Zhou H. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets. 2014:1–3. doi: 10.3109/09537104.2014.948838. [DOI] [PubMed] [Google Scholar]
- 91.Kaplan C. Immune thrombocytopenia in the foetus and the newborn: diagnosis and therapy. Transfus Clin Biol. 2001;8:311–314. doi: 10.1016/s1246-7820(01)00114-8. [DOI] [PubMed] [Google Scholar]
- 92.Bussel J.B. Immune thrombocytopenia in pregnancy: autoimmune and alloimmune. J Reprod Immunol. 1997;37:35–61. doi: 10.1016/s0165-0378(97)00072-7. [DOI] [PubMed] [Google Scholar]
- 93.Hachisuga K., Hidaka N., Fujita Y., Fukushima K., Kato K. Can we predict neonatal thrombocytopenia in offspring of women with idiopathic thrombocytopenic purpura? Blood Res. 2014;49:259–264. doi: 10.5045/br.2014.49.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Webert K.E., Mittal R., Sigouin C., Heddle N.M., Kelton J.G. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood. 2003;102:4306–4311. doi: 10.1182/blood-2002-10-3317. [DOI] [PubMed] [Google Scholar]
- 95.Kutuk M.S., Croisille L., Gorkem S.B. Fetal intracranial hemorrhage related to maternal autoimmune thrombocytopenic purpura. Childs Nerv Syst. 2014;30:2147–2150. doi: 10.1007/s00381-014-2473-9. [DOI] [PubMed] [Google Scholar]
- 96.Roberts I., Stanworth S., Murray N.A. Thrombocytopenia in the neonate. Blood Rev. 2008;22:173–186. doi: 10.1016/j.blre.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 97.Murray N.A., Roberts I.A. Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates. Pediatr Res. 1996;40:112–119. doi: 10.1203/00006450-199607000-00020. [DOI] [PubMed] [Google Scholar]
- 98.Watts T.L., Murray N.A., Roberts I.A. Thrombopoietin has a primary role in the regulation of platelet production in preterm babies. Pediatr Res. 1999;46:28–32. doi: 10.1203/00006450-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 99.Kaplan C., Ni H., Freedman J. Alloimmune Thrombocytopenia (CHAPTER 46) In: Michelson A.D., editor. Platelets. 3rd ed. Elsevier/Academic Press; San Diego, CA: 2013. pp. 953–970. [Google Scholar]
- 100.Bussel J.B., Primiani A. Fetal and neonatal alloimmune thrombocytopenia: progress and ongoing debates. Blood Rev. 2008;22:33–52. doi: 10.1016/j.blre.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Mueller-Eckhardt C., Kiefel V., Grubert A. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989;1:363–366. doi: 10.1016/s0140-6736(89)91733-9. [DOI] [PubMed] [Google Scholar]
- 102.Bussel J.B., Zabusky M.R., Berkowitz R.L., McFarland J.G. Fetal alloimmune thrombocytopenia. N Engl J Med. 1997;337:22–26. doi: 10.1056/NEJM199707033370104. [DOI] [PubMed] [Google Scholar]
- 103.Kaplan C. Foetal and neonatal alloimmune thrombocytopaenia. Orphanet J Rare Dis. 2006;1:39. doi: 10.1186/1750-1172-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harrington W.J., Sprague C.C., Minnich V., Moore C.V., Aulvin R.C., Dubach R. Immunologic mechanisms in idiopathic and neonatal thrombocytopenic purpura. Ann Intern Med. 1953;38:433–469. doi: 10.7326/0003-4819-38-3-433. [DOI] [PubMed] [Google Scholar]
- 105.Shulman N.R., Aster R.H., Pearson H.A., Hiller M.C. Immunoreactions involving platelet. VI. Reactions of maternal isoantibodies responsible for neonatal purpura. Differentiation of a second platelet antigen system. J Clin Invest. 1962;41:1059–1069. doi: 10.1172/JCI104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dreyfus M., Kaplan C., Verdy E., Schlegel N., Durand-Zaleski I., Tchernia G. Frequency of immune thrombocytopenia in newborns: a prospective study. Immune Thrombocytopenia Working Group. Blood. 1997;89:4402–4406. [PubMed] [Google Scholar]
- 107.Chakravorty S., Roberts I. How I manage neonatal thrombocytopenia. Br J Haematol. 2012;156:155–162. doi: 10.1111/j.1365-2141.2011.08892.x. [DOI] [PubMed] [Google Scholar]
- 108.Herman J.H., Jumbelic M.I., Ancona R.J., Kickler T.S. In utero cerebral hemorrhage in alloimmune thrombocytopenia. Am J Pediatr Hematol Oncol. 1986;8:312–317. doi: 10.1097/00043426-198624000-00008. [DOI] [PubMed] [Google Scholar]
- 109.Tiller H., Kamphuis M.M., Flodmark O. Fetal intracranial haemorrhages caused by fetal and neonatal alloimmune thrombocytopenia: an observational cohort study of 43 cases from an international multicentre registry. BMJ Open. 2013;3:e002490. doi: 10.1136/bmjopen-2012-002490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silva F., Morais S., Sevivas T., Veiga R., Salvado R., Taborda A. Severe intracranial haemorrhage in neonatal alloimmune thrombocytopenia. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.07.2011.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murphy M.F., Manley R., Roberts D. Neonatal alloimmune thrombocytopenia. Haematologica. 1999;84(suppl EHA-4):110–114. [PubMed] [Google Scholar]
- 112.Giovangrandi Y., Daffos F., Kaplan C., Forestier F., Mac Aleese J., Moirot M. Very early intracranial haemorrhage in alloimmune fetal thrombocytopenia. Lancet. 1990;336:310. doi: 10.1016/0140-6736(90)91842-x. [DOI] [PubMed] [Google Scholar]
- 113.Bussel J.B., Berkowitz R.L., Hung C. Intracranial hemorrhage in alloimmune thrombocytopenia: stratified management to prevent recurrence in the subsequent affected fetus. Am J Obstet Gynecol. 2010;203 doi: 10.1016/j.ajog.2010.03.011. e131–114. [DOI] [PubMed] [Google Scholar]
- 114.Butros L.J., Bussel J.B. Intracranial hemorrhage in immune thrombocytopenic purpura: a retrospective analysis. J Pediatr Hematol Oncol. 2003;25:660–664. doi: 10.1097/00043426-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 115.von Lindern J.S., van den Bruele T., Lopriore E., Walther F.J. Thrombocytopenia in neonates and the risk of intraventricular hemorrhage: a retrospective cohort study. BMC Pediatr. 2011;11:16. doi: 10.1186/1471-2431-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kjeldsen-Kragh J., Killie M.K., Tomter G. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110:833–839. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]
- 117.Davoren A., McParland P., Barnes C.A., Murphy W.G. Neonatal alloimmune thrombocytopenia in the Irish population: a discrepancy between observed and expected cases. J Clin Pathol. 2002;55:289–292. doi: 10.1136/jcp.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bertrand G., Petermann R., Kaplan C. Prediction of IVIG treatment efficiency in fetal/neonatal alloimmune thrombocytopenia. Blood. 2014;124:654–655. doi: 10.1182/blood-2014-04-569541. [DOI] [PubMed] [Google Scholar]
- 119.Murphy M.F., Hambley H., Nicolaides K., Waters A.H. Severe fetomaternal alloimmune thrombocytopenia presenting with fetal hydrocephalus. Prenat Diagn. 1996;16:1152–1155. doi: 10.1002/(SICI)1097-0223(199612)16:12<1152::AID-PD8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 120.Bertrand G., Drame M., Martageix C., Kaplan C. Prediction of the fetal status in non-invasive management of alloimmune thrombocytopenia. Blood. 2011;117:3209–3213. doi: 10.1182/blood-2010-08-302463. [DOI] [PubMed] [Google Scholar]
- 121.Forestier F., Daffos F., Galacteros F., Bardakjian J., Rainaut M., Beuzard Y. Hematological values of 163 normal fetuses between 18 and 30 weeks of gestation. Pediatr Res. 1986;20:342–346. doi: 10.1203/00006450-198604000-00017. [DOI] [PubMed] [Google Scholar]
- 122.Chen P., Li C., Lang S. Animal model of fetal and neonatal immune thrombocytopenia: role of neonatal Fc receptor in the pathogenesis and therapy. Blood. 2010;116:3660–3668. doi: 10.1182/blood-2010-05-284919. [DOI] [PubMed] [Google Scholar]
- 123.Roopenian D.C., Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 124.McMillan R., Longmire R.L., Tavassoli M., Armstrong S., Yelenosky R. In vitro platelet phagocytosis by splenic leukocytes in idiopathic thrombocytopenic purpura. N Engl J Med. 1974;290:249–251. doi: 10.1056/NEJM197401312900505. [DOI] [PubMed] [Google Scholar]
- 125.von dem Borne A.E., Decary F. ICSH/ISBT working party on platelet serology. Nomenclature of platelet-specific antigens. Vox Sang. 1990;58:176. doi: 10.1111/j.1423-0410.1990.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 126.Metcalfe P., Watkins N.A., Ouwehand W.H. Nomenclature of human platelet antigens. Vox Sang. 2003;85:240–245. doi: 10.1046/j.1423-0410.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 127.Santoso S., Kiefel V., Richter I.G. A functional platelet fibrinogen receptor with a deletion in the cysteine-rich repeat region of the beta(3) integrin: the Oe(a) alloantigen in neonatal alloimmune thrombocytopenia. Blood. 2002;99:1205–1214. doi: 10.1182/blood.v99.4.1205. [DOI] [PubMed] [Google Scholar]
- 128.Curtis B.R., McFarland J.G. Human platelet antigens – 2013. Vox Sang. 2014;106:93–102. doi: 10.1111/vox.12085. [DOI] [PubMed] [Google Scholar]
- 129.Salomon O., Rosenberg N. Predicting risk severity and response of fetal neonatal alloimmune thrombocytopenia. Br J Haematol. 2013;162:304–312. doi: 10.1111/bjh.12372. [DOI] [PubMed] [Google Scholar]
- 130.Newman P.J., Derbes R.S., Aster R.H. The human platelet alloantigens, PlA1 and PlA2, are associated with a leucine33/proline33 amino acid polymorphism in membrane glycoprotein IIIa, and are distinguishable by DNA typing. J Clin Invest. 1989;83:1778–1781. doi: 10.1172/JCI114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knight M., Pierce M., Allen D. The incidence and outcomes of fetomaternal alloimmune thrombocytopenia: a UK national study using three data sources. Br J Haematol. 2011;152:460–468. doi: 10.1111/j.1365-2141.2010.08540.x. [DOI] [PubMed] [Google Scholar]
- 132.Ramsey G., Salamon D.J. Frequency of PLA1 in blacks. Transfusion. 1986;26:531–532. doi: 10.1046/j.1537-2995.1986.26687043619.x. [DOI] [PubMed] [Google Scholar]
- 133.Brouk H., Halle L., Bertrand G., Neche F.Z., Ouelaa H., Kaplan C. Human platelet antigen allele frequencies in different Algerian populations. Tissue Antigens. 2010;75:673–678. doi: 10.1111/j.1399-0039.2009.01429.x. [DOI] [PubMed] [Google Scholar]
- 134.Mueller-Eckhardt C., Santoso S., Kiefel V. Platelet alloantigens – molecular, genetic, and clinical aspects. Vox Sang. 1994;67(suppl 3):89–93. doi: 10.1111/j.1423-0410.1994.tb04551.x. [DOI] [PubMed] [Google Scholar]
- 135.Santoso S., Kiefel V., Masri R., Mueller-Eckhardt C. Frequency of platelet-specific antigens among Indonesians. Transfusion. 1993;33:739–741. doi: 10.1046/j.1537-2995.1993.33994025024.x. [DOI] [PubMed] [Google Scholar]
- 136.Smaoui M., Hadjkacem B., Ben Amor I. Allelic polymorphisms of human platelets-specific alloantigens in South Tunisian population. Hematology. 2013;18:365–369. doi: 10.1179/1607845413Y.0000000087. [DOI] [PubMed] [Google Scholar]
- 137.Koh Y., Ishii H., Amakishi E. The first two cases of neonatal alloimmune thrombocytopenia associated with the low-frequency platelet antigen HPA-21bw (Nos) in Japan. Transfusion. 2012;52:1468–1475. doi: 10.1111/j.1537-2995.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 138.Ghevaert C., Rankin A., Huiskes E. Alloantibodies against low-frequency human platelet antigens do not account for a significant proportion of cases of fetomaternal alloimmune thrombocytopenia: evidence from 1054 cases. Transfusion. 2009;49:2084–2089. doi: 10.1111/j.1537-2995.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 139.Rousseau J., Goldman M., David M. HPA-5b (Bra) neonatal alloimmune thrombocytopenia in Quebec: incidence and clinical outcome in 31 cases. Transfusion. 2004;44:844–848. doi: 10.1111/j.1537-2995.2004.03304.x. [DOI] [PubMed] [Google Scholar]
- 140.Goldman M., Trudel E., Richard L., Khalife S., Spurll G.M. Neonatal alloimmune thrombocytopenia due to anti-HPA-2b (anti-Koa) Immunohematology/American Red Cross. 2003;19:43–46. [PubMed] [Google Scholar]
- 141.Al-Sheikh I.H., Khalifa M., Rahi A., Qadri M.I., Al Abad K. A rare case of neonatal alloimmune thrombocytopenia due to ANTI-HPA-2b. Ann Saudi Med. 1998;18:547–549. doi: 10.5144/0256-4947.1998.547. [DOI] [PubMed] [Google Scholar]
- 142.Kaplan C., Porcelijn L., Vanlieferinghen P. Anti-HPA-9bw (Maxa) fetomaternal alloimmunization, a clinically severe neonatal thrombocytopenia: difficulties in diagnosis and therapy and report on eight families. Transfusion. 2005;45:1799–1803. doi: 10.1111/j.1537-2995.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 143.Glade-Bender J., McFarland J.G., Kaplan C., Porcelijn L., Bussel J.B. Anti-HPA-3A induces severe neonatal alloimmune thrombocytopenia. J Pediatr. 2001;138:862–867. doi: 10.1067/mpd.2001.114029. [DOI] [PubMed] [Google Scholar]
- 144.Peterson J.A., Pechauer S.M., Gitter M.L. New platelet glycoprotein polymorphisms causing maternal immunization and neonatal alloimmune thrombocytopenia. Transfusion. 2012;52:1117–1124. doi: 10.1111/j.1537-2995.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sachs U.J., Eva O., Giptner A., Bein G., Peterson A., Santoso S. A new low-frequency polymorphism on platelet glycoprotein IIIa (sec) is associated with neonatal alloimmune thrombocytopenia and with impaired GP IIb/IIIa function. Vox Sang. 2010;99(suppl 2):19–23. [Google Scholar]
- 146.Gerhardt A., Howe N., Krussel J.S., Scharf R.E., Zotz R.B. Elevated lipoprotein(a) levels and homozygous human platelet antigen 1b (HPA-1b) genotype are risk factors for intrauterine growth restriction (IUGR) J Thromb Thrombolysis. 2014;37:107–117. doi: 10.1007/s11239-013-0902-3. [DOI] [PubMed] [Google Scholar]
- 147.Bray P.F. Integrin polymorphisms as risk factors for thrombosis. Thromb Haemost. 1999;82:337–344. [PubMed] [Google Scholar]
- 148.Curtis B.R., Ali S., Glazier A.M., Ebert D.D., Aitman T.J., Aster R.H. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion. 2002;42:1173–1179. doi: 10.1046/j.1537-2995.2002.00176.x. [DOI] [PubMed] [Google Scholar]
- 149.Xu X., Ye X., Xia W. Studies on CD36 deficiency in South China: two cases demonstrating the clinical impact of anti-CD36 antibodies. Thromb Haemost. 2013;110:1199–1206. doi: 10.1160/TH13-05-0435. [DOI] [PubMed] [Google Scholar]
- 150.Ikeda H., Mitani T., Ohnuma M. A new platelet-specific antigen, Naka, involved in the refractoriness of HLA-matched platelet transfusion. Vox Sang. 1989;57:213–217. doi: 10.1111/j.1423-0410.1989.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 151.Kjeldsen-Kragh J., Ni H., Skogen B. Towards a prophylactic treatment of HPA-related foetal and neonatal alloimmune thrombocytopenia. Curr Opin Hematol. 2012;19:469–474. doi: 10.1097/MOH.0b013e328358f86c. [DOI] [PubMed] [Google Scholar]
- 152.Chakraborty C., Gleeson L.M., McKinnon T., Lala P.K. Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol. 2002;80:116–124. doi: 10.1139/y02-016. [DOI] [PubMed] [Google Scholar]
- 153.Ni H., Chen P., Spring C.M. A novel murine model of fetal and neonatal alloimmune thrombocytopenia: response to intravenous IgG therapy. Blood. 2006;107:2976–2983. doi: 10.1182/blood-2005-06-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu S., Maslanka K., Gorski J. An integrin polymorphism that defines reactivity with alloantibodies generates an anchor for MHC class II peptide binding: a model for unidirectional alloimmune responses. J Immunol. 1997;158:3221–3226. [PubMed] [Google Scholar]
- 155.Loewenthal R., Rosenberg N., Kalt R. Compound heterozygosity of HLA-DRB3*01:01 and HLA-DRB4*01:01 as a potential predictor of fetal neonatal alloimmune thrombocytopenia. Transfusion. 2013;53:344–352. doi: 10.1111/j.1537-2995.2012.03734.x. [DOI] [PubMed] [Google Scholar]
- 156.Li C., Chen P., Vadasz B. Co-stimulation with LPS or Poly I: C markedly enhances the anti-platelet immune response and severity of fetal and neonatal alloimmune thrombocytopenia. Thromb Haemost. 2013;110:1250–1258. doi: 10.1160/TH13-04-0354. [DOI] [PubMed] [Google Scholar]
- 157.Mohapatra S., Cao Y., Ni H., Salo D. In pursuit of the “holy grail”: recombinant allergens and peptides as catalysts for the allergen-specific immunotherapy. Allergy. 1995;50(25 suppl):37–44. doi: 10.1111/j.1398-9995.1995.tb04275.x. [DOI] [PubMed] [Google Scholar]
- 158.Xu W., Banchereau J. The antigen presenting cells instruct plasma cell differentiation. Front Immunol. 2014;4:504. doi: 10.3389/fimmu.2013.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blanchette V.S., Chen L., de Friedberg Z.S., Hogan V.A., Trudel E., Decary F. Alloimmunization to the PlA1 platelet antigen: results of a prospective study. Br J Haematol. 1990;74:209–215. doi: 10.1111/j.1365-2141.1990.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 160.Williamson L.M., Hackett G., Rennie J. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–2287. [PubMed] [Google Scholar]
- 161.Kamphuis M.M., Oepkes D. Fetal and neonatal alloimmune thrombocytopenia: prenatal interventions. Prenat Diagn. 2011;31:712–719. doi: 10.1002/pd.2779. [DOI] [PubMed] [Google Scholar]
- 162.Bussel J. Diagnosis and management of the fetus and neonate with alloimmune thrombocytopenia. J Thromb Haemost. 2009;7(suppl 1):253–257. doi: 10.1111/j.1538-7836.2009.03380.x. [DOI] [PubMed] [Google Scholar]
- 163.Vinograd C.A., Bussel J.B. Antenatal treatment of fetal alloimmune thrombocytopenia: a current perspective. Haematologica. 2010;95:1807–1811. doi: 10.3324/haematol.2010.030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paidas M.J., Berkowitz R.L., Lynch L. Alloimmune thrombocytopenia: fetal and neonatal losses related to cordocentesis. Am J Obstet Gynecol. 1995;172(2 Pt 1):475–479. doi: 10.1016/0002-9378(95)90559-6. [DOI] [PubMed] [Google Scholar]
- 165.Shivdasani R.A., Rosenblatt M.F., Zucker-Franklin D. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 166.Palumbo J.S., Zogg M., Talmage K.E., Degen J.L., Weiler H., Isermann B.H. Role of fibrinogen- and platelet-mediated hemostasis in mouse embryogenesis and reproduction. J Thromb Haemost. 2004;2:1368–1379. doi: 10.1111/j.1538-7836.2004.00788.x. [DOI] [PubMed] [Google Scholar]
- 167.Kiefel V., Bassler D., Kroll H. Antigen-positive platelet transfusion in neonatal alloimmune thrombocytopenia (NAIT) Blood. 2006;107:3761–3763. doi: 10.1182/blood-2005-06-2235. [DOI] [PubMed] [Google Scholar]
- 168.Kaplan C., Murphy M.F., Kroll H., Waters A.H. Feto-maternal alloimmune thrombocytopenia: antenatal therapy with IvIgG and steroids–more questions than answers. European Working Group on FMAIT. Br J Haematol. 1998;100:62–65. doi: 10.1046/j.1365-2141.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- 169.Pacheco L.D., Berkowitz R.L., Moise K.J., Jr., Bussel J.B., McFarland J.G., Saade G.R. Fetal and neonatal alloimmune thrombocytopenia: a management algorithm based on risk stratification. Obstet Gynecol. 2011;118:1157–1163. doi: 10.1097/AOG.0b013e31823403f4. [DOI] [PubMed] [Google Scholar]
- 170.Bertrand G., Kaplan C. How do we treat fetal and neonatal alloimmune thrombocytopenia? Transfusion. 2014;54:1698–1703. doi: 10.1111/trf.12671. [DOI] [PubMed] [Google Scholar]
- 171.Bussel J.B., Zacharoulis S., Kramer K., McFarland J.G., Pauliny J., Kaplan C. Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatr Blood Cancer. 2005;45:176–183. doi: 10.1002/pbc.20282. [DOI] [PubMed] [Google Scholar]
- 172.Nishimoto T., Satoh T., Simpson E.K., Ni H., Kuwana M. Predominant autoantibody response to GPIb/IX in a regulatory T-cell-deficient mouse model for immune thrombocytopenia. J Thromb Haemost. 2013;11:369–372. doi: 10.1111/jth.12079. [DOI] [PubMed] [Google Scholar]
- 173.Oyaizu N., Yasumizu R., Miyama-Inaba M. (NZW x BXSB)F1 mouse. A new animal model of idiopathic thrombocytopenic purpura. J Exp Med. 1988;167:2017–2022. doi: 10.1084/jem.167.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dekel B., Marcus H., Shenkman B. Human/BALB radiation chimera engrafted with splenocytes from patients with idiopathic thrombocytopenic purpura produce human platelet antibodies. Immunology. 1998;94:410–416. doi: 10.1046/j.1365-2567.1998.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hansen R.J., Balthasar J.P. Pharmacokinetics, pharmacodynamics, and platelet binding of an anti-glycoprotein IIb/IIIa monoclonal antibody (7E3) in the rat: a quantitative rat model of immune thrombocytopenic purpura. J Pharmacol Exp Ther. 2001;298:165–171. [PubMed] [Google Scholar]
- 176.Hosono M., Sone N., Endo K. Kinetics of platelets in dogs with thrombocytopenia induced by antiglycoprotein IIb/IIIa receptor monoclonal antibody. Nucl Med Biol. 1995;22:71–76. doi: 10.1016/0969-8051(94)00072-r. [DOI] [PubMed] [Google Scholar]
- 177.Coetzee L.M., Pieters H., van Wyk V. The effect of monoclonal anti-human-platelet antibodies on platelet kinetics in a baboon model: IgG subclass dependency. Thromb Haemost. 2000;83:148–156. [PubMed] [Google Scholar]
- 178.Leytin V., Mykhaylov S., Starkey A.F. Intravenous immunoglobulin inhibits anti-glycoprotein IIb-induced platelet apoptosis in a murine model of immune thrombocytopenia. Br J Haematol. 2006;133:78–82. doi: 10.1111/j.1365-2141.2006.05981.x. [DOI] [PubMed] [Google Scholar]
- 179.Crow A.R., Song S., Semple J.W., Freedman J., Lazarus A.H. IVIg inhibits reticuloendothelial system function and ameliorates murine passive-immune thrombocytopenia independent of anti-idiotype reactivity. Br J Haematol. 2001;115:679–686. doi: 10.1046/j.1365-2141.2001.03136.x. [DOI] [PubMed] [Google Scholar]
- 180.Ghevaert C., Wilcox D.A., Fang J. Developing recombinant HPA-1a-specific antibodies with abrogated Fcgamma receptor binding for the treatment of fetomaternal alloimmune thrombocytopenia. J Clin Invest. 2008;118:2929–2938. doi: 10.1172/JCI34708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bakchoul T., Boylan B., Sachs U.J. Blockade of maternal anti-HPA-1a-mediated platelet clearance by an HPA-1a epitope-specific F(ab') in an in vivo mouse model of alloimmune thrombocytopenia. Transfusion. 2009;49:265–270. doi: 10.1111/j.1537-2995.2008.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lang S., Yang H., Boyd S. Impaired angiogenesis contributes to pathogenesis of fetal and neonatal immune thrombocytopenia. J Thromb Haemost. 2011 XXIII Congress of the International Society on Thrombosis and Haemostasis. [Google Scholar]
- 183.Yougbaré I., Lang S., Yang H. Maternal anti-platelet β3 integrins impair angiogenesis and cause intracranial hemorrhage. J Clin Invest. 2015 doi: 10.1172/JCI77820. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Firan M., Bawdon R., Radu C. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 185.Killie M.K., Husebekk A., Kjeldsen-Kragh J., Skogen B. A prospective study of maternal anti-HPA 1a antibody level as a potential predictor of alloimmune thrombocytopenia in the newborn. Haematologica. 2008;93:870–877. doi: 10.3324/haematol.12515. [DOI] [PubMed] [Google Scholar]
- 186.Kjeldsen-Kragh J., Skogen B. Mechanisms and prevention of alloimmunization in pregnancy. Obstet Gynecol Surv. 2013;68:526–532. doi: 10.1097/OGX.0b013e3182947ce4. [DOI] [PubMed] [Google Scholar]
- 187.Tiller H., Killie M.K., Chen P. Toward a prophylaxis against fetal and neonatal alloimmune thrombocytopenia: induction of antibody-mediated immune suppression and prevention of severe clinical complications in a murine model. Transfusion. 2012;52:1446–1457. doi: 10.1111/j.1537-2995.2011.03480.x. [DOI] [PubMed] [Google Scholar]