Abstract
The consequences of treatment for the kidney at the molecular level have not been explored in human lupus nephritis (LN). In this investigation, changes in intra-renal transcript expression were measured and correlated with response in a LN cohort that underwent serial kidney biopsies. The intra-renal transcript expression of 19 patients with proliferative LN (Class III or IV) was measured at diagnostic biopsy (Bx1) and after induction therapy was completed (Bx2) using Nanostring® technology. Patients were segregated by clinical response into complete responders (n=5, CR) or nonresponders (n=4, NR). Transcript expression for each biopsy was compared to normal controls (n=4) and the change in expression was compared in each responder group and between groups. Compared to controls, the CR group had 21 and 28 while NR had 45 and 103 differentially-expressed transcripts at Bx1 and Bx2, respectively. The profiles of these differentially-expressed genes indicated that the type I and II interferon, alternative complement and T cell signaling pathways discriminated CR from NR. Comparing the change in transcript expression from Bx1 to Bx2 revealed a 5-gene signature that differentiated NR from CR and included increased IL1RAP and FCAR in NR and increased NCAM1 in CR. In summary, molecular imaging of serial kidney biopsies from LN patients shows several immune and inflammatory pathways that are dysregulated in the kidneys during active disease that may serve as therapeutic targets to improve clinical response. This approach to LN biomarker development may facilitate personalized medicine in LN and improve long-term kidney outcomes.
Keywords: Lupus Nephritis, Systemic Lupus Erythematosus, transcriptional profiling, kidney biopsy
INTRODUCTION
The short-term goal of lupus nephritis (LN) induction therapy is to achieve a clinical response, assessed mainly as a reduction in proteinuria and stabilization or improvement in kidney function. In human LN the intra-renal molecular correlates of clinical response or non-response are not known, although such data are emerging in animal models of lupus (1, 2). Understanding the kidney’s molecular response to treatment may have therapeutic implications. For example, it is becoming increasingly clear that a clinical renal response may not reflect histologic resolution of kidney injury (3). This raises the possibility that despite treatment and improvement of clinical signs of kidney injury, there may be ongoing activity of the intra-renal immune and inflammatory pathways that were engaged at the time of LN flare. Knowledge of these active pathways could facilitate maintenance therapy. Furthermore, patients who completely respond to treatment often develop chronic kidney damage, suggesting the activation of additional mechanisms of kidney injury, like fibrosis pathways, during therapy (4). Such mechanisms, if known may be amenable to treatment, and this could attenuate the development of chronic kidney disease in lupus. For patients who do not respond to conventional immunosuppressive therapy it is reasonable to assume that certain intra-renal immune and inflammatory pathways are still active, but without identifying these pathways the choice of an alternative treatment regimen remains uninformed.
To characterize the molecular correlates of clinical renal responses we have examined intra-renal gene expression in serial biopsies from a well-phenotyped LN cohort. Transcript expression of genes relevant to immunity and inflammation were measured in kidney biopsy material taken at the time of LN flare and after completion of induction therapy in patients who responded completely to induction and patients who did not respond to induction. Differentially-expressed transcripts in LN biopsies relative to normal kidney were identified, and changes in transcript expression between responders and non-responders during treatment were compared.
MATERIALS AND METHODS
Kidney Biopsies
Kidney biopsies were done on 19 patients with proliferative (Class III or IV ± V) LN between 2007 and 2011. The biopsies had been archived after all clinical testing was completed. Biopsy 1 (Bx1) was done to diagnose LN flare and biopsy 2 (Bx2) was done after LN induction therapy was completed. Patients were segregated by clinical response and only those who achieved a complete clinical response (n=5) or had a non-response (n=4) were included in the analysis. Ten patients achieved a partial clinical response and were not analyzed further in this study. Normal control kidney tissue was from archived kidney biopsies of living-donor kidneys (n=4), and was analyzed in parallel with the LN biopsies. The investigation of the kidney biopsies was approved by the Hospital Fernandez (Buenos Aires) ethics board and The Ohio State University institutional review board.
Treatment Protocols and Outcomes
All LN patients were treated with a tapering course of prednisone starting at 1mg/kg/d and either mycophenolate mofetil (MMF, 63% of patients) or intravenous cyclophosphamide (37% of patients). The induction period generally lasted 6 months. Clinical responses were assessed using achieved levels of serum creatinine concentration (SCr) and 24-hour urine protein when induction was finished. A complete clinical renal response (CR) was defined as having an improvement in proteinuria to < 0.5 g/d with normalization of SCr. A partial clinical renal response (PR) was defined as a reduction in proteinuria of >50% and to a level <3 g/d but > 0.5 g/d, with stable or improved SCr (5). A non-response (NR) was defined as less than 50% reduction in proteinuria or proteinuria that remained >3g/d with stable or worsening Scr.
RNA Extraction and Analysis
The complete protocol for RNA isolation and analysis was previously described (6). In brief, formalin-fixed and paraffin-embedded (FFPE) kidney biopsy tissue blocks were sectioned. The sections were deparaffinized and whole tissue RNA was extracted from each biopsy using the RNeasy FFPE kit (Qiagen Valencia, CA). Transcript expression was analyzed from 250 ng of extracted RNA using the Nanostring ncounter® platform and the GX human immunology transcript panel (Nanostring Technologies, Seattle, WA) (7-9). The human immunology panel consisted of 511 immune response genes, 6 positive control genes and 6 negative control genes. A complete list of these genes can be found here http://www.nanostring.com/products/gene_expression_panels.
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation or as a percentage. For clinical variables t-tests, ANOVA or Wilcoxon rank sum tests were applied as appropriate. For categorical clinical variables, Fisher’s exact test was used. For Nanostring data, raw counts were normalized to the positive spike-in controls and then log2 transformed. To reduce technical bias, genes with an expression level below the mean plus 2xSD of the negative controls’ expression for most samples were filtered out. Then quantile normalization method was employed to the remaining 468 genes across samples. Linear mixed effects models were used to identify differentially expressed genes by taking into account the correlation between repeated measures before and after treatment. In order to improve the stability of variance estimation, variance smoothing methods and moderated t-tests were employed (10). Since samples were profiled in two batches of different time points, batch effects were estimated in the model and adjusted for group comparisons as well. A total of seven comparisons were performed based on five patients groups: Bx1 and Bx2 of CR patients and NR patients and normal kidney tissue as control. To determine differential expression, each group (Bx1 or Bx2 of CR or NR) was compared to normal control. To evaluate the change in transcript expression over time, Bx2 was compared to Bx1 within CR and within NR separately. The change in expression from Bx1 to Bx2 was also compared between the CR and NR groups. P values were adjusted by controlling the mean number of false positives at 5 out of 500 tests (i.e. α = 0.01). For any specific gene to be considered differentially-expressed at least a 2-fold difference in transcript level and a p-value < 0.01 must have been achieved.
Ingenuity Pathway Analysis® (IPA®, Qiagen) was used to evaluate the change in pathway expression with treatment in each group and to compare pathway expression after treatment between groups. Pathway analysis was done as described previously (6). In brief, pathway analysis was conducted using less stringent criteria than those used to identify differentially expressed genes. This was done to enrich the analysis for the targeted dataset. Transcripts that met inclusion criteria for pathway analysis had at least a 1.5-fold-change compared to normal controls and a p < 0.05. Using these parameters, 68 transcripts in CR and 189 transcripts in NR were included in the analysis.
RESULTS
The clinical, demographic and pathologic characteristics of the LN kidney biopsy cohort are provided in Table 1. All patients were White and Hispanic. The median time between Bx1 and Bx2 was 13 months, with a range of 6-37 months. The median time between Bx1 and Bx2 in the CR group was 13 months (6-37) and the median time between Bx1 and Bx2 in the NR group was 12.5 months (6-28). The clinical variables and follow up time between biopsies for each patient are provided in Table 2. Although clinical characteristics were generally similar between groups, NR had more proteinuria than CR. While not statistically significant, there were more first time LN flares in the CR group (60%) compared to the NR group (25%). The histologic activity and chronicity index was similar between the groups at flare and after treatment. After treatment, serum creatinine and proteinuria improved in CR but were worse in NR.
Table 1.
Baseline Demographics and Clinical Data1
| Complete Response Group (n=5) |
No Response Group (n=4) |
P (CR v NR) | |
|---|---|---|---|
| Age (years, ±SD) | 24.8±4.97 | 30±8.16 | 0.2 |
| Female (%) | 4 (80) | 4 (100) | 1.0 |
| LN Class at Flare (% Class IV) | 3 (60) | 4 (100) | 0.4 |
| First LN Flare (%) | 60% | 25% | 0.4 |
| Induction Therapy CYC (%) | 2 (40) | 2 (50) | 1.0 |
| Induction Therapy MMF (%) | 3 (60) | 2 (50) | 0.8 |
| SCr2 at B×13 (±SD) | 0.94±0.31 | 1.08±0.28 | 0.7 |
| SCr at B×24 (±SD) | 0.74±0.15 | 1.18±0.36 | 0.004 |
| Proteinuria5 at B×1 (±SD) | 3.04±1.11 | 5.5±2.08 | 0.014 |
| Proteinuria at B×2 (±SD) | 0.24±0.09 | 3.33±1.27 | <0.0001 |
| Activity Index at B×1 (range) | 6(4-8) | 5 (4-12) | 0.1 |
| Chronicity Index at B×1 (range) | 2 (0-4) | 5 (0-6) | 0.3 |
| Activity Index at B×2 (range) | 3 (0-8) | 3.5 (0-9) | 0.7 |
| Chronicity Index at B×2 (range) | 4(0-5) | 5 (4-6) | 0.1 |
p-value calculation: Fisher’s exact test was applied to categorical demographic and clinical parameters: Age, sex, LN Class, and induction therapy. ANOVA was used to look for significant trends among all groups, and t-tests were used to compare differences between two specific groups
Serum creatinine in mg/dl
Biopsy 1 (B×1), done at LN flare
Biopsy 2 (B×2), done after completion of induction therapy
24-hour Urine protein in g/d
Table 2.
Clinical Variables for each patient at Biopsy 1 and Biopsy 2
| Patient number |
Clinical response Group |
SCr at flare (mg/dl) |
Proteinuria at flare (g/d) |
Induction therapy |
Maintenance treatment |
SCr after treatment (mg/dl) |
Proteinuria after treatment (g/d) |
Time to Repeat biopsy |
|---|---|---|---|---|---|---|---|---|
| 1 | CR | 1.4 | 4.2 | CYC1 | N/A | 0.7 | 0.2 | 6 |
| 2 | CR | 0.6 | 1.8 | CYC | N/A | 0.8 | 0.2 | 6 |
| 3 | CR | 0.8 | 3.2 | MMF2 | Azathioprine | 0.5 | 0.2 | 36 |
| 4 | CR | 1.1 | 2.0 | MMF | MMF | 0.8 | 0.2 | 37 |
| 5 | CR | 0.8 | 4 | MMF | MMF | 0.9 | 0.4 | 13 |
| 6 | NR | 0.8 | 6.0 | CYC | Azathioprine | 1.1 | 3.5 | 28 |
| 7 | NR | 1.2 | 5.0 | MMF | N/A | 1.4 | 2.8 | 6 |
| 8 | NR | 0.9 | 8.0 | CYC | N/A | 0.7 | 5.0 | 10 |
| 9 | NR | 1.4 | 3.0 | MMF | MMF | 1.5 | 2.0 | 15 |
CYC – Cyclophosphamide,
MMF – Mycophenolate Mofetil
We approached the analysis of intra-renal transcript expression changes during LN induction therapy in two ways. First, to determine how well treatment reverses the altered pattern of gene expression that occurs in the kidney when LN flares, transcript expression profiles from Bx1 and Bx2 of CR were compared to transcript expression profiles from normal kidney tissue. Similarly, to determine whether the altered pattern of gene expression that occurs in the kidney when LN flares continues in patients who do not experience a clinical response, transcript expression profiles from Bx1 and Bx2 from NR were compared to transcript expression profiles from normal kidney tissue. Second, to develop a molecular signature of response the changes in transcript expression from Bx1 to Bx2 in CR were directly compared to the changes in transcript expression from Bx1 to Bx2 in NR.
Transcript expression at flare and after treatment compared to normal kidney
At Bx1, 21 intra-renal transcripts were differentially-expressed relative to control in patients who achieved a CR after induction compared to 45 differentially-expressed transcripts in those who had NR (Tables 3 and 4). At Bx2, there were 28 differentially expressed transcripts in the CR group compared to 103 differentially-expressed transcripts in the NR group (Tables 3 and 4). For ease of comparison, in each column of Tables 3 and 4 the bolded transcripts were differentially-expressed in both CR and NR.
Table 3.
Differentially Expressed Transcripts at Flare and After Treatment in Complete Responders
| Transcripts Differentially-Expressed Compared to Normal Kidney at Biopsy 1 |
Change in Transcript Expression from Biopsy 1 to Biopsy 2 |
Transcripts that Become Differentially- Expressed Compared to Control After Treatment at Biopsy 2 |
|||||
|---|---|---|---|---|---|---|---|
| Gene | Fold Change1 | P2 | Percent Change3 | P4 | Gene | Fold Change1 | P2 |
| MX1 | 6.96 | 0.0000 | −47% | 0.1475 | TGFBI | 2.57 | 0.001 |
| STAT1 | 4.03 | 0.0000 | −22% | 0.3158 | FN1 | 2.23 | 0.007 |
| HLA-DRB1 | 3.91 | 0.003 | −6% | 0.8138 | LCK | 2.19 | 0.005 |
| BST2 | 3.89 | 0.0000 | −34% | 0.0694 | ENTPD1 | 2.11 | 0.008 |
| IFITM1 | 3.71 | 0.001 | −20% | 0.5124 | CX3CR1 | 2.09 | 0.002 |
| HLA-C | 3.00 | 0.0004 | −19% | 0.2562 | NFKBIA | 0.49 | 0.0001 |
| C1QB | 2.60 | 0.002 | −29% | 0.1041 | IL8 | 0.48 | 0.004 |
| FCER1G | 2.58 | 0.003 | −26% | 0.1990 | IL6R | 0.48 | 0.0003 |
| HLA-A | 2.50 | 0.0007 | −27% | 0.1276 | NFKBIZ | 0.47 | 0.008 |
| IRF7 | 2.43 | 0.003 | −31% | 0.1855 | TAL1 | 0.42 | 0.0002 |
| CYBB | 2.37 | 0.0007 | 18% | 0.4603 | SOCS3 | 0.31 | 0.004 |
| ITGAL | 2.35 | 0.006 | −1% | 0.9793 | MME | 0.28 | 0.002 |
| LILRB1 | 2.09 | 0.008 | −19% | 0.43 | FKBP5 | 0.11 | 0.0000 |
| TNF | 2.05 | 0.001 | −10% | 0.66 | |||
| NFATC1 | 0.48 | 0.002 | 25% | 0.27 | |||
| SELE | 0.47 | 0.003 | 33% | 0.27 | |||
| CEBPB | 0.44 | 0.0005 | −35% | 0.04 | |||
| NFIL3 | 0.44 | 0.009 | −5% | 0.87 | |||
| RORC | 0.43 | 0.002 | 12% | 0.63 | |||
| CDKN1A | 0.32 | 0.0000 | −25% | 0.13 | |||
| CR2 | 0.10 | 0.0000 | 28% | 0.50 | |||
Transcript expression in LN compared to normal controls
LN versus normal; p≤0.01 and fold change compared to normal >2 or <0.5 to be considered differentially expressed
Transcript expression from biopsy 1 to biopsy 2
Biopsy 1 versus biopsy 2
Table 4.
Differentially Expressed Transcripts at Flare and After Treatment in Non-Responders
| Transcripts Differentially-Expressed Compared to Normal Kidney at Biopsy 1 |
Change in Transcript Expression from Biopsy 1 to Biopsy 2 |
Transcripts that Become Differentially-Expressed Compared to Normal After Treatment at Biopsy 2 |
|||||
|---|---|---|---|---|---|---|---|
| Gene | Fold Change1 | P2 | Percent Change3 | P4 | Gene | Fold Change1 | P2 |
| C1QB | 4.52 | 0.0000 | −38% | 0.04 | CFD | 8.01 | 0.0002 |
| MX1 | 3.90 | 0.004 | 67% | 0.29 | CCL19 | 5.94 | 0.0006 |
| ITGAL | 3.73 | 0.0001 | 66% | 0.09 | C3 | 4.96 | 0.001 |
| ITGB2 | 3.48 | 0.0000 | 25% | 0.39 | SELL | 4.22 | 0.003 |
| CYBB | 3.41 | 0.0000 | 59% | 0.07 | CX3CR1 | 3.74 | 0.0000 |
| FCER1G | 3.41 | 0.0004 | 33% | 0.27 | CASP1 | 3.24 | 0.0000 |
| FCGR3A | 3.35 | 0.006 | 35% | 0.48 | IFITM1 | 3.09 | 0.006 |
| STAT1 | 3.09 | 0.0008 | 39% | 0.25 | ITGAX | 3.08 | 0.0007 |
| C1S | 3.00 | 0.0000 | 10% | 0.60 | B2M | 2.87 | 0.009 |
| BST2 | 2.88 | 0.0000 | 8% | 0.75 | CD40 | 2.87 | 0.009 |
| C1R | 2.79 | 0.001 | −5% | 0.85 | LCK | 2.82 | 0.0005 |
| FN1 | 2.75 | 0.001 | −25% | 0.36 | LCP2 | 2.77 | 0.0001 |
| LAIR1 | 2.68 | 0.0000 | −5% | 0.78 | C2 | 2.77 | 0.008 |
| TGFBI | 2.62 | 0.002 | 17% | 0.59 | SLAMF7 | 2.76 | 0.008 |
| KIR2DL3 | 2.51 | 0.009 | −50% | 0.04 | IL1RN | 2.73 | 0.001 |
| GZMA | 2.48 | 0.002 | 24% | 0.34 | IRF8 | 2.72 | 0.005 |
| HLA-B | 2.44 | 0.0001 | 29% | 0.27 | TAGAP | 2.66 | 0.009 |
| HLA-DRB3 | 2.44 | 0.002 | 37% | 0.28 | IFI16 | 2.66 | 0.0000 |
| LILRA3 | 2.39 | 0.001 | 21% | 0.46 | JAK3 | 2.61 | 0.01 |
| IRF7 | 2.23 | 0.009 | 34% | 0.36 | FCGR2B | 2.61 | 0.003 |
| HLA-A | 2.23 | 0.004 | 3% | 0.90 | CD5 | 2.55 | 0.009 |
| BTK | 2.20 | −1% | 0.10 | PTPN6 | 2.42 | ||
| 0.001 | 0.0001 | ||||||
| PTPRC | 2.20 | 0.01 | 115% | 0.01 | ZAP70 | 2.34 | 0.006 |
| CSF2RB | 2.19 | 0.002 | 85% | 0.01 | NCF4 | 2.34 | 0.0009 |
| TNF | 2.18 | 0.0009 | 48% | 0.15 | TGFB1 | 2.34 | 0.0002 |
| CD6 | 2.05 | 0.005 | −10% | 0.64 | ITGAM | 2.27 | 0.0007 |
| MR1 | 0.50 | 0.001 | 10% | 0.68 | GZMB | 2.26 | 0.003 |
| NOS2 | 0.49 | 0.008 | 45% | 0.10 | ARHGDIB | 2.26 | 0.0000 |
| CEBPB | 0.48 | 0.002 | 13% | 0.58 | CD24 | 2.25 | 0.002 |
| ITGA6 | 0.46 | 0.0006 | −13% | 0.51 | TLR1 | 2.21 | 0.0000 |
| IGF2R | 0.45 | 0.006 | −9% | 0.74 | IKZF1 | 2.19 | 0.008 |
| IL6R | 0.44 | 0.0001 | 4% | 0.88 | LILRB1 | 2.18 | 0.007 |
| C9 | 0.43 | 0.0007 | −8% | 0.71 | PRF1 | 2.15 | 0.003 |
| ICOSLG | 0.43 | 0.0001 | −35% | 0.03 | CXCL13 | 2.11 | 0.008 |
| TFRC | 0.42 | 0.0002 | −23% | 0.21 | CD97 | 2.11 | 0.001 |
| CR1 | 0.42 | 0.002 | 33% | 0.32 | STAT2 | 2.07 | 0.0000 |
| BST1 | 0.41 | 0.001 | 32% | 0.29 | NFATC1 | 0.50 | 0.004 |
| SELE | 0.40 | 0.0008 | 15% | 0.61 | LTB4R2 | 0.50 | 0.003 |
| CISH | 0.38 | 0.0000 | −6% | 0.73 | MAPKAPK2 | 0.49 | 0.001 |
| CDKN1 A | 0.30 | 0.0000 | −3% | 0.90 | GPI | 0.49 | 0.001 |
| RORC | 0.22 | 0.0000 | −6% | 0.81 | IL8 | 0.47 | 0.004 |
| DPP4 | 0.22 | 0.003 | −17% | 0.67 | PDGFB | 0.46 | 0.003 |
| NFIL3 | 0.21 | 0.0000 | 4% | 0.90 | TRAF4 | 0.46 | 0.002 |
| MME | 0.15 | 0.0000 | −5% | 0.88 | CTNNB1 | 0.45 | 0.0002 |
| CR2 | 0.12 | 0.0000 | 76% | 0.17 | CRADD | 0.45 | 0.0006 |
| TAL1 | 0.45 | 0.001 | |||||
| CD8B | 0.44 | 0.003 | |||||
| NFKBIA | 0.44 | 0.0000 | |||||
| TOLLIP | 0.40 | 0.001 | |||||
| CTSC | 0.40 | 0.0002 | |||||
| CD274 | 0.40 | 0.009 | |||||
| C5 | 0.38 | 0.0000 | |||||
| KIT | 0.37 | 0.002 | |||||
| IL12RB1 | 0.35 | 0.008 | |||||
| BLNK | 0.35 | 0.0003 | |||||
| C6 | 0.35 | 0.0000 | |||||
| CD81 | 0.34 | 0.008 | |||||
| C1QBP | 0.34 | 0.003 | |||||
| CEACAM6 | 0.33 | 0.005 | |||||
| CFI | 0.29 | 0.0001 | |||||
| CX3CL1 | 0.28 | 0.0001 | |||||
| VTN | 0.25 | 0.0001 | |||||
| ABCB1 | 0.25 | 0.0005 | |||||
| DEFB1 | 0.23 | 0.002 | |||||
| SPP1 | 0.17 | 0.001 | |||||
| FKBP5 | 0.14 | 0.0004 | |||||
Transcript expression in LN compared to normal controls
LN versus normal; p≤0.01 and fold change compared to normal >2 or <0.5 to be considered differentially expressed
Transcript expression from biopsy 1 to biopsy 2
Biopsy 1 versus biopsy 2
Several general patterns of gene expression changes during LN induction treatment became apparent. Despite complete clinical remission, 66% of the differentially-expressed transcripts at flare remained differentially-expressed at Bx2 compared to normal tissue. Most of these transcripts (86%) either did not change expression level or tended to shift their expression level toward normal (Table 3). Additionally, 13 transcripts that were not differentially-expressed at flare became so after treatment. Newly upregulated transcripts included, CX3CR1 (a chemokine receptor), LCK (lymphocyte-specific tyrosine kinase, a T cell signaling protein) and FN1 and TGFBI (genes for the pro-fibrotic proteins fibronectin and transforming-growth factor beta-induced protein). Newly down-regulated transcripts included, CISH, (a regulator of T cell receptor cytokine signaling), SOCS3 (STAT-induced cytokine signaling and suppressor of cytokine signaling-3), NKFBIA and NFKBIZ (NF-κB inhibitors), MME (a brush border neutral endopeptidase) and FKBP5 (an immunophilin).
In contrast, in the NR group 85% of the transcripts that had altered expression at flare continued to show altered expression after induction, and the expression level of 39% of these genes continued to increase relative to normal controls, while 55% did not change expression between Bx1 and Bx2 (Table 4). Furthermore, an additional 66 transcripts became activated or suppressed at Bx2 (Table 4). Six of these newly differentially expressed transcripts after treatment in NR overlapped with the 13 transcripts uniquely expressed after treatment in CR and included CX3CR1, LCK, IL8, NKBIA, TAL1, and FKBP5. Additionally, 3 transcripts (TGFBI, IL6R, and MME) that were newly expressed in CR after treatment were already differentially expressed at flare in NR and remained so after treatment in NR.
Considering the gene products of the differentially-expressed transcripts in CR and NR, a molecular definition of response and non-response begins to emerge. In this regard the type I and II interferon, alternative complement, and T cell signaling pathways appear to discriminate between CR and NR during treatment (Figure 1).
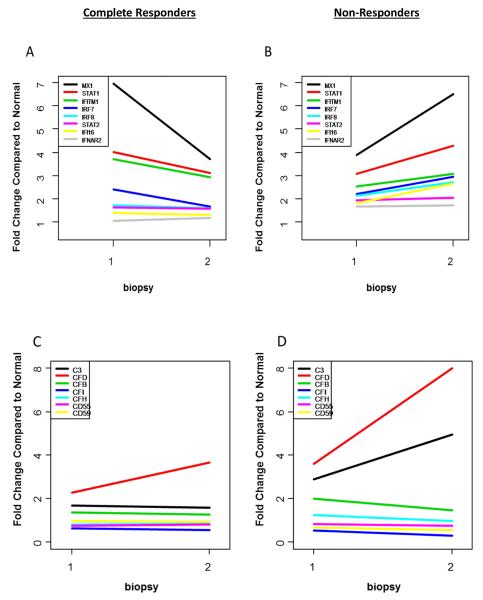
Figure 1. Intra-renal expression of type 1 interferon, complement and T cell-related genes before and after treatment for lupus nephritis.
The change in transcript expression from biopsy 1 and biopsy 2 of genes relevant to the type 1 interferon pathway (A and B), alternative complement pathway (C and D), and T cell activation pathway (E and F) were compared between complete responders and non-responders. A level of 1 on the y-axis indicates expression equivalent to normal kidney.
The Interferon Pathways
The intra-renal interferon signature was significantly upregulated at flare in CR and NR (Figure 1A and 1B). Specifically, at Bx1, MX1, STAT1, IRF7, IFIH1, and IFITM1 levels were increased compared to control in CR. After treatment, MX1, IRF7, STAT1, and IFITM1 expression remained significantly elevated but their levels declined by 47%, 31%, 22%, and 20% respectively, relative to Bx1 (Figure 1A). In NR, MX1, STAT1, and IRF7 were significantly upregulated at flare, but in contrast to CR their expression levels increased by 67%, 39%, and 34%, respectively, after treatment relative to Bx1 (Figure 1B). Furthermore, several additional type I and II interferon-related genes became significantly upregulated after treatment in NR and include increased expression of IFITM1 (P=0.006), IRF8 (P=0.005), IFI16 (P=0.000), and STAT2 (P=0.000) (Table 4).
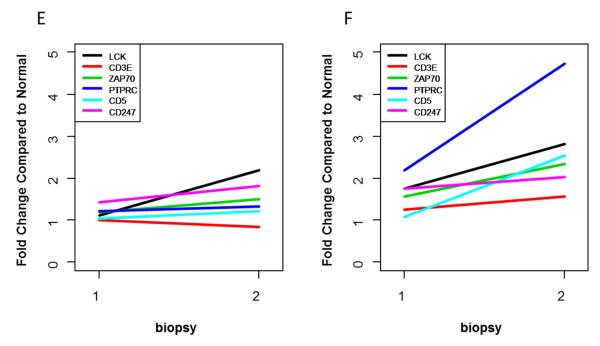
Because of the importance of interferon in SLE and LN the interferon pathways in the kidneys were investigated in more detail by IPA® upstream transcription analysis. This showed that the interferon-α, interferon-β, and interferon-γ pathways were upregulated at flare in CR, but after treatment only the interferon-β pathway remained upregulated (Figure 2A and B). In contrast, in NR the interferon-α and interferon-β pathways were upregulated at flare and after treatment, and the interferon-γ pathway became upregulated post-treatment (Figure 2C and 2D).
Figure 2. Network analysis of intra-renal interferon pathways in lupus nephritis.
Network analysis was done to determine the extent of interferon pathway activation in lupus nephritis kidneys at flare and after treatment. In flares that ended in complete response, the interferon-α, β and γ pathways were predicted to be upregulated (A). After treatment, only the interferon-β pathway remained upregulated (B). In flares that ended in non-response, the interferon-α and β pathways were predicted to be upregulated (C). After treatment, the interferon-α, β and γ pathways were all predicted to be upregulated (D).
Complement Pathways
Intra-renal alternative complement pathway-related genes were upregulated in CR and NR at flare, but changed discordantly with treatment (Fig. 1C,D). In NR, the expression of complement component 3 (C3) and complement factor D (CFD), key activators of the alternative pathway, increased 80% and 122% after treatment, respectively, while the expression of complement factor I (CFI), an important regulator of the alternative pathway, decreased by 44% (Fig. 1C, D). Compared to normal kidney, C3 expression was 5-fold higher (P=0.001), CFD expression was 8-fold higher (P=0.0002) and CFI expression was 3.5-fold lower (P=0.0001) after treatment in NR. In CR, CFD expression did increase after treatment, but did not reach significance, while C3 and CFI expression were similar to flare levels.
T Cell Signaling Pathways
Intra-renal expression of transcripts that facilitate T cell activation and proliferation increased at Bx2 in NR compared to normal controls (Fig. 1E,F). For example, the transcript for the protein tyrosine phosphatase receptor type C (PTPRC/CD45) was significantly increased at flare in NR (fold change (FC)=2.2, P=0.001), and expression further increased by 115% after treatment (FC=4.74, P=0.000) (Table 4). T cell signaling and activation transcripts that became significantly upregulated in NR after treatment included LCP2 (FC=2.8, P=0.001), LCK (FC=2.8, P=0.0001), ZAP70 (FC=2.34, P=0.006), and CD5 (FC=2.6, P=0.009) (Fig. 1E,F).
Markers of Leukocyte Infiltration
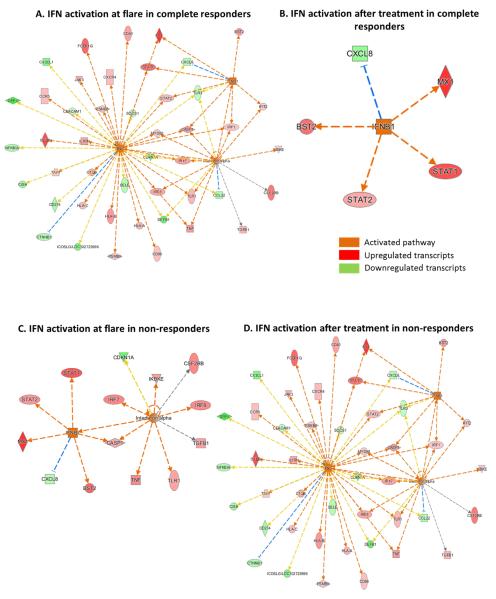
The transcripts of several pro-inflammatory proteins associated with leukocyte infiltration were upregulated in NR compared to CR (Fig. 3). ITGAL and ITGB2, encode for the alpha and beta chains of the leukocyte integrin lymphocyte function-associated antigen 1 (LFA-1). LFA-1 facilitates adhesion of infiltrating leukocytes to renal endothelium. In NR, ITGAL (FC=3.7, P=0.0001) and ITGB2 (FC=3.5, P=0.000) levels were significantly increased at flare and post-treatment levels increased by 81% and 22% respectively, compared to flare levels (Table 4). Additionally, the alpha-integrin genes ITGAX (FC=3.1, P=0.0007 and ITGAM (FC=2.3, P=0.0007) were differentially upregulated after treatment in NR only.
Figure 3. Change in intra-renal expression of pro-inflammatory genes after induction treatment for LN.
The values for each gene are given as a fold-change relative to normal controls at biopsy 1 and 2. A level of 1 on the y-axis indicates expression equivalent to normal kidney.
In addition to integrin-mediated leukocyte infiltration, several chemokines were upregulated after induction therapy in NR. CCL19 increased 6-fold compared to normal (P=0.00006), and CXCL13 expression increased 2-fold relative to normal (P=0.008).
Colony stimulating factor receptor transcripts CSF2RB and CSF3R are differentially upregulated after treatment in NR. CSF2RB expression was upregulated at flare compared to controls (FC=2.2, P=0.002) and its expression increased 86% relative to flare levels after treatment (P=0.01). Although it did not quite reach significance, after treatment, CSF3R expression increased 3-fold above controls levels in NR (P=0.01)
Comparison of transcript changes in response to treatment in responders and non-responders
In a second approach to evaluate how gene expression is altered in the kidney during treatment, the change in transcript levels between Bx1 and Bx2 from CR were directly compared to changes between Bx1 and Bx2 from NR. Overall, 5 transcripts were different between CR and NR (Table 5, Column 1). The origin of these differences is best understood by examining how each transcript changed from Bx1 to Bx2 in CR and NR separately (Table 5, Columns 2 and 3). For example, C7, which encodes for complement component C7, a membrane anchor protein of the terminal complement pathway, was 4-fold higher in CR relative to NR with treatment (Table 5, Column 1). This occurred because C7 levels remained stable in CR from Bx1 to Bx2 (Table 5, Column 2), but decreased by 72% from Bx1 to Bx2 in NR. NCAM1 expression fell between biopsies in NR but increased between biopsies in CR, accounting for a very large difference in NCAM1 expression between CR and NR. Several additional transcripts followed a similar pattern of expression after treatment. Most of these transcripts increased in expression in NR and stayed constant or decreased in CR (Figure 3).
Table 5.
Differentially expressed transcripts from B×1 to B×2
| Complete vs No Response | Complete Response | No Response3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Genes | Fold change1 | P2 | Genes | Fold change3 | P4 | Genes | Fold change3 | P4 |
| C7 | 4.00 | 0.003 | C7 | 1.15 | 0.63 | C7 | 0.29 | 0.0006 |
| IL28B | 3.33 | 0.007 | IL28B | 1.22 | 0.48 | IL28B | 0.36 | 0.003 |
| NCAM1 | 2.94 | 0.005 | NCAM1 | 2.22 | 0.002 | NCAM1 | 0.75 | 0.30 |
| IL1RAP | 0.39 | 0.003 | IL1RAP | 0.85 | 0.39 | IL1RAP | 2.19 | 0.001 |
| FCAR | 0.24 | 0.0009 | FCAR | 0.49 | 0.01 | FCAR | 2.04 | 0.02 |
Ratio between complete and non-responders
Complete responder vs non-responder
Biopsy 1 versus biopsy 2
Biopsy 1 versus biopsy 2
DISCUSSION
This is the first study to profile the expression of immune system genes in the kidneys of patients with LN before and after induction therapy, and associate changes in transcript expression with clinical responses to treatment. Several general observations emerged from this investigation. Most importantly, despite complete clinical remission after induction, intra-renal immune and inflammatory gene expression did not return to levels seen in normal kidneys, although most of these up or down-regulated (compared to normal) transcripts did decrease or increase expression toward normal, respectively. These findings are consistent with the lack of improvement in the activity index at Bx2 in clinical responders.
Patients who did not achieve a clinical response had many more immune genes activated or suppressed at flare than CR. In contrast to the changes observed in CR kidneys, in NR kidneys the expression of most of these genes tended to move further away from normal expression with treatment. Additionally, during treatment, a handful of additional genes became up or down-regulated in responders, but many more became up or down-regulated in NR.
Overall, several conclusions may be drawn from these data. First, the results suggest that inflammatory and immune-injury pathways are highly dysregulated in the kidneys of patients at LN flare, and that standard-of-care induction therapy is not adequate to normalize these pathways even in patients who respond well clinically. These data highlight the importance of maintenance immunosuppressive therapy after induction therapy has been completed for all LN patients. Furthermore, the extent of gene dysregulation is far greater in patients who do not respond to induction. While not statistically significant, the chronicity index at flare in NR was higher than CR and may account for some of the difference seen in transcript expression at flare between the 2 groups, however both the activity and chronicity indices after treatment were similar between the two groups reinforcing the limitations of histology to recapitulate molecular changes. Thus, the highly dysregulated state of NR kidneys cannot be readily detected clinically at flare, and although proteinuria was greater in NR than CR, both groups presented with nephrotic-range proteinuria. Thus, molecular imaging of the kidney biopsy can provide clinically-useful information not available by routine histology or clinical measurements.
Comparing the specific transcripts differentially-expressed in CR and NR at flare, and how these transcripts changed with treatment identified several immune pathways that could be therapeutically targeted to improve clinical response in LN. These include the type 1 interferon pathway, the alternative complement pathway, and T-cell activation pathways.
Type I interferon genes were upregulated at flare in CR and NR. CR tended to downregulate interferon gene expression with treatment, but in NR, interferon-associated transcripts continued to increase. This suggests that anti-interferon therapy, now in clinical trial (NCT # NCT02547922), may be useful both in the initial treatment of LN and to potentially salvage patients who have not responded to induction therapy. Similarly, in both CR and NR, activators of the alternative complement pathway were upregulated in the kidney at flare, while regulators of the alternative complement pathway were down-regulated. As with interferon-inducible transcripts, many of the complement genes tended to normalize expression with treatment in CR, but continued to be abnormally activated or suppressed in NR, suggesting that inhibitors of the alternative complement pathway, such as eculizumab or a C5a receptor antagonist (11, 12), could be effective during the induction phase of LN treatment. Finally, several T cell activation transcripts appeared to be regulated differently in CR and NR kidneys. The most striking difference was the enhanced activation of these genes in NR after treatment. Specifically, transcripts important for T cell receptor recognition and engagement of antigen and kinases responsible for signal transduction and ultimately T cell activation were upregulated in NR only after treatment (13). This enhanced T cell activation profile in NR may provide a mechanistic explanation for the apparent effectiveness of adding a calcineurin inhibitor to standard of care therapy in increasing the short term CR rates (14).
The specific transcripts upregulated in NR and CR kidneys relative to normal suggest that treatment resistance may be explained, in part, by activation of a program of tissue inflammation in NR kidneys that overwhelms standard-of-care therapy. For example, leukocyte chemotactic factors and their receptors are over-expressed in NR kidneys. CCL19 codes for macrophage-inflammatory protein beta-3, a T, B and dendritic cell chemoattractant previously shown to be expressed in SLE (15, 16). Transcripts for the B cell chemoattractant CXCL13, which is selective for B cells and follicular T cells were upregulated. CXCL13 has also previously been implicated in the pathogenesis of SLE in experimental models (17, 18), and serum levels correlated with SLE and LN disease activity (19).
Once recruited to the kidney, the persistence and activation of inflammatory cells may be facilitated by leukocyte integrins, colony stimulating factors and receptors, whose transcripts (ITGAL, ITGB2, ITGAM, ITGAX, CSF2RB, CSF3R, FCAR/CD89, IL1RAP, PTPRC/CD45) are upregulated in NR. Consistent with our observations, genome-wide association studies demonstrated that single nucleotide polymorphisms of ITGAM and ITGAX strongly associated with SLE (20), and FCAR has also been shown to be overexpressed in peripheral blood mononuclear cells of SLE patients (21). PTPRC is expressed on all leukocytes and PTPRC levels are not increased at flare or after treatment in CR, but were increased in NR at flare and continued to rise after treatment.
We also directly compared changes in transcript expression between Bx1 and Bx2 in CR and NR. Several additional transcript differences were seen by this analysis that was not apparent during the comparisons to normal kidneys. NCAM1, FCAR, and IL1RAP are of particular interest. NCAM1 expression increased with treatment in responders only while FCAR and IL1RAP expression increased in NR but decreased in CR with treatment.
NCAM1 is an adhesion molecule largely found in developing kidneys, but minimally expressed in adult kidneys (22). NCAM1 expression increases when the adult kidney is damaged and may facilitate tubular regeneration (23). It is also present in the early stages of fibrosis (24). In LN, activation of NCAM1 may be important in renal healing by mediating repair of tubular epithelium and regulating the early stages of interstitial fibrosis (23). In an experimental model of acute tubular necrosis, NCAM1+ interstitial cells increased in the early phase of repair, and kidney injury was prolonged when these cells were eliminated (23). Additionally, NCAM1+ interstitial cells increase in the early stages of interstitial fibrosis but are not present at late stages of fibrosis (22). Increased tissue expression of NCAM1 after treatment in CR may signify ongoing repair that is not occurring in NR.
FCAR is the Fc receptor for IgA and encodes a transmembrane glycoprotein present on myeloid cells, including neutrophils, eosinophils, and macrophages, which activates several immune processes including endocytosis, phagocytosis, antibody-dependent cell-mediated cytotoxicity, and release of inflammatory mediators (25). Increased expression of FCAR may reflect infiltration of myeloid cells such as neutrophils and macrophages and suggests ongoing inflammation in non-responders.
IL1RAP forms a complex with the interleukin-1 receptor and activates pro-inflammatory cytokines through NF-κB (26). This complex is responsible for IL-1-dependent activation of NF-κB and through NF-κB, pro-inflammatory cytokines are expressed in areas of tissue damage. Increased expression of IL1RAP after treatment in non-responders is likely a response to ongoing kidney injury and reflects increased NF-κB-mediated cytokine expression in areas of active inflammation within the kidney.
This study has limitations. Sample size is small, however these are clinical samples and the availability of serial biopsies for LN is limited. Additionally, our data are similar to previous studies showing the molecular heterogeneity of LN (1, 27). For example, a recent investigation of LN using microarray analysis of murine kidneys at different disease stages showed a significant increase in inflammatory gene expression at the onset of proteinuria that improved with treatment and returned to baseline levels at clinical remission. The LN cohort was Hispanic and from Argentina, and the controls were from Ohio. It is possible that some of the molecular heterogeneity of the kidney in LN is influenced by race/ethnicity. Therefore, these results may not be generally applicable to all LN patients. Finally, whole kidney cortex was used, and because the cortex represents mostly the tubulointerstitial space the data may not accurately reflect glomerular events.
In summary, molecular imaging of serial kidney biopsies from LN patients identifies several immune and inflammatory pathways that are dysregulated in the kidneys during active disease. Many of these pathways are consistent with what is known of the pathogenesis of kidney injury in LN. Importantly, molecular imaging shows how these pathways respond to standard-of-care immunosuppression. It is clear that even with aggressive immunosuppression it is very difficult to turn off the dsyregulated inflammatory and immune pathways active in LN kidneys. However the data do suggest several therapeutic targets that could facilitate earlier and more complete disease resolution at the tissue level in clinical responders, and rescue clinical non-responders. Some of these targets have not yet been tested or are just undergoing clinical trials in human LN. Molecular imaging of the kidney in LN provides insights into disease mechanisms that cannot be appreciated by histology alone. This approach to LN biomarker development that may facilitate personalized medicine in LN and improve long-term kidney outcomes.
Background.
The diagnosis of lupus nephritis relies on clinical and histologic findings. Unfortunately, these findings do not inform treatment or determine prognosis. Additionally, there are no markers to differentiate treatment responders from non-responders.
Translational Significance.
We evaluated the molecular profile of serial kidney biopsies in human LN to identify intra-renal transcripts that differentiate treatment responders from non-responders. This has not been previously explored and provides insights into disease pathogenesis that cannot be appreciated by histology alone. We identify pathways that affect treatment response and may serve as novel therapeutic targets to improve response rates and facilitate personalized medicine in LN.
Acknowledgements
This work was supported by NIDDK U01: DK096927 (BHR) and Mallinckrodt/Questcor Fellowship Grant: 00033990 (SVP),
The Genomics Shared Resource (at Comprehensive Cancer Center, Department of Molecular Virology, Immunology and Medical Genetics at The Ohio State University Wexner Medical Center) assisted with nanostring analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Rovin is a Scientific Advisor for Biogen Idec, Mallinckrodt, Centocor, Lilly, GlaxoSmithKline, Abbivie, and Genentech. Dr. Rovin has received research funding from Biogen Idec and Malinckrodt
Dr. Parikh has received a Research Fellowship Grant from Mallinckrodt and served as an Consultant for Alexion Pharmaceuticals
There are no other conflicts of interest to disclose
All of the Authors have read the Journal’s policy on Conflict of Interest
All of the Authors have read the Journal’s Authorship Agreement
REFERENCES
- 1.Berthier CC, Bethunaickan R, Gonzalez-Rivera T, Nair V, Ramanujam M, Zhang W, Bottinger EP, Segerer S, Lindenmeyer M, Cohen CD, Davidson A, Kretzler M. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. Journal of immunology. 2012;189:988–1001. doi: 10.4049/jimmunol.1103031. Available at http://www.ncbi.nlm.nih.gov/pubmed/22723521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethunaickan R, Berthier CC, Zhang W, Eksi R, Li HD, Guan Y, Kretzler M, Davidson A. Identification of stage-specific genes associated with lupus nephritis and response to remission induction in (NZB × NZW)F1 and NZM2410 mice. Arthritis & rheumatology. 2014;66:2246–2258. doi: 10.1002/art.38679. Available at http://www.ncbi.nlm.nih.gov/pubmed/24757019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarado A, Malvar A, Lococo B, Alberton V, Toniolo F, Nagaraja H, Rovin B. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus. 2014;23:840–847. doi: 10.1177/0961203313518625. Available at http://www.ncbi.nlm.nih.gov/pubmed/24401872. [DOI] [PubMed] [Google Scholar]
- 4.Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, Nagaraja H, Indrakanti D, Rovin BH. Histologic versus clinical remission in proliferative lupus nephritis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association; 2015. Available at http://www.ncbi.nlm.nih.gov/pubmed/26250434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM, American College of R American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis care & research. 2012;64:797–808. doi: 10.1002/acr.21664. Available at http://www.ncbi.nlm.nih.gov/pubmed/22556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh SV, Malvar A, Song H, Alberton V, Lococo B, Vance J, Zhang J, Yu L, Rovin BH. Characterising the immune profile of the kidney biopsy at lupus nephritis flare differentiates early treatment responders from non-responders. Lupus science & medicine. 2015;2:e000112. doi: 10.1136/lupus-2015-000112. Available at http://www.ncbi.nlm.nih.gov/pubmed/26629350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dave V, Volberg V, Huen K, Holland N. Use of the nCounter System for the Analysis of Multiple RNA Expression Profiles in Peripheral Blood Mononuclear Cells. Environ Mol Mutagen. 2013;54:S29–S29. [Google Scholar]
- 8.Golubeva Y, Salcedo R, Mueller C, Liotta LA, Espina V. Laser capture microdissection for protein and NanoString RNA analysis. Methods in molecular biology. 2013;931:213–257. doi: 10.1007/978-1-62703-056-4_12. Available at http://www.ncbi.nlm.nih.gov/pubmed/23027006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaes E, Khan M, Mombaerts P. Statistical analysis of differential gene expression relative to a fold change threshold on NanoString data of mouse odorant receptor genes. BMC bioinformatics. 2014;15:39. doi: 10.1186/1471-2105-15-39. Available at http://www.ncbi.nlm.nih.gov/pubmed/24495268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartor MA, Tomlinson CR, Wesselkamper SC, Sivaganesan S, Leikauf GD, Medvedovic M. Intensity-based hierarchical Bayes method improves testing for differentially expressed genes in microarray experiments. BMC bioinformatics. 2006;7:538. doi: 10.1186/1471-2105-7-538. Available at http://www.ncbi.nlm.nih.gov/pubmed/17177995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. European journal of immunology. 2005;35:2496–2506. doi: 10.1002/eji.200526327. Available at http://www.ncbi.nlm.nih.gov/pubmed/16052609. [DOI] [PubMed] [Google Scholar]
- 12.Pickering MC, Ismajli M, Condon MB, McKenna N, Hall AE, Lightstone L, Terence Cook H, Cairns TD. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology. 2015;54:2286–2288. doi: 10.1093/rheumatology/kev307. Available at http://www.ncbi.nlm.nih.gov/pubmed/26316577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak A, Kow NY. The pathology of T cells in systemic lupus erythematosus. Journal of immunology research. 2014;2014:419029. doi: 10.1155/2014/419029. Available at http://www.ncbi.nlm.nih.gov/pubmed/24864268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, Chen J, Lin H, Liu F, He Y, He Y, Miao L, Chen N, Li Y, Gu Y, Shi W, Hu W, Liu Z, Bao H, Zeng C, Zhou M. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Annals of internal medicine. 2015;162:18–26. doi: 10.7326/M14-1030. Available at http://www.ncbi.nlm.nih.gov/pubmed/25383558. [DOI] [PubMed] [Google Scholar]
- 15.Adhya Z, Borozdenkova S, Karim MY. The role of cytokines as biomarkers in systemic lupus erythematosus and lupus nephritis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:3273–3280. doi: 10.1093/ndt/gfq860. Available at http://www.ncbi.nlm.nih.gov/pubmed/21372259. [DOI] [PubMed] [Google Scholar]
- 16.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. Journal of immunology. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. Available at http://www.ncbi.nlm.nih.gov/pubmed/12077273. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa S, Sato T, Abe M, Nagai S, Onai N, Yoneyama H, Zhang Y, Suzuki T, Hashimoto S, Shirai T, Lipp M, Matsushima K. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. The Journal of experimental medicine. 2001;193:1393–1402. doi: 10.1084/jem.193.12.1393. Available at http://www.ncbi.nlm.nih.gov/pubmed/11413194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worthmann K, Gueler F, von Vietinghoff S, Davalos-Misslitz A, Wiehler F, Davidson A, Witte T, Haller H, Schiffer M, Falk CS, Schiffer L. Pathogenetic role of glomerular CXCL13 expression in lupus nephritis. Clinical and experimental immunology. 2014;178:20–27. doi: 10.1111/cei.12380. Available at http://www.ncbi.nlm.nih.gov/pubmed/24827905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffer L, Worthmann K, Haller H, Schiffer M. CXCL13 as a new biomarker of systemic lupus erythematosus and lupus nephritis - from bench to bedside? Clinical and experimental immunology. 2015;179:85–89. doi: 10.1111/cei.12439. Available at http://www.ncbi.nlm.nih.gov/pubmed/25138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, Ballinger DG, Kosoy R, Demirci FY, Kamboh MI, Kao AH, Tian C, Gunnarsson I, Bengtsson AA, Rantapaa-Dahlqvist S, Petri M, Manzi S, Seldin MF, Ronnblom L, Syvanen AC, Criswell LA, Gregersen PK, Behrens TW. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. The New England journal of medicine. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. Available at http://www.ncbi.nlm.nih.gov/pubmed/18204098. [DOI] [PubMed] [Google Scholar]
- 21.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. Available at http://www.ncbi.nlm.nih.gov/pubmed/12604793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markovic-Lipkovski J, Muller CA, Klein G, Flad T, Klatt T, Blaschke S, Wessels JT, Muller GA. Neural cell adhesion molecule expression on renal interstitial cells. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:1558–1566. doi: 10.1093/ndt/gfm006. Available at http://www.ncbi.nlm.nih.gov/pubmed/17337466. [DOI] [PubMed] [Google Scholar]
- 23.Buzhor E, Omer D, Harari-Steinberg O, Dotan Z, Vax E, Pri-Chen S, Metsuyanim S, Pleniceanu O, Goldstein RS, Dekel B. Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. The American journal of pathology. 2013;183:1621–1633. doi: 10.1016/j.ajpath.2013.07.034. Available at http://www.ncbi.nlm.nih.gov/pubmed/24055371. [DOI] [PubMed] [Google Scholar]
- 24.Markovic-Lipkovski J, Zivotic M, Muller CA, Tampe B, Cirovic S, Vjestica J, Tomanovic N, Zeisberg M, Muller GA. Variable Expression of Neural Cell Adhesion Molecule Isoforms in Renal Tissue: Possible Role in Incipient Renal Fibrosis. PloS one. 2015;10:e0137028. doi: 10.1371/journal.pone.0137028. Available at http://www.ncbi.nlm.nih.gov/pubmed/26327314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otten MA, van Egmond M. The Fc receptor for IgA (FcalphaRI, CD89) Immunology letters. 2004;92:23–31. doi: 10.1016/j.imlet.2003.11.018. Available at http://www.ncbi.nlm.nih.gov/pubmed/15081523. [DOI] [PubMed] [Google Scholar]
- 26.Lang D, Knop J, Wesche H, Raffetseder U, Kurrle R, Boraschi D, Martin MU. The type II IL-1 receptor interacts with the IL-1 receptor accessory protein: a novel mechanism of regulation of IL-1 responsiveness. Journal of immunology. 1998;161:6871–6877. Available at http://www.ncbi.nlm.nih.gov/pubmed/9862719. [PubMed] [Google Scholar]
- 27.Peterson KS, Huang JF, Zhu J, D'Agati V, Liu X, Miller N, Erlander MG, Jackson MR, Winchester RJ. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. The Journal of clinical investigation. 2004;113:1722–1733. doi: 10.1172/JCI19139. Available at http://www.ncbi.nlm.nih.gov/pubmed/15199407. [DOI] [PMC free article] [PubMed] [Google Scholar]