Abstract
Adenosine has an important role in inflammation and tissue remodeling and promotes dermal fibrosis by adenosine receptor (A2AR) activation. Adenosine may be formed intracellularly from adenine nucleotides or extracellularly through sequential phosphohydrolysis of released ATP by nucleoside triphosphate diphosphohydrolase (CD39) and ecto-5′-nucleotidase (CD73). Because the role of these ecto-enzymes in fibrosis appears to be tissue specific, we determined whether these ectonucleotidases were directly involved in diffuse dermal fibrosis. Wild-type and mice globally deficient in CD39 knockout (CD39KO), CD73 (CD73KO), or both (CD39/CD73DKO) were challenged with bleomycin. Extracellular adenosine levels and dermal fibrosis were quantitated. Adenosine release from skin cultured ex vivo was increased in wild-type mice after bleomycin treatment but remained low in skin from CD39KO, CD73KO, or CD39/CD73DKO bleomycin-treated mice. Deletion of CD39 and/or CD73 decreased the collagen content, and prevented skin thickening and tensile strength increase after bleomycin challenge. Decreased dermal fibrotic features were associated with reduced expression of the profibrotic mediators, transforming growth factor-β1 and connective tissue growth factor, and diminished myofibroblast population in CD39- and/or CD73-deficient mice. Our work supports the hypothesis that extracellular adenosine, generated in tandem by ecto-enzymes CD39 and CD73, promotes dermal fibrogenesis. We suggest that biochemical or biological inhibitors of CD39 and/or CD73 may hold promise in the treatment of dermal fibrosis in diseases such as scleroderma.
Tissue damage leads to the release of the signaling nucleoside adenosine, which, by engaging specific adenosine receptors (A1R, A2AR, A2BR, and A3R), exhibits both tissue-protective and tissue-destructive effects.1, 2, 3, 4 In particular, adenosine is a potent regulator of tissue repair, and we have previously reported that adenosine promotes dermal fibrosis via the A2AR receptor, as shown in vitro,5 in a bleomycin-induced dermal injury model of scleroderma,6 and in a model of elevated tissue adenosine.7 Similarly, we found that pharmacological blockade of A2AR diminishes skin scarring.8
Elevations in extracellular adenosine can result from either an increase in intracellular adenosine, followed by release into the extracellular space, or the release of adenine nucleotides, followed by their extracellular catabolism into adenosine.9 The main source of extracellular adenosine stems from the enzymatic phosphohydrolysis of precursor nucleotides to adenosine.10, 11, 12, 13 This is achieved by a two-step enzymatic process involving the ecto-apyrase, CD39 (conversion of ATP/ADP to AMP) and the ecto-5′-nucleotidase, CD73 (conversion of AMP to adenosine).14 It is widely accepted that CD39 and CD73 promote anti-inflammatory effects of adenosine in the immune system,15, 16, 17 and both enzymes have been previously shown to attenuate acute injury and inflammation in models of ambient hypoxia,18, 19 cyclic mechanical stretch,20 and bleomycin-induced lung injury.2 However, CD39 and CD73 promote fibrosis in murine models of pancreatitis21 and hepatic fibrosis,22 respectively, suggesting an important role for CD39 and CD73 in the regulation of fibrogenesis in vivo.
We hypothesized that limiting extracellular adenosine levels by CD39 and/or CD73 gene deletion may protect against bleomycin-induced dermal fibrosis, a model of scleroderma. CD39-deficient, CD73-deficient, and CD39/73 double-deficient mice were subjected to bleomycin-induced skin injury, and the extent of skin fibrosis was compared with the wild-type (WT) mice. Our results show that, after bleomycin injection, mice globally null for CD39 and/or CD79 released lower levels of adenosine and concurrently developed less dermal fibrosis, indicating that adenosine generation by CD39 and CD73 is highly likely to be a critical regulator of fibrogenesis in skin.
Materials and Methods
Animals
The WT C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). CD39 (ENTPD1) knockout mice (CD39KO) and ecto-5′-nucleotidase knockout mice (CD73KO) were generated as described previously23, 24, 25 and backcrossed >10 generations onto a C57BL/6 background. Double-knockout mice null for both CD39 and CD73 were generated by intercrossing the single knockouts.
Animals were bred in the animal facilities of the School of Medicine of New York University (New York City). All experimental mice used were 6- to 8-week-old male mice. All experimental procedures were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the School of Medicine of New York University.
Morphometric Dermal Measurements in Bleomycin-Treated Mice
Mice were injected with either 0.1 mL PBS or 10 mg/mL bleomycin (0.1 mL s.c. on alternate days) for 21 days, and were sacrificed at the end of the experimental period. The backs of the animals were shaved before morphometric measurements. Skinfold (pinch) thickness was measured using skin calipers on the same area over the middle to upper back of the mice. Breaking strength of the skin was measured on the 6-mm punch biopsy specimens using a tensiometer (Series EG Digital Force Gauge; Mark-10, Copiague, NY), and the point of maximal stress before tearing of the biopsy specimen was recorded, as we have previously reported.6 All measurements were taken in a blinded manner.
Quantification of Adenosine Levels by High-Performance Liquid Chromatography
Skin biopsy specimens were washed in PBS containing antibiotics (penicillin, 200 U/L; streptomycin, 200 μg/L; and amphotericin B 50 μg/L), cut into small pieces, and incubated in Dulbecco's modified Eagle's medium (containing the same antibiotic concentration as before) at 37°C, 5% CO2. After 2 hours of incubation, supernatants were collected and adenosine was extracted and quantified by high-performance liquid chromatography, as previously described.26 Results were expressed as pmol adenosine/mg tissue.
Quantification of Dermal Hydroxyproline Content
Hydroxyproline content in tissue specimens was measured colorimetrically, as described previously.7 Results were expressed as μg hydroxyproline/mg tissue.
Western Blot Analysis
Skin biopsy specimens were lysed in T-PER tissue protein extraction reagent (Pierce Biotechnology, Rockford, IL). Total protein was determined spectrophotometrically by a bicinchoninic acid assay kit (Pierce Biotechnology), using bovine serum albumin as standard protein. Skin homogenates (20 μg protein/lane) were electrophoresed (4% to 20% SDS Tris-glycine) and transferred onto nitrocellulose membranes. The nitrocellulose membranes were blocked for 2 hours at 4°C in blocking solution [3% bovine serum albumin in 1× Tween 20 Tris-buffered saline (TTBS), which consists of 20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, and 0.1% Tween 20]. After blocking, the membranes were incubated with primary antibodies using the following dilutions: 1:1000 for α-smooth muscle actin (SMA; Abcam Cambridge, MA), 1:1000 for transforming growth factor-β1 [TGF-β1; a gift of Dr. Leslie B. Gold (New York University, NY)], and 1:5000 for β-actin (Sigma, St. Louis, MO), and incubated for 2 hours at 37°C with gentle shaking on a platform shaker. After incubation with secondary antibody, proteins were visualized using the enhanced chemifluorescene kit (Amersham Biosciences, Piscataway, NJ). Band intensities were analyzed by the Adobe Photoshop CS2 (Adobe Systems, Mountain View, CA) and normalized to the β-actin level.
Real-Time RT-PCR
Total RNA from skin biopsy specimens was isolated using an RNeasy Fibrous Tissue kit (Qiagen, Valencia, CA), according to the manufacturer's protocol. RNA was quantified using spectrophotometric OD260 measurements, and quality was assessed by the OD260/OD280 ratio. Reverse transcription was performed using the GeneAmp RNA Core Kit (Applied Biosystems, Carlsbad, CA) in a volume of 25 μL using oligo dT primers and MuLV Reverse Transcriptase (Applied Biosystems), according to the manufacturer's protocol. Real-time PCRs were performed using the SYBR Green PCR Master Mix (Stratagene, Santa Clara, CA), following the manufacturer's instructions, and performed on the Mx3005P Q-PCR system (Stratagene). Aliquots of reverse transcription reactions were subjected to PCR in 25-μL reactions with SYBR Green using primers for CTGF: forward, 5′-TCCTACCGCGTCCCGATCAT-3′ and reverse, 5′-GCTTTACGCCATGTCTCCGT-3′; TGFβ1: forward, 5′-GTCAGACATTCGGGAAGCAG-3′ and reverse, 5′-GCGTATCAGTGGGGGTCA-3′; and GAPDH: forward, 5′-CTACACTGAGGACCAGGTTGTCT-3′ and reverse, 5′-GGTCTGGGATGGAAATTGTG-3′. For each assay, standards, no-template, and no-RT controls were included to verify the quality and cDNA specificity of the primers. Comparison of the expression of each gene between its control and stimulated states was determined with ΔΔCT, according to the following formula:
| (1) |
where the gene of interest (GOI) corresponds to the CTGF or TGFβ1 and the housekeeping gene (HKG) corresponds to GAPDH. Fold increase was calculated according to the formula:
| (2) |
Immunohistochemistry
After deparaffination and rehydration of tissue sections (5 μm thick), antigen retrieval was performed for 15 minutes at 98°C with 0.01 mol/L citrate buffer, pH 6.0. To block non-specific binding, the slides were incubated for 30 minutes with 5% normal goat serum in TTBS buffer (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, and 0.1% Tween 20). The 1:100 anti–α-SMA primary antibody in TTBS containing 1.5% normal goat serum was incubated overnight at 4°C. After washing, secondary antibody (alkaline phosphatase– or peroxidase-conjugated goat anti-rabbit immunoglobulins) in TTBS containing 1.5% normal goat serum was incubated for 60 minutes at room temperature. Fast Red substrate system (Dako, Carpinteria, CA) or diaminobenzidine (DAB) substrate (Vector, Burlington, ON, Canada) was used according to reagent availability to detect positive staining; no appreciable differences were observed in control slides (from bleomycin-treated WT mice) stained with either Fast Red or DAB substrate. Counterstaining was performed with Gill's hematoxylin. Negative staining control experiments were performed according to the previously described protocol, with omission of the primary antibody. Images were obtained with a Q-Imaging (Surrey, BC, Canada) Retiga digital camera mounted on an Olympus BX51 microscope (Olympus America Inc., Center Valley, PA). The number of myofibroblasts was determined by quantifying positive cells in six independent fields for each skin section (n = 3 mice per group).
Statistical Analysis
Results are represented as means ± SEM. Data were analyzed by one-way analysis of variance, and post hoc analyses of significance of differences between groups were determined by Dunnett's multiple comparison tests. All statistical analyses were performed with Graphpad Prism software version 4.02 (Graphpad, San Diego, CA).
Results
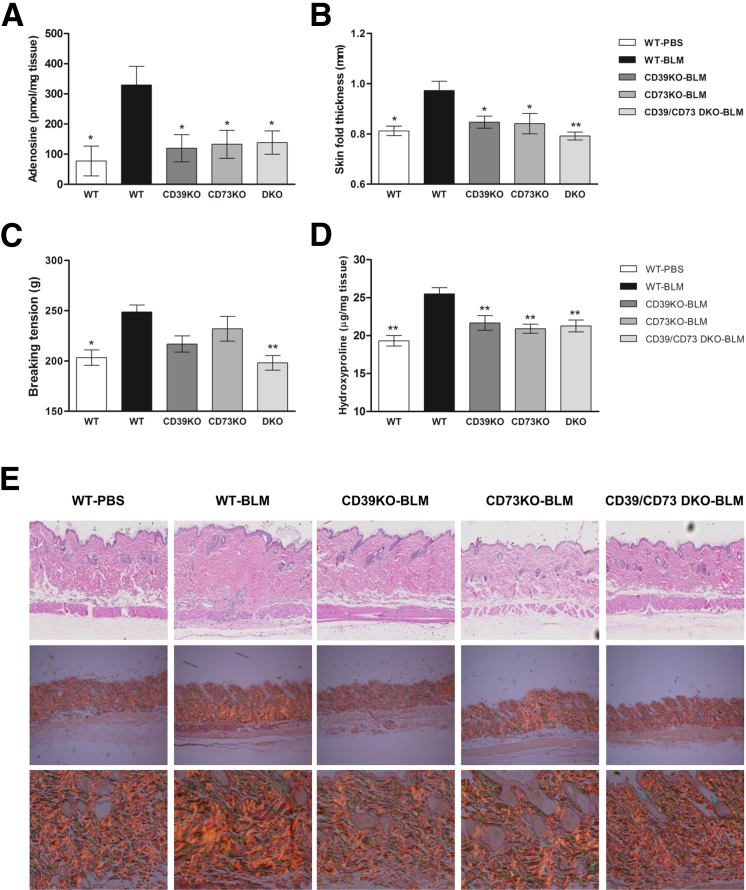
It has been previously reported that adenosine critically contributes to bleomycin-induced dermal fibrosis6 and that CD39 and CD73 play an important role in fibrogenesis after pancreatitis and in hepatic fibrosis, respectively.21, 22 However, to our knowledge, the contributions of CD39 and CD73 on the progression of skin fibrosis have not been studied. We, therefore, challenged WT mice and mice deficient for CD39 (CD39KO), CD73 (CD73KO), and double CD39/CD73 (CD39/CD73DKO)–deficient mice with the known sclerosant, bleomycin. As expected, bleomycin treatment significantly increased the level of adenosine release from skin of WT mice (more than threefold increase, P < 0.01), but not in mice deficient for CD39 and/or CD73 (Figure 1A), indicating that bleomycin promotion of adenosine generation proceeds via the two-step enzymatic process involving both CD39 and CD73.
Figure 1.
Deficiency of CD39 and/or CD73 limits adenosine levels and dermal fibrosis after bleomycin treatment. A: Skin adenosine levels were measured by high-performance liquid chromatography in supernates after 2 hours of skin culture. ∗P < 0.05 (n = 6 to 12 skin samples per group). Skinfold thickness (B) and breaking tension (C) measurements were performed on freshly excised skin and 6-mm skin punch biopsy specimens. D: Dermal hydroxyproline content was assessed on 6-mm skin biopsy specimens. Data represent means ± SEM. ∗P < 0.05, ∗∗P < 0.01, comparisons versus WT + bleomycin (BLM); analysis of variance, followed by Dunnett's post-test analyses. E: Skin histological sections were stained with H&E (top row) and picrosirius red, viewed under polarized microscopy (middle and bottom rows). Original magnifications: ×20 (E, top row); ×10 (E, middle row); ×40 (E, bottom row).
Morphometric measurements in skin after 21 days of bleomycin treatment revealed a significant increase of skin thickness in WT mice, but not in CD39KO, CD73KO, and DKO mice (Figure 1B), which is also reflected in H&E skin sections (Figure 1E). Breaking tension of the skin, an indicator of tensile strength, was increased in bleomycin-treated WT mice, whereas this change was less pronounced in CD39KO and CD73KO mice. In CD39/CD73DKO mice, tensile strength was significantly inhibited when compared with WT mice after bleomycin treatment (Figure 1C).
Collagen production was assessed by measuring hydroxyproline content in skin biopsy specimens and by picrosirius red staining of skin sections. Interestingly, deletion of CD39 and/or CD73 completely prevented the bleomycin-induced increase in dermal collagen (Figure 1D), because hydroxyproline levels remain at low levels, similar to those of PBS-treated WT mice. No difference in basal collagen content was found in PBS-treated KO mice compared with WT mice (Supplemental Figure S1). Picrosirius red stains of collagen impart an intense yellow to red birefringence to thick and densely packed fibrils when viewed under polarized light.27, 28 As shown in Figure 1E, a denser packaging of the collagen fibrils, indicated by an increase of the yellow to red birefringence, is observed in skin sections of bleomycin-treated WT, but not in CD39KO, CD73KO, and CD39/CD73DKO, mice.
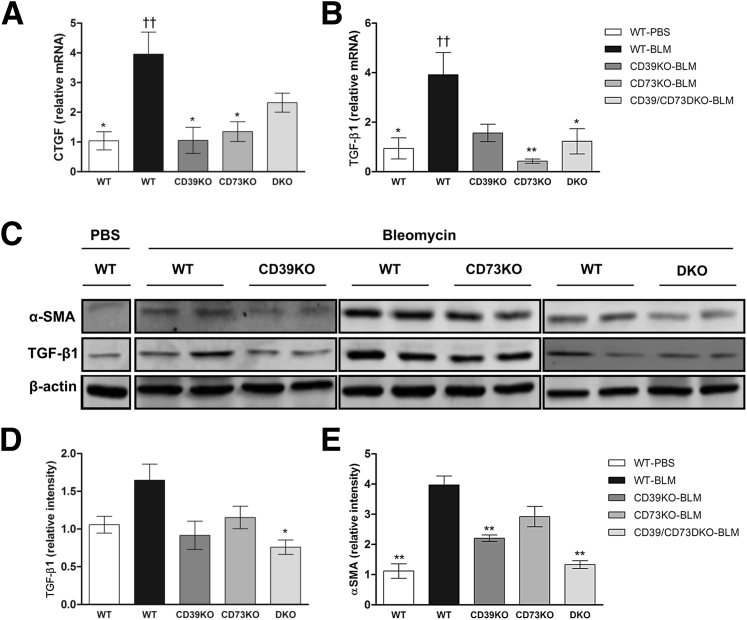
We have recently shown that CTGF is an important mediator of the fibrogenic actions of adenosine29 in human dermal fibroblasts and in a mouse model of elevated tissue adenosine, where skin fibrosis mediated by A2AR activation is associated with increased levels of CTGF and TGF-β.7 In agreement, we found that mRNA levels of CTGF and TGF-β are increased by bleomycin only in the WT mice (bleomycin- versus PBS-treated WT mice; P < 0.01), but not in the CD39KO, CD73KO, or CD39/CD73DKO mice (comparisons versus PBS-treated WT mice; P > 0.05) (Figure 2, A and B). Low levels of TGF-β in CD39- and/or CD73-deficient mice were also corroborated at the protein level (Figure 2, C and D).
Figure 2.
Deficiency of CD39 and/or CD73 prevents synthesis of profibrotic mediators after bleomycin (BLM) treatment. mRNA levels of connective tissue growth factor (CTGF) (A) and TGF-β1 (B) were measured by quantitative RT-PCR in skin lysates. mRNA levels of CTGF and TGF-β are increased by bleomycin only in the WT mice. ††P < 0.01, bleomycin- versus PBS-treated WT mice. Protein levels of α-SMA and TGF-β1 were assessed by using Western blot analysis, and representative blots are shown (C). Band intensities were quantified and normalized to β-actin as loading control (D and E). Data represent means ± SEM (A, B, D, and E). ∗P < 0.05, ∗∗P < 0.01 for comparisons made versus WT-bleomycin (BLM) by analysis of variance followed by Dunnett's post-test analyses.
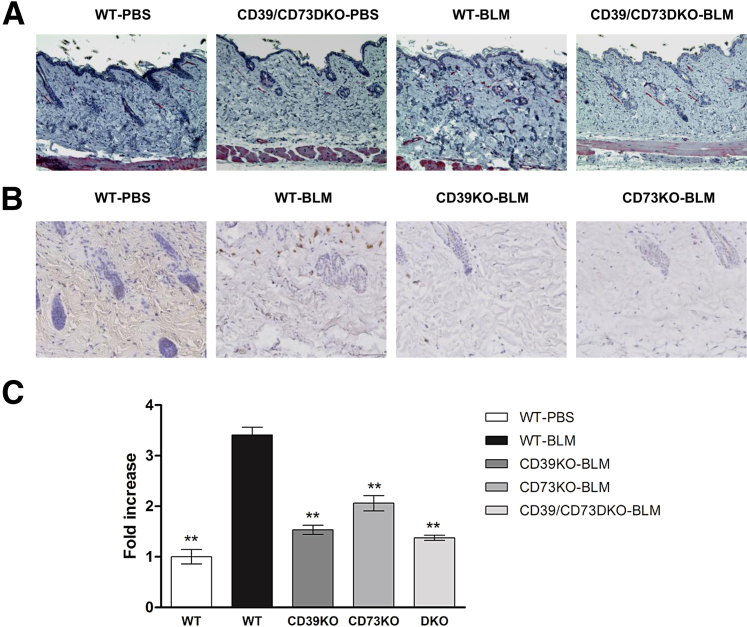
Myofibroblasts play a central role in the accumulation of excessive collagen and the contraction of the extracellular matrix in fibrosing diseases of the skin. These cells have a phenotype characterized by excessive production of collagenous extracellular matrix and tensile force,30 playing a pivotal role in the establishment of fibrotic conditions.31 It has become accepted that neo-expression of α-SMA in myofibroblasts regulates essential phenomena for organ fibrosis,30, 31 and myofibroblast differentiation and organ fibrosis are predominantly controlled by TGF-β.32 Therefore, we first analyzed the expression of α-SMA in skin lysates. As shown in Figure 2, C and E, α-SMA protein levels were increased in WT mice after bleomycin treatment, but remained low in CD39- and/or CD73-deficient mice. We confirmed that the myofibroblast population was significantly reduced in bleomycin-treated CD39KO, CD73KO, and CD39/CD73DKO mice compared with bleomycin-treated WT mice by quantification of α-SMA+ cells in skin sections (Figure 3).
Figure 3.
Deficiency of CD39 and/or CD73 prevents myofibroblast accumulation after bleomycin treatment. Anti–α-SMA immunohistochemistry was performed on skin histological sections on CD39/CD73DKO mice (A) and on CD39- or CD73-deficient mice (B). Fast Red or DAB substrates were used to develop immunostaining, respectively. Representative photomicrographs are shown for each group. C: Number of α-SMA+ cells were counted in skin cross sections, and data represent means ± SEM. Original magnification, ×20. ∗∗P < 0.01. All comparisons were made versus WT-bleomycin (BLM) by analysis of variance, followed by Dunnett's post-test analyses.
Discussion
In the present work, we investigated the role of CD39 and CD73 in an established model of dermal fibrosis induced by bleomycin. WT, CD39, and CD73 KO mice, as well as CD39/CD73DKO mice, were challenged with bleomycin, and dermal fibrosis was analyzed. As expected, bleomycin promoted a dramatic increase in skin adenosine production, but skin from CD39KO, CD73KO, and CD39/CD73DKO mice generated significantly lower levels of adenosine when compared with WT mice (Figure 1A). As previously found,6 bleomycin caused severe fibrotic changes in the skin, as indicated by increased skin thickening, tensile strength, and collagen content (Figure 1, B–D), as well as increased collagen density in the dermis, as measured by picrosirius red stain (Figure 1E). Increased collagen deposition was accompanied by increased levels of mediators of fibrosis, including TGF-β1, CTGF, and fibroblast transformation to myofibroblasts (Figures 2 and 3). Interestingly, in support of the hypothesis that extracellular adenosine generation by the enzymatic process involving CD39 and CD73 plays a critical role in the development of fibrosis in the skin, the fibrotic response elicited by bleomycin was significantly diminished in the CD39KO, CD73KO, and CD39/CD73DKO mice, strongly suggesting that extracellular generation of adenosine is needed for the development of dermal fibrosis in response to bleomycin injection.
Extracellular adenosine signals via binding to one or more of four adenosine receptors (A1R, A2AR, A2BR, and A3R)10 and adenosine activation of its A2AR receptor promote synthesis of collagen from dermal fibroblasts6, 29; therefore, it accelerates wound healing by stimulating collagen matrix production.33, 34, 35, 36, 37 However, the A2AR may promote excessive collagen deposition in the healed wound and continued A2AR activation contributes to scar progression; its pharmacological blockade diminishes scar size and dysfunctional matrix remodeling in a murine model that mimics human scarring.8 Similarly, we have previously shown that A2AR blockade or A2AR-deficient mice were protected from developing bleomycin-induced dermal fibrosis,6 suggesting that A2AR contributes to the pathogenesis of dermal fibrosis in diseases such as scleroderma. To further confirm the fibrogenic effects of adenosine, we have found that deficiency in adenosine deaminase (the principal catabolic enzyme for adenosine in vivo) leads to elevated adenosine levels and spontaneous pulmonary38 and skin fibrosis in mice, accompanied by increased collagen deposition, which is significantly prevented by A2AR pharmacological blockade in skin.7
Levels of adenosine are rapidly elevated in response to tissue injury, and the main source of extracellular adenosine is the stepwise dephosphorylation of ATP by the coordinated action of ecto-apyrase (CD39) and ecto-5′-nucleotidase (CD73).33 It has been well documented that CD39 and CD73 favor the anti-inflammatory effects of adenosine in the immune system,15, 16, 17 CD39 plays a major role in modulating extracellular matrix remodeling in inflammatory diseases of the pancreas,21 and CD73 is critical for progression of hepatic fibrosis.22 However, less is known about the roles of both ecto-enzymes in adenosine-mediated skin fibrosis.
Prior work indicates that adenosine can serve as a profibrotic signal in the lung38, 39 and that CD39 and CD73 promote fibrosis in the pancreas and in the liver,21, 22 respectively. But paradoxically, decreased levels of adenosine after bleomycin challenge in CD73KO mice enhance pulmonary fibrosis.2
These diverse responses to tissue adenosine could be influenced by the levels of ligand produced, the pattern of receptor expression on various cells, the effector systems coupled to these receptors, and the cytokine or growth factor environment.2, 3, 40 Further investigations are, therefore, needed to decipher the mechanisms by which CD73 ensures proper tissue remodeling in the lungs, as opposed to propagation of fibrosis in the liver22 and skin, as suggested by the present work.
In addition, CD39, such as CD73, has organ-specific effects: ectonucleoside triphosphate diphosphohydrolase activity seems to inhibit fibrosis in liver, and effects may be related to aberrant ATP signaling (S.C.R.).
In summary, our results support the hypothesis that increased levels of extracellular adenosine, generated by the two-step enzymatic process involving the ecto-apyrase (CD39) and the ecto-5′-nucleotidase (CD73), mediate dermal fibrogenesis, as we have shown in a murine model of bleomycin-induced dermal fibrosis. Therefore, modulation of extracellular adenosine production by CD39 and CD73 may represent a useful therapeutic means to regulate dermal fibrogenesis in conditions such as scleroderma.
Footnotes
Supported by NIH grants AR057544 (E.S.L.C.), AR56672, AR56672S1, AR54897, and AR046121 (B.N.C.) and P01 HL087203 with R01 HL094400 (S.C.R.), the Scleroderma Foundation (E.S.L.C.), the Arthritis Foundation (E.S.L.C.), the Spanish Ministry of Health (P.F.), and the New York University HHC Clinical and Translational Science Institute (UL1 TR000038).
Disclosures: B.N.C. holds patents on the use of adenosine A2A receptor agonists to promote wound healing and the use of A2A receptor antagonists to inhibit dermal fibrosis, the use of adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone, the use of adenosine A1 and A2B receptor antagonists to treat fatty liver, and the use of adenosine A2A receptor agonists to prevent prosthesis loosening. E.S.L.C. holds a patent on the use of A2A receptor antagonists to inhibit fibrosis. S.C.R. has developed intellectual property with Nanopharma, receives royalties from BioLegend, MSD, Mersano, and eBioscience for monoclonal antibodies to CD39, and holds patents on the therapeutic use of CD39 in inflammation and transplantation. B.N.C. is a consultant for Bristol-Myers Squibb, Novartis, CanFite Biopharmaceuticals, Cypress Laboratories, Regeneron (Westat, DSMB), Endocyte, Protalex, Allos, Inc., Savient, Gismo Therapeutics, Antares Pharmaceutical, and Medivector.
Current address of P.F., National Cancer Institute, NIH, Bethesda, MD.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.08.024.
Supplemental Data
PBS-treated mice do not show a difference in skin collagen content. Dermal hydroxyproline content was assessed on 6-mm skin biopsy specimens. Data represent means ± SEM.
References
- 1.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Ann Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 2.Volmer J.B., Thompson L.F., Blackburn M.R. Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 3.Hasko G., Cronstein B.N. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn M.R. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Aso M., Mediero A., Cronstein B.N. Adenosine A2A receptor is a fine-tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 2013 doi: 10.1007/s11302-013-9368-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan E.S., Fernandez P., Merchant A.A., Montesinos M.C., Trzaska S., Desai A., Tung C.F., Khoa D.N., Pillinger M.H., Reiss A.B., Tomic-Canic M., Chen J.F., Schwarzschild M.A., Cronstein B.N. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez P., Trzaska S., Wilder T., Chiriboga L., Blackburn M.R., Cronstein B.N., Chan E.S. Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am J Pathol. 2008;172:1675–1682. doi: 10.2353/ajpath.2008.070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Aso M., Chiriboga L., Cronstein B.N. Pharmacological blockade of adenosine A2A receptors diminishes scarring. FASEB J. 2012;26:4254–4263. doi: 10.1096/fj.12-209627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 10.Fredholm B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 11.Sitkovsky M., Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 12.Sitkovsky M.V., Lukashev D., Apasov S., Kojima H., Koshiba M., Caldwell C., Ohta A., Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Ann Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 13.Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- 14.Reutershan J., Vollmer I., Stark S., Wagner R., Ngamsri K.C., Eltzschig H.K. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 15.Bonner F., Borg N., Burghoff S., Schrader J. Resident cardiac immune cells and expression of the ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PLoS One. 2012;7:e34730. doi: 10.1371/journal.pone.0034730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deaglio S., Dwyer K.M., Gao W., Friedman D., Usheva A., Erat A., Chen J.F., Enjyoji K., Linden J., Oukka M., Kuchroo V.K., Strom T.B., Robson S.C. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwyer K.M., Deaglio S., Gao W., Friedman D., Strom T.B., Robson S.C. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltzschig H.K., Ibla J.C., Furuta G.T., Leonard M.O., Jacobson K.A., Enjyoji K., Robson S.C., Colgan S.P. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eltzschig H.K., Thompson L.F., Karhausen J., Cotta R.J., Ibla J.C., Robson S.C., Colgan S.P. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 20.Eckle T., Fullbier L., Wehrmann M., Khoury J., Mittelbronn M., Ibla J., Rosenberger P., Eltzschig H.K. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 21.Kunzli B.M., Nuhn P., Enjyoji K., Banz Y., Smith R.N., Csizmadia E., Schuppan D., Berberat P.O., Friess H., Robson S.C. Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice. Gastroenterology. 2008;134:292–305. doi: 10.1053/j.gastro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Z., Fernandez P., Wilder T., Yee H., Chiriboga L., Chan E.S., Cronstein B.N. Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 23.Enjyoji K., Sevigny J., Lin Y., Frenette P.S., Christie P.D., Esch J.S., 2nd, Imai M., Edelberg J.M., Rayburn H., Lech M., Beeler D.L., Csizmadia E., Wagner D.D., Robson S.C., Rosenberg R.D. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 24.Thompson L.F., Eltzschig H.K., Ibla J.C., Van De Wiele C.J., Resta R., Morote-Garcia J.C., Colgan S.P. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goepfert C., Sundberg C., Sevigny J., Enjyoji K., Hoshi T., Csizmadia E., Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 26.Cronstein B.N., Naime D., Ostad E. The antiinflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junqueira L.C., Montes G.S., Krisztan R.M. The collagen of the vertebrate peripheral nervous system. Cell Tissue Res. 1979;202:453–460. doi: 10.1007/BF00220437. [DOI] [PubMed] [Google Scholar]
- 28.Montes G.S., Krisztan R.M., Shigihara K.M., Tokoro R., Mourao P.A., Junqueira L.C. Histochemical and morphological characterization of reticular fibers. Histochemistry. 1980;65:131–141. doi: 10.1007/BF00493161. [DOI] [PubMed] [Google Scholar]
- 29.Chan E.S., Liu H., Fernandez P., Luna A., Perez-Aso M., Bujor A.M., Trojanowska M., Cronstein B.N. Adenosine A2A receptors promote collagen production by a Fli1- and CTGF-mediated mechanism. Arthritis Res Ther. 2013;15:R58. doi: 10.1186/ar4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 32.Wipff P.J., Hinz B. Integrins and the activation of latent transforming growth factor beta1: an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Valls M.D., Cronstein B.N., Montesinos M.C. Adenosine receptor agonists for promotion of dermal wound healing. Biochem Pharmacol. 2009;77:1117–1124. doi: 10.1016/j.bcp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montesinos M.C., Shaw J.P., Yee H., Shamamian P., Cronstein B.N. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montesinos M.C., Desai A., Chen J.F., Yee H., Schwarzschild M.A., Fink J.S., Cronstein B.N. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Victor-Vega C., Desai A., Montesinos M.C., Cronstein B.N. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel) Inflammation. 2002;26:19–24. doi: 10.1023/a:1014417728325. [DOI] [PubMed] [Google Scholar]
- 37.Montesinos M.C., Gadangi P., Longaker M., Sung J., Levine J., Nilsen D., Reibman J., Li M., Jiang C.K., Hirschhorn R., Recht P.A., Ostad E., Levin R.I., Cronstein B.N. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chunn J.L., Molina J.G., Mi T., Xia Y., Kellems R.E., Blackburn M.R. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 39.Blackburn M.R., Lee C.G., Young H.W., Zhu Z., Chunn J.L., Kang M.J., Banerjee S.K., Elias J.A. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn M.R., Kellems R.E. Adenosine deaminase deficiency: metabolic basis of immune deficiency and pulmonary inflammation. Adv Immunol. 2005;86:1–41. doi: 10.1016/S0065-2776(04)86001-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PBS-treated mice do not show a difference in skin collagen content. Dermal hydroxyproline content was assessed on 6-mm skin biopsy specimens. Data represent means ± SEM.