Abstract
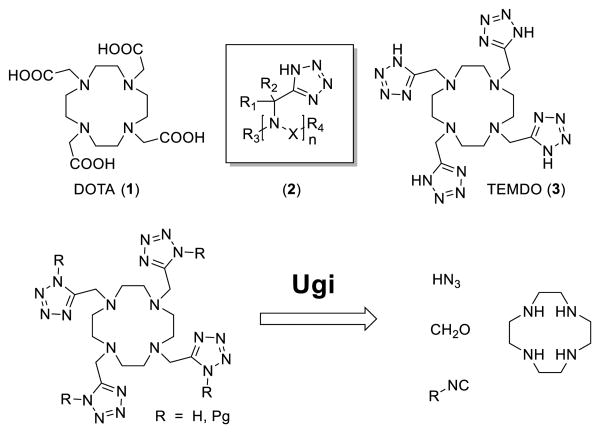
A simple Ugi tetrazole multicomponent reaction allows the synthesis of a novel macrocyclic cyclen derivative with four appendant tetrazole arms in just two steps in excellent yields. This ligand, called TEMDO, turns out to be a very good complexator of lanthanoid metals. Here we describe the design, synthesis, solid state structure, binding constant and some MRI applications of the Gd-TEMDO complex as the first example of a congeneric family of oligo-amino tetrazoles.
Keywords: Magnetic resonance imaging, Gadolinium, Ugi, oligoamino tetrazole, TEMDO
Since its discovery in 1971 magnetic resonance imaging (MRI) has evolved as a major medical imaging technique and has rapidly found its entry into daily clinical diagnostics.[1] Today more than 20.000 MRI scanners are operating worldwide in hospitals and more than 50 million clinical MRI examinations are performed every year. The impact of this breakthrough technology for mankind was honored by the Nobel Prize in Physiology or Medicine in 2003.[2] As opposed to other imaging techniques, MRI is a radiation-less method and is therefore widely used in medical diagnosis and staging of disease. While MRI in principle does not require contrast agents, its use dramatically accelerates acquisition times and signal intensity. MRI contrast agents work by accelerating the relaxation of water protons in the surrounding tissues. Paramagnetic ions are suitable contrast agents and amongst them gadolinium (Gd3+) complexes are by far the most widely used MRI contrast agents due to its seven unpaired electrons, its slow electronic relaxation and its exquisite complex stability. Although clinically approved MRI contrast agents are generally considered as safe, a small number of fatalities were reported likely due to nephron- and neurotoxicity by Gd-leaking.[3] In addition, not only patients with pre-existing renal failure are affected by Gd-leakage, but also patients with other conditions showed considerable neuronal tissue concentrations of Gd3+ after being subjected to Gd-based contrast agents (GBCA's).[4]
Here we introduce the first example of the novel class of oligoamino tetrazoles as chelating agent useful in imaging: 1,4,7,10-tetrakis((1H-tetrazol-5-yl)methyl)-1,4,7,10-tetraazacyclo-dodecane (TEMDO, 3) (scheme 1). We describe the design, synthesis, solid state structure, binding constant and some MRI applications of the Gd-TEMDO complex.
Scheme 1.
DOTA, general formula of oligo-amino tetrazoles, TEMDO and retro synthesis thereof.
The tetrazole is a known bioisostere of the carboxylic acid with often superior PKPD properties.[5] For example the tetrazolate allows for a more widely delocalization of the negative charge and could thus facilitate better penetration into tissue. In the context of MRI a tetrazolate ligand could also help to increase proton relaxivity through changes in the metal-H distance by electron delocalization towards the ligand. Moreover through subtle changes in the ligand composition and geometry eventually higher tilt angles between the plane of the bound water and the metal–O bond could be induced by hydrogen bonding of the coordinated water to an appropriate side group of the chelate, which could potentially result in a significant decrease of the metal–proton distance. Thus we reasoned that oligoamino tetrazoles are suitable for MRI, exhibiting different and eventually better physicochemical and biological properties compared to their carboxylic acid analogues. Surprisingly oligoamino tetrazoles in general and specifically as MRI agents are unknown.[6] Based on our longstanding expertise in multicomponent reaction (MCR) chemistry and its versatility, speed and ease-to-perform we choose MCR as a perfect tool to assemble this functional material class of MRI agents.[7] As a first synthetic target of the class of the oligoamino tetrazoles (2) we chose the tetrazole analogue (3) of DOTA[8] (1) (scheme 1).
In our retrosynthesis we envisioned that TEMDO can be fast and convergently synthesized by applying a Ugi tetrazole reaction from available cyclen.[9] The unprotected TEMDO ligand suitable for metal complexation has to be generated by cleavage of the isocyanide substituent. In principle several cleavable isocyanides such as Walborsky's, benzyl- or tert-butyl are suitable, however we have chosen here β-cyanoethyl isocyanide 4 due to its mild cleavage conditions.
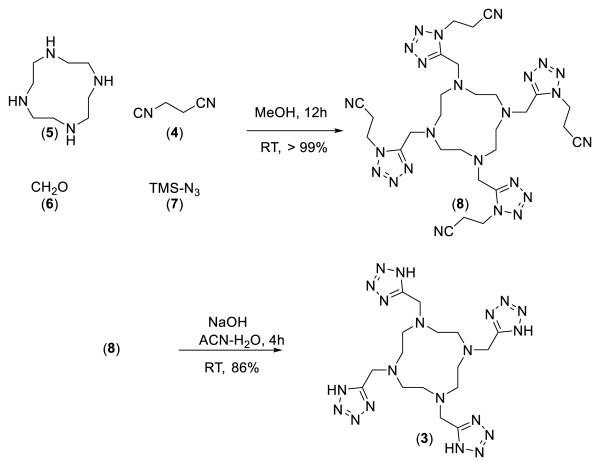
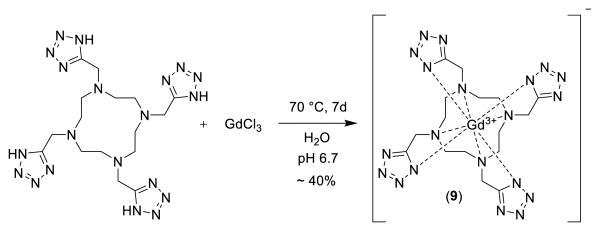
In fact after some optimization of the reaction conditions we could obtain the Ugi tetrazole product 8 in quantitative yields by reacting the commercially available starting materials cyclen 5, paraformaldehyde 6, and TMS azide 7, as a safe hydrogen azide source and β-cyanoethyl isocyanide 4 (scheme 2). The four β-cyanoethyl groups can be cleaved under mild conditions using NaOH in acetonitrile/water at room temperature. The crude product was purified by precipitation at pH = 7.75, to give neutral TEMDO ligand (86%) in high purity. Next the chelating property of TEMDO towards the lanthanide element Gadolinium was assessed. The Gd3+ complex of TEMDO was prepared by either a) heating the 1:1.1 mixture of TEMDO ligand and GdCl3 in water at 70 °C for 7 days at pH 6.7, or by b) heating TEMDO (in acidic form), the approved excipient meglumin and GdCl3 1:1:1 in water for 7 days at 70 °C. The remaining free Gd3+ was removed using ion-exchange resin Chelex®-100 and the clean liquid was lyophilized to get pure Gd-TEMDO complex (>98%) as a white bench stable solid.
Scheme 2.
Two step UT-MCR synthesis of the TEMDO ligand 3
Then we investigated the complex-behaviour of TEMDO towards the lanthanide element Gadolinium by determining its solid state 3D structure using X-ray crystallography. The complex crystallized in small rhombic shapes from water and the solved structure is shown in figure 1. The side by side comparison of the obtained crystal structure of Gd-TEMDO with the Gd-DOTA complex shows a surprisingly high isosterism (figure 1, SI-movie).
Figure 1.
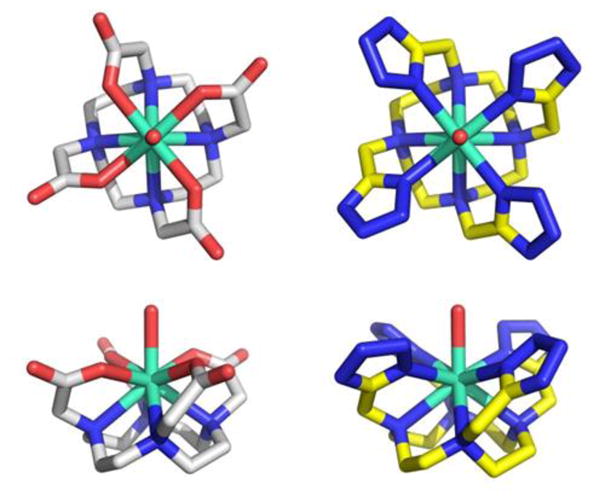
Comparison of the solid state structure of Gd-DOTA (left column) and Gd-TEMDO (right column). Stick representation of the crystal structures (unbound crystal waters and counterions are omitted for clarity). Above row: top down view; below row: side on view.
The central Gd3+ ion is surrounded by the four basal cyclen-N and apical by the four N1 of the appendant tetrazoles in a highly symmetrical twisted quadratic coordination sphere. In addition a water molecule is coordinated on top of the twisted cube in-between the four tetrazole ligands. The coordination number for Gd3+ is therefore 9 and the idealized complex belongs to the chiral point group C4. In principle 9-fold coordinated Ln-complexes with C4 symmetry can present a square antiprismatic (SAP) or twisted square antiprismatic (TSAP) geometry. Moreover due to their membership to the chiral C4 point group Ln-TEMDO complexes can show the absolute chirality Λ (left-handed) or Δ (right-handed). The coordination geometry of the Gd-TEMDO and Eu-TEMDO complexes is square antiprismatic (SAP), whereas in the La-TEMDO we have found twisted square antiprismatic (TSAP) geometry. For example SAP geometry is the predominant form found for the Gd-DOTA complex in aqueous solution, besides some twisted square antiprism.[6a] Interestingly the X-ray structures of the Gd- and Eu-TEMDO reveal only one SAP enantiomer per crystal, while La-TEMDO is present in a TSAP geometry and both Λ and Δ enantiomers are present in one crystal (SI). Similarly in Ln-DOTA complexes SAP geometry is preferred over TSAP with increasing ionic lanthanide radius.[10] The complex geometry is an important parameter in MRI active Ln complexes as the water exchange kinetics are linked to its geometry and relaxivity.[11] Compared to TSAP, a SAP geometry will result in slower water exchange, but surprisingly a faster relaxation.[12] The comparable size of the macrocyclic cavity of DOTA and TEMDO indicates that suitable metal ions will very well fit to form thermodynamically stable chelates.[13] Qualitative assessment of free lanthanide ions with indicator xylenol orange showed that chelating generally occurs for the lanthanides. In table 1 we compare some key distances between Gd3+ with DOTA and TEMDO and its coordination towards N, and O as shown in solid state. The similarity of the alignment between Gd3+ and the both complexators DOTA and TEMDO expressed in distances gives an average difference of 0.09 Å RMSD. Such minor changes in bond length and angle, however, can have profound effects on how well the ion is held, the residence time of the water molecule and its exchange rate.[14]
Table 1.
M-O, M-N and M-N’ bond lengths in the 9-coordinated Gd-DOTA and TEMDO complexes.
| Complex | M-N (Å)[a] | M-O (Å) [b] | M-N’ (Å) [c] | M-O’ [d] |
|---|---|---|---|---|
| [Gd(TEMDO)(H2O)]− | 2,712 | - | 2,475 | 2.434 |
| [La(TEMDO)(H2O)]− | 2,780 | - | 2,616 | 2.507 |
| [Gd(DOTA)(H2O)]− | 2,648 | 2,377 | - | 2.458 |
N represents the basal rim nitrogen's.
Metal coordination to the four surrounding carboxylates
Metal coordination to the tetrazoles nitrogen N’
Average distance between the metal and water molecule.
The chelating properties of Gd-TEMDO were accessed by determination of the thermodynamic stability constant using colorimetry (table 1).[15] The stability constant 16.6 confirms the strong chelating properties of the macrocyclic TEMDO, similar to Gd-(DTPA-BMA), a marketed MRI contrast agent (Omniscan, 16.8), however lower than Gd-DOTA. Studies on Gd3+ release revealed that not only thermodynamic stability, but also kinetic inertness and elimination rate of the complex plays an important role in the amount of free Gd3+ in vivo.[12] Kinetic inertness expressed as dissociation rate is therefore important to predict toxicity through Gd3+ leakage (table 2). However, due to the general observed fast excretion of Gd-based contrast agents the complex is not long enough in the body to establish thermodynamic equilibrium. The lower stability constant of Gd-TEMDO as compared to the DOTA complex is thus compensated by a two orders of magnitude slower release of Gd3+ from the TEMDO complex
Table 2.
Binding constants and kinetic data of Gd3+ complexes.
| Metal Complex | Log K | kOH(M−1s−1) [a] | kH(M−1s−1) [b] |
|---|---|---|---|
| [Gd(TEMDO)(H2O)]− | 16.6 | 3.47 ± 0.5 × 103 | 1.20 ± 0.5 × 10-8 |
| [Gd(DOTA)(H2O)]− | 24.1[c] | 5.16 ± 0.5 × 103 | 3.20 ± 0.5 × 10-6 |
Formation rate, experimentally determined for both TEMDO and DOTA by UV/VIS spectrometry
Dissociation rate
Value under identical conditions determined as for TEMDO (lit. value for Gd-DOTA 24.7[16]).
Finally we investigated the usefulness of Gd-TEMDO for magnetic resonance imaging. In a Bruker 9.4 T 400 MHz small bore MRI apparatus, we ran relaxation experiments resulting in phantom images of Gd-TEMDO (figure 2). From the phantom images we calculated a relaxivity r1 of 2.0 mM-1 s-1 which is in comparison with Gd-DOTA 6.0 mM-1 s-1 (measured under identical conditions at ambient temperature) somewhat lower, but being in the same range a promising starting point for further introduction of variations on TEMDO. The T1-weighted images as expected show, however a concentration dependent increase of the relaxation rate of the solvent water protons associated with Gd-based contrast agents. In-vivo assessment of Gd-TEMDO was performed via delayed contrast-enhanced MRI. Some Gd-based contrast agents such as Gd-DOTA accumulate in damaged myocardium tissues, diffusing from the intravascular into the interstitial space, unable to enter intact cells. Delay in measurement causes the majority of agent to wash out, and contrast agent absorbed in damaged tissue provides enhanced contrast.[17] In a left-coronary-artery occlusion murine animal model we investigated Gd-TEMDO to image the myocardial infarcted tissue. This clearly shows that the TEMDO contrast agent is absorbed in damaged tissue and the enhanced contrast is shown in figure 2.
Figure 2.
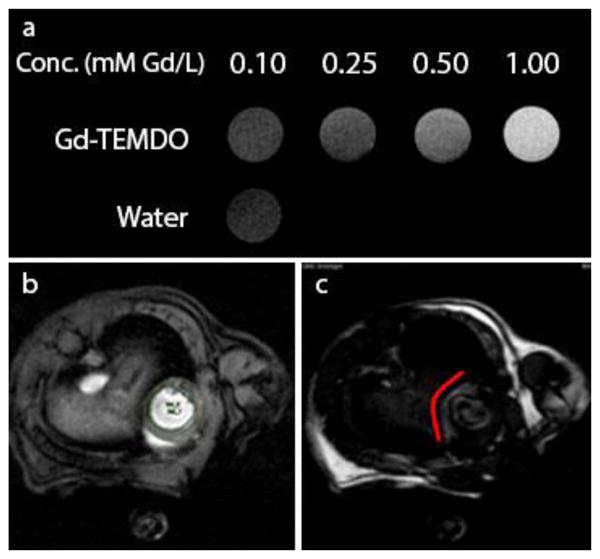
Gd-TEMDO MRI. (a) T1-weighted MRI phantoms of Gd-TEMDO proving concentration dependent T1 shortening. (b) MRI obtained from isoflurane-anaesthetized mice, (c) taken 30 minutes after I.P. administration of Gd-TEMDO (0.6 mmol/kg). Left: the heart fully visible; right: heart with reduced brightness, the damaged tissue remains visible due to absorbed Gd-TEMDO following the red line.
In summary, we have described design, synthesis, X-ray structure, binding and some applications of the first example of a new class of Ln chelators with potential for use in MRI as Gd-based contrast agents. The macrocyclic TEMDO ligand is easy accessible by only two synthetic steps, in excellent yields employing a Ugi tetrazole multicomponent reaction. The thermodynamic stability constant of our first Gd3+ complex is comparable to clinically used Gd-(DTPA-BMA). So far we were able to grow diffractable crystals of the Gd(III), Eu(III) and La(III) complexes. Moreover we were able to show proof-of-principle, utilizing the Gd-TEMDO complex as contrast agent and visualizing myocardial infarcts in mice. Further analysis of the complex confirms comparable structural features as compared to marketed Gd-based contrast agents, a very good starting point for future development of this new class of oligotetrazolo-based metal complexes.
Supplementary Material
Scheme 3.
Reaction scheme of the formation of Gd-TEMDO complex, the counter ion sodium or meglumine is present depending on the method of preparation.
Acknowledgments
We want to thank Prof. Petr Vanura, Department of Analytical Chemistry, Institute of Chemical Technology, Prague, Czech Republic for providing computer programs LTGW ETITR and LTGW-SPEFO and helpful discussions. This work was financially supported from the NIH (1R01GM097082-01).
Footnotes
To Ivar Ugi, the father of modern multicomponent reaction chemistry
Supporting information for this article is given via a link at the end of the document.
References
- 1.Helm L, Merbach AE, Tóth Ev. The chemistry of contrast agents in medical magnetic resonance imaging. (Second edition) 2013 [Google Scholar]
- 2.a) Lauterbur PC. Angew Chem Int Ed. 2005;44:1004–1011. doi: 10.1002/anie.200462400. [DOI] [PubMed] [Google Scholar]; b) Mansfield P. Angew Chem Int Ed. 2004;43:5456–5464. doi: 10.1002/anie.200460078. [DOI] [PubMed] [Google Scholar]
- 3.Hermann P, Kotek J, Kubicek V, Lukes I. Dalton Trans. 2008:3027–3047. doi: 10.1039/b719704g. [DOI] [PubMed] [Google Scholar]
- 4.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Radiology. 2015;275:772–782. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 5.Herr RJ. Bioorgan Med Chem. 2002;10:3379–3393. doi: 10.1016/s0968-0896(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 6.a) Aime S, Botta M, Fasano M, Marques MPM, Geraldes CFGC, Pubanz D, Merbach AE. Inorg Chem. 1997;36:2059–2068. doi: 10.1021/ic961364o. [DOI] [PubMed] [Google Scholar]; b) Dömling A. Vol WO/2015/117626. Patentscope; 2015. [Google Scholar]
- 7.a) Dömling A. Chem Rev. 2005;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; b) Dömling A, Wang W, Wang K. Chem Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Main M, Snaith JS, Meloni MM, Jauregui M, Sykes D, Faulkner S, Kenwright AM. Chem Commun. 2008:5212–5214. doi: 10.1039/b810083g. For an example of cyclen derivatization by Ugi reaction, see: [DOI] [PubMed] [Google Scholar]
- 8.a Stetter H, Frank W. Angew Chem Int Ed. 1976;15:686–686. [Google Scholar]; b Desreux JF. Inorg Chem. 1980;19:1319–1324. [Google Scholar]
- 9.Ugi I, Steinbruckner C. Chem Ber. 1961;94:734–742. [Google Scholar]
- 10.Meyer M, Dahaoui-Gindrey V, Lecomte C, Guilard L. Coordin Chem Rev. 1998;178:1313–1405. [Google Scholar]
- 11.Milne M, Chicas K, Li A, Bartha R, Hudson RHE. Org Biomol Chem. 2012;10:287–292. doi: 10.1039/c1ob06162c. [DOI] [PubMed] [Google Scholar]
- 12.Avedano S, Botta M, Haigh JS, Longo DL, Woods M. Inorg Chem. 2013;52:8436–8450. doi: 10.1021/ic400308a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado R, Felix V, Lima LMP, Price DW. Dalton Trans. 2007:2734–2745. doi: 10.1039/b704360k. [DOI] [PubMed] [Google Scholar]
- 14.Cacheris WP, Nickle SK, Sherry AD. Inorg Chem. 1987;26:958–960. [Google Scholar]
- 15.Baranyai Z, Gianolio E, Ramalingam K, Swenson R, Ranganathan R, Brucher E, Aime S. Contrast Media Mol Imaging. 2007;2:94–102. doi: 10.1002/cmmi.131. [DOI] [PubMed] [Google Scholar]
- 16.Reková M, Vañura P, Jedináková-Křížová V. The Open Inorganic Chemistry Journal. 2009;3:26–32. [Google Scholar]
- 17.Bohl S, Lygate CA, Barnes H, Medway D, Stork LA, Schulz-Menger J, Neubauer S, Schneider JE. Am J Physiol Heart Circ Physiol. 2009;296:H1200–H1208. doi: 10.1152/ajpheart.01294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.