Abstract
Retinal neovascularization (NV) due to retinal ischemia remains one of the principal causes of vision impairment in patients with ischemic retinal diseases. We recently reported that periostin (POSTN) may play a role in the development of preretinal fibrovascular membranes, but its role in retinal NV has not been determined. The purpose of this study was to examine the expression of POSTN in the ischemic retinas of a mouse model of oxygen-induced retinal NV. We also studied the function of POSTN on retinal NV using Postn KO mice and human retinal endothelial cells (HRECs) in culture. In addition, we used a novel RNAi agent, NK0144, which targets POSTN to determine its effect on the development of retinal NV. Our results showed that the expression of POSTN was increased in the vascular endothelial cells, pericytes, and M2 macrophages in ischemic retinas. POSTN promoted the ischemia-induced retinal NV by Akt phosphorylation through integrin αvβ3. NK0144 had a greater inhibitory effect than canonical double-stranded siRNA on preretinal pathological NV in vivo and in vitro. These findings suggest a causal relationship between POSTN and retinal NV, and indicate a potential therapeutic role of intravitreal injection of NK0144 for retinal neovascular diseases.
Keywords: periostin, RNAi, neovascularization, diabetic retinopathy
Introduction
Retinal neovascularization (NV) due to retinal ischemia is one of the principal causes of vision impairment in patients with ischemic retinal diseases such as proliferative diabetic retinopathy (PDR), retinal vein occlusion, and retinopathy of prematurity.1 Retinal NV can result in macular edema, vitreous hemorrhage, and tractional retinal detachment, which are the causes of the vision reduction. Despite recent progress in pharmacological therapies and surgical techniques, there are still cases of treatment-resistant retinal NV.
Retinal NV is an example of excessive angiogenesis that is characterized by proliferation, migration, and tube formation of the retinal vascular endothelial cells. In this process, the extracellular matrix (ECM) components play a critical role in regulating retinal NV.2 Previous studies have shown that several growth factors, including the vascular endothelial growth factors (VEGFs), platelet-derived growth factors, transforming growth factor βs, and placenta growth factor, are involved in this process.3, 4, 5, 6 Among these factors, the VEGFs have been well characterized and are known to play a causal role in retinal NV. Although anti-VEGF therapies have been shown to inhibit the progression of retinal NV to some degree, it was recently reported that the intravitreal injection of anti-VEGF antibodies can cause retinal damage and might promote retinal fibrosis.7, 8, 9 Therefore, retinal NV still remains a vision-threatening pathological condition.
Several laboratories including ours have demonstrated a higher expression of periostin (POSTN) in the fibrovascular membranes (FVMs) of patients with PDR than in those of patients with normal retinas by comprehensive gene and protein expression profiling.10, 11, 12 We have also detected increased levels of POSTN protein in the vitreous of patients with PDR.13
POSTN is a 90-kDa matricellular protein belonging to the fasciclin family that interacts not only with other ECMs but also with the integrins, such as αvβ3 and αvβ5, as a ligand.14, 15 POSTN is associated with tissue development and remodeling through these interactions. POSTN has been reported to promote angiogenesis in an ischemic limb, in the choroid, in cancerous tissues, in keloids, and in cardiac valve degeneration.16, 17, 18, 19, 20 These findings suggest that POSTN may play a role in the development of pathological retinal NV. However, whether POSTN is involved in retinal NV has not been examined.
The silencing of post-transcriptional genes by RNAi is an excellent method of inhibiting gene expressions because of its high selectivity and potency, which are advantages over conventional therapies using antibodies and small molecules.21 Moreover, RNAi agents have other advantages due to their easy synthesis and rapid identification and optimization. However, previous investigations have shown that canonical double-stranded small interfering RNAs (siRNAs) have several problems for their use, including the need of a drug delivery system (DDS), low biological stability, off-target gene silencing, and immunostimulatory effects through the activation of Toll-like receptor 3 (TLR3).22, 23, 24, 25 We used a novel single-stranded RNAi agent that can overcome these obstacles, and we found that the naked single-stranded RNAi agent had an inhibitory effect on choroidal FVM formation with good stability, no sequence-independent choroidal neovascularization (CNV) suppression through TLR3, and no serious toxicity.17
Based on these findings, we hypothesized that POSTN is involved in retinal NV and can be used as a therapeutic target. To test this hypothesis, we investigated the role played by POSTN in retinal NV using a mouse model of oxygen-induced retinopathy (OIR) in vivo and human retinal microvascular endothelial cells (HRECs) in vitro. We also studied the therapeutic effect of the single-stranded RNAi agent targeting POSTN in retinal NV.
Results
Expression and Localization of POSTN in OIR Retina
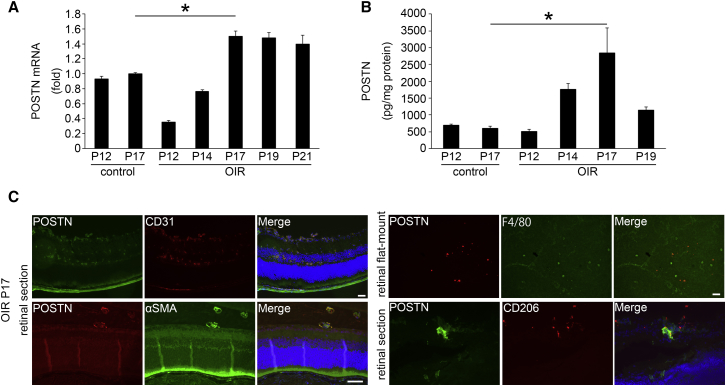
To determine whether POSTN is involved in retinal NV, the expression of the mRNA of Postn in OIR retinas was determined by real-time RT-PCR at several time points. The expression of the mRNA of Postn in the OIR retinas was significantly upregulated compared to that in the retinas of control mice and reached a peak on postnatal day 17 (P17) when the retinal NV reaches its peak (p < 0.05, n = 4; Figure 1A).
Figure 1.
Expression of Periostin in OIR Retina
Expression and localization of POSTN determined by real-time RT-PCR (A), ELISA (B), and immunohistochemistry (C) in OIR retina. The mouse model of OIR was induced by exposure of pups to 75% ± 2% oxygen for 5 days from P7 to P12 and then returned to room air. Mice in the control group were kept in room air during the entire postnatal period. (A) The expression of the Postn mRNA in OIR retinas was significantly upregulated compared to that in control mice retinas and reached a peak on P17 when retinal NV reaches its peak (n = 4/group). *p < 0.05. (B) The level of POSTN protein in OIR retinas was significantly higher at P17 than that in the control group (n = 4/group). *p < 0.001. (C) Retinal sections showing that POSTN (green) co-stained with CD31 and αSMA in the preretinal pathological NV and CD206 around the preretinal pathological NV at P17 when the expression of POSTN in retina is maximal. Retinal flat-mounts show that POSTN (red) staining can be seen in some of the F4/80-positive (green) cells. Error bars are SEM.
To confirm the results of real-time RT-PCR, we performed ELISA and determined the protein concentration of POSTN. ELISA showed that the level of POSTN protein in the OIR retinas was significantly higher at P17 (2,835.54 ± 750.11 pg POSTN/mg total protein, p < 0.001; n = 4) than that in the control retinas (602.59 ± 52.07 pg POSTN/mg total protein; n = 4; Figure 1B).
Next, we stained retinal sections and retinal flat-mounts with antibodies to determine the location of the POSTN in the OIR retinas. Immunohistochemical analyses of retinal sections showed that POSTN-positive cells were co-stained with both CD31 and α-smooth muscle actin (αSMA) in the preretinal pathological NVs. In the retinal flat-mounts, POSTN co-stained with F4/80. In addition, POSTN co-stained the preretinal pathological NVs with CD206 (Figure 1C). These findings indicated that the expression of POSTN was increased in the vascular endothelial cells, pericytes, and M2 macrophages in the preretinal pathological NV of OIR retinas.
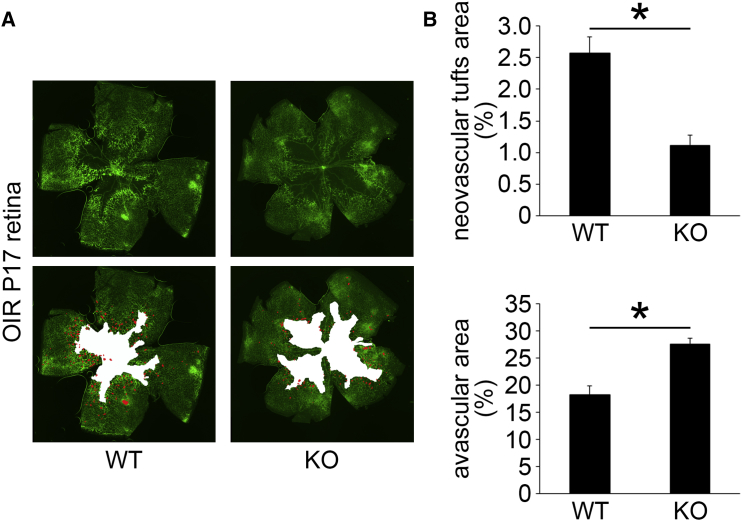
Attenuation of Ischemia-Induced Retinal Angiogenesis in OIR Retinas of Postn Knockout Mice
To investigate whether POSTN alters the ischemia-induced retinal NV, we quantified the size of the neovascular tufts and avascular areas in the OIR retinas of wild-type (WT) mice and Postn knockout (KO) mice stained with isolectin B4 at P17. In the OIR retinas, the neovascular tufts indicate preretinal pathological NV, whereas the avascular areas represent the physiological revascularization.26 The size of the neovascular tufts was significantly smaller in the OIR retinas of Postn KO mice than in WT mice (p < 0.01, n = 6; Figures 2A and 2B). The mean avascular area was significantly larger in Postn KO mice than that in WT mice (p < 0.01, n = 6; Figures 2A and 2B). These results indicated that POSTN promotes both preretinal pathological NV and physiological revascularization in OIR retinas.
Figure 2.
The Mean Size of the Neovascular Tufts and Avascular Areas in the OIR Retinas of WT and Postn KO Mice
The retinas were stained by FITC-conjugated isolectin B4 to observe the vessels, and flat-mounted retinas were examined with a fluorescence microscope. (A) Representative images of the neovascular tufts and avascular areas at P17 in WT and Postn KO mice. (B) The percentage of the neovascular tufts in the OIR retinas of POSTN KO mice was significantly smaller than that of WT mice, whereas the mean avascular area in POSTN KO mice was significantly larger than that in WT mice (n = 6/group). *p < 0.01. Error bars are SEM.
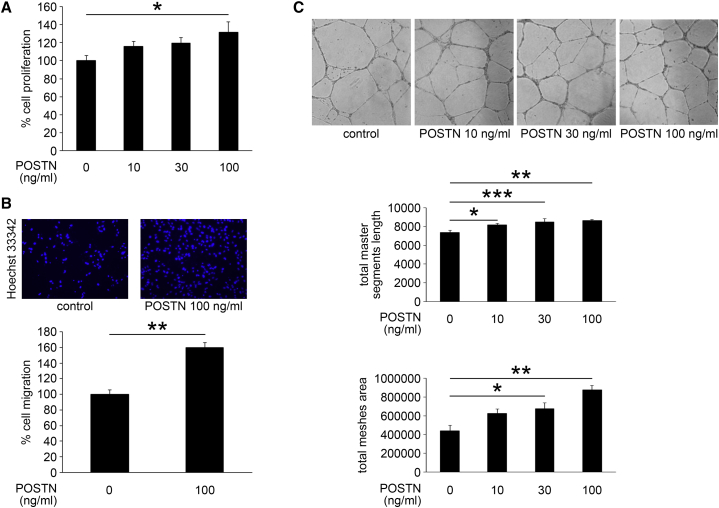
In Vitro Effects of POSTN on Proliferation, Migration, and Tube Formation of HRECs
To test the functional role of POSTN in retinal NV, we examined the effect of POSTN on proliferation, migration, and tube formation of HRECs, which are key steps of angiogenesis. POSTN promoted the proliferation of HRECs in a dose-dependent manner as determined by bromodeoxyuridine (BrdU) incorporation, and the difference of proliferation between the 100 ng/mL POSTN group and the control group was significant (p < 0.05, n = 6; Figure 3A).
Figure 3.
In Vitro Effect of POSTN on Proliferation, Migration, and Tube Formation of HRECs
Proliferation, migration, and tube formation were determined using BrdU, Boyden chamber, and Matrigel, respectively. (A) POSTN promoted the proliferation of HRECs in a dose-dependent manner (n = 6/group). *p < 0.05. (B) The migration of HRECs was significantly promoted by POSTN (n = 12/group). **p < 0.0001. (C) POSTN significantly promoted the tube formation of HRECs in a dose-dependent manner (n = 12/group). *p < 0.05, **p < 0.0001, ***p < 0.01. Error bars are SEM.
The migration of HRECs examined in Boyden chambers was also significantly promoted by POSTN (p < 0.0001, n = 12; Figure 3B). Furthermore, POSTN significantly increased the tube formation of HRECs in a dose-dependent manner (p < 0.0001, n = 12; Figure 3C). These results indicated a causal role of POSTN in retinal NV.
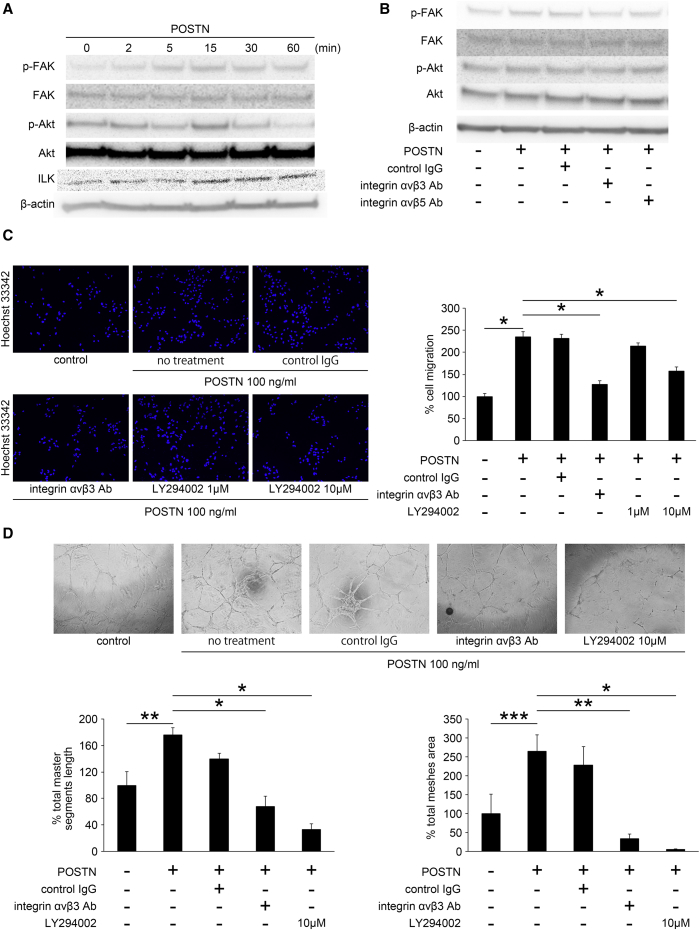
Signaling Pathway for Retinal NV Promoted by POSTN
We next investigated the signaling pathway for the retinal NV regulated by POSTN. Because POSTN can bind to integrin αvβ3 or αvβ5 as a ligand, and because FAK/Akt phosphorylation is involved in cell proliferation, migration, and tube formation through integrin αvβ3 or αvβ5, we examined the effect of POSTN on FAK/Akt phosphorylation in HRECs. FAK-Tyr397 and Akt-Ser473 phosphorylation was increased at 15 min after POSTN stimulation (Figures 4A and S1A). In addition, we observed the integrin-linked kinase 1 (ILK1) phosphorylation, which is a key mediator between ECMs and integrins. ILK1 phosphorylation was also increased 15 min after POSTN stimulation (Figure 4A). To confirm that the FAK/Akt phosphorylation induced by POSTN was mediated through integrin αvβ3 or αvβ5, we used antibodies against integrin αvβ3 or αvβ5 in HRECs 30 min before the POSTN stimulation. Inhibition of integrin αvβ3 reduced the FAK/Akt phosphorylation, whereas inhibition of integrin αvβ5 had no influence on the Akt phosphorylation (Figures 4B and S1B). These results indicated that POSTN stimulated FAK/Akt phosphorylation through integrin αvβ3 and ILK1 in HRECs.
Figure 4.
Signaling Pathway in Retinal NV Promoted by POSTN
HRECs were stimulated with POSTN and treated with or without control IgG, integrin αvβ3 Ab, αvβ5 Ab, or LY294002 (Akt inhibitor). (A) The phosphorylation of FAK-Tyr397, Akt-Ser473, ILK1 was increased at 15 min after POSTN stimulation. (B) Inhibition of integrin αvβ3 effectively reduced the FAK/Akt phosphorylation promoted by POSTN, whereas inhibition of integrin αvβ5 had no influence on the Akt phosphorylation. (C) The inhibition of integrin αvβ3 and Akt significantly suppressed the POSTN-induced migration (n = 12/group). *p < 0.0001. (D) Tube formation of HRECs was significantly inhibited by integrin αvβ3 Ab and Akt inhibitor (n = 4/group). *p < 0.0001, **p < 0.01, ***p < 0.05. Error bars are SEM.
To confirm that the FAK/Akt phosphorylation enhanced by POSTN through integrin αvβ3 is involved in key steps of angiogenesis, we inhibited integrin αvβ3 and Akt by the antibody (Ab) and the inhibitor (LY294002). The inhibition of integrin αvβ3 and Akt significantly suppressed the POSTN-induced migration (p < 0.0001, n = 12; Figure 4C) and the tube formation of HRECs (p < 0.01 or 0.0001, n = 4; Figure 4D). These results demonstrated that POSTN stimulated Akt phosphorylation through integrin αvβ3, which then resulted in the promotion of migration and tube formation of HRECs.
In Vitro NK0144-Mediated Inhibition of POSTN Expression in HRECs
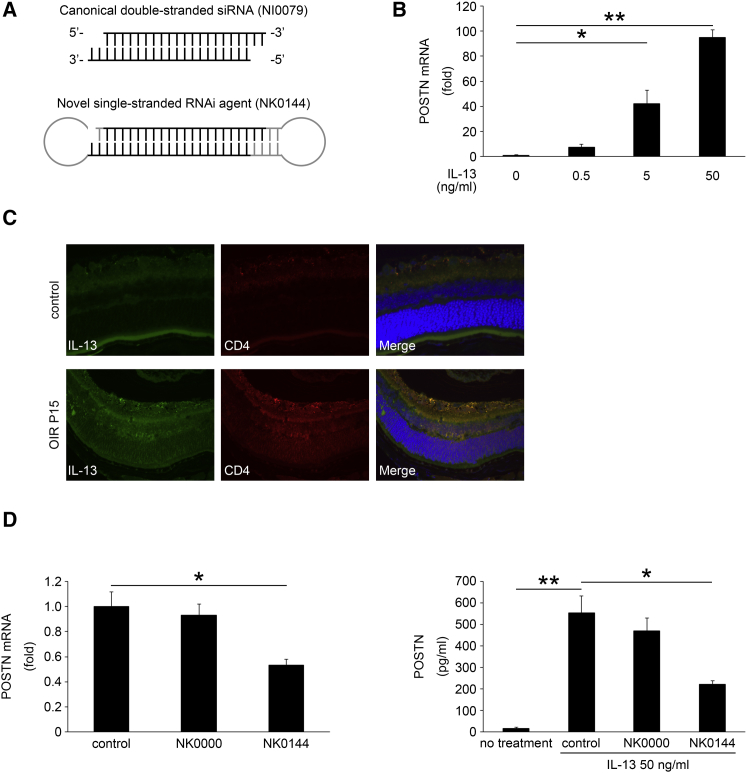
A novel single-stranded RNAi agent targeting POSTN (NK0144) was used as described in Materials and Methods. The difference in the structure of canonical double-stranded siRNA (NI0079) and NK0144 is shown in Figure 5A.
Figure 5.
In Vitro Effect of IL-13 and Novel Single-Stranded RNAi Agent on the Expression of POSTN of HRECs
Structure of canonical double-stranded siRNA and novel single-stranded RNAi agent (A), expression of IL-13 determined by immunohistochemistry in OIR retina (C), and the NK0144-mediated effects on POSTN expression induced by IL-13 in HRECs (B and D). Cultured HRECs transfected or not transfected with NK0000 or NK0144 were stimulated by IL-13. Total RNA was extracted at 24 hr after IL-13 stimulation and was analyzed by real-time RT-PCR (C) and ELISA (D). (A) The novel single-stranded RNAi agent was prepared as a single-stranded RNA oligomers that had the sequences of canonical double-stranded siRNA. (B) The expression of POSTN in HRECs stimulated by IL-13 was significantly increased in a dose-dependent manner (n = 4/group). *p < 0.01, **p < 0.0001. (C) Retinal sections show that IL-13 (green) is increased in CD4-positive cells (red) in OIR retina compared with control retina. (D) The expression of POSTN mRNA in HRECs induced by IL-13 was significantly reduced following transfection with 10 nM NK0144, whereas transfection with 10 nM of single-stranded scramble RNAi agent (NK0000) as a negative control RNAi agent had no significant inhibitory effect on the expression (n = 4/group). *p < 0.01. The protein level of POSTN in the supernatant from HRECs stimulated by IL-13 after the transfection with NK0144 was significantly decreased compared to that in the control group (n = 4/group). *p < 0.01, **p < 0.001. Error bars are SEM.
First, we tested the effects of inflammatory cytokines related to PDR on the synthesis of POSTN by HRECs. Interleukin-6 (IL-6), IL-8, IL-13, MCP-1, and VEGF were used for stimulation.27, 28 The expression of POSTN in HRECs was significantly increased in a dose-dependent manner only by IL-13 (p < 0.01 or 0.0001, n = 4; Figure 5B). IL-13 was also expressed by CD4-positive cells in OIR retinas (Figure 5C). HRECs exposed to the other cytokines did not show a significant induction in the expression of POSTN (data not shown).
Next, the knockdown effect of NK0144 on the expression of the mRNA of POSTN in HRECs was examined. Real-time RT-PCR showed that the expression of POSTN mRNA in HRECs induced by IL-13 was significantly decreased following transfection with 10 nM NK0144 (p < 0.01, n = 4; Figure 5D). Transfection with 10 nM of single-stranded scramble RNAi agent (NK0000) as a negative control RNAi agent had no significant inhibitory effect on the expression of POSTN mRNA.
To confirm the results of real-time RT-PCR, we performed ELISA to examine whether the protein level of POSTN was also decreased in the supernatant after transfection with NK0144. ELISA showed that the level of POSTN protein in the supernatant from HRECs transfected with NK0144 was significantly decreased (222.4 ± 14.0 pg/mL, p < 0.01; n = 4) compared with that in the control group (554.0 ± 77.9 pg/mL; n = 4; Figure 5D). These results indicated that NK0144 can inhibit the expression of POSTN from vascular endothelial cells induced by IL-13.
In Vitro Inhibitory Effect of NK0144 on Migration and Tube Formation of HRECs
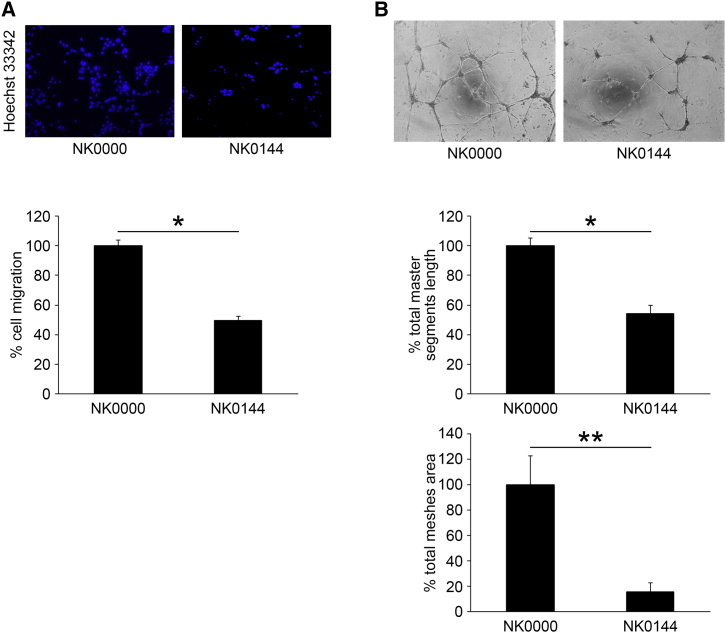
We next investigated whether NK0144 affected the migration and tube formation of HRECs facilitated by IL-13. The migration of HRECs induced by 50 ng/mL IL-13 was significantly inhibited by 10 nM NK0144 transfection (p < 0.0001, n = 18; Figure 6A) compared to that with 10 nM NK0000 transfection. The tube formation of HRECs was also significantly reduced by 10 nM NK0144 transfection (p < 0.01, n = 6; Figure 6B). These results indicated that NK0144 can inhibit the angiogenesis of HREC in vitro.
Figure 6.
In Vitro Inhibitory Effect of NK0144 on Migration and Tube Formation of HRECs
(A) The migration of HRECs induced by 50 ng/mL IL-13 was significantly inhibited by 10 nM NK0144 transfection compared to 10 nM NK0000 transfection (n = 18/group). *p < 0.0001. (B) The tube formation of HRECs induced by 50 ng/mL IL-13 was significantly impaired by 10 nM NK0144 transfection (n = 6/group). *p < 0.0001, **p < 0.01. Error bars are SEM.
In Vivo NK0144-Mediated Inhibition of Ischemia-Induced Retinal Angiogenesis in OIR Retinas
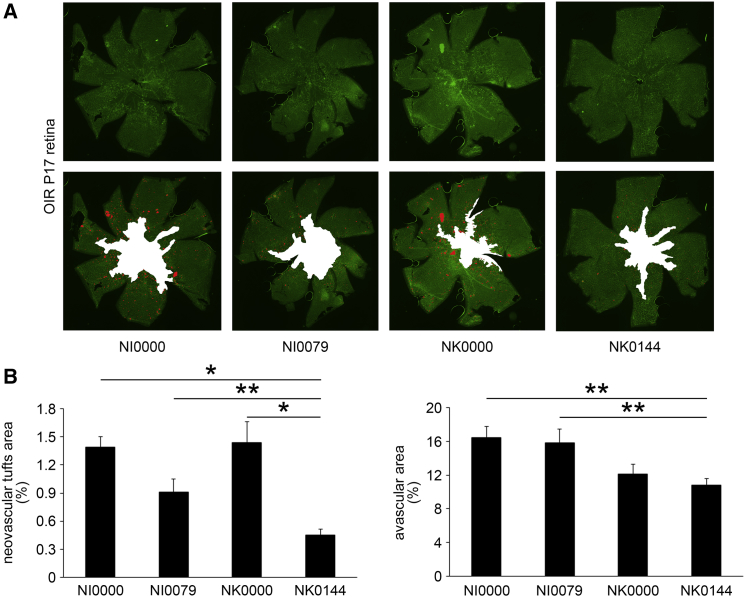
To determine whether NK0144 also inhibits the ischemia-induced retinal NV in vivo, NK0144 was injected intravitreally in OIR mice at P12. At P17, the eyes that received an intravitreal injection of 1 μL of 10 μM NK0144 had smaller preretinal pathological NV area than eyes after 1 μL of 10 μM NK0000 injections (p < 0.001, n = 4; Figures 7A and 7B). In addition, the inhibitory effect of NK0144 on preretinal pathological NV was significantly greater than that of the canonical double-stranded siRNA (NI0079; p < 0.05, n = 4; Figures 7A and 7B).
Figure 7.
In Vivo NK0144-Mediated Inhibition of Ischemia-Induced Retinal NV in OIR Retinas
Each RNAi agent was intravitreally injected at P12 soon after returning to room air. (A) Representative images of the neovascular tufts and avascular areas at P17 in each treatment group. (B) The percentage of the neovascular tufts in NK0144 treatment group was significantly smaller than that of scramble RNAi agents. The inhibitory effect of NK0144 on preretinal pathological NV is significantly greater than that of NI0079. The avascular area of novel single-stranded RNAi agents (NK0000 and NK0144) treatment group was significantly smaller than canonical double-stranded siRNAs (NI0000 and NI0079) treatment group (n = 4/group). *p < 0.001, **p < 0.05. Error bars are SEM.
We further investigated the effect of NK0144 on the size of the avascular area. At P17, there was no significant difference in the avascular area between the NK0000 treatment group and the NK0144 treatment group, whereas the difference between the novel single-stranded RNAi agents (NK0000 and NK0144) treatment groups and canonical double-stranded siRNAs (NI0000 and NI0079) treatment groups was significant (p < 0.05, n = 4; Figures 7A and 7B). These results indicated that the single-stranded RNAi agent (NK0144) had a greater inhibitory effect on preretinal pathological NV than the canonical double-stranded siRNA, whereas the inhibitory effect of NK0144 on physiological revascularization was less than that of canonical double-stranded siRNA.
Discussion
The results of this study showed the functional role of POSTN in the pathogenesis of ischemia-induced retinal NV, and the possible therapeutic effect of a novel single-stranded RNAi agent targeting POSTN in retinal NV. We showed that POSTN promoted Akt phosphorylation through integrin αvβ3, which can promote retinal NV. We also demonstrated that a novel single-stranded RNAi agent targeting POSTN (NK0144) can inhibit retinal NV more than a canonical double-stranded siRNA targeting POSTN (NI0079) both in vivo and in vitro. Our studies thus raise the possibility that this single-stranded RNAi agent targeting POSTN can be considered as a potential therapeutic agent for pathological retinal NV.
Our results demonstrated that POSTN was expressed in vascular endothelial cells during ischemia-induced preretinal pathological NV. In addition, IL-13 stimulation enhanced the expression of POSTN in the vascular endothelial cells. These results are in line with earlier reports showing that IL-13 induced POSTN production by human microvascular endothelial cells from lung and skin blood vessels.19 In this study, IL-13 was increased in CD4-positive cells in OIR retinas compared with normal retinas. IL-13 is a Th2 cytokine and has angiogenic activities.29 A previous study has shown that Th2 cells are one of the cellular components in FVMs of PDR patients.30 We have also reported an increase of the IL-13 level in the vitreous of patients with PDR.27, 28 Based on these findings, the IL-13 produced by CD4-positive Th2 cells may be the inducer of POSTN secreted by the retinal vascular endothelial cells in ischemic retinas.
Our results showed that POSTN also co-stained with markers of pericytes and M2 macrophages in ischemic retinas. Earlier studies including ours have reported that αSMA-positive cells can secrete POSTN.13, 31, 32 In addition, our earlier studies have shown a strong positive correlation between M2 macrophage markers and POSTN in the vitreous of PDR patients.33 We have subsequently shown that macrophage polarization toward M2 phenotype by IL-13 resulted in the production of POSTN.28 Thus, αSMA-positive pericytes and M2 macrophages polarized by IL-13 may also produce POSTN in ischemic retinas.
Our data demonstrated that genetic ablation of POSTN resulted in a reduction of both preretinal pathological NV and physiological revascularization. Additionally, we observed the promotion effects of POSTN on the proliferation, migration, and tube formation of retinal vascular endothelial cells and Akt phosphorylation through integrin αvβ3. These findings are consistent with our previous reports showing that POSTN promotes CNV.17 In addition, several reports have shown that POSTN can promote NV through integrin αvβ3 in ischemic limbs, cancerous cells, and keloid cells.16, 18, 19 Furthermore, the expression of integrin αvβ3 on the vascular cells in tissues from patients with PDR has been reported.34 Previous papers including ours also showed the FAK/Akt phosphorylation through integrin αv by POSTN in endothelial cells and epithelial cells.31, 35 Our laboratory has also shown that M2 macrophages enhance preretinal pathological NV in OIR retinas.36 Thus, an elevated expression of POSTN by retinal vascular endothelial cells, pericytes, and M2 macrophages may promote the development of ischemia-induced retinal NV mediated by Akt phosphorylation through integrin αvβ3 in both an autocrine and paracrine fashion.
Although recent studies have shown the attractive and promising aspects of canonical double-stranded siRNAs as a new therapy for retinal NV, some hurdles still remain to be overcome before their clinical application.37 The obstacles are the lack of a safe DDS, adverse off-target effects through TLR3 activation, and the lack of stability. We used a novel single-stranded RNAi agent that self-anneals into a unique structure containing a canonical double-stranded RNA to overcome these obstacles. Our results demonstrated that this single-stranded RNAi agent targeting POSTN can significantly inhibit the preretinal pathological NV following an intravitreal injection without any DDS. Moreover, the inhibitory effect of the single-stranded RNAi agent was greater than the canonical double-stranded siRNA, whereas treatment with the single-stranded RNAi agent led to a significant decrease in the avascular areas in OIR retinas compared to treatment with canonical double-stranded siRNAs. Furthermore, the sequence used for POSTN knockdown is present in not only human POSTN but also in mouse, rat, rabbit, and rhesus macaque POSTN.17 This indicates that NK0144 can be used for both in vitro and in vivo experiments and would also be suitable for future human clinical trials. Previous studies including ours have shown that, compared with canonical double-stranded siRNA, the single-stranded RNAi agent has a greater effect without target sequence-independent NV suppression through TLR3 activation.17, 38, 39, 40 The mechanisms causing the differences of the effect on ischemia-induced retinal NV between the single-stranded RNAi agent and the canonical double-stranded siRNA were not completely determined. However, we suggest that these are because the single-stranded RNAi agent has better stability against nuclease, no off-target gene silencing, and no immunostimulatory effects through TLR3 activation.17, 38, 39, 40 Thus, intravitreal injection of naked single-stranded RNAi agent targeting POSTN may be a safer and a more efficient therapeutic method of inhibiting preretinal pathological NV.
Although anti-VEGF therapy for PDR is now a mainstream therapy to prevent retinal NV, it was recently reported that anti-VEGF therapy might be associated with impairment of the function of normal retina and the maintenance of the choriocapillaris.8 This is partly because VEGF plays an important role in retinal homeostasis. Thus, therapies that block VEGF to inhibit pathological NV could result in unexpected complications of the normal retina and should be used cautiously. In contrast to VEGF, we have reported that POSTN was barely detectable in the normal retina.10, 13 We also reported that the correlation between the vitreous concentration of POSTN and VEGF was weak in patients with PDR.13 In addition, previous studies have shown that the binding of VEGF with VEGF receptor-2 (VEGFR2) promoted angiogenesis mainly through the PLCγ/PKC/MAPK pathway,41 whereas the binding of POSTN with integrin αvβ3 promotes angiogenesis mainly through the FAK/Akt pathway. This is good evidence of the concept that anti-POSTN therapy might have independent effect on retinal NV from anti-VEGF therapy. Together with the results of anti-POSTN therapy, POSTN might be an interesting therapeutic target to regulate “disease-specific” pathways involved in the development of retinal NV while minimizing the unfavorable side effects on the normal retina.
In conclusion, our results show a causal link between POSTN and retinal NV, and the effects of a naked, unmodified single-stranded RNAi agent targeting POSTN. Although additional preclinical studies on the toxicity, stability, and effect on duration are underway, a POSTN-targeting RNAi agent may be a new therapeutic agent against preretinal pathological NV.
Materials and Methods
Animals
All animal experiments were performed following the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Kyushu University.
WT C57BL/6J mice (CLEA) and Postn KO mice were used for the animal experiments. PCR was used to determine the genotype of the experimental mice as described in detail.17
Mouse Model of OIR
The OIR mice were generated as described in detail.26 Briefly, pups were exposed to 75 ± 2% oxygen from P7 to P12 and then returned to room air. Mice in the control group were kept in room air continuously.
qRT-PCR
Total RNAs were extracted from homogenized retinas at the selected time points using a MagDEA RNA kit (Precision System Science) according to the manufacturer’s protocol. Total RNAs were also extracted from HRECs treated with RNAi agents. cDNAs were synthesized by reverse transcription with a First Strand cDNA Synthesis Kit (Roche) following the quantification of the RNAs concentration. qRT-PCR was performed and analyzed using a LightCycler 96 PCR system (Roche) and a SYBR Premix Ex Taq (Takara). The primer sequences were as follows: for mouse Postn, 5′-CTTTCGAGAAACTGCCACGAG-3′ and 5′-CCTTCCATGGTCTCAAACACG-3′; for mouse β-actin, 5′-GATGACCCAGATCATGTTTGA-3′ and 5′-GGAGAGCATAGCCCTCGTAG-3′; for human POSTN, 5′-TGCCCAGCAGTTTTGCCCAT-3′ and 5′-CGTTGCTCTCCAAACCTCTA-3′; and for human β-actin, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and 5′-CACCTTCTACAATGAGCTGCGTGTG-3′.
The quality and specificity of the PCR were determined by melting curves, and the relative expression levels by standard curves.
ELISA
Total protein was isolated from sonicated retinas using Tissue Protein Extraction Reagent with protease inhibitor (T-PER; Thermo). The concentrations of POSTN in the mouse retinas and the supernatants from the cultured HRECs were measured with a mouse POSTN immunoassay kit (R&D Systems) and human POSTN ELISA according to the manufacturer’s instructions.
Immunohistochemistry
Eyes enucleated from OIR mice were fixed in 4% paraformaldehyde (PFA), embedded in paraffin, and cut at 3-μm thickness. After deparaffinization, rehydration, antigen retrieval by citric acid, and blocking with 5% skim milk, the sections were incubated with the primary antibodies overnight at 4°C, and the secondary antibodies were added for 1 hr at room temperature. Nuclei were counterstained with Hoechst 33342 (Molecular Probes). After washing with PBS, the slides were coverslipped with an aqueous mounting medium (Thermo). A fluorescent microscope (BZ-9000; Keyence) was used to examine and analyze the slides.
The primary antibodies were POSTN (MAB 3548: 5 μg/mL; R&D Systems), CD31 (550274: 1:50 dilution; BD Biosciences), αSMA (F3777: 1:250 dilution; Sigma-Aldrich), F4/80 (MCA497: 1:100 dilution; AbD Serotec), CD206 (1:100 dilution; Biolegend), IL-13 (ab106732: 1:100 dilution; Abcam), and CD4 (sc-1140: 1:100 dilution; Santa Cruz). The secondary antibodies were Alexa Fluor 488 and 647 (1:100 dilution; Molecular Probes).
Quantification of Neovascular Tufts and Avascular Areas
Both preretinal pathological NV and physiological revascularization in the OIR retinas were quantified at P17 as described in detail.26 Briefly, after fixation in 4% PFA, the neurosensory retinas were isolated from the eye cups. The retinas were washed with PBS and placed in 50% and 100% methanol for 10 min each at room temperature. The retinas were then blocked by PBS containing 1% BSA and 0.5% Triton X-100 for 1 hr at room temperature. This was followed by incubation with fluorescein-labeled isolectin B4 (FL1201; 1:200 dilution; Vector Laboratories) overnight at 4°C. The retinal flat mounts were mounted with mounting medium and were examined with a fluorescent microscope (BZ-9000; Keyence), and each area was quantified with Adobe Photoshop CS6.
Cell Cultures
HRECs were purchased from Applied Cell Biology Research Institute and used for the in vitro studies. Cells were grown in complete medium of the kit with serum and Cultureboost-R (4Z0-500-R; Cell Systems) containing 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in 5% CO2. Cultured HRECs with normal morphology at the fifth to seventh passages were used for these experiments.
Cell Proliferation Assay
Starved HRECs were seeded in each well of 96-well plates at 1 × 104 cells and incubated with recombinant POSTN. After 48 hr, the degree of proliferation was assessed using BrdU ELISA (Roche) according to the manufacturer’s instructions.
Cell Migration Assay
Migration of HRECs was determined using a modified Boyden chamber containing polycarbonate membranes (Transwell, 8-μm pore size; Corning) coated with 10 μg/mL of collagen for 1 hr at 37°C. Starved HRECs were seeded into the upper chamber at 2 × 104 cells/insert in medium containing recombinant POSTN (R&D Systems) or recombinant IL-13 (50 ng/mL; R&D Systems). The lower chamber was filled with medium without serum and growth factors. After 16 hr of incubation at 37°C, non-migrated cells on the upper surface of the membrane were removed by gentle scraping with cotton swabs, and the migrated cells on the lower surface of the membrane were stained with Hoechst 33342 (Molecular Probes). Three photographs were taken of each insert at randomly selected sites with a fluorescence microscope (BZ-9000; Keyence). The number of migrated cells was counted using Adobe Photoshop CS6.
Tube Formation Assay
Tube formation assay was performed as described in detail.36 In brief, starved HRECs were suspended in 96-well plate coated with growth factor-reduced Matrigel matrix (BD Biosciences) at 2 × 104 cells. The cells were cultured with recombinant POSTN or recombinant IL-13 (50 ng/mL). After culturing for 24 hr, the cells were photographed with a phase contrast microscope (Olympus CK2; Olympus). The images were analyzed using the Angiogenesis Analyzer toolset for NIH ImageJ software.
Western Blot Analysis
HRECs were seeded in collagen-coated six-well plates. After starvation with serum-free medium for 24 hr, the cells were cultured with POSTN following treatment with control IgG, integrin αvβ3 Ab, integrin αvβ5 Ab, or LY294002 for 30 min. Total cell lysates of HRECs were extracted using lysis buffer with protease inhibitor and phosphatase inhibitor (Thermo). The extracted cell lysates were added to 4%–12% SDS-NuPAGE, and the blots were incubated with antibodies against phosphorylated FAK (Tyr397, 3283: 1:1,000 dilution; Cell Signaling Technology), FAK (3285: 1:1,000 dilution; Cell Signaling Technology), phosphorylated Akt (Ser473, 4060: 1:2,000 dilution; Cell Signaling Technology), Akt (4691: 1:1,000 dilution; Cell Signaling Technology), or ILK1 (3862: 1:1,000 dilution; Cell Signaling Technology). The signals were made visible with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo) detection system. Differences in lane loading were determined by blotting the membranes with an Ab against β-actin (4970: 1:1,000; Cell Signaling Technology).
RNAi Agent Targeting POSTN
The novel single-stranded RNAi agent (NK) and the canonical double-stranded siRNA (NI) targeting POSTN that were used were as follows: canonical double-stranded siRNA (NI0079), sense 5′-GCACCAAAAAGAAAUACUUTT-3′, antisense 5′-AAGUAUUUCUUUUUGGUGCTT-3′; canonical double-stranded scramble siRNA (NI0000), sense 5′-UACUAUUCGACACGCGAAGTT-3′, antisense 5′-CUUCGCGUGUCGAAUAGUATT-3′; novel single-stranded RNAi agent (NK0144), 5′-AGCACCAAAAAGAAAUACUUUUCCCCACACCGGAAAAGUAUUUCUUUUUGGUGCUUCUUCGG-3′, novel single-stranded scramble RNAi agent (NK0000), 5′-AUACUAUUCGACACGCGAAGUUCCCCACACCGGAACUUCGCGUGUCGAAUAGUAUUCUUCGG-3′.
The sequence for the POSTN knockdown was designed to target the mRNA of both human and mouse POSTN. The novel single-stranded RNAi agent was constructed by incorporating the sense and antisense nucleotides of the canonical double-stranded siRNA into the scaffold of a unique RNAi platform named nkRNA. The nkRNAs spontaneously anneal to form a helical structure containing a double-stranded central stem and two loops within a molecule.
In Vitro Transfection
HRECs were cultured to a confluence of 50%–70%. RNAi agents were mixed (10 nM, final concentration) with RNA transfection reagent (Lipofectamine RNAiMAX; Invitrogen) according to the manufacturer’s protocol. The mixtures for transfection were replaced after 24 hr by medium containing IL-13 at a final concentration of 50 ng/mL as the inducer of POSTN followed by each assay.
In Vivo RNAi Treatment
Immediately after returning the pups to room air at P12, they were given an intravitreal injection of 1 μL of PBS containing 10 μM scramble RNAi agent in one eye and RNAi agent targeting POSTN in the other eye. The intravitreal injections were performed 0.5 mm away from the limbus using a 1-mL Hamilton syringe (Hamilton) and a 33-gauge needle under a surgical microscope.
Statistical Analyses
All results were expressed as means ± SEMs. The statistical significance of differences between groups was determined by two-tailed t tests. For comparisons with the control group, Dunnett’s test was used. Differences were considered significant with p < 0.05. Statistical analyses were performed using JMP, version 11.0.0 (SAS Institute).
Author Contributions
S.Y. designed the study, and T.N. wrote the initial draft of the manuscript. T.N., S.Y., K. Ishikawa, and S.N. contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. The final version of the manuscript was approved by all authors.
Conflicts of Interest
The patent on periostin (WPO Patent WO/2013/147140) became public, and in these patents, the names of T.N., S.Y., K. Ishikawa, and T.I. are included. K.T. and K.Y. are employees of AQUA Therapeutics and hold equity. The other authors have no conflict of interest.
Acknowledgments
The authors thank Masayo Eto, Kinuko Sasada, and Hiroko Miura for their excellent technical assistance. This work was supported in part by JSPS KAKENHI grants 26293374, 26670757, and 15H04995, and Takeda Science Foundation. T.N. is supported by a fellowship from The Japan Society for the Promotion of Science for Young Scientists.
Footnotes
Supplemental Information includes one figure and can be found with this article online at http://dx.doi.org/10.1016/j.omtn.2017.01.004.
Supplemental Information
References
- 1.Campochiaro P.A. Retinal and choroidal neovascularization. J. Cell. Physiol. 2000;184:301–310. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Bishop P.N. The role of extracellular matrix in retinal vascular development and preretinal neovascularization. Exp. Eye Res. 2015;133:30–36. doi: 10.1016/j.exer.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Aiello L.P., Avery R.L., Arrigg P.G., Keyt B.A., Jampel H.D., Shah S.T., Pasquale L.R., Thieme H., Iwamoto M.A., Park J.E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson-Berka J.L., Babic S., De Gooyer T., Stitt A.W., Jaworski K., Ong L.G., Kelly D.J., Gilbert R.E. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am. J. Pathol. 2004;164:1263–1273. doi: 10.1016/s0002-9440(10)63214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braunger B.M., Leimbeck S.V., Schlecht A., Volz C., Jägle H., Tamm E.R. Deletion of ocular transforming growth factor β signaling mimics essential characteristics of diabetic retinopathy. Am. J. Pathol. 2015;185:1749–1768. doi: 10.1016/j.ajpath.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Huang H., He J., Johnson D., Wei Y., Liu Y., Wang S., Lutty G.A., Duh E.J., Semba R.D. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1α-VEGF pathway inhibition. Diabetes. 2015;64:200–212. doi: 10.2337/db14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuiper E.J., Van Nieuwenhoven F.A., de Smet M.D., van Meurs J.C., Tanck M.W., Oliver N., Klaassen I., Van Noorden C.J., Goldschmeding R., Schlingemann R.O. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saint-Geniez M., Kurihara T., Sekiyama E., Maldonado A.E., D’Amore P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Geest R.J., Lesnik-Oberstein S.Y., Tan H.S., Mura M., Goldschmeding R., Van Noorden C.J., Klaassen I., Schlingemann R.O. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br. J. Ophthalmol. 2012;96:587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa K., Yoshida S., Kobayashi Y., Zhou Y., Nakama T., Nakao S., Sassa Y., Oshima Y., Niiro H., Akashi K. Microarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2015;56:932–946. doi: 10.1167/iovs.14-15589. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida S., Ogura A., Ishikawa K., Yoshida A., Kohno R., Yamaji Y., Ikeo K., Gojobori T., Kono T., Ishibashi T. Gene expression profile of fibrovascular membranes from patients with proliferative diabetic retinopathy. Br. J. Ophthalmol. 2010;94:795–801. doi: 10.1136/bjo.2009.167072. [DOI] [PubMed] [Google Scholar]
- 12.Takada M., Ban Y., Yamamoto G., Ueda T., Saito Y., Nishimura E., Fujisawa K., Koide R., Mizutani M., Kozawa T. Periostin, discovered by nano-flow liquid chromatography and mass spectrometry, is a novel marker of diabetic retinopathy. Biochem. Biophys. Res. Commun. 2010;399:221–226. doi: 10.1016/j.bbrc.2010.07.058. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S., Ishikawa K., Asato R., Arima M., Sassa Y., Yoshida A., Yoshikawa H., Narukawa K., Obika S., Ono J. Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2011;52:5670–5678. doi: 10.1167/iovs.10-6625. [DOI] [PubMed] [Google Scholar]
- 14.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell. Mol. Life Sci. 2011;68:3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway S.J., Izuhara K., Kudo Y., Litvin J., Markwald R., Ouyang G., Arron J.R., Holweg C.T., Kudo A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014;71:1279–1288. doi: 10.1007/s00018-013-1494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim B.R., Jang I.H., Shin S.H., Kwon Y.W., Heo S.C., Choi E.J., Lee J.S., Kim J.H. Therapeutic angiogenesis in a murine model of limb ischemia by recombinant periostin and its fasciclin I domain. Biochim. Biophys. Acta. 2014;1842:1324–1332. doi: 10.1016/j.bbadis.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Nakama T., Yoshida S., Ishikawa K., Kobayashi Y., Zhou Y., Nakao S., Sassa Y., Oshima Y., Takao K., Shimahara A. Inhibition of choroidal fibrovascular membrane formation by new class of RNA interference therapeutic agent targeting periostin. Gene Ther. 2015;22:127–137. doi: 10.1038/gt.2014.112. [DOI] [PubMed] [Google Scholar]
- 18.Kyutoku M., Taniyama Y., Katsuragi N., Shimizu H., Kunugiza Y., Iekushi K., Koibuchi N., Sanada F., Oshita Y., Morishita R. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int. J. Mol. Med. 2011;28:181–186. doi: 10.3892/ijmm.2011.712. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z., Nie F., Chen X., Qin Z., Kang C., Chen B., Ma J., Pan B., Ma Y. Upregulated periostin promotes angiogenesis in keloids through activation of the ERK 1/2 and focal adhesion kinase pathways, as well as the upregulated expression of VEGF and angiopoietin-1. Mol. Med. Rep. 2015;11:857–864. doi: 10.3892/mmr.2014.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakuno D., Kimura N., Yoshioka M., Mukai M., Kimura T., Okada Y., Yozu R., Shukunami C., Hiraki Y., Kudo A. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Invest. 2010;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 22.Karikó K., Bhuyan P., Capodici J., Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through Toll-like receptor 3. J. Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 23.Cho W.G., Albuquerque R.J., Kleinman M.E., Tarallo V., Greco A., Nozaki M., Green M.G., Baffi J.Z., Ambati B.K., De Falco M. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc. Natl. Acad. Sci. USA. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinman M.E., Yamada K., Takeda A., Chandrasekaran V., Nozaki M., Baffi J.Z., Albuquerque R.J., Yamasaki S., Itaya M., Pan Y. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinman M.E., Kaneko H., Cho W.G., Dridi S., Fowler B.J., Blandford A.D., Albuquerque R.J., Hirano Y., Terasaki H., Kondo M. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol. Ther. 2012;20:101–108. doi: 10.1038/mt.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor K.M., Krah N.M., Dennison R.J., Aderman C.M., Chen J., Guerin K.I., Sapieha P., Stahl A., Willett K.L., Smith L.E. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki Y., Nakazawa M., Suzuki K., Yamazaki H., Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn. J. Ophthalmol. 2011;55:256–263. doi: 10.1007/s10384-011-0004-8. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida S., Kobayashi Y., Nakama T., Zhou Y., Ishikawa K., Arita R., Nakao S., Miyazaki M., Sassa Y., Oshima Y. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formation. Br. J. Ophthalmol. 2015;99:629–634. doi: 10.1136/bjophthalmol-2014-305860. [DOI] [PubMed] [Google Scholar]
- 29.Fukushi J., Ono M., Morikawa W., Iwamoto Y., Kuwano M. The activity of soluble VCAM-1 in angiogenesis stimulated by IL-4 and IL-13. J. Immunol. 2000;165:2818–2823. doi: 10.4049/jimmunol.165.5.2818. [DOI] [PubMed] [Google Scholar]
- 30.Tang S., Le-Ruppert K.C. Activated T lymphocytes in epiretinal membranes from eyes of patients with proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 1995;233:21–25. doi: 10.1007/BF00177781. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa K., Yoshida S., Nakao S., Nakama T., Kita T., Asato R., Sassa Y., Arita R., Miyazaki M., Enaida H. Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J. 2014;28:131–142. doi: 10.1096/fj.13-229740. [DOI] [PubMed] [Google Scholar]
- 32.Shimazaki M., Nakamura K., Kii I., Kashima T., Amizuka N., Li M., Saito M., Fukuda K., Nishiyama T., Kitajima S. Periostin is essential for cardiac healing after acute myocardial infarction. J. Exp. Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi Y., Yoshida S., Nakama T., Zhou Y., Ishikawa K., Arita R., Nakao S., Miyazaki M., Sassa Y., Oshima Y. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: possible involvement of periostin. Br. J. Ophthalmol. 2015;99:451–456. doi: 10.1136/bjophthalmol-2014-305321. [DOI] [PubMed] [Google Scholar]
- 34.Friedlander M., Theesfeld C.L., Sugita M., Fruttiger M., Thomas M.A., Chang S., Cheresh D.A. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita O., Yoshimura K., Nagasawa A., Ueda K., Morikage N., Ikeda Y., Hamano K. Periostin links mechanical strain to inflammation in abdominal aortic aneurysm. PLoS One. 2013;8:e79753. doi: 10.1371/journal.pone.0079753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Yoshida S., Nakao S., Yoshimura T., Kobayashi Y., Nakama T., Kubo Y., Miyawaki K., Yamaguchi M., Ishikawa K. M2 macrophages enhance pathological neovascularization in the mouse model of oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 2015;56:4767–4777. doi: 10.1167/iovs.14-16012. [DOI] [PubMed] [Google Scholar]
- 37.Pecot C.V., Calin G.A., Coleman R.L., Lopez-Berestein G., Sood A.K. RNA interference in the clinic: challenges and future directions. Nat. Rev. Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamasaki T., Suzuki H., Shirohzu H., Matsumoto T., D’Alessandro-Gabazza C.N., Gil-Bernabe P., Boveda-Ruiz D., Naito M., Kobayashi T., Toda M. Efficacy of a novel class of RNA interference therapeutic agents. PLoS One. 2012;7:e42655. doi: 10.1371/journal.pone.0042655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita Y., Takeshita F., Mizutani T., Ohgi T., Kuwano K., Ochiya T. A novel platform to enable inhaled naked RNAi medicine for lung cancer. Sci. Rep. 2013;3:3325. doi: 10.1038/srep03325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takanashi M., Sudo K., Ueda S., Ohno S., Yamada Y., Osakabe Y., Goto H., Matsunaga Y., Ishikawa A., Usui Y., Kuroda M. Novel types of small RNA exhibit sequence- and target-dependent angiogenesis suppression without activation of Toll-like receptor 3 in an age-related macular degeneration (AMD) mouse model. Mol. Ther. Nucleic Acids. 2015;4:e258. doi: 10.1038/mtna.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.