Abstract
Hypoxia-inducible factors mediate adaptive responses to ischemia, among others, by induction of anti- and pro-survival genes. Thus, the impact of HIF on neuronal survival upon stroke is controversial. Therefore, neuron-specific knockout mice deficient for Hif1a and Hif2a were exposed to inspiratory hypoxia or ischemia–reperfusion injury. Both Hif1a- and Hif2a-deficient mice showed no altered infarct and edema size, suggesting that both HIF-α subunits might compensate for each other. Accordingly, hypoxic HIF-target gene regulation was marginally affected with exception of anti-survival Bnip3 and pro-survival erythropoietin. In the early acute stage upon stroke, Hif1a/Hif2a double knockout mice exhibited significantly reduced expression of the anti-survival Bnip3, Bnip3L, and Pmaip1. Accordingly, global cell death and edema were significantly reduced upon 24 h but not 72 h reperfusion. Behavioral assessment indicated that Hif1a/Hif2a-deficient mice initially performed better, but became significantly more impaired after 72 h accompanied by increased apoptosis and reduced angiogenesis. Our findings suggest that in neurons HIF-1 and HIF-2 have redundant functions for cellular survival under ischemic conditions. By contrast, lack of anti-survival factors in Hif1a/Hif2a-deficient mice might protect from early acute neuronal cell death and neurological impairment, indicating a benefit of HIF-pathway inhibition in neurons in the very acute phase after ischemic stroke.
Keywords: Erythropoietin, hypoxia-inducible factor, ischemia, stroke, vascular endothelial growth factor
Introduction
In response to low tissue oxygenation during ischemic stroke, cells activate hypoxia-inducible factors (HIFs) to enhance the transcription of genes involved in glycolysis, angiogenesis, and cell survival, promoting adaptation to hypoxic/ischemic stress.1 However, also anti-survival factors have been identified as HIF-target genes.2–4 Accordingly, results concerning the role of HIF-1 during cerebral ischemia are controversial. While Baranova et al.5 found that neuron-specific loss of HIF-1 increases ischemic brain injury, Helton et al.6 reported decreased tissue damage upon cerebral ischemia. Furthermore, nothing is known about the function of neuronal HIF-2.
HIFs are heterodimers consisting of an α and a β subunit. HIF-1α/HIF-1β and HIF-2α/HIF-1β dimers are the primary factors regulating hypoxic transcriptional responses in most mammalian cells. HIF-1α and HIF-2α exhibit similar functional domain structures, containing DNA binding and dimerization domains at their N termini and transactivation domains at their C termini.7 HIFs are primarily regulated by changes in protein stability and transcriptional activity in an oxygen-dependent manner. Under normoxic conditions, both α subunits are hydroxylated on conserved proline and asparagine residues by the family of prolyl-4-hydroxylase domain (PHD) proteins and factor inhibiting HIF (FIH), respectively, whose activity is dependent on molecular oxygen, ferrous iron, 2-oxoglutarate, and reducing compounds like ascorbate. While prolyl-4-hydroxylation results in recruitment of the von Hippel-Lindau protein E3 ubiquitin ligase and immediate proteasomal degradation of HIF-1α and -2α, asparaginyl hydroxylation blocks interaction with the transcriptional co-activators p300 and CBP, reducing its transcriptional activity.1,8–12 By contrast, when oxygenation declines during hypoxia or ischemia, PHDs and FIH are less active. As a result, hypohydroxylated HIF-α accumulates and translocates into the nucleus to form the HIF complex by dimerization with HIF-1β (also known as aryl hydrocarbon receptor nuclear translocator, ARNT). Subsequently, HIF specifically binds to hypoxia response elements (HREs) in the promoters and enhancers of numerous genes followed by recruitment of p300/CBP resulting in enhanced transcription of HIF-responsive genes.1,8–12
A growing number of pre-clinical studies in rodents strongly suggests that activation of the HIF signaling pathway prior or shortly after ischemic stroke by application of low-molecular weight hydroxylase inhibitors5,13–19 or brief exposure to mild systemic hypoxia,20–23 reduces tissue damage and increases functional recovery from ischemic stroke. However, as these therapeutic strategies unavoidably lead to pleiotropic activation of HIF signaling throughout all cell types of the central nervous system (CNS), it is difficult to draw conclusions about the definite significance of the HIF signaling pathway in neurons and their viability upon ischemia–reperfusion injury. Furthermore, although HIF-1α and HIF-2α are closely related and share numerous target genes, they also regulate unique target genes, suggesting that HIF-1α and HIF-2α may exhibit distinct roles in the neuronal response to ischemic conditions. Thus, in the present study we generated transgenic mice bearing neuron-specific deficiencies of Hif1a and/or Hif2a, and studied the histological and functional outcome upon mild and severe focal cerebral ischemia.
Materials and methods
Animal experiments
All animal experiments were approved by the local animal welfare committee (Regierungspräsidium Karlsruhe, Germany, approval number: 35-9185.81/G-103/12, 35-9185.81/G-210/14), conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were performed in accordance with the recently published Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (http://www.nc3rs.org/ARRIVE). All mice used in the animal experiments were randomized. The operator or investigator was blinded for the respective mouse genotypes throughout the study. Evaluation of all read-out parameters was done independently and in a blinded fashion.
Generation of neuron-specific Hif1a, Hif2a, and Hif1a/Hif2a knockout mice
All mice were maintained at the animal facility of the University of Heidelberg under specific pathogen-free conditions, a controlled 12:12 h light-dark cycle, constant room temperature (22 ± 2℃) with food and water ad libitum. Hif1aflox/flox 24 and Hif2aflox/flox mice25 were obtained from Dr. Ben Wielockx (Department of Clinical Pathobiochemistry, Technical University of Dresden). Hif1af/f and Hif2af/f mice were intercrossed to generate Hif1a/Hif2aff/ff mice. Subsequently, Hif1af/f, Hif2af/f, and Hif1a/Hif2aff/ff mice were bred with a transgenic mouse line expressing Cre recombinase under control of the Ca2+/calmodulin-dependent protein kinase IIα promotor (CaMKIIα:cre)26,27 to obtain homozygous forebrain neuron-restricted knockout mice (nHif1aΔ/Δ, nHif2aΔ/Δ, nHif1a/Hif2aΔΔ/ΔΔ). All mouse strains were at least nine generations backcrossed to C57BL/6. Mice were genotyped using the primers (Eurofins Genomics, Ebersberg, Germany) described in Table S1.
Middle cerebral artery occlusion
Male mice (8–10 weeks old) were anesthetized by a mixture of 2% isoflurane, 70% N2O and remainder O2, and were maintained by reducing the isoflurane concentration to 1.0–1.5%. Core body temperature was maintained at 37℃ throughout surgery by using a feedback-controlled heating device. To induce focal cerebral ischemia, a 7-0 silicon rubber-coated nylon monofilament (Doccol Corporation, Redlands, CA, USA) was introduced into the left internal carotid artery (ICA) and pushed toward the left MCA as described.27 The intraluminal suture was left for 30–60 min. Then, animals were re-anesthetized and the occluding monofilament was withdrawn to allow reperfusion for 24–72 h. Subsequently, animals were sacrificed by decapitation, and brains were removed and embedded into Tissue-Tek (Sakura Finetek, Staufen, Germany) for histological analyses. From each brain, 24 coronal cryosections (10 µm thickness; 0.4 mm distance) were prepared and submitted to Nissl staining for quantification of infarct and edema size as described previously.17,27 Briefly, brain slices were digitized, and infarct and edema volume was measured using the image analysis software ImageJ (National Institutes of Health, Bethesda, MD, USA). Animals that met the following criteria were excluded from end-point analyses: (i) death after induction of middle cerebral artery occlusion (MCAO) and (ii) intracerebral hemorrhage (macroscopically assessed during brain sampling; Table S2).
Systemic hypoxia
Mice (10–14 weeks old) were exposed to normobaric hypoxia at 6% oxygen (corresponding to 9500 m altitude) for 6 h or were kept at room air pressure (∼21% oxygen). During the entire procedure mice had free access to food and water. Hypoxia was achieved by substituting nitrogen for oxygen using a Digamix 5SA 18/3 a pump (H. Wösthoff, Bochum, Germany) as described.28 Then, mice were sacrificed by decapitation, brains were isolated, shock frozen in liquid nitrogen and stored at −80℃ until use.
Assessment of functional outcome
Sensorimotor function was assessed by using the corner test and the latency to move test as described previously.29,30 Details are specified in the Supplementary Information. In addition, neurological function before and after MCAO was studied using a modified Bederson neurological deficit score,31 according to the following scoring system: 0, no deficit; 1, whole body bent upon hanging from tail; 2, forelimb flexion; 3, unidirectional turning; 4, unidirectional circling; 5, no movement.
Analysis of cerebrovascular anatomy
Mice were sacrificed by CO2 asphyxiation, the skin and the ribs were cut at the midline of the chest, and the thorax cavity was opened. The right atrium was incised to allow venous outflow followed by insertion of the perfusion cannula into the left ventricle. Animals were perfused at a rate of ∼10–15 ml/min with pre-warmed Ringer's solution containing 0.1 mM sodium nitroprusside (Sigma-Aldrich, Steinheim, Germany) and 0.1 mM adenosine (Sigma) until blood was completely replaced by Ringer's solution. Then, perfusion was continued with 10 ml of a pre-warmed gouache pigment solution that was prepared as described previously.32 Subsequently, brains were carefully harvested and incubated overnight in 4% paraformaldehyde at 4℃, and digital images were taken under a dissecting microscope.
TdT-mediated dUTP nick-end labeling assay
Apoptotic cells were detected in brain sections using the TdT-mediated dUTP nick-end labeling (TUNEL) assay. Details are specified in the Supplementary Information.
Immunofluorescence staining, image acquisition, and analysis
Immunofluorescent staining was used to detect endothelial cells and microglia/macrophages in brain sections. For a detailed description see the Supplementary Information.
RNA isolation and real-time polymerase chain reaction
A detailed description can be found in the Supplementary Information. Oligonucleotide primers were purchased from Eurofins Genomics (Ebersberg, Germany; for primer sequences, see Table S3).
Protein extraction and immunoblotting
A detailed description is provided in the Supplementary Information.
Statistical analysis
If not stated otherwise, the results are presented as mean + standard error of the mean (SEM). Kolmogorov–Smirnov test (n = 5–7) and D'Agostino-Pearson omnibus test (n ≥ 8) were applied to analyze data sets for Gaussian distribution. Grubb's test was used on normal distributed data sets (n ≥ 5) to identify statistical outliers (http://www.graphpad.com/quickcalcs/grubbs1/). Unpaired and paired two-tailed Student's t-test or two-way analysis of variance (ANOVA) combined with Holm-Sidak's multiple comparison test were applied to determine statistical significance. Prism 6 (GraphPad Software, San Diego, CA, USA) was used for data plotting and statistical analyses.
Results
Characterization of neuron-specific Hif1a and Hif2a knockout mice
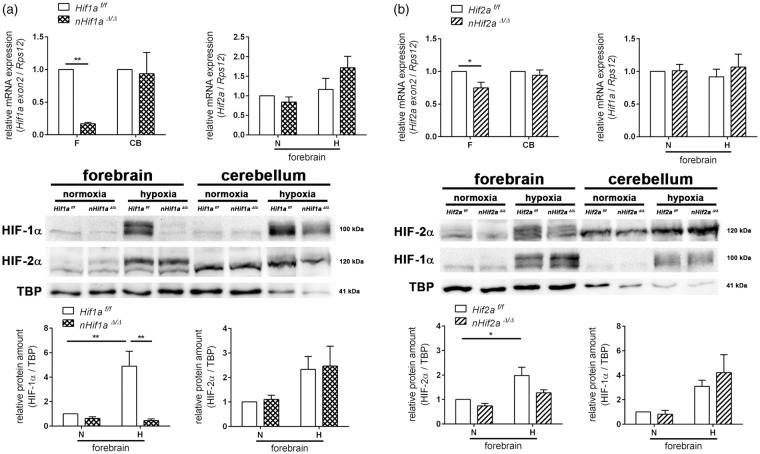
In the forebrain of nHif1aΔ/Δ mice, Hif1a mRNA was markedly reduced by 80% in comparison to wild-type (WT) littermates (Figure 1(a)). Accordingly, hypoxia-induced stabilization of HIF-1α protein in the forebrain was absent, as hypoxic HIF-1α levels were significantly reduced by about 90% in nHif1aΔ/Δ mice (Figure 1(a)), suggesting that neurons are the main cellular source of HIF-1α in the CNS. Our data further reveal that neuron-restricted Hif1a knockout did not cause a compensatory up-regulation of Hif2a mRNA and protein in the forebrain of animals kept under normoxic or hypoxic conditions (Figure 1(a)). By contrast, neuronal ablation of Hif2a reduced total Hif2a mRNA in the murine forebrain by only 25% (Figure 1(b)). At protein level, HIF-2α in the forebrain of nHif2aΔ/Δ mice exposed to systemic hypoxia was decreased by roughly 35% as compared to WT animals under the same conditions and was not significantly different from normoxic levels (Figure 1(b)). These results together with a previous study indicate that HIF-2α in brain tissue predominantly derives from non-neuronal cells such as astrocytes and endothelial cells.33 Under both normal and declined oxygen tensions, Hif1a gene expression was not significantly different between WT and nHif2aΔ/Δ mice (Figure 1(b)).
Figure 1.
Characterization of neuron-specific Hif1a and Hif2a knockout. Hif1af/f and nHif1aΔ/Δ (a) or Hif2af/f and nHif2aΔ/Δ (b) mice were exposed to normobaric hypoxia (6% oxygen) for 6 h or were kept at normoxic conditions. Nuclear proteins and RNA were prepared from forebrain and cerebellum. Gene expression was determined by real-time polymerase chain reaction (PCR) (n = 5, per group) and Western blotting (n = 3–4, per group). Values are normalized to Rps12 (mRNA) or TBP (protein) and expressed as fold change to normoxic Hif1af/f and Hif2af/f, respectively. Significant differences determined by two-way ANOVA with Holm-Sidak post-hoc test are indicated with *(p < 0.05) or **(p < 0.01).
CB: cerebellum; F: forebrain; H: hypoxia; N: normoxia.
Reduced neuronal HIF-1α or HIF-2α levels do not affect infarct or edema size during ischemic stroke
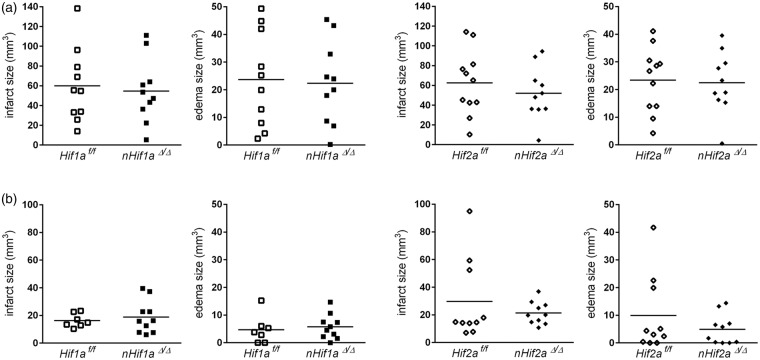
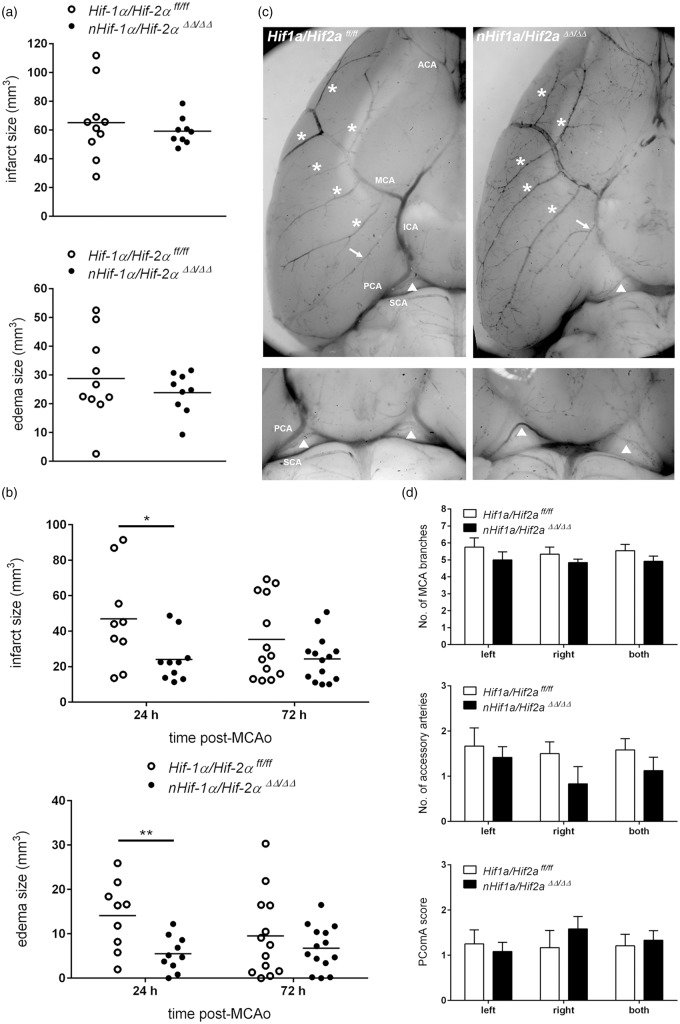
Next, we subjected mice to either 60 min or 30 min of MCAO provoking severe or mild brain tissue damage, respectively. Following one or three days of reperfusion, global infarct and edema size were histologically determined. Irrespective of mild or severe ischemic stroke, infarct and edema volume of nHif1aΔ/Δ mice was comparable to that obtained for WT animals (Figure 2(a) and (b), Table S4). Similarly, neuronal Hif2a deficiency did not affect the lesion or edema size in mice subjected to either mild or severe cerebral ischemia (Figure 2(a) and (b), Table S4). Despite similar lesion size, WT animals undergoing 30 min of MCAO showed an increased, but non-significant, mortality rate during prolonged reperfusion phase as compared to nHif1aΔ/Δ and nHif2aΔ/Δ mice, respectively (Table S2, Figure S1).
Figure 2.
Neuronal Hif1a or Hif2a deficiency does not affect global brain tissue injury following mild or severe ischemic stroke. Mice were subjected to transient focal ischemic stroke: (a) 60 min of MCAO followed by 24 h reperfusion (n = 10–11, per group) or (b) 30 min of MCAO followed by 72 h reperfusion (n = 7–10, per group). Brains were removed and from each brain, 24 coronal cryosections (10 µm thickness; 0.4 mm distance) were prepared and submitted to cresyl violet staining for quantification of infarct and edema size. Unpaired two-tailed t-test was applied to determine statistical significance.
Neuronal HIF-α deletion maintains hypoxic gene induction but shows subunit dependency
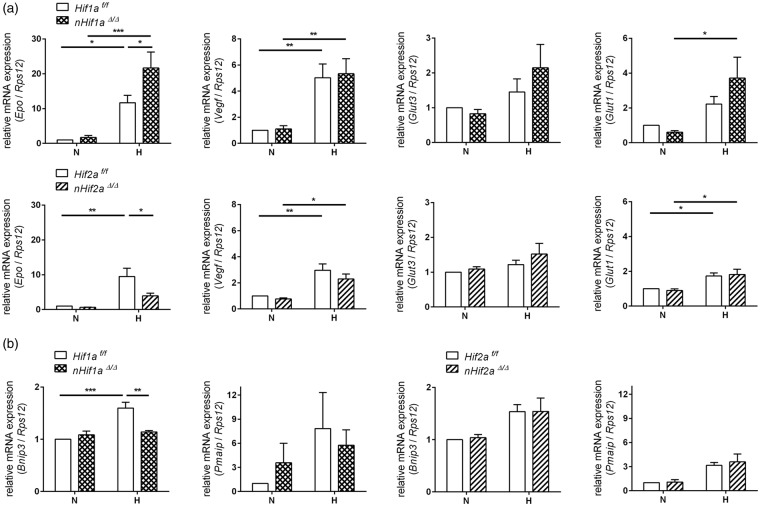
HIF-independent infarct and edema size might be due to a mutual compensation in Hifa single knockout mice. Alternatively, it may also result from a compensatory co-expression of both pro- and anti-survival HIF-target genes after a hypoxic/ischemic stimulus. To address the latter, we analyzed the expression of HIF-regulated anti-survival genes such as Bnip3 and Pmaip1 (also known as Noxa),2–4 and compared them to prominent protective factors such as vascular endothelial growth factor (Vegf) and erythropoietin (Epo), which not only directly protect neurons upon cerebral ischemia through antiapoptotic pathways, but also contribute to long-term regeneration via induction of angiogenesis and neurogenesis,34,35 and to Glut-1 and Glut-3, which increase glucose transport.36,37 Overall, hypoxic induction of anti- and pro-survival factors was comparable. Irrespective of genotype, exposure to a short episode of cerebral hypoxia resulted in significant up-regulation of Vegf and Epo within the forebrain, and also Glut-1 and Glut-3 expression was moderately enhanced after cerebral hypoxia (Figure 3(a)). On the other hand, also anti-survival genes were induced to a similar degree. Cerebral hypoxia enhanced Pmaip1 mRNA expression irrespective of genotype and Bnip3 transcription was increased by 50% in hypoxic WT mice (Figure 3(b)), suggesting that pro- and anti-survival factors could indeed lead to a mutual counterbalance.
Figure 3.
Expression of HIF-targeted genes in the forebrain of neuron-specific Hif1a and Hif2a knockout mice. Hif1af/f, nHif1aΔ/Δ, Hif2af/f, and nHif2aΔ/Δ mice (n = 5, per group) were exposed to normobaric hypoxia (6% oxygen) for 6 h or were kept at normoxic conditions. Expression of (a) pro-survival and (b) anti-survival HIF-target genes within forebrain was analyzed by real-time PCR. Values are normalized to Rps12 mRNA and expressed as fold change to normoxic Hif1af/f and Hif2af/f, respectively. Significant differences determined by two-way ANOVA with Holm-Sidak post-hoc test are indicated with *(p < 0.05), **(p < 0.01) or ***(p < 0.001).
H: hypoxia; N: normoxia.
With respect to redundant HIF-α function, some differences were seen. Although normoxic and hypoxic Vegf as well as Glut-1 and Glut-3 expression was not significantly altered in both nHif1aΔ/Δ or nHif2aΔ/Δ mice in comparison to WT animals (Figure 3(a)), Epo and Bnip3 showed a clear HIF-α subunit dependency. Upon hypoxia, neuronal loss of Hif1a caused a significant increase in total Epo mRNA expression, while loss of Hif2a resulted in reduced expression as compared to WT controls (Figure 3(a)). It suggests that similar to other cell types,38 neuronal Epo transcription is preferentially controlled by HIF-2 and not HIF-1. Basal expression of Bnip3 and Pmaip1 was not significantly different in both nHif1aΔ/Δ and nHif2aΔ/Δ mice as compared to WT animals (Figure 3(b)). Although hypoxic up-regulation of Bnip3 was comparable in nHif2aΔ/Δ, it was completely absent in nHif1aΔ/Δ mice, suggesting that neuronal Bnip3 expression is regulated by HIF-1 rather than HIF-2 (Figure 3(b)).
In the cerebellum of both Hifa knockout mouse lines, even under hypoxic conditions, mRNA expression of all studied genes was comparable to that obtained in WT littermates (Figure S2), reflecting low recombination efficiency due to low CaMKIIα promoter activity in this tissue. Overall, these data support our hypothesis that HIF-1α and HIF-2α functions in neurons are to a certain degree redundant.
Inactivation of HIF-1 and HIF-2 in neurons partially impairs expression of oxygen sensitive genes in hypoxic brain tissue
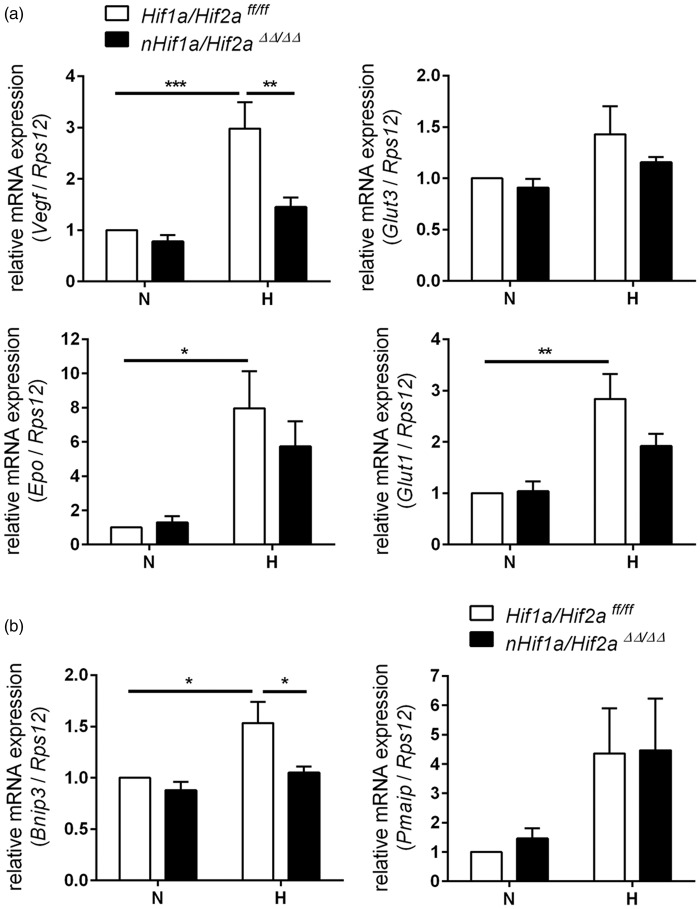
In order to circumvent potential HIF-α redundancy, we generated neuron-specific Hif1a/Hif2a double knockout mice, and studied HIF-target gene expression within the forebrain. Similar to Hifa single knockout, basal expression of all studied genes was not significantly different between nHif1a/Hif2aΔΔ/ΔΔ mice and WT animals (Figure 4). However, Vegf and Bnip3 up-regulation found in WT upon cerebral hypoxia was completely abrogated in nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 4), indicating neurons as major CNS source for both factors upon global hypoxia. By contrast, hypoxic mRNA levels of Epo (7.9 vs. 5.7, p = 0.446), Glut-1 (2.8 vs. 1.9, p = 0.142), Glut-3 (1.4 vs. 1.1, p = 0.608), and Pmaip1 (4.4 vs. 4.5, p = 0.954) were not or only slightly attenuated in forebrains of hypoxic nHif1a/Hif2aΔΔ/ΔΔ animals when compared to WT littermates (Figure 4). This might reflect the contribution of other resident brain cells like astrocytes, endothelial cells, oligodendrocytes, or microglia to total mRNA levels of these genes during the hypoxic adaptive response.
Figure 4.
Expression of HIF-regulated genes in the forebrain of neuron-specific Hif1a/Hif2a knockout mice. Hif1a/Hif2aff/ff and nHif1a/Hif2aΔΔ/ΔΔ mice (n = 5, per group) were exposed to normobaric hypoxia (6% oxygen) for 6 h or were kept at normoxic conditions. Expression of (a) pro-survival and (b) anti-survival HIF-target genes within forebrain was analyzed by real-time PCR. Values are normalized to Rps12 mRNA levels and expressed as fold change to normoxic Hif1a/Hif2aff/ff. Significant differences determined by two-way ANOVA with Holm-Sidak post-hoc test are indicated with *(p < 0.05), **(p < 0.01) or ***(p < 0.001).
H: hypoxia; N: normoxia.
Neuronal loss of HIF-1 and HIF-2 decreases infarct and edema size in the early acute stage of mild ischemic stroke
We next investigated whether attenuation of the HIF pathway in neurons by cell type-specific knockout of both Hif1a and Hif2a influences global brain damage upon mild or severe ischemic stroke. Upon 60 min of MCAO and 24 h reperfusion, massive cell death occurred within cortical and striatal brain regions, and a pronounced hemispheric swelling was determined. Under these conditions, quantification of global lesion and edema volume did not reveal significant differences between Hif1a/Hif2aff/ff and nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 5(a), Table S4). In animals subjected to 30 min of MCAO and up to 72 h reperfusion, the loss of cells was almost restricted to the striatum, and edema formation was moderate. Upon 24h of reperfusion, both infarct and edema size were significantly reduced in nHif1a/Hif2aΔΔ/ΔΔ mice as compared to WT (Figure 5(b), Table S4). By contrast, in the acute (72 h after onset of reperfusion) stage, global lesion and edema volume were similar in Hifa deficient and control mice (Figure 5(b), Table S4). Furthermore, a non-significant tendency toward reduced post-stroke survival was determined in Hif1a/Hif2aff/ff mice as compared to Hifa deficient animals (Table S2, Figure S1). In order to analyze whether the lack of neuronal HIF activity affects the cerebrovascular anatomy which might either enhance or aggravate tissue injury upon MCAO, the cerebral vasculature at the circle of Willis was visualized by pigment particle perfusion (Figure 5(c)). The number of arterial branches that arise from the MCA main trunk was comparable between WT and nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 5(c) and (d)). In most of the animals, we also found accessory arteries that arise near the MCA from the ICA, and potentially supply some of the MCA territory. In comparison to Hif1a/Hif2aff/ff mice, the number of accessory arteries was not statistically different in nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 5(c) and (d)). In our experimental model of focal cerebral ischemia, a nylon monofilament is introduced in the external carotid artery and is advanced through the ICA until blood flow to the MCA is impeded, and may also lead to hypoperfusion of regions (e.g. hippocampus and thalamus) supplied by the posterior cerebral artery (PCA). However, if the blood flow from the carotid circulation is shut down, the ipsilateral PCA could be supplied by the superior cerebellar artery (SCA) through the posterior communicating artery (PComA). Development of the PComA is highly variable in mice from different strains and within the same strain ranging from complete absence to bilateral presence, and from hypo to hyper plasticity.39–41 Along this line, in mice lacking functional PComA, the PCA territory has an enhanced susceptibility to focal ischemia caused by the intraluminal filament technique.40,41 Thus, we evaluated the PComA plasticity according to the following scoring system: 0, no anastomosis between PCA and SCA; 1, hypoplastic PComA; 2, well-developed PComA. Although we determined interindividual differences in accordance with previously published studies, the mean PComA score in Hifa deficient and control mice was quite comparable (Figure 5(c) and (d)).
Figure 5.
Neuron-restricted ablation of Hif1a and Hif2a decreases global damage of cerebral tissue in the early acute phase after mild ischemic stroke, which is not due to altered cerebrovascular architecture. Mice underwent transient focal ischemic stroke using different conditions: (a) 60 min of MCAO followed by 24 h reperfusion (n = 9–10, per group) or (b) 30 min of MCAO followed by 24 h (n = 9–10, per group) and 72 h (n = 13–14, per group) reperfusion, respectively. Brains were removed, coronal cryosections were prepared and submitted to cresyl violet staining for quantification of infarct and edema size. Significant differences determined by unpaired two-tailed Student's t-test are indicated with *(p < 0.05) or **(p < 0.01). (c,d) In adult Hif1a/Hif2a ff/ff and nHif1a/Hif2a ΔΔ/ΔΔ mice (n = 6, per group), the cerebral vasculature was stained through transcardial pigment particle perfusion. For each mouse arterial branches (labeled with asterisks) that arise from the left or right MCA trunk as well as accessory arteries (labeled with arrows) that run more or less parallel to the course of the left or right MCA were quantified. The PCA can be supplied by the SCA through the PComA (labeled with arrowheads). Accordingly, PComA plasticity was scored on scale of 0 to 2 with 0 = no anastomosis between PCA and SCA, 1 = hypoplastic PComA, and 2 = well-developed PComA. Two-way ANOVA with Holm-Sidak's multiple comparison test was applied to determine statistical significance.
ACA: anterior cerebral artery; ICA: internal carotid artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; PComA: posterior communicating artery; SCA: superior cerebellar artery.
Blockage of HIF pathway in neurons improves sensorimotor function in the early acute phase, while it impairs functional outcome in the acute stage of mild ischemic stroke
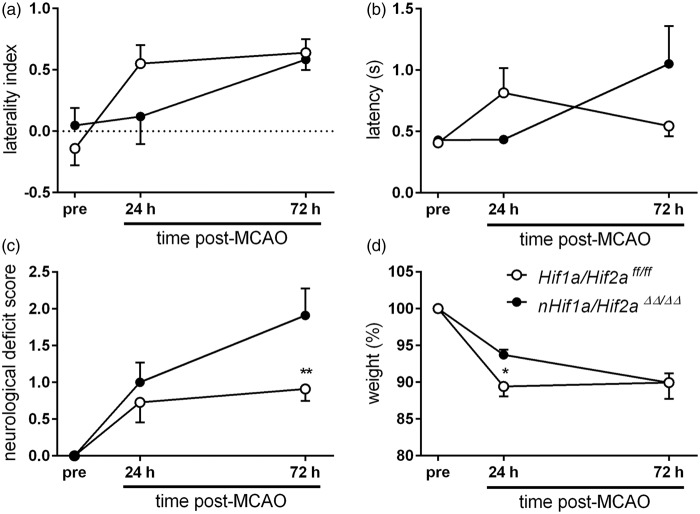
We tested sensorimotor and general neurological function of Hif1a/Hif2aff/ff and nHif1a/Hif2aΔΔ/ΔΔ mice suffering from mild ischemic stroke. Irrespective of genotype, mice studied with corner test before induction of stroke turned equally to the left and right side. However, in both the early acute (24 h after onset of reperfusion) and acute (72 h after onset of reperfusion) stage of stroke, WT mice turned preferentially to the ipsilateral left side, which is typically not affected by paralysis (Figure 6(a), Table S5). By contrast, nHif1a/Hif2aΔΔ/ΔΔ mice showed no side preference in the early acute phase similar to pre-testing results, pointing to an early protective effect of HIF deficiency. However, later on the behavior worsened, and the animals now turned mainly to the ipsilateral left side (Figure 6(a), Table S5). These results were sustained by the latency to move test, demonstrating that WT but not nHif1a/Hif2aΔΔ/ΔΔ mice showed a strongly reduced motor activity at the early acute stage as compared to the pre-test (Figure 6(b), Table S5). However, in the acute phase motor activity in WT animals returned to baseline, while latency to move markedly increased in nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 6(b), Table S5). Similarly, at the acute stage nHif1a/Hif2aΔΔ/ΔΔ mice exhibited a significantly higher neurological deficit score in comparison to WT (Figure 6(c), Table S5). Accordingly, early body weight loss as result of stroke was significantly attenuated in Hifa deficient mice (Figure 6(d), Table S5). Later, body weight remained constant in WT mice, but further decreased in nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 6(d), Table S5).
Figure 6.
Neuron-specific Hif1a/Hif2a knockout mice show improved and impaired sensorimotor function in the early acute and acute phase after mild ischemic stroke, respectively. Mice were subjected to 30 min of MCAO followed by 72 h reperfusion (n = 13–14, per group). Neurological function was assessed 24 h before, and 24 and 72 h after transient MCAO by using (a) Corner test, (b) Latency to move test, and (c) a modified Bederson neurological deficit score. (a) The laterality index (LI) was calculated according to the formula: LI = (turns to the left side – turns to the right side)/total number of turnings. (d) shows the body weight before, 24 and 72 h after onset of reperfusion. Significant differences determined by two-way repeated measures ANOVA with Holm-Sidak post-hoc test are indicated with *(p < 0.05) or **(p < 0.01).
Overall, our functional evaluation suggests that blockage of the HIF pathway in neurons improves early acute outcome from ischemic stroke, while it impairs neuronal recovery in the acute phase.
Neuronal loss of HIF-1 and HIF-2 reduces expression of anti-survival genes in the early acute phase, but increases apoptotic cell death and decreases angiogenesis in the acute phase of mild ischemic stroke
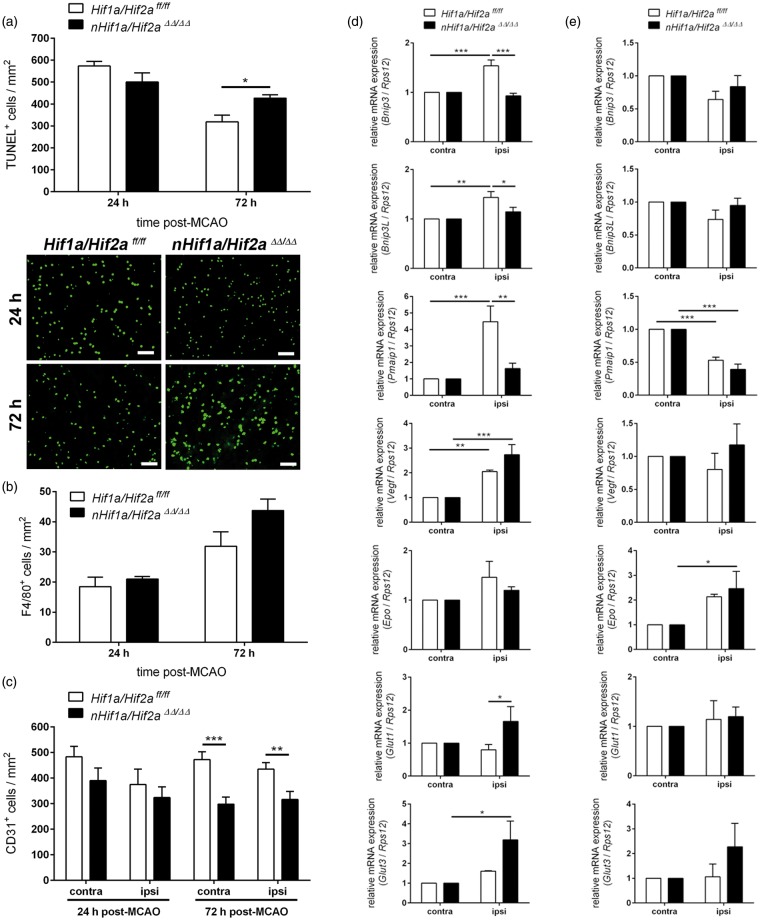
While neurons predominantly undergo necrotic cell death in the early acute phase, the frequency of apoptosis strongly increases in acute stages of ischemic stroke. Thus, we analyzed whether the observed changes in behavior in Hifa deficient mice may correlate with the apoptosis rate after cerebral ischemia. In the early phase, in animals subjected to 30 min of MCAO and 24 h reperfusion, the number of apoptotic cells within the entire infarcted tissue was slightly reduced in nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 7(a)). Sub-regional analysis revealed decreased apoptosis in ipsilateral cortex (55 ± 18 vs. 170 ± 54 cells/mm2, p = 0.069), but not striatum (422 ± 24 vs. 476 ± 79 cells/mm2, p = 0.564) of Hif1a/Hif2a double knockout as compared to Hif1a/Hif2aff/ff mice. However, at later stages, i.e. 72 h after mild ischemic stroke, apoptotic cell death within the entire infarct area was significantly higher in nHif1a/Hif2aΔΔ/ΔΔ mice (Figure 7(a)). Sub-regional quantification in cortex (239 ± 18 vs. 165 ± 34 cells/mm2, p = 0.081) and striatum (438 ± 75 vs. 310 ± 15 cells/mm2, p = 0.124) revealed a likewise contribution of both areas for enhanced apoptosis in Hifa deficient animals. We further showed that the number of microglia/macrophages was not significantly different between WT and Hifa deficient mice during both early acute and acute stages, suggesting that the enhanced number of apoptotic cells in brains of nHif1a/Hif2aΔΔ/ΔΔ mice is not due to attenuated phagocytosis through activated microglia/macrophages (Figure 7(b)). In order to identify potential underlying molecular mechanisms, we investigated the expression of anti- and pro-survial HIF-target genes in Hif1a/Hif2a deficient mice and WT littermates upon mild cerebral ischemia; 6 h after the onset of reoxygenation, the anti-survival HIF-target genes Bnip3, Bnip3L, and Pmaip1 as well as Vegf were significantly up-regulated in the ipsilateral hemisphere of WT animals (Figure 7(d)). In Hif1a/Hif2a double knockout mice, the induction of Bnip3, Bnip3L (also known as Nix),42 and Pmaip1 in ischemic brain tissue was only marginal, and significantly lower as those in WT (Figure 7(d)). In contrast to the attenuated Vegf expression in Hif1a/Hif2a deficient mice upon inspiratory hypoxia (Figure 4), its expression in the very acute stage after ischemic stroke was comparable to that in WT mice (Figure 7(d)). Glucose transporters (Glut1, Glut3) were up-regulated in the ipsilateral hemisphere of Hif1a/Hif2a deficient, but not in WT mice, and Glut1 expression in KO was significantly higher as compared to WT. Irrespective of genotype, during early acute stage of stroke Epo expression was not enhanced within injured brain tissue (Figure 7(d)); 72 h upon ischemic insult during transition from acute toward sub-acute stages, none of the studied HIF-targeted factors were significantly up-regulated in the ipsilateral hemisphere as compared to contralateral control tissue in both Hif1a/Hif2a deficient and WT animals (Figure 7(e)). The only exception was Epo that showed significantly increased transcription in Hif1a/Hif2a deficient mice and a tendency toward up-regulation in WT (Figure 7(e)). In spite of comparable Vegf expression, we determined a significantly reduced density of microvascular endothelial cells in both ipsi- and contralateral hemisphere of Hif1a/Hif2a deficient mice in the acute phase, while during the early acute stage, the number of endothelial cells was not significantly different between Hif1a/Hif2a deficient and WT animals that suffered from mild ischemic stroke (Figure 7(c)).
Figure 7.
Neuronal deficiency for Hif1a and Hif2a reduces expression of anti-survival genes in the early acute phase, while it increases apoptosis and decreases angiogenesis at the acute stage of mild ischemic stroke. (a) Mice were subjected to 30 min of MCAO followed by 24 h (n = 5–6, per group) and 72 h (n = 5, per group) reperfusion, respectively. Brains were removed, coronal cryosections were prepared, and the number of apoptotic cells within the ischemic ipsilateral hemisphere was determined using TUNEL assay. Scale bar = 50 µm. Significant differences determined by unpaired two-tailed t-test are indicated with *(p < 0.05). (b,c) Mice underwent 30 min of MCAO followed by 24 h (n = 6, per group) and 72 h (n = 6, per group) reperfusion, respectively. Brains were removed, coronal cryosections were prepared, and the number of CD31-immunoreactive endothelial cells and F4/80-immunoreactive microglia/macrophages within non-ischemic contralateral and ischemic ipsilateral hemisphere was determined using immunofluorescence microscopy. (b) Unpaired two-tailed t-test was applied to determine statistical significance. (c) Significant differences determined by two-way ANOVA with Holm-Sidak post-hoc test are indicated with **(p < 0.01) or ***(p < 0.001). Mice were subjected to 30 min of MCAO followed by (d) 6 h (n = 5, per group) and (e) 72 h (n = 4–5, per group) reperfusion, respectively. Gene expression in the contralateral and ipsilateral hemispheres was analyzed by real-time PCR. Values are normalized to Rps12 mRNA levels and expressed as fold change to contralateral Hif1a/Hif2aff/ff and nHif1a/Hif2a ΔΔ/ΔΔ, respectively. Significant differences determined by two-way ANOVA with Holm-Sidak post-hoc test are indicated with *(p < 0.05), **(p < 0.01) or ***(p < 0.001).
These results support our hypothesis that neuronal HIF deficiency is favorable early after stroke but becomes awkward at later stages.
Discussion
Hypoxia-/ischemia-induced factors play a major role in the pathogenesis of stroke recovery. Many of them are HIF-target genes such as Epo and VEGF both of which are neuroprotective and result in reduced infarct size after cerebral ischemia.34,35,43,44 However, both factors also have undesired side effects when given as treatment, such as erythrocytosis and vascular leakage leading to brain edema.34,45 Understanding the in vivo situation where not only a single factor but the whole hypoxia-induced pathway is activated might help to find alternative therapeutic strategies. We recently showed that neuronal inactivation of PHD2 results in up-regulation of HIF-α, Epo, VEGF, glucose transporters, and glycolytic enzymes after cerebral ischemia, thereby leading to smaller infarct size but also reduced edema formation, suggesting that simultaneous activation of several HIF targets could counteract negative side effects of single target genes27 and might represent the pathophysiological basis for stroke recovery.
Role of HIF-1α and HIF-2α
To further characterize the PHD-HIF-axis in stroke recovery, we generated mice with single or combined neuronal deficiency for HIF-1α and HIF-2α. Our current in vivo findings do not indicate that neuron-specific lack of HIF-1 function alters neuronal cell death upon mild or severe ischemic insult, and may reflect discrepant results in the literature. While Helton et al.6 reported decreased tissue damage upon cerebral ischemia, Baranova et al.5 found increased ischemic brain injury due to the loss of HIF-1 in neurons. The observed differences might be due to the use of diverse murine stroke models, i.e. global brain ischemia using transient bilateral occlusion of the common carotid artery6 or focal ischemia induced by transient MCAO.5 Although we also applied the intraluminal filament technique to transiently occlude the MCA, our Cre recombinase driver mouse line26 used to generate neuron-specific gene knockout was different from that described in the previous reports, which might also explain differing observations.
Our current results indicate that HIF-2 may substitute for HIF-1 in Hif1a deficient neurons. Indeed, up-regulation of HIF-regulated genes including Vegf, Glut1, Glut3, and Pmaip1 in response to a short hypoxic episode was unaffected in brain tissue of Hif1a deficient mice and comparable to WT mice. Similar results were reported by Helton et al.,6 when studying overall gene expression through microarray analysis in brain tissue upon hypoxic insult. However, we also found opposed responses. Hypoxic induction of Epo in nervous tissue of neuronal Hif1a knockout mice was not only not reduced, but even enhanced compared to WT, and Bnip3 expression was clearly attenuated under hypoxia, implying that Epo transcription in neurons is primarily regulated by HIF-2, while Bnip3 is a HIF-1 target. Similar to our observation, two previous studies showed that transcriptional activation of Bnip3, a pro-apoptotic member of the Bcl-2 protein family, in renal cell carcinoma and hepatocellular tumor spheroids is predominantly induced by HIF-1.46,47 Along the same line, chromatin immunoprecipitation analyses in mice exposed to mild hypoxia demonstrated that Epo expression in brain tissue is induced by HIF-2.48 Moreover, abrogation of HIF-2α but not HIF-1α expression in cultured astrocytes led to a drastic decrease of hypoxic Epo expression.49 Accordingly, multiple genetic studies in mice provided evidence that, in the adult, renal and hepatic Epo synthesis is predominantly HIF-2- and not HIF-1-regulated.38 Interestingly, Baranova et al.5 also observed enhanced Epo expression, which was accompanied with higher HIF-2α protein levels in cerebral tissue of Hif1a deficient animals. The phenomenon of increased HIF-2α protein levels in the absence of HIF-1α was also found by others in several cancer cell lines.47,50 By contrast, we did not find any compensatory up-regulation of Hif2a on mRNA or protein level in neuronal Hif1a knockout mice, which could have explained increased Epo expression. However, on cellular level we cannot exclude a slight up-regulation of HIF-2 in Hif1a deficient neurons.
To further study potential redundancy of HIF-1 and HIF-2 for the neuronal adaptive response to hypoxic/ischemic insults, we generated transgenic mice with neuronal deficiency for Hif2a. In contrast to 90% overall reduction of hypoxic HIF-1α levels in brains of Hif1a deficient mice, indicative of neurons as major CNS source of HIF-1, hypoxic HIF-2α levels in cerebral tissue of Hif2a deficient animals were only slightly decreased by approximately 35%, suggesting HIF-2 rather derives from non-neuronal cells such as glial and endothelial cells. Gene expression of classical HIF-target genes in hypoxic brain parenchyma of Hif2a knockout mice was not significantly different from that in WT with the exception of Epo. Hypoxia-induced up-regulation of Epo was abrogated due to neuronal Hif2a deficiency, further underlining the importance of HIF-2 for transcriptional activation of the Epo gene and the significant neuronal origin of this factor in the CNS. Accordingly, irrespective of severity of the ischemic insult, global brain tissue damage and edema expansion in neuron-specific Hif2a deficient mice were unaltered as compared to WT littermates. Overall, we provide evidence that in animals exposed to hypoxic/ischemic conditions neuronal loss of HIF-1 can be compensated by HIF-2, and vice versa.
Tissue protection after completely impairing neuronal HIF function
In order to circumvent potential compensatory mechanisms, we used conditional knockout mice deficient for both Hif1a and Hif2a. Hypoxic up-regulation of several HIF-regulated factors such as Epo, Glut-1, and Pmaip1 was only slightly attenuated in Hif1/2 a double deficient mice, which might reflect the contribution of other resident brain cells like astrocytes, endothelial cells, oligodendrocytes, or microglia to total mRNA levels of these genes during the hypoxic adaptive response. However, in comparison to WT mice subjected to short global inspiratory hypoxia, ablation of both HIF-α subunits in neurons almost completely prevented up-regulation of Vegf and Bnip3 in the hypoxic forebrain indicating neurons as substantial cellular source for VEGF and BNIP3, respectively. In accordance with our findings, several previous studies identified neurons as important source for VEGF and BNIP3 expression in brains of rodents and human patients suffering from ischemic stroke.2,51–53 However, it should be considered that during focal brain ischemia caused by occlusion of a cerebral artery, in contrast to global inspiratory hypoxia, the oxygen availability gradually decreases from the center to the periphery of the ischemic lesion leading to a broad spectrum of HIF activity levels, highest in the center toward lower levels in the periphery. Furthermore, additional lack of supply with glucose and further energy substrates might directly or indirectly affect stability and/or activity of HIFs which could influence the spectrum and strength of HIF-target gene induction. In accordance with these assumptions, in the very acute stage of mild ischemic stroke, neuronal Hifa deficiency not only attenuated up-regulation of Bnip3 but also prevented stroke-induced expression of further HIF-targeted cell death factors such as Bnip3L and Pmaip1. Moreover, in contrast to global hypoxia, stroke-induced Vegf up-regulation was not affected in Hifa deficient mice. Our comprehensive histological and functional investigations revealed that in Hifa deficient animals subjected to mild ischemic stroke, infarct, and edema size as well as sensorimotor deficit in the early acute (24 h after onset of reperfusion) stage of stroke were clearly reduced in comparison to WT. By contrast, in the acute stage (72 h after onset of reperfusion) this protective effect was completely lost, and global lesion and edema volume were no longer different from WT. Furthermore, sensorimotor dysfunction was now markedly aggravated, suggesting that blockage of the HIF pathway in neurons enhances early acute outcome from ischemic stroke, while it impairs neuronal recovery in the acute and likely sub-acute phase.
One might speculate that diminished up-regulation of anti-survival factors like BNIP3, BNIP3L, and PMAIP1 in neurons in response to an ischemic insult might contribute to diminished early cell death and sensorimotor dysfunction in Hifa deficient mice. Accordingly, BNIP3 has been demonstrated to be up-regulated predominantly in neurons after ischemic stroke leading to neuronal cell death by activating excessive mitophagy.2,54 Furthermore, mice deficient for BNIP3 show significantly reduced neuronal mitophagy and apoptosis following ischemic/hypoxic insult.54 Similarly, Chen et al.55,56 demonstrated in a rat model of transient cerebral ischemia that early inhibition of HIF-1 by application of inhibitors or small interfering RNA attenuated up-regulation of VEGF and BNIP3, reduced the infarct volume and blood–brain barrier (BBB) hyperpermeability, decreased mortality and improved neurological deficits. Moreover, acutely enhanced expression of PMAIP1 and BNIP3L has been shown in a rodent model of ischemic stroke, and suppression of PMAIP1 expression by delivery of antisense oligonucleotides markedly reduced cerebral infarction.4,57
In contrast, global Vegf expression was not altered within the entire ischemic lesion of Hif1/2 a double knockout mice at both early acute and acute stage of mild ischemic stroke. However, we cannot fully exclude that post-ischemic Vegf expression is at least focally attenuated in Hif1a/Hif2a deficient neurons, which might enhance neuronal survival during the early acute phase of ischemic stroke by attenuating post-ischemic BBB dysfunction and vasogenic edema formation. Contrary, in late acute and sub-acute stages, the lack of autocrine/paracrine VEGF as potent antiapoptotic mediator35,44 might enhance the delayed programmed cell death of compromised neurons located in peri-infarct regions that may contribute to impaired functional outcome. Accordingly, we have shown that in the acute phase after mild ischemic stroke the apoptosis rate was substantially enhanced in Hif1a/Hif2a deficient mice. Moreover, angiogenesis and neurogenesis, adaptive cellular mechanisms strongly contributing to long-term regeneration from ischemic stroke and known to be potently induced by VEGF,35,58 might be impaired due to the loss of neuronal VEGF production. Indeed, the capillary density in ischemic brains of Hif1a/Hif2a deficient mice was markedly reduced in the acute phase, whereas cerebral microvasculature in the early acute stage was not different between knockout and WT animals. In accordance with our findings, Zhang et al.45showed in a rat model of focal cerebral embolic ischemia that early post-ischemic (1 h) administration of recombinant human VEGF significantly increased BBB leakage, hemorrhagic transformation, and ischemic lesions, while late (48 h) administration of VEGF enhanced angiogenesis in the ischemic penumbra and significantly improved neurological recovery.45
In summary, our results suggest that neuronal HIF-1α and HIF-2α can partially compensate for each other, although specific target genes are differentially regulated after hypoxia/ischemia. We also show that combined loss of neuronal HIF-1α and HIF-2α impairs functional recovery after cerebral ischemia, although it may be beneficial in the early acute phase after stroke, indicating that a timely regulated activation/inhibition of hypoxia-regulated cytoprotective and damaging factors might be of major importance for the functional outcome after stroke. This should be considered when using HIF activators or repressors for therapeutic purposes.
Supplementary Material
Acknowledgements
The expert technical assistance of Maria Harlacher and Inge Keller is gratefully acknowledged. The authors would like to thank Dr. Ben Wielockx for providing floxed Hif1a and Hif2a mice. The authors thank Dr. Roland H. Wenger for his careful and critical reading of the manuscript.
Funding
This work was supported by a grant from the Else Kröner-Fresenius-Stiftung (2012_A171; RK, HHM).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
PB performed the experiments, analyzed data, and contributed to the writing of the manuscript. LL, ASE, and LIB performed experiments. HHM contributed to the experimental design and the writing of the manuscript. RK conducted experimental design of the study, analyzed data, and wrote the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402. [DOI] [PubMed] [Google Scholar]
- 2.Althaus J, Bernaudin M, Petit E, et al. Expression of the gene encoding the pro-apoptotic BNIP3 protein and stimulation of hypoxia-inducible factor-1alpha (HIF-1alpha) protein following focal cerebral ischemia in rats. Neurochem Int 2006; 48: 687–695. [DOI] [PubMed] [Google Scholar]
- 3.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A 2000; 97: 9082–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JY, Ahn HJ, Ryu JH, et al. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med 2004; 199: 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baranova O, Miranda LF, Pichiule P, et al. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 2007; 27: 6320–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helton R, Cui J, Scheel JR, et al. Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci 2005; 25: 4099–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu CJ, Sataur A, Wang L, et al. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell 2007; 18: 4528–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54. [DOI] [PubMed] [Google Scholar]
- 9.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292: 464–468. [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001; 292: 468–472. [DOI] [PubMed] [Google Scholar]
- 11.Lando D, Peet DJ, Gorman JJ, et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 2002; 16: 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lando D, Peet DJ, Whelan DA, et al. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 2002; 295: 858–861. [DOI] [PubMed] [Google Scholar]
- 13.Chen RL, Ogunshola OO, Yeoh KK, et al. HIF prolyl hydroxylase inhibition prior to transient focal cerebral ischaemia is neuroprotective in mice. J Neurochem 2014; 131: 177–189. [DOI] [PubMed] [Google Scholar]
- 14.Nagel S, Papadakis M, Chen R, et al. Neuroprotection by dimethyloxalylglycine following permanent and transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2011; 31: 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogle ME, Gu X, Espinera AR, et al. Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1alpha. Neurobiol Dis 2012; 45: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prass K, Ruscher K, Karsch M, et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab 2002; 22: 520–525. [DOI] [PubMed] [Google Scholar]
- 17.Reischl S, Li L, Walkinshaw G, et al. Inhibition of HIF prolyl-4-hydroxylases by FG-4497 reduces brain tissue injury and edema formation during ischemic stroke. PLoS One 2014; 9: e84767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiq A, Ayoub IA, Chavez JC, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem 2005; 280: 41732–41743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Rempe DA. Prophylactic neuroprotection against stroke: low-dose, prolonged treatment with deferoxamine or deferasirox establishes prolonged neuroprotection independent of HIF-1 function. J Cereb Blood Flow Metab 2011; 31: 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernaudin M, Nedelec AS, Divoux D, et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab 2002; 22: 393–403. [DOI] [PubMed] [Google Scholar]
- 21.Leconte C, Tixier E, Freret T, et al. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke 2009; 40: 3349–3355. [DOI] [PubMed] [Google Scholar]
- 22.Miller BA, Perez RS, Shah AR, et al. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport 2001; 12: 1663–1669. [DOI] [PubMed] [Google Scholar]
- 23.Prass K, Scharff A, Ruscher K, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke 2003; 34: 1981–1986. [DOI] [PubMed] [Google Scholar]
- 24.Ryan HE, Poloni M, McNulty W, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 2000; 60: 4010–4015. [PubMed] [Google Scholar]
- 25.Gruber M, Hu CJ, Johnson RS, et al. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A 2007; 104: 2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanova E, Fehsenfeld S, Mantamadiotis T, et al. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis 2001; 31: 37–42. [DOI] [PubMed] [Google Scholar]
- 27.Kunze R, Zhou W, Veltkamp R, et al. Neuron-specific prolyl-4-hydroxylase domain 2 knockout reduces brain injury after transient cerebral ischemia. Stroke 2012; 43: 2748–2756. [DOI] [PubMed] [Google Scholar]
- 28.Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 2002; 125(Pt 11): 2549–2557. [DOI] [PubMed] [Google Scholar]
- 29.Lubjuhn J, Gastens A, von Wilpert G, et al. Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J Neurosci Methods 2009; 184: 95–103. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Schallert T, Zhang ZG, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods 2002; 117: 207–214. [DOI] [PubMed] [Google Scholar]
- 31.Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 1986; 17: 472–476. [DOI] [PubMed] [Google Scholar]
- 32.Limbourg A, Korff T, Napp LC, et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc 2009; 4: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 33.Wiesener MS, Jurgensen JS, Rosenberger C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 2003; 17: 271–273. [DOI] [PubMed] [Google Scholar]
- 34.Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology (Bethesda) 2008; 23: 263–274. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 2003; 111: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergeron M, Yu AY, Solway KE, et al. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 1999; 11: 4159–4170. [DOI] [PubMed] [Google Scholar]
- 37.Sharp FR, Bergeron M, Bernaudin M. Hypoxia-inducible factor in brain. Adv Exp Med Biol 2001; 502: 273–291. [DOI] [PubMed] [Google Scholar]
- 38.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013; 27: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barone FC, Knudsen DJ, Nelson AH, et al. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab 1993; 13: 683–692. [DOI] [PubMed] [Google Scholar]
- 40.Kitagawa K, Matsumoto M, Yang G, et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab 1998; 18: 570–579. [DOI] [PubMed] [Google Scholar]
- 41.Ozdemir YG, Bolay H, Erdem E, et al. Occlusion of the MCA by an intraluminal filament may cause disturbances in the hippocampal blood flow due to anomalies of circle of Willis and filament thickness. Brain Res 1999; 822: 260–264. [DOI] [PubMed] [Google Scholar]
- 42.Sowter HM, Ratcliffe PJ, Watson P, et al. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 2001; 61: 6669–6673. [PubMed] [Google Scholar]
- 43.Bernaudin M, Marti HH, Roussel S, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab 1999; 19: 643–651. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Kilic E, Kilic U, et al. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain 2005; 128(Pt 1): 52–63. [DOI] [PubMed] [Google Scholar]
- 45.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000; 106: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menrad H, Werno C, Schmid T, et al. Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology 2010; 51: 2183–2192. [DOI] [PubMed] [Google Scholar]
- 47.Raval RR, Lau KW, Tran MG, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 2005; 25: 5675–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo EJ, Cho YS, Kim MS, et al. Contribution of HIF-1alpha or HIF-2alpha to erythropoietin expression: in vivo evidence based on chromatin immunoprecipitation. Ann Hematol 2008; 87: 11–17. [DOI] [PubMed] [Google Scholar]
- 49.Chavez JC, Baranova O, Lin J, et al. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 2006; 26: 9471–9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stiehl DP, Bordoli, Abreu-Rodriguez I, et al. Non-canonical HIF-2alpha function drives autonomous breast cancer cell growth via an AREG-EGFR/ErbB4 autocrine loop. Oncogene 2012; 31: 2283–2297. [DOI] [PubMed] [Google Scholar]
- 51.Issa R, Krupinski J, Bujny T, et al. Vascular endothelial growth factor and its receptor, KDR, in human brain tissue after ischemic stroke. Lab Invest 1999; 79: 417–425. [PubMed] [Google Scholar]
- 52.Kovacs Z, Ikezaki K, Samoto K, et al. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke 1996; 27: 1865–1873. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, Yang X, Zhang S, et al. BNIP3 upregulation and EndoG translocation in delayed neuronal death in stroke and in hypoxia. Stroke 2007; 38: 1606–1613. [DOI] [PubMed] [Google Scholar]
- 54.Shi RY, Zhu SH, Li V, et al. BNIP3 interacting with LC3 triggers excessive mitophagy in delayed neuronal death in stroke. CNS Neurosci Ther 2014; 20: 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C, Hu Q, Yan J, et al. Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem 2007; 102: 1831–1841. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Hu Q, Yan J, et al. Early inhibition of HIF-1alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol Dis 2009; 33: 509–517. [DOI] [PubMed] [Google Scholar]
- 57.Birse-Archbold JL, Kerr LE, Jones PA, et al. Differential profile of Nix upregulation and translocation during hypoxia/ischaemia in vivo versus in vitro. J Cereb Blood Flow Metab 2005; 25: 1356–1365. [DOI] [PubMed] [Google Scholar]
- 58.Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci 2013; 70: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.