Abstract
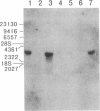
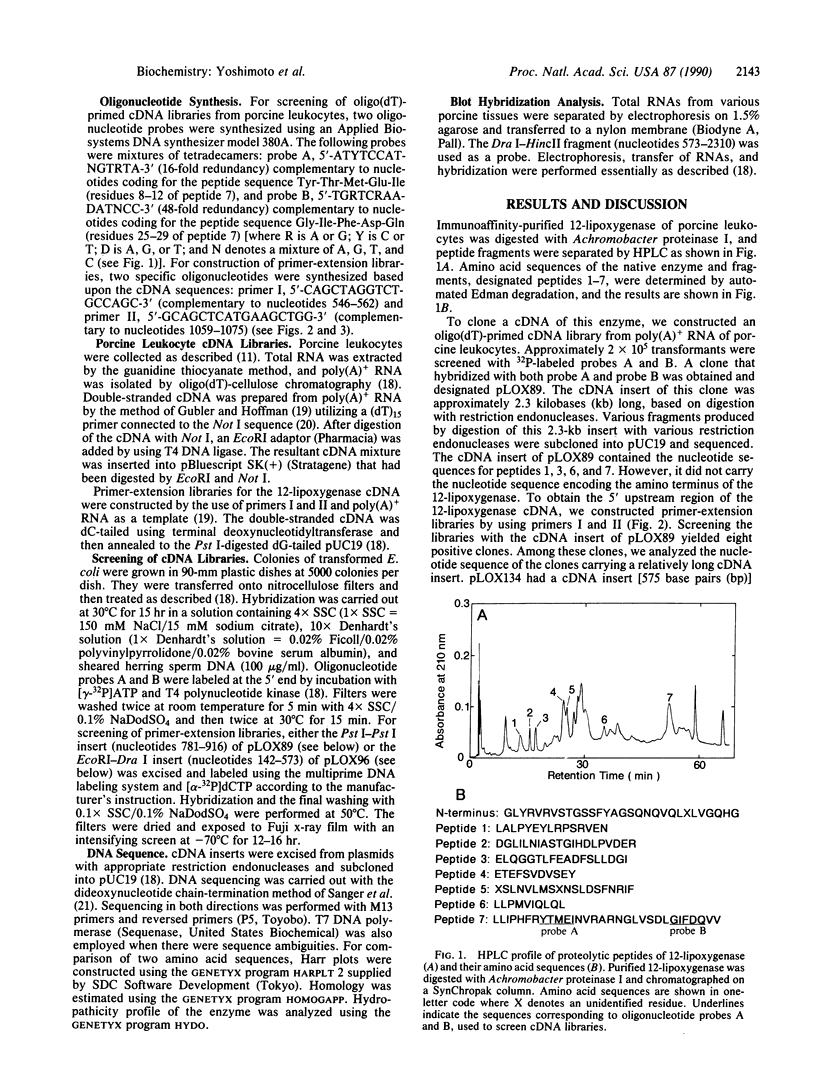
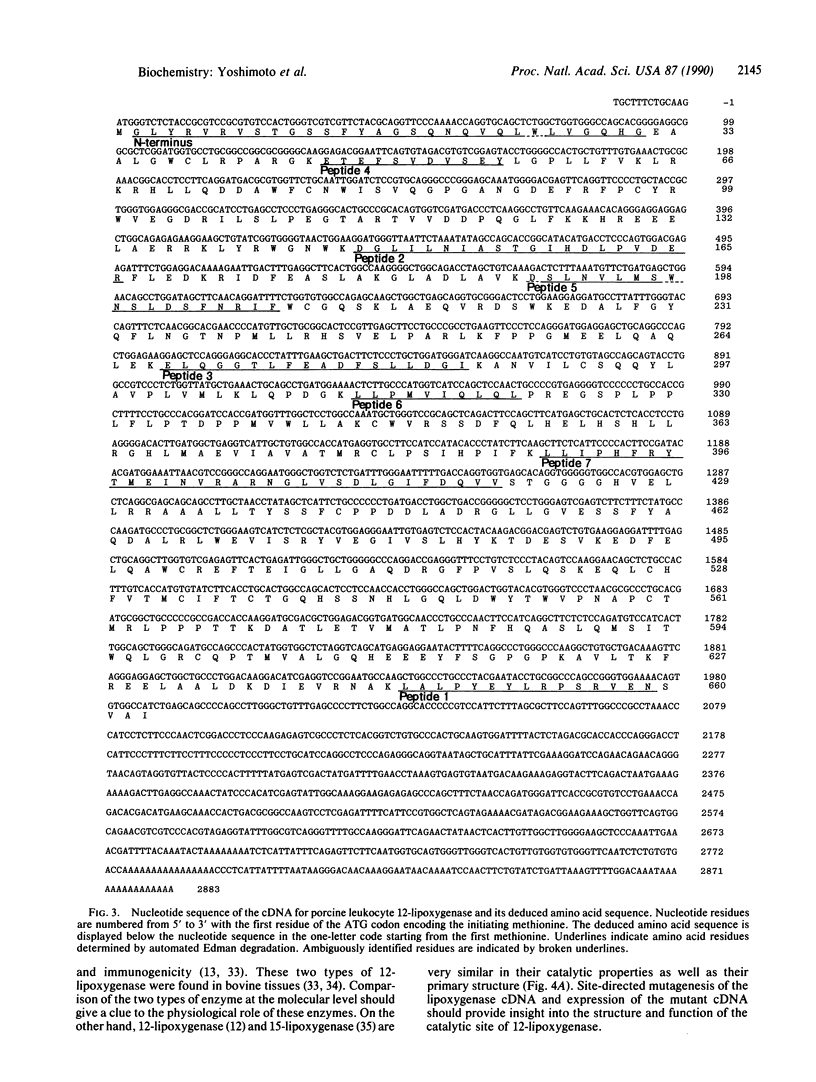
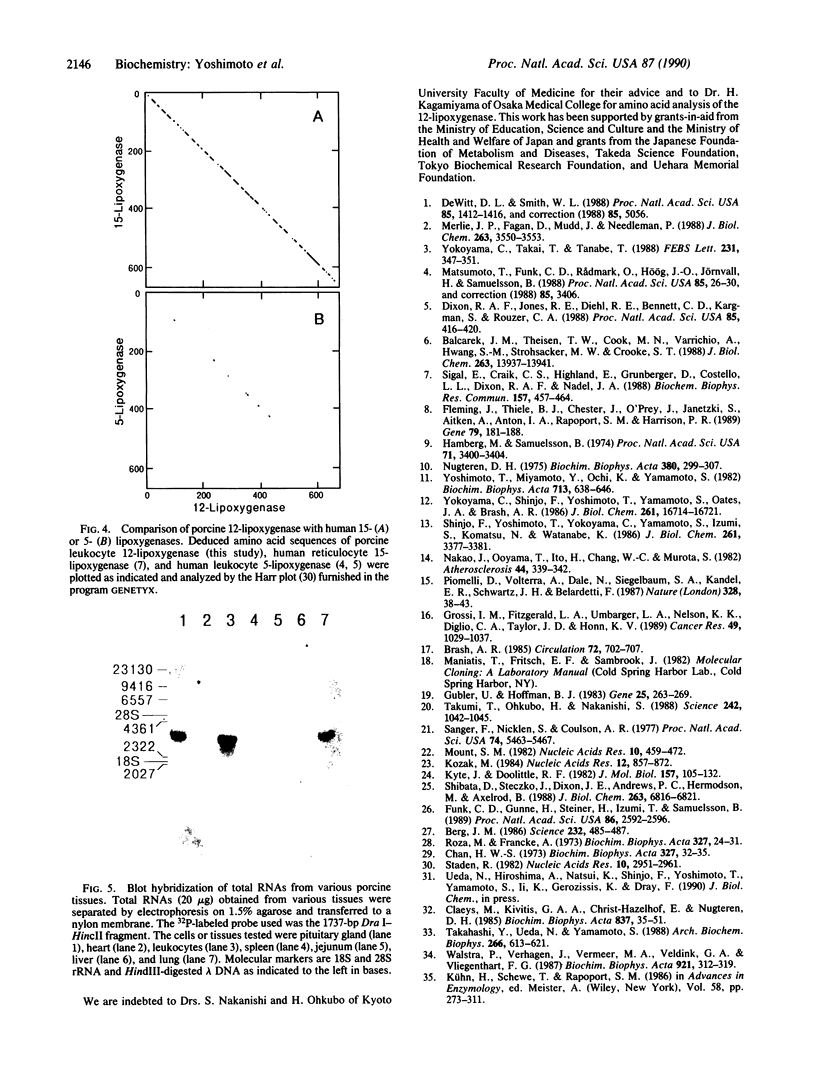
The complete amino acid sequence of arachidonate 12-lipoxygenase (EC 1.13.11.31) of porcine leukocytes was deduced by cloning and sequence analysis of DNA complementary to its mRNA. The sequence was confirmed by automated Edman degradation of the N-terminal regions of the native enzyme and its proteolytic fragments. The cDNA had an open reading frame encoding 662 amino acid residues with a calculated molecular weight of 74,911. Amino acid residues 533-545, Cys-(Xaa)3-Cys-(Xaa)3-His-(Xaa)3-His, showed significant homology to the short cysteine- or histidine-containing sequences proposed as the metal-binding domains of transcription factors and various metal-containing proteins [Berg, J. M. (1986) Science 232, 485-487]. The amino acid sequence of 12-lipoxygenase exhibited 86% identity with human reticulocyte 15-lipoxygenase and showed 41% identity with human leukocyte 5-lipoxygenase. The 12-lipoxygenase cDNA recognized a 3.4-kilobase mRNA species in various porcine cell types, with the largest amount in leukocytes, followed by pituitary, lung, jejunum, and spleen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcarek J. M., Theisen T. W., Cook M. N., Varrichio A., Hwang S. M., Strohsacker M. W., Crooke S. T. Isolation and characterization of a cDNA clone encoding rat 5-lipoxygenase. J Biol Chem. 1988 Sep 25;263(27):13937–13941. [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Brash A. R. A review of possible roles of the platelet 12-lipoxygenase. Circulation. 1985 Oct;72(4):702–707. doi: 10.1161/01.cir.72.4.702. [DOI] [PubMed] [Google Scholar]
- Chan H. W. Soya-bean lipoxygenase: an iron-containing dioxygenase. Biochim Biophys Acta. 1973 Nov 15;327(1):32–35. doi: 10.1016/0005-2744(73)90100-9. [DOI] [PubMed] [Google Scholar]
- Claeys M., Kivits G. A., Christ-Hazelhof E., Nugteren D. H. Metabolic profile of linoleic acid in porcine leukocytes through the lipoxygenase pathway. Biochim Biophys Acta. 1985 Oct 23;837(1):35–51. doi: 10.1016/0005-2760(85)90083-9. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Jones R. E., Diehl R. E., Bennett C. D., Kargman S., Rouzer C. A. Cloning of the cDNA for human 5-lipoxygenase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):416–420. doi: 10.1073/pnas.85.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J., Thiele B. J., Chester J., O'Prey J., Janetzki S., Aitken A., Anton I. A., Rapoport S. M., Harrison P. R. The complete sequence of the rabbit erythroid cell-specific 15-lipoxygenase mRNA: comparison of the predicted amino acid sequence of the erythrocyte lipoxygenase with other lipoxygenases. Gene. 1989 Jun 30;79(1):181–188. doi: 10.1016/0378-1119(89)90103-0. [DOI] [PubMed] [Google Scholar]
- Funk C. D., Gunne H., Steiner H., Izumi T., Samuelsson B. Native and mutant 5-lipoxygenase expression in a baculovirus/insect cell system. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2592–2596. doi: 10.1073/pnas.86.8.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi I. M., Fitzgerald L. A., Umbarger L. A., Nelson K. K., Diglio C. A., Taylor J. D., Honn K. V. Bidirectional control of membrane expression and/or activation of the tumor cell IRGpIIb/IIIa receptor and tumor cell adhesion by lipoxygenase products of arachidonic acid and linoleic acid. Cancer Res. 1989 Feb 15;49(4):1029–1037. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Funk C. D., Rådmark O., Hög J. O., Jörnvall H., Samuelsson B. Molecular cloning and amino acid sequence of human 5-lipoxygenase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):26–30. doi: 10.1073/pnas.85.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Fagan D., Mudd J., Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao J., Ooyama T., Ito H., Chang W. C., Murota S. Comparative effect of lipoxygenase products of arachidonic acid on rat aortic smooth muscle cell migration. Atherosclerosis. 1982 Sep;44(3):339–342. doi: 10.1016/0021-9150(82)90008-9. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Roza M., Francke A. Soyabean lipoxygenase: an iron-containing enzyme. Biochim Biophys Acta. 1973 Nov 15;327(1):24–31. doi: 10.1016/0005-2744(73)90099-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Steczko J., Dixon J. E., Andrews P. C., Hermodson M., Axelrod B. Primary structure of soybean lipoxygenase L-2. J Biol Chem. 1988 May 15;263(14):6816–6821. [PubMed] [Google Scholar]
- Shinjo F., Yoshimoto T., Yokoyama C., Yamamoto S., Izumi S., Komatsu N., Watanabe K. Studies on porcine arachidonate 12-lipoxygenase using its monoclonal antibodies. J Biol Chem. 1986 Mar 5;261(7):3377–3381. [PubMed] [Google Scholar]
- Sigal E., Craik C. S., Highland E., Grunberger D., Costello L. L., Dixon R. A., Nadel J. A. Molecular cloning and primary structure of human 15-lipoxygenase. Biochem Biophys Res Commun. 1988 Dec 15;157(2):457–464. doi: 10.1016/s0006-291x(88)80271-7. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Ueda N., Yamamoto S. Two immunologically and catalytically distinct arachidonate 12-lipoxygenases of bovine platelets and leukocytes. Arch Biochem Biophys. 1988 Nov 1;266(2):613–621. doi: 10.1016/0003-9861(88)90294-9. [DOI] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Walstra P., Verhagen J., Vermeer M. A., Veldink G. A., Vliegenthart J. F. Demonstration of a 12-lipoxygenase activity in bovine polymorphonuclear leukocytes. Biochim Biophys Acta. 1987 Sep 25;921(2):312–319. doi: 10.1016/0005-2760(87)90032-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama C., Shinjo F., Yoshimoto T., Yamamoto S., Oates J. A., Brash A. R. Arachidonate 12-lipoxygenase purified from porcine leukocytes by immunoaffinity chromatography and its reactivity with hydroperoxyeicosatetraenoic acids. J Biol Chem. 1986 Dec 15;261(35):16714–16721. [PubMed] [Google Scholar]
- Yokoyama C., Takai T., Tanabe T. Primary structure of sheep prostaglandin endoperoxide synthase deduced from cDNA sequence. FEBS Lett. 1988 Apr 25;231(2):347–351. doi: 10.1016/0014-5793(88)80847-0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Miyamoto Y., Ochi K., Yamamoto S. Arachidonate 12-lipoxygenase of porcine leukocyte with activity for 5-hydroxyeicosatetraenoic acid. Biochim Biophys Acta. 1982 Dec 13;713(3):638–646. [PubMed] [Google Scholar]