ABSTRACT
Maternal vaccination represents a potential strategy to protect both the mother and the offspring against life-threatening infections. This protective role has mainly been associated with antibodies, but the role of cell-mediated immunity, in particular passively transferred cytokines, is not well understood. Here, using a pertussis model, we have demonstrated that immunization of pregnant sows with heat-inactivated bacteria leads to induction of a wide range of cytokines (e.g., tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], interleukin-6 [IL-6], IL-8, and IL-12/IL-23p40) in addition to pertussis-specific antibodies. These cytokines can be detected in the sera and colostrum/milk of vaccinated sows and subsequently were detected at significant levels in the serum and bronchoalveolar lavage fluid of piglets born to vaccinated sows together with pertussis-specific antibodies. In contrast, active vaccination of newborn piglets with heat-inactivated bacteria induced high levels of specific IgG and IgA but no cytokines. Although the levels of antibodies in vaccinated piglets were comparable to those of passively transferred antibodies, no protection against Bordetella pertussis infection was observed. Thus, our results demonstrate that a combination of passively transferred cytokines and antibodies is crucial for disease protection. The presence of passively transferred cytokines/antibodies influences the cytokine secretion ability of splenocytes in the neonate, which provides novel evidence that maternal immunization can influence the newborn's cytokine milieu and may impact immune cell differentiation (e.g., Th1/Th2 phenotype). Therefore, these maternally derived cytokines may play an essential role both as mediators of early defense against infections and possibly as modulators of the immune repertoire of the offspring.
KEYWORDS: pertussis, maternal immunization, neonatal immunization, passive transfer, Th1 type cytokines, Bordetella pertussis, Th1 responses, antibodies, passive immunity
INTRODUCTION
Newborns are highly susceptible to infection, and infectious disease is a major cause of human infant mortality (1, 2). This defect in host defense has been ascribed to the immaturity of neonatal immune cells and a skewed T-helper-cell differentiation (Th2 bias) of their immune system (3–5). However, we recently challenged this notion and demonstrated that the presence of a transient active immunosuppression, rather than immune cell intrinsic defects mediated by physiologically enriched immunosuppressive CD71+ erythroid cells, compromises neonatal host defense against infection (6). It is possible to conclude that the course of an immune response to vaccination in newborns is influenced by the presence of these immunosuppressor cells until they gradually decay (7). The question is what is the best approach to protect newborns from the risk of vaccine-preventable diseases in the first few months of life before the start and completion of their required vaccination regimens.
Maternal immunization using inactivated vaccines either before or during pregnancy might be a strategy to overcome this problem. Vulnerability of this group most recently has been stressed with the higher mortality from pandemic H1N1 influenza in pregnant women and fetuses than in the general population (8, 9). Universal administration of tetanus toxoid (TT) during pregnancy is the best example of how this approach can be effectively utilized (10, 11). Vaccination with TT induces a specific antibody response that is transported across the placenta with 100% efficiency and provides protection against neonatal tetanus (12, 13). In addition, vaccinations against Haemophilus influenzae type b and pneumococcal infections are other examples of success in maternal immunization strategies (14).
It is well established that maternal antibody can effectively neutralize specific bacterial and viral colonization that generally cannot be cleared by the innate immune system of the neonate (15). Therefore, maternal antibody plays an essential role in shaping the specific antibody repertoire and peripheral B cell development in the neonate long after the maternal antibodies themselves become undetectable (16, 17).
However, the possible role of maternal cytokines/cells transferred to the fetus or the newborn via colostrum and milk, and how these immune components could impact the immune system development of the offspring, has not been fully elucidated. Although cellular components of the maternal and fetal immune systems are generally separated by the placenta, compelling evidence indicates a bidirectional transfer of maternal and fetal cells during gestation. For example, long-term effects of noninherited maternal antigens (NIMA) on immune programing have been well documented (18, 19). Furthermore, several lines of evidence support the notion of fetal and newborn immune imprinting. In animal models, maternal Th1 type cytokines during gestation were shown to contribute to the reduction of experimental allergic airway disease in the newborn (20). Similarly, in humans, maternal exposure to Th1 type cytokines during gestation alleviates atopic sensitization of the offspring (21, 22). Intriguingly, maternal cytokine levels (e.g., tumor necrosis factor alpha [TNF-α], monocyte chemoattractant protein 1 [MCP-1], and interleukin-10 [IL-10]) during gestation correlate with the newborn's cytokine levels at up to 1 year of age (23), reinforcing the synchronized polarization of the maternal and fetal immune systems. More recent studies indicated higher levels of immune proteins, such as host defense peptides and cytokines, in preterm mothers' breast milk and the potential influence of these cytokines on the immune system of the newborn (24).
Pertussis is a highly infectious bacterial disease caused primarily by Bordetella pertussis and occasionally by Bordetella parapertussis. More recently, cases of Bordetella holmesii have been identified during pertussis outbreaks that have mainly affected adolescents (25). Pertussis has had a substantial resurgence in recent years and continues to be a major global health concern (26, 27). Unfortunately, the highest attack rates and pertussis-related mortality are consistently seen in young infants who are too young to be vaccinated or who have not completed their primary immunization series (3, 28, 29). Therefore, maternal immunization might be an effective approach in generating an early and temporal immune response against this disease. However, despite extensive research on this disease, the nature of protective immunity is not very well understood.
While it is believed that antibodies play a role in bacterial toxin neutralization and in the prevention of bacterial attachment, it has been difficult to establish a direct correlation between serum antibody titers and protection from disease (30, 31). Thus, other factors, such as CD4+ T cells and the production of Th1-like cytokines, might play a role in protection, particularly with the whole-cell pertussis vaccines (Pw) (32, 33). Type 1 cytokines are strong activators of natural antimicrobial effector cells, such as macrophages and neutrophils, which are critical for B. pertussis elimination (34, 35). T-cell responses in immunized children, as well as in a mouse model in which protection is associated with vaccine efficacy in children, have indicated that immunization with Pw induces a Th1 type immune response (36). This contrasts with immunization with acellular pertussis vaccine (Pa), which generates a Th2-biased or mixed Th1/Th2 cytokine profile (37, 38). Several reports have indicated that gamma interferon (IFN-γ) plays a critical role in innate and adaptive immunity to B. pertussis, since IFN-γ−/− or IFN-γ-defective mice developed disseminating lethal infections following challenge (39, 40). More recent studies demonstrated that both Th1 and Th17, but not Th2, cells contribute to clearance of B. pertussis and that IFN-γ has an instrumental role in adaptive immunity to bacterial clearance (41).
To our knowledge, there is no report on the role of maternal cytokines following vaccination against pertussis using whole pertussis vaccine, except for a recent study using acellular vaccine. Interestingly, that study indicated that vaccine-specific cellular response was less pronounced in pregnant women than in control women (42). In contrast, another study has reported robust cellular immune response to inactivated influenza vaccine during pregnancy (43). We previously demonstrated that maternal immunity provides protection against B. pertussis in newborn piglets (44, 45). Here, we build on our previous observations and demonstrate that in addition to maternal antibodies, maternal cytokines play an essential role in protection against B. pertussis. Moreover, the contribution of maternal antibody and cytokines in polarization and programing of the newborn's immune system is further studied.
RESULTS
Vaccination of newborn piglets with heat-inactivated B. pertussis. (i) Immunization of newborn piglets results in elevated serum levels of B. pertussis-specific antibodies.
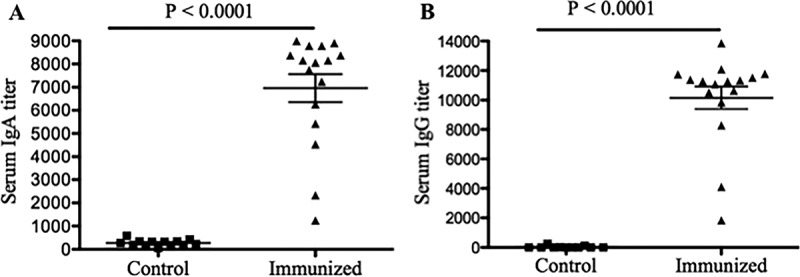
Newborn piglets were immunized with 1 × 109 to 2 × 109 CFU heat-inactivated B. pertussis in order to assess protection against subsequent infection with B. pertussis. Sera of piglets were collected prior to priming, boosting, challenge, and euthanization and tested for the presence of B. pertussis-specific antibodies (IgA and IgG). Significant levels of B. pertussis-specific antibodies, of both IgG and IgA isotypes, were detected in serum from vaccinated piglets prior to challenge (Fig. 1A and B). All vaccinated piglets displayed significantly (P < 0.0001) elevated levels of B. pertussis-specific IgA and IgG in their sera compared to control animals (phosphate-buffered saline [PBS] treated) at the time of challenge.
FIG 1.
Induction of IgA (A) and IgG (B) antibodies in the serum of piglets vaccinated with heat-inactivated B. pertussis or PBS. Piglets were vaccinated at 3 to 5 days of age and boosted 2 weeks later. Sera were collected before challenge, and levels of antibodies were analyzed by ELISA. Each point represents data from an individual piglet, representative of three independent experiments. Bars indicate means ± one standard error.
(ii) Immunization with heat-inactivated B. pertussis does not induce detectable levels of cytokines in the serum of newborn piglets.
Newborn piglets were bled 1 week postboosting, and their sera were analyzed for the presence of TNF-α, IFN-γ, IL-2, IL-12/IL-23p40, IL-6, IL-4, and IL-8. Surprisingly, these cytokines were not detected in the serum of either immunized or control piglets at the time of challenge or euthanasia (data not shown), demonstrating that immunization of newborn piglets with heat-inactivated B. pertussis did not result in the induction of detectable cytokines in their serum.
(iii) Active immunization does not provide protection against infection with B. pertussis in newborn piglets.
Piglets of both vaccinated and control groups were challenged with 2 × 1010 to 5 × 1010 CFU of live bacteria at 24 to 26 days of age as we described elsewhere (46–48). Piglets from either control or vaccinated groups displayed clinical symptoms, including mild fever, nasal discharge, nonparoxysmal cough, and breathing difficulties, as we previously showed (46, 48). There was no significant difference in the clinical symptoms between vaccinated piglets and control animals. In addition, postmortem investigation revealed pathological alterations, such as hemorrhagic and necrotizing bronchopneumonia, in both animal groups at day 4 postinfection (Fig. 2A and B), demonstrating that active immunization failed to provide protection against infection with B. pertussis in vaccinated piglets.
FIG 2.
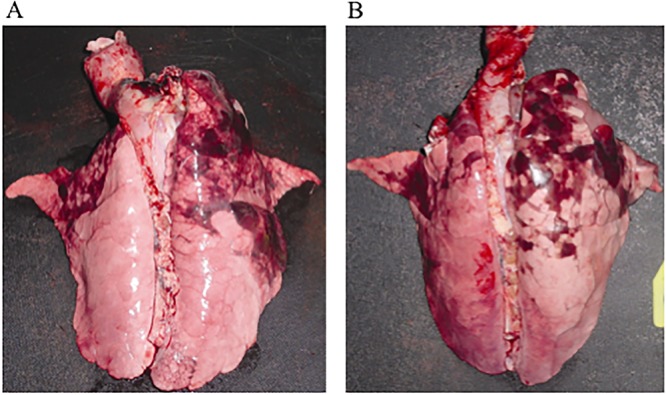
Gross pathology of lungs obtained from vaccinated newborn piglets. (A) Representative lung of piglet vaccinated with heat-inactivated bacteria, infected with 2 × 1010 to 5 × 1010 CFU, and harvested at day 4 postchallenge. (B) Lung of piglet vaccinated with PBS (control), infected with 2 × 1010 to 5 × 1010 CFU, and harvested at day 4 postchallenge. Results are representative of three independent experiments.
Vaccination of pregnant sows with heat-inactivated B. pertussis. (i) Vaccination of pregnant sows results in high levels of serum and colostrum B. pertussis-specific antibodies.
To determine the induction of specific anti-B. pertussis antibodies in the serum of vaccinated sows, serum was collected prior to priming, boosting, and farrowing. As we have previously shown (44), high levels of both IgG and IgA isotypes were detected in the serum obtained from vaccinated sows prior to farrowing (P < 0.0001) but not in the serum from control sows (data not shown). In addition, colostrum was collected after farrowing and analyzed for the presence of B. pertussis-specific secretory IgA (S-IgA) and IgG antibodies by enzyme-linked immunosorbent assay (ELISA). In agreement with our previous reports (44), all vaccinated sows displayed high levels of both IgG and S-IgA antibodies (P < 0.003), whereas these antibodies were not detectable in colostrum from control sows (data not shown). Passive transfer of these antibodies to newborn piglets was demonstrated by analyzing the serum of piglets born either to vaccinated or nonvaccinated sows. As shown in Fig. 3A and B, piglets born to vaccinated sows had significantly elevated levels of both IgA and IgG serum antibodies (P < 0.0001). In contrast, serum obtained from piglets born to nonvaccinated control sows did not contain detectable levels of specific antibodies against B. pertussis (Fig. 3).
FIG 3.
Induction of IgA (A) and IgG (B) antibodies in the serum of piglets born to either vaccinated or nonvaccinated control sows. Sows were vaccinated at 4 weeks prior to farrowing and boosted 2 weeks later. Sera of piglets were collected before challenge, and levels of antibodies were analyzed by ELISA. Each point represents data from an individual piglet. Results are from piglets born to 8 different sows (four vaccinated and four control sows) and are representative of three independent experiments. Bars indicate means ± one standard error.
(ii) Vaccination with heat-inactivated bacteria induces cytokine production in sows.
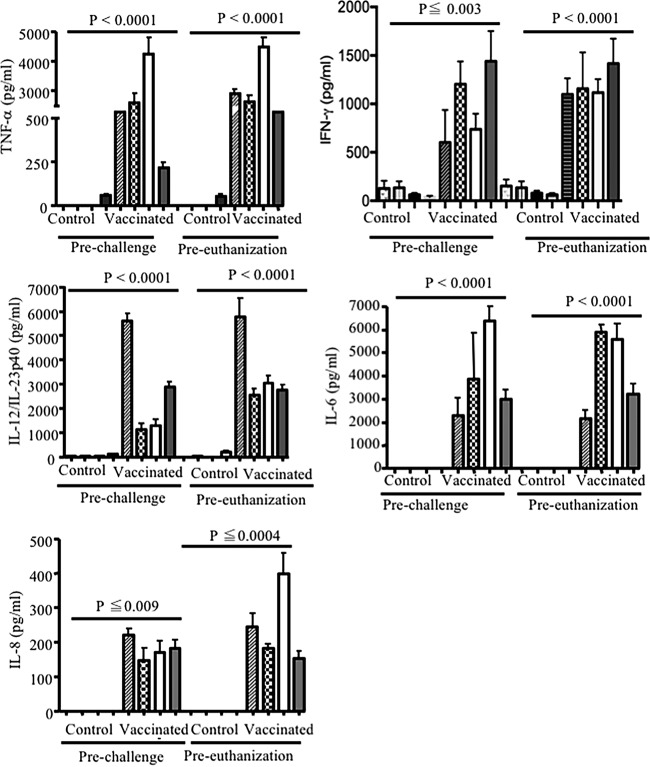
To assess the effect of immunization with heat-inactivated bacteria on the induction of cytokine, serum and colostrum were collected prior to farrowing and cytokine production was examined by ELISA. Immunization with heat-inactivated bacteria elicited high levels of TNF-α, IFN-γ, IL-12/IL-23p40, IL-6, and IL-8 in the serum (Fig. 4) and colostrum (Fig. 5) of vaccinated sows. The highest concentration of cytokine in serum was for TNF-α, and the lowest was for IFN-γ (TNF-α > IL-12/IL-23p40 > IL-8 > IL-6 > IFN-γ). In contrast, cytokine levels in the colostrum and serum of control sows were undetectable or very low (Fig. 4 and 5). Very low levels of IFN-γ were detected in the serum and colostrum of control animals; however, their levels were significantly (P < 0.0001) lower than those in immunized sows. As shown in Fig. 5, IL-8 was detectable in the colostrum of two control sows. Interestingly, we were not able to detect any IL-4 in either colostrum or serum of vaccinated and nonvaccinated sows throughout this study (data not shown).
FIG 4.
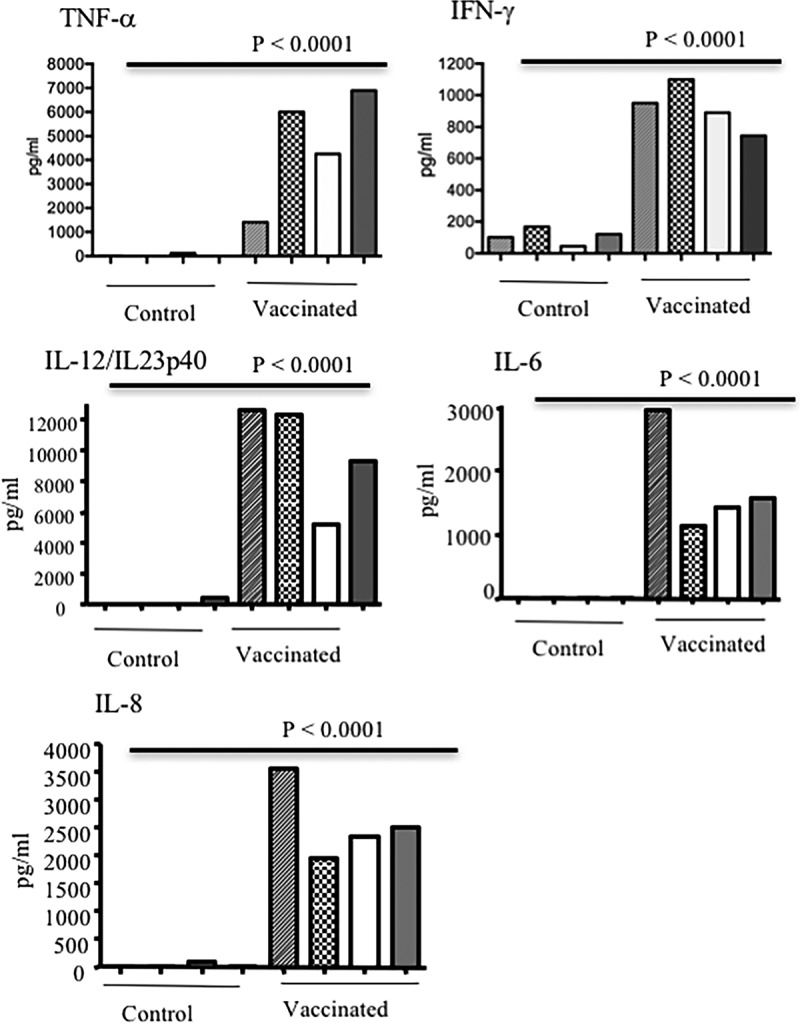
Induction of TNF-α, IFN-γ, IL-6, IL-8, and IL-12/IL-23p40 in serum of sows either vaccinated with heat-inactivated bacteria or treated with PBS. Sows were vaccinated at 4 weeks prior to farrowing and boosted 2 weeks later. Sera were collected prior to farrowing. ELISA results are shown from eight different sows and from three separate experiments, with each bar representing one sow.
FIG 5.
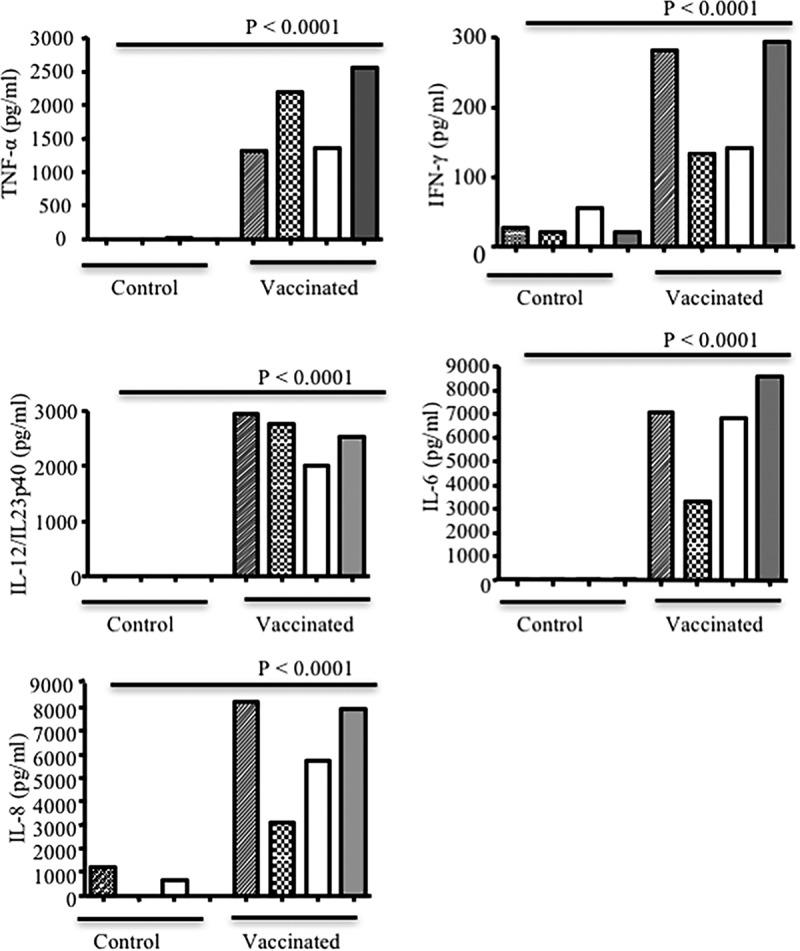
Concentrations of TNF-α, IFN-γ, IL-6, IL-8, and IL-12/IL-23p40 in the sow colostrum. Sows were vaccinated with heat-inactivated bacteria, and control animals were treated with PBS. ELISA results are shown from eight different sows from three separate experiments, with each bar representing one sow.
(iii) Transfer of maternal cytokines to suckling piglets.
In order to examine the possibility that cytokines transfer from mother to the neonate via colostrum and milk, piglets were bled prior to suckling, prior to challenge, and at the time of euthanasia. Piglets born to vaccinated sows had significantly high levels of detectable TNF-α, IFN-γ, IL-12/IL-23p40, IL-6, and IL-8 in their sera prior to and after challenge (Fig. 6). The concentrations of these cytokines were generally elevated over the course of study, suggesting that these cytokines were constitutively transferred to the offspring. However, we cannot exclude production of some cytokines by the neonatal immune system, since our assays are unable to distinguish between passively transferred and actively produced cytokine in the neonate. In contrast, IL-12/IL-23p40, TNF-α, IL-6, and IL-8 cytokines were undetectable in the sera of piglets born to control sows, except TNF-α was detectable in piglets born to one sow (Fig. 6). Interestingly, IFN-γ was present in the sera of piglets born to control sows prior to challenge and at the time of euthanization. However, IFN-γ levels were significantly lower in sera of piglets born to control sows than in sera of piglets born to vaccinated sows at the time of challenge and euthanization (P ≤ 0.003 and P < 0.0001, respectively) (Fig. 6). Of note, cytokine concentrations in the sera of piglets do not correlate with their respective colostrum concentrations. Substantially higher levels of TNF-α, IL-12/IL-23p40, and IFN-γ were observed in sera than in colostrum of piglets born to vaccinated sows, which suggests two possible mechanisms: (i) accumulation and stability of transferred cytokines via suckling in the newborn, and (ii) induction of cytokines in piglets born to vaccinated sows by unknown mechanisms.
FIG 6.
Passive transfer of TNF-α, IFN-γ, IL-6, IL-8, and IL-12/IL-23p40 via colostrum/milk. Cytokine concentrations were measured in sera of piglets, born to either vaccinated or PBS-treated sows, prior to challenge (3 to 4 days old). The results shown are the means ± standard errors of the cytokine concentrations detected by ELISA. Each bar pattern represents piglets born to one sow.
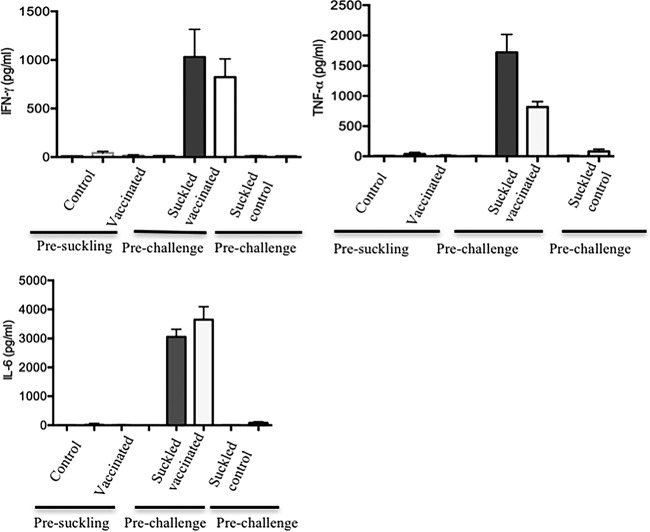
(iv) Maternal cytokine transfer occurs via suckling.
In order to determine the source of these cytokines, piglets were bled prior to suckling, and their serum was subjected to cytokine assays. Interestingly, no cytokines were detected in the serum of newborn piglets born to either control or vaccinated sows prior to suckling, demonstrating that these cytokines were transferred exclusively via colostrum and milk (Fig. 7), which is in agreement with other studies (49). To reconfirm our observations, some piglets born to vaccinated sows were transferred to control sows right after birth, prior to suckling, and vice versa. We found that piglets born to vaccinated sows and then transferred to control sows had no detectable levels of cytokines in their serum. In contrast, piglets born to control sows and then nursed by vaccinated sows had significantly higher levels of cytokines (TNF-α, IFN-γ, and IL-6) in their serum (Fig. 7). A similar pattern was observed for IL-12/IL23p40 and IL-8 (data not shown).
FIG 7.
Maternal cytokine transfer occurs via suckling. Detection of cytokines in the serum of piglets born to either control or vaccinated (heat-inactivated bacteria) sows presuckling, in piglets born to control sows but nursed by vaccinated sows (suckled vaccinated), and in piglets born to vaccinated sows but nursed by control sows (suckled control). The results shown are the means ± standard errors for the cytokine concentrations detected by ELISA. Each bar pattern represents piglets born to one sow.
(v) Maternally derived cytokines in BAL fluid of newborn piglets.
The presence of TNF-α, IFN-γ, IL-12/IL-23p40, IL-6, IL-8, and IL-4 cytokines was assessed in the bronchoalveolar lavage (BAL) fluids obtained from newborn piglets at days 2, 4, and 7 postchallenge. Piglets born to vaccinated sows had significantly higher levels of TNF-α, IFN-γ, IL-12/IL-23p40, and IL-8 cytokines in their BAL fluids at days 2, 4, and 7 postchallenge than piglets born to control sows (P ≤ 0.05) (Fig. 8). In contrast, there was no significant difference in detectable IL-6 levels between piglets born to vaccinated and nonvaccinated sows (data not shown). While TNF-α was not detectable in BAL fluids from piglets born to nonvaccinated sows at these time points, there were detectable but very low levels of IFN-γ and IL-8 in the BAL fluids obtained from piglets born to control sows (Fig. 8). Although piglets born to vaccinated sows had significant levels of cytokines in their BAL fluid, the levels of these cytokines tended to decline by day 7 postinfection.
FIG 8.
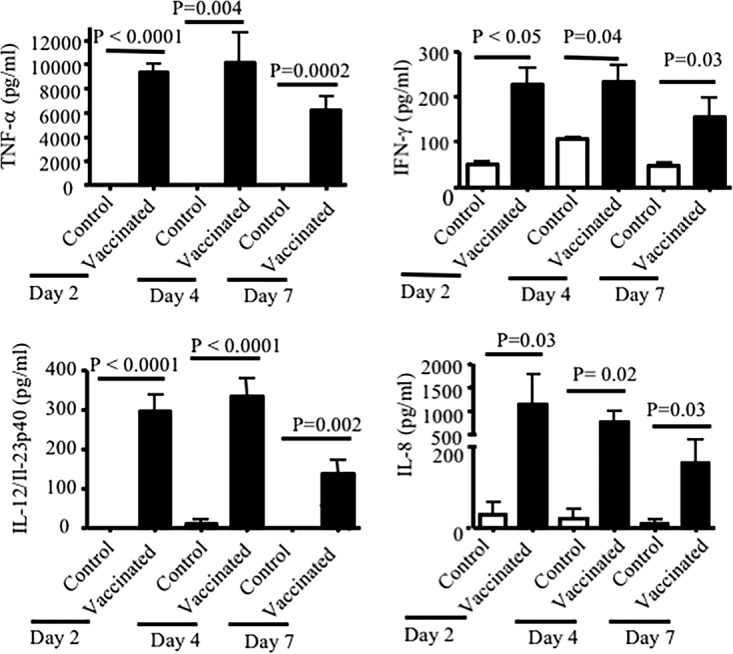
Detected cytokine (TNF-α, IFN-γ, IL-6, IL-8, IL-10, and IL-12/IL-23p40) in the BAL fluid of newborn piglets. BAL fluid samples were collected in SS medium (15 ml) and tested for the presence of cytokines at days 2, 4, and 7 postinfection. BAL fluid from piglets born to immunized sows had significantly higher levels of cytokines than BAL fluid from piglets born to control sows. The results shown are the means ± standard errors for cytokine concentrations detected by ELISA from 3 separate experiments. Each bar pattern represents piglets born to one sow.
(vi) Maternal immunity transiently enhances cytokine secretion by splenocytes obtained from piglets born to vaccinated sows.
To assess the potential effects of passively transferred cytokines on induction of cytokines by the recipient's immune cells, splenocytes obtained from piglets born to vaccinated sows rather than the control group were restimulated in vitro. As indicated in Fig. 9, splenocytes obtained from piglets born to vaccinated sows produced significantly higher levels of IFN-γ (P < 0.001), TNF-α (P < 0.01), IL-12 (P < 0.001), IL-8 (P < 0.001), and IL-6 (P < 0.05) at day 3 postsuckling than splenocytes from control piglets following stimulation with concanavalin A (ConA). In contrast, splenocytes from piglets born to control sows secreted significantly lower levels of cytokines at the same time point following restimulation in vitro. Interestingly, splenocytes from piglets born to vaccinated sows continued to exhibit similar cytokine secretion capabilities even at day 5 postsuckling (data not shown).
FIG 9.
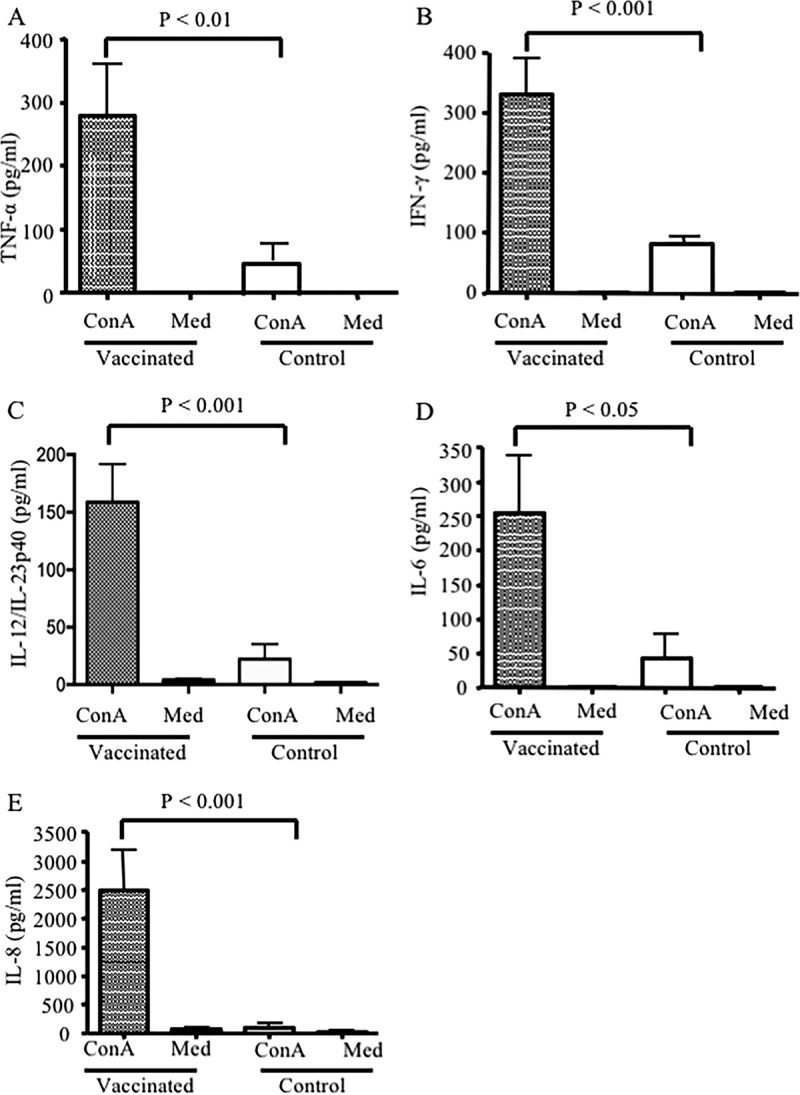
Cytokine secretion by piglet splenocytes stimulated with ConA. Three days postsuckling, spleen cells were isolated (from piglets born to vaccinated or nonvaccinated sows), and 1 × 106 cells/ml were stimulated with 5 μg/ml ConA. Supernatants were collected after 48 h, and concentrations of TNF-α (A), IFN-γ (B), IL-12 (C), IL-6 (D), and IL-8 (E) were determined by ELISA. Results are representative of two independent experiments and expressed as the means from duplicate assays from 7 piglets per group. P values on each graph indicate statistically significant associations. Cultured cells without ConA (Med) were used as a negative control.
(vii) Maternal immunity provides protection against challenge infection with B. pertussis in newborn piglets born to vaccinated sows.
Newborn piglets born to either vaccinated or control sows were challenged with 5 × 109 CFU of live bacteria at 3 to 5 days of age. All of the piglets born to nonvaccinated control sows displayed severe clinical symptoms and pathological alterations similar to findings of our previously published studies (44, 46). In contrast, piglets born to vaccinated sows showed no or significantly milder fever and respiratory symptoms (44). Postmortem examination on days 2, 4, and 7 postchallenge revealed severe pathological alterations, such as hemorrhagic and necrotizing bronchopneumonia, in piglets born to control sows (Fig. 10A). In contrast, pathological alterations in piglets born to vaccinated sows were either absent or significantly reduced in size (Fig. 10B). In addition, the lung lesions from piglets born to vaccinated or control sows were removed and quantified. As shown in Fig. 10C, the sizes of lesions in the lungs of piglets born to vaccinated sows were significantly reduced.
FIG 10.
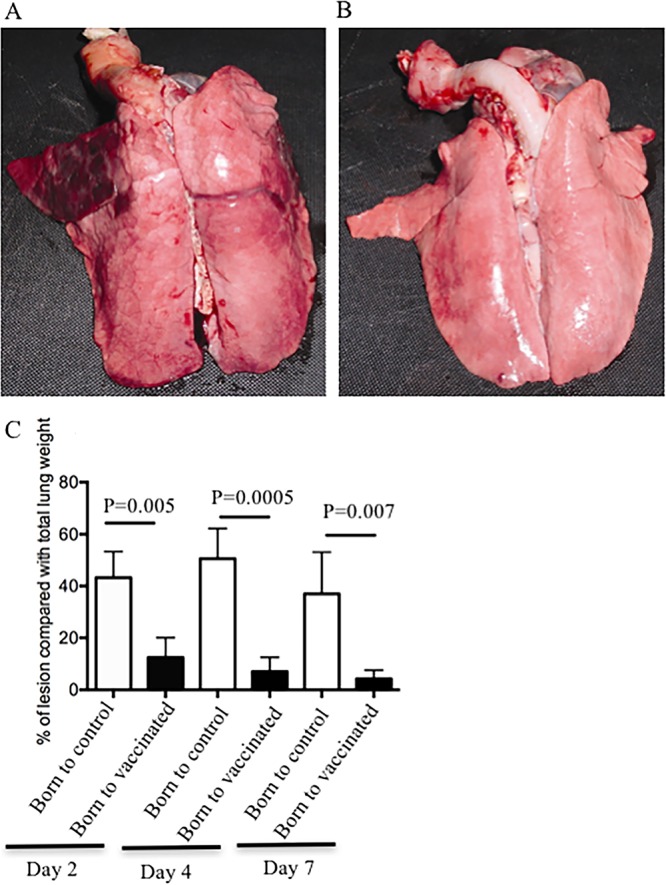
Gross pathology of lungs obtained from piglets born to vaccinated sows during pregnancy. (A) Lung of piglet born to sow vaccinated with PBS (control) and obtained at day 4 postinfection. (B) Lung of piglet born to vaccinated sow with heat-inactivated B. pertussis, infected with 5 × 109 CFU, and harvested at day 4 postinfection. These are representative pictures from four vaccinated sows with the heat-killed bacteria and four control (PBS-vaccinated) sows. (C) Lungs were harvested at days 2, 4, and 7 postchallenge, and both lesions and remaining nonlesion tissues were weighed. The size of the lesion is expressed as a percentage of the total lung weight. These data were obtained from piglets born to three vaccinated sows and three control sows.
DISCUSSION
Resurgence of pertussis has been reported globally in the past 2 decades, with the highest incidence and death rates in young infants less than 3 months of age (50, 51). Pertussis vaccination during pregnancy offers an ideal strategy to protect the mother and the newborn against potentially life-threatening disease (42). Therefore, better understanding the nature of maternal immunity to vaccines is of interest not only for the knowledge of basic mechanisms but also in designing better vaccines and implementing vaccination strategies to protect the fetus, the mother, and the newborn. We have previously demonstrated that maternal immunization may represent an alternative strategy for protecting the neonate against pertussis (44). However, so far the focus of maternal immunization studies has been on the induction of antibodies (14). Here, in addition to maternal antibodies, we further examined the role of maternal cytokines following immunization. We show that vaccination with heat-inactivated B. pertussis, in addition to antibodies, induces significant levels of mainly Th1 type cytokines which are detectable in the colostrum/milk of vaccinated sows. Subsequently, these cytokines are transferred into the offspring via suckling and are detected in serum and BAL fluids of piglets born to vaccinated sows. Although the highest rate of antibody absorption from colostrum into the piglet circulation has been shown to occur prior to the gut closure period (49), it is not very well known whether intestinal permeability to colostral cytokines is similar to that of colostral antibodies. It is believed that cytokines have a very short half-life, ranging from 20 min to a few hours under physiological conditions (52–54). However, transfer of cytokines and leukocytes via colostrum and milk in other animal models was reported previously (55, 56). In our studies, we observed that vaccinated sows possess a high level of mainly proinflammatory cytokines in their serum and colostrum/milk in addition to antibodies. Interestingly, these passively transferred cytokines can be detected in significant levels in the serum and even BAL fluid of suckling piglets born to vaccinated sows up to 7 days of age. Although investigating the mechanism of cytokine absorption is beyond the scope of this study, it is reasonable to speculate possible absorption of these cytokines even after the gut closure period through cytokine receptors expressed on the gastrointestinal (GI) tract epithelium (57). Alternatively, colostral lymphocytes (CL) migrating across the epithelial lining of the GI tract of piglets may initiate specific positive signals into the lymphoid system of the neonate for the release of cytokines. Our findings are consistent with a large body of evidence from experimental animal models indicating that leukocytes in breast milk penetrate the tissues of suckling neonates, including primates, and survive for extended periods of time (58–60). Thus, immune cells passively transferred via milk may maintain their capacity to participate in the production of cytokines well after the colostral phase of lactation is over (61). Although the distribution and function of these cells in the body of piglets were not determined in the current study, they merit further investigation. Additionally, intestinal epithelial cells (IEC) may contribute to the induction of cytokines and chemokines important for the recruitment and activation of immune cells. Several cytokines and chemokines, including TNF-α, IL-1, IL-6, IL-8, IL-10, MCP-1, and CCL20, are expressed by normal epithelial cells and are upregulated in response to different stimuli (62, 63). Therefore, it may be possible for passively transferred cytokines or CL to induce an inflammatory response in the adjacent intestinal mucosa for the initiation and amplification of a mucosal response and subsequent production of cytokines. Interestingly, these cytokines are also observed in significant levels in BAL fluids of piglets born to vaccinated sows. It is not known how these cytokines can be transferred into specific sites such as the lung. It is possible that circulating cytokines and CL taken up by epithelial lining of the GI tract migrate from the blood to other tissues, such as the respiratory tract. This has been shown for CL via a process that occurs at the mucosal system associated with maternal lactiferous glands and transfer of leukocytes transepithelially into the alveoli (64).
In this study, newborn piglets, in contrast to adult sows, were not able to mount a sufficient cytokine response following immunization with heat-inactivated bacteria. The status of cell-mediated immunity (CMI) in the newborn piglets is not clearly understood. It is believed that the neonatal pig is immunocompetent; however, their gut/mucosa-associated lymphoid tissue develops later than that of other species (64). Furthermore, studies in both humans and mice indicated a significant limitation of functional capacity of neonatal immune cells (65–67), possibly due to the presence of immunosuppressive CD71+ erythroid cells (6), which may account for the hyporesponsiveness of the innate and adaptive immune repertoire of newborn piglets to mount cytokine responses following vaccination. Therefore, it is possible to speculate that the presence of transient immunosuppression in piglets can delay the dynamic interaction of antigen-presenting cells with T and B cells and negatively impact the production of soluble mediators by these cells.
Despite difficulties in defining quantitative serological correlates of protection, increasing evidence suggests that immunity to B. pertussis is mediated by a combination of both humoral and cellular immunity (32, 33, 38). Most recent findings demonstrate that both Th1 and Th17 cells contribute to the clearance of infection with B. pertussis in mice and that IFN-γ is an instrumental element in adaptive immunity to B. pertussis infection (41). In agreement, our recent studies demonstrated a role for Th1 and IFN-γ in disease protection against pertussis (68). Although the direct contribution of these maternally derived cytokines to protection has not been investigated, it is pertinent to note in this context that Th1-type cytokines are necessary for providing optimal protection against B. pertussis (36, 40, 69–71). Of note, although our studies were conducted using whole heat-inactivated B. pertussis cells, further studies using acellular vaccine are required to determine whether such a cytokine response can be detected in pregnancy. It is also important to address the potential impact of these vaccine-induced cytokines on the mothers' and newborns' immune systems.
While the precise role of maternal cytokines and CL in postnatal differentiation of immune cells (Th1/Th2 phenotype) has not been established, our results clearly demonstrate skewed Th1-type cytokine profiles in piglets suckled from vaccinated sows. Although our data indicate that this is a transient phenomenon, it merits further investigations to determine how this type of response is initiated and whether these animals have an advantage later in their lives in terms of immune response to different stimuli and infections. In this context, it will be important to investigate the possible long-term impacts of maternal cytokines (mainly Th1 type) on the newborn's susceptibility to allergic and autoimmune diseases later in life.
Despite the fact that antibodies play a key role in limiting infection and disease by preventing initial bacterial adherence to ciliated cells in the respiratory tract (72), we observed here that the presence of specific IgA and IgG antibodies alone failed to provide sufficient protection following active immunization with heat-inactivated bacteria in newborn piglets. Surprisingly, these vaccinated piglets had levels of specific IgA and IgG antibodies in their serum prior to challenge comparable to those of the passively transferred antibodies in piglets born to vaccinated sows. Although our results are in contrast to other studies demonstrating a protective role for passively transferred pertussis-specific antibodies (73, 74), we cannot exclude the role of these antibodies and believe that the humoral immune response is involved in several aspects of protection against pertussis. However, our data demonstrate that protection against pertussis depends on a combination of antibody and cellular immune response, as has been reported elsewhere (75). Our data suggest that the presence of significant levels of passively transferred cytokines, in particular Th1 type cytokines, in the serum and BAL fluid contributes to the antibacterial activity of macrophages and neutrophils against B. pertussis (70). Therefore, direct cellular immune responses to B. pertussis may be necessary for complete elimination of B. pertussis (33, 36, 37, 40).
Taken together, our findings demonstrated a crucial role for maternally derived cytokines in providing protection against infection with B. pertussis. Moreover, since clinical trials particularly for maternal studies are focused exclusively on antibody responses, our findings suggest that maternal cytokines need to be considered in evaluating the outcome of future maternal immunization studies. Our findings may have implications for maternal immunization policy in relation to the importance of this approach in providing the newborn with optimal protection against infectious diseases.
MATERIALS AND METHODS
Animals.
Pregnant Landrace sows were purchased from the Prairie Swine Centre, University of Saskatchewan. Sows were induced to farrow by intramuscular (i.m.) injection of prostaglandin (Planate; Schering, Quebec, Canada) at day 113 of gestation. Piglets were born at day 114 to 115 of gestation. Nursing piglets were kept in the same room but in separate pens and were monitored closely. The piglets were challenged at 3 to 5 days of age for investigating the role of maternal immunity. To study the effects of active immunization, newborn piglets were immunized at 3 to 5 days of age. All experiments were conducted in accordance with the ethical guidelines of the University of Saskatchewan and the Canadian Council for Animal Care (CCAC).
Bacterial culture.
Bacterial suspensions of strain Tohama I were stored at −80°C in Casamino Acids plus 10% glycerol. Organisms were initially grown on the surface of Bordet-Gengou (BG; Becton, Dickinson and Company) agar containing 15% (vol/vol) defibrinated sheep blood and 40 μg/ml of cephalexin (Sigma-Aldrich) at 37°C for 72 h. After incubation, heavy inocula of bacteria were transferred to Stainer-Scholte (SS) medium and grown aerobically at 37°C overnight as liquid cultures, as we described elsewhere (46).
Intrapulmonary challenge.
Piglets were anesthetized with isoflurane and intubated using a laryngoscope and an endotracheal tube, and bacteria (5 × 109 CFU) were delivered through the tube at a level of 1.5 ml/lung craniodorsal to the bronchial bifurcation, as we have previously described elsewhere (44, 46, 47).
Collection of samples.
Colostrum and milk samples were collected and the solid fraction was removed by adding a rennet tablet (125 μg/ml; Redco Foods, Inc., Windsor, CT). Samples were stirred and incubated for 3 to 4 h at 37°C for clot formation. In order to collect the whey, samples were centrifuged at 3,000 rpm for 20 min. This resulted in the formation of three layers, the top layer (fat), the middle layer (whey), and the bottom solid layer. The middle layer was carefully removed and stored at −20°C until use. Sows were bled prior to priming, boosting, and farrowing. Newborn piglets were bled before suckling, at the time of challenge, and prior to euthanization. Serum was collected from the blood samples and stored at −20°C until used.
Vaccination of pregnant sows.
Pregnant sows were prescreened prior to vaccination by measuring IgA and IgG antibodies against B. bronchiseptica that could cross-react with B. pertussis. Selected sows were vaccinated i.m. in each side of the neck (trapezius muscle) behind the ear with 2 × 1010 CFU of heat-inactivated B. pertussis (by heating at 56°C for 60 min; inactivation was confirmed by plating onto BG plates). The same B. pertussis strain (Tohama I) in 2 ml of PBS without adjuvants was used as the vaccine. At the same time, sows were also fed with the same number of heat-inactivated bacteria. Control sows received PBS instead. Sows were boosted after 2 weeks in the same manner.
Vaccination of newborn piglets.
Piglets at 3 to 5 days of age were vaccinated (i.m.) in one side of the neck (trapezius muscle) behind the ear with 2 × 108 CFU of heat-inactivated B. pertussis in 1 ml of PBS. Piglets were boosted after 2 weeks in the same manner. Adjuvants were not added to the vaccine, and control piglets received PBS instead.
Prescreening of sows for mastitis.
Selected sows had no clinical signs of mastitis, and subclinical mastitis was monitored by performing colostrum/milk cell count (cytospin) and bacterial culture onto blood and Luria-Bertani (LB) agar plates.
Cytokine secretion by splenocytes.
Splenocytes (2 × 106/ml) were cultured at 37°C and 5% CO2 in 24-well plates and stimulated either with 5 μg/ml of heat-inactivated bacteria or 5 μg/ml of ConA for 48 h. The culture supernatants were collected and stored at −20°C until they were used for detection of cytokines, including IFN-γ, TNF-α, IL-12, IL-10, IL-8, and IL-6, by ELISA.
B. pertussis-specific ELISA.
Polystyrene microtiter plates (Immulon 2 HB; Dynex Technologies, Chantilly, VA) were coated with 2 μg/ml of sonicated heat-inactivated B. pertussis and incubated with serially diluted serum. Alkaline phosphatase-conjugated goat anti-pig IgG (1:5,000 dilution; Kirkegaard and Perry Laboratories, Gaithersburg, MD) was used to detect B. pertussis-specific IgG. Mouse anti-pig IgA (1:300 dilution; Serotec) was used to detect B. pertussis-specific IgA in samples. The reaction was amplified using biotinylated goat anti-mouse IgG (1:5,000 dilution; Zymed). Detection was carried out using streptavidin peroxidase (1:5,000 dilution; Jackson Laboratories), and the reaction was visualized with p-nitrophenyl phosphate (PNPP; Sigma-Aldrich). B. pertussis-specific antibody titers were determined using Microplate Manager 5.0 (Bio-Rad Laboratories Ltd.) with the assay read at 450 nm using a microplate reader (Bio-Rad Laboratories Ltd.).
Detection of cytokine levels by ELISA.
Concentrations of porcine IFN-γ, TNF-α, IL-6, IL-8, IL-10, IL-4, and IL-12/IL-23p40 were measured in serum, colostrum, and BAL fluid using a capture sandwich ELISA. Immulon 2 HB 96-well plates were coated with anti-porcine IFN-γ (0.5 μg/ml), anti-porcine TNF-α (1 μg/ml), anti-porcine IL-6 (1 μg/ml), anti-porcine IL-8 (2 μg/ml), anti-porcine IL-10 (1 μg/ml), and anti-porcine IL-4 (1 μg/ml), and Nunc Immulon 96-well plates were used for anti-porcine IL-12/IL-23p40 (2 μg/ml) (all from R&D Systems) overnight at 4°C. Prior to use, the plates were blocked with PBS–1% bovine serum albumin (BSA) for 1 h at room temperature (RT). Samples were added to the wells in a volume of 50 μl plus 50 μl of PBS–1% BSA and incubated for 2 h at RT. The reaction was amplified with biotinylated monoclonal antibodies to porcine IFN-γ (1 μg/ml), IL-12/IL-23p40 (250 μg/ml), TNF-α (250 μg/ml), IL-6 (0.2 μg/ml), IL-8 (25 μg/ml), IL-4 (50 μg/ml), and IL-10 (100 μg/ml) (all from R&D Systems). Plates were incubated for 1 h at RT. Detection was carried out with peroxidase-conjugated streptavidin (1:5,000; Jackson Laboratories) following 60 min of incubation at RT, and the reaction was visualized with PNPP (Sigma-Aldrich). Standard curves were generated using recombinant porcine IFN-γ and IL-12/IL-23p40, IL-6, IL-8, IL-4, IL-10, and TNF-α (R&D Systems).
Statistical analysis.
All outcome data from this study followed nonnormal distributions. To account for this outcome, data were ranked and then analysis of variance (ANOVA) or Student's t test was used to detect differences among the experimental groups. The distribution of the ranked data and the residuals from each ANOVA were consistent with the assumptions of procedure. If there were more than two experimental groups in the analysis and the ANOVA was significant, the means of the ranks were compared using Tukey's test. Probabilities less than or equal to 0.05 were considered significant.
ACKNOWLEDGMENTS
We are thankful to the VIDO Animal Care Staff for their assistance with housing, challenging, and monitoring the animals. We are especially thankful to Don Wilson, Kuldip Mirakhur, Amanda Giesbrecht, Lucas Wirth, and Sherry Tetland.
This study was funded by a grant from the Bill & Melinda Gates Foundation and the Canadian Institutes of Health Research (CIHR) through the Grand Challenges in Global Health Initiative (http://www.grandchallengesgh.org/) and the Krembil Foundation.
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE, Child Health Epidemiology Reference Group of WHO, Unicef. 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Schmitz G, Levy O. 2011. Development of newborn and infant vaccines. Sci Transl Med 3:90ps27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healy CM, Munoz FM, Rench MA, Halasa NB, Edwards KM, Baker CJ. 2004. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J Infect Dis 190:335–340. doi: 10.1086/421033. [DOI] [PubMed] [Google Scholar]
- 4.Lichty JR, Slavin B, Bradford WL. 1938. Attempt to increase resistance to pertussis in newborn infants by immunizing their mothers during pregnancy. J Clin Investig 17:613–621. doi: 10.1172/JCI100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendrick P, Thompson M, Elderling G. 1945. Immunity response of mothers and babies to injections of pertussis vaccine during pregnancy. Am J Dis Child 70:25–28. [Google Scholar]
- 6.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. 2013. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elahi S. 2014. New insight into an old concept: role of immature erythroid cells in immune pathogenesis of neonatal infection. Front Immunol 5:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, Skrondal A, Cappelen I, Engeland A, Aavitsland P, Madsen S, Buajordet I, Furu K, Nafstad P, Vollset SE, Feiring B, Nokleby H, Magnus P, Stoltenberg C. 2013. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med 368:333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ, Pandemic H1N1 Influenza in Pregnancy Working Group . 2010. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anonymous. 1996. Progress toward elimination of neonatal tetanus–Egypt, 1988-1994. JAMA 275:679–680. [PubMed] [Google Scholar]
- 11.Schofield FD, Tucker VM, Westbrook GR. 1961. Neonatal tetanus in New Guinea. Effect of active immunization in pregnancy. Br Med J 2:785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu HY, Englund JA. 2014. Maternal immunization. Clin Infect Dis 59:560–568. doi: 10.1093/cid/ciu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. 1993. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis 168:647–656. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 14.Esposito S, Bosis S, Morlacchi L, Baggi E, Sabatini C, Principi N. 2012. Can infants be protected by means of maternal vaccination? Clin Microbiol Infect 18:85–92. doi: 10.1111/j.1469-0691.2012.03936.x. [DOI] [PubMed] [Google Scholar]
- 15.Van de Perre P. 2003. Transfer of antibody via mother's milk. Vaccine 21:3374–3376. doi: 10.1016/S0264-410X(03)00336-0. [DOI] [PubMed] [Google Scholar]
- 16.Fink K, Zellweger R, Weber J, Manjarrez-Orduno N, Holdener M, Senn BM, Hengartner H, Zinkernagel RM, Macpherson AJ. 2008. Long-term maternal imprinting of the specific B cell repertoire by maternal antibodies. Eur J Immunol 38:90–101. doi: 10.1002/eji.200737872. [DOI] [PubMed] [Google Scholar]
- 17.Zinkernagel RM. 2003. On natural and artificial vaccinations. Annu Rev Immunol 21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]
- 18.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD, Sollinger HW, Bean MA. 1998. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med 339:1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 19.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matson AP, Zhu L, Lingenheld EG, Schramm CM, Clark RB, Selander DM, Thrall RS, Breen E, Puddington L. 2007. Maternal transmission of resistance to development of allergic airway disease. J Immunol 179:1282–1291. doi: 10.4049/jimmunol.179.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ege MJ, Herzum I, Buchele G, Krauss-Etschmann S, Lauener RP, Roponen M, Hyvarinen A, Vuitton DA, Riedler J, Brunekreef B, Dalphin JC, Braun-Fahrlander C, Pekkanen J, Renz H, von Mutius E, Protection Against Allergy Study in Rural Environments (PASTURE) Study Group. 2008. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J Allergy Clin Immunol 122:407–412. doi: 10.1016/j.jaci.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 22.von Mutius E, Vercelli D. 2010. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 23.Herberth G, Hinz D, Roder S, Schlink U, Sack U, Diez U, Borte M, Lehmann I. 2011. Maternal immune status in pregnancy is related to offspring's immune responses and atopy risk. Allergy 66:1065–1074. doi: 10.1111/j.1398-9995.2011.02587.x. [DOI] [PubMed] [Google Scholar]
- 24.Trend S, Strunk T, Lloyd ML, Kok CH, Metcalfe J, Geddes DT, Lai CT, Richmond P, Doherty DA, Simmer K, Currie A. 2016. Levels of innate immune factors in preterm and term mothers' breast milk during the 1st month postpartum. Br J Nutr 115:1178–1193. doi: 10.1017/S0007114516000234. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers L, Martin SW, Cohn A, Budd J, Marcon M, Terranella A, Mandal S, Salamon D, Leber A, Tondella ML, Tatti K, Spicer K, Emanuel A, Koch E, McGlone L, Pawloski L, LeMaile-Williams M, Tucker N, Iyer R, Clark TA, DiOrio M. 2013. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with cocirculating Bordetella holmesii and Bordetella pertussis-Ohio, 2010-2011. Clin Infect Dis 56:322–331. doi: 10.1093/cid/cis888. [DOI] [PubMed] [Google Scholar]
- 26.Guiso N. 2014. Bordetella pertussis: why is it still circulating? J Infect 68(Suppl 1):S119–S124. doi: 10.1016/j.jinf.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Hartzell JD, Blaylock JM. 2014. Whooping cough in 2014 and beyond: an update and review. Chest 146:205–214. doi: 10.1378/chest.13-2942. [DOI] [PubMed] [Google Scholar]
- 28.CDC. 2002. Pertussis–United States, 1997-2000. MMWR Morb Mortal Wkly Rep 51:73–76. [PubMed] [Google Scholar]
- 29.CDC. 2002. Pertussis deaths–United States, 2000. MMWR Morb Mortal Wkly Rep 51:616–618. [PubMed] [Google Scholar]
- 30.Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, Ciofi degli Atti ML, Giammanco A, Panei P, Blackwelder WC, Klein DL, Wassilak SG. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N Engl J Med 334:341–348. [DOI] [PubMed] [Google Scholar]
- 31.Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 32.Mahon BP, Brady MT, Mills KH. 2000. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J Infect Dis 181:2087–2091. doi: 10.1086/315527. [DOI] [PubMed] [Google Scholar]
- 33.Mills KH, Barnard A, Watkins J, Redhead K. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun 61:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassatella MA, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. 1992. IL-8 production by human polymorphonuclear leukocytes. The chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol 148:3216–3220. [PubMed] [Google Scholar]
- 35.Petersen JW, Ibsen PH, Haslov K, Heron I. 1992. Proliferative responses and gamma interferon and tumor necrosis factor production by lymphocytes isolated from tracheobroncheal lymph nodes and spleen of mice aerosol infected with Bordetella pertussis. Infect Immun 60:4563–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills KH, Ryan M, Ryan E, Mahon BP. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun 66:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redhead K, Watkins J, Barnard A, Mills KH. 1993. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun 61:3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ausiello CM, Urbani F, La Sala A, Lande R, Piscitelli A, Cassone A. 1997. Acellular vaccines induce cell-mediated immunity to Bordetella pertussis antigens in infants undergoing primary vaccination against pertussis. Dev Biol Stand 89:315–320. [PubMed] [Google Scholar]
- 39.Barbic J, Leef MF, Burns DL, Shahin RD. 1997. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect Immun 65:4904–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahon BP, Sheahan BJ, Griffin F, Murphy G, Mills KH. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J Exp Med 186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. 2013. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 9:e1003264. doi: 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huygen K, Cabore RN, Maertens K, Van Damme P, Leuridan E. 2015. Humoral and cell mediated immune responses to a pertussis containing vaccine in pregnant and nonpregnant women. Vaccine 33:4117–4123. doi: 10.1016/j.vaccine.2015.06.108. [DOI] [PubMed] [Google Scholar]
- 43.Kay AW, Fukuyama J, Aziz N, Dekker CL, Mackey S, Swan GE, Davis MM, Holmes S, Blish CA. 2014. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci U S A 111:14506–14511. doi: 10.1073/pnas.1416569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elahi S, Buchanan RM, Babiuk LA, Gerdts V. 2006. Maternal immunity provides protection against pertussis in newborn piglets. Infect Immun 74:2619–2627. doi: 10.1128/IAI.74.5.2619-2627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elahi S, Holmstrom J, Gerdts V. 2007. The benefits of using diverse animal models for studying pertussis. Trends Microbiol 15:462–468. doi: 10.1016/j.tim.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Elahi S, Brownlie R, Korzeniowski J, Buchanan R, O'Connor B, Peppler MS, Halperin SA, Lee SF, Babiuk LA, Gerdts V. 2005. Infection of newborn piglets with Bordetella pertussis: a new model for pertussis. Infect Immun 73:3636–3645. doi: 10.1128/IAI.73.6.3636-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elahi S, Buchanan R, Attah-Poku S, Townsend H, Babiuk LA, Gerdts V. 2006. The host defense peptide beta-defensin 1 confers protection against Bordetella pertussis in newborn piglets. Infect Immun 74:2338–2352. doi: 10.1128/IAI.74.4.2338-2352.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elahi S, Thompson DR, Strom S, O'Connor B, Babiuk LA, Gerdts V. 2008. Infection with Bordetella parapertussis but not Bordetella pertussis causes pertussis-like disease in older pigs. J Infect Dis 198:384–392. doi: 10.1086/589713. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen TV, Yuan L, Azevedo MS, Jeong KI, Gonzalez AM, Saif LJ. 2007. Transfer of maternal cytokines to suckling piglets: in vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet Immunol Immunopathol 117:236–248. doi: 10.1016/j.vetimm.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cherry JD. 2012. Epidemic pertussis in 2012–the resurgence of a vaccine-preventable disease. N Engl J Med 367:785–787. doi: 10.1056/NEJMp1209051. [DOI] [PubMed] [Google Scholar]
- 51.Healy CM. 2016. Pertussis vaccination in pregnancy. Hum Vaccin Immunother 12:1972–1981. doi: 10.1080/21645515.2016.1171948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. 1990. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Investig 86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shechter Y, Preciado-Patt L, Schreiber G, Fridkin M. 2001. Prolonging the half-life of human interferon-alpha 2 in circulation: design, preparation, and analysis of (2-sulfo-9-fluorenylmethoxycarbonyl)7-interferon-alpha 2. Proc Natl Acad Sci U S A 98:1212–1217. doi: 10.1073/pnas.98.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pietersz GA, Toohey B, McKenzie IF. 1998. In vitro and in vivo evaluation of human tumor necrosis factor-alpha (hTNFalpha) chemically conjugated to monoclonal antibody. J Drug Target 5:109–120. doi: 10.3109/10611869808995864. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez DD, Rimondi A, Aguirreburualde MSP, Mozgovoj M, Bellido D, Wigdorovitz A, Santos MJD. 2013. Quantitation of cytokine gene expression by real time PCR in bovine milk and colostrum cells from cows immunized with a bovine rotavirus VP6 experimental vaccine. Res Vet Sci 95:703–708. doi: 10.1016/j.rvsc.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Riedelcaspari G. 1993. The influence of colostral leukocytes on the course of an experimental Escherichia-Coli infection and serum antibodies in neonatal calves. Vet Immunol Immunopathol 35:275–288. doi: 10.1016/0165-2427(93)90039-7. [DOI] [PubMed] [Google Scholar]
- 57.Calhoun DA, Lunoe M, Du Y, Christensen RD. 2000. Granulocyte colony-stimulating factor is present in human milk and its receptor is present in human fetal intestine. Pediatrics 105:e7. doi: 10.1542/peds.105.1.e7. [DOI] [PubMed] [Google Scholar]
- 58.Jain L, Vidyasagar D, Xanthou M, Ghai V, Shimada S, Blend M. 1989. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch Dis Child 64:930–933. doi: 10.1136/adc.64.7_Spec_No.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiler IJ, Hickler W, Sprenger R. 1983. Demonstration that milk cells invade the suckling neonatal mouse. Am J Reprod Immunol 4:95–98. doi: 10.1111/j.1600-0897.1983.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, Shimamura M. 2000. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology 101:570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hawkes JS, Bryan DL, Gibson RA. 2002. Cytokine production by human milk cells and peripheral blood mononuclear cells from the same mothers. J Clin Immunol 22:338–344. doi: 10.1023/A:1020652215048. [DOI] [PubMed] [Google Scholar]
- 62.Stadnyk AW. 2002. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol 16:241–246. doi: 10.1155/2002/941087. [DOI] [PubMed] [Google Scholar]
- 63.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig 95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams PP. 1993. Immunomodulating effects of intestinal absorbed maternal colostral leukocytes by neonatal pigs. Can J Vet Res 57:1–8. [PMC free article] [PubMed] [Google Scholar]
- 65.Hayward AR, Lawton AR. 1977. Induction of plasma cell differentiation of human fetal lymphocytes: evidence for functional immaturity of T and B cells. J Immunol 119:1213–1217. [PubMed] [Google Scholar]
- 66.Lewis DB, Yu CC, Meyer J, English BK, Kahn SJ, Wilson CB. 1991. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Investig 87:194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zola H, Fusco M, Weedon H, Macardle PJ, Ridings J, Roberton DM. 1996. Reduced expression of the interleukin-2-receptor gamma chain on cord blood lymphocytes: relationship to functional immaturity of the neonatal immune response. Immunology 87:86–91. [PMC free article] [PubMed] [Google Scholar]
- 68.Elahi S, Van Kessel J, Kiros TG, Strom S, Hayakawa Y, Hyodo M, Babiuk LA, Gerdts V. 2014. c-di-GMP enhances protective innate immunity in a murine model of pertussis. PLoS One 9:e109778. doi: 10.1371/journal.pone.0109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahon BP, Ryan MS, Griffin F, Mills KH. 1996. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect Immun 64:5295–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mills KH. 2001. Immunity to Bordetella pertussis. Microbes Infect 3:655–677. doi: 10.1016/S1286-4579(01)01421-6. [DOI] [PubMed] [Google Scholar]
- 71.Wolfe DN, Mann PB, Buboltz AM, Harvill ET. 2007. Delayed role of tumor necrosis factor-alpha in overcoming the effects of pertussis toxin. J Infect Dis 196:1228–1236. doi: 10.1086/521303. [DOI] [PubMed] [Google Scholar]
- 72.Tuomanen EI, Zapiain LA, Galvan P, Hewlett EL. 1984. Characterization of antibody inhibiting adherence of Bordetella pertussis to human respiratory epithelial cells. J Clin Microbiol 20:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shahin RD, Hamel J, Leef MF, Brodeur BR. 1994. Analysis of protective and nonprotective monoclonal antibodies specific for Bordetella pertussis lipooligosaccharide. Infect Immun 62:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahin RD, Brennan MJ, Li ZM, Meade BD, Manclark CR. 1990. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med 171:63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mills KHG, Ross PJ, Allen AC, Wilk MM. 2014. Do we need a new vaccine to control the re-emergence of pertussis? Trends Microbiol 22:49–52. doi: 10.1016/j.tim.2013.11.007. [DOI] [PubMed] [Google Scholar]