Abstract
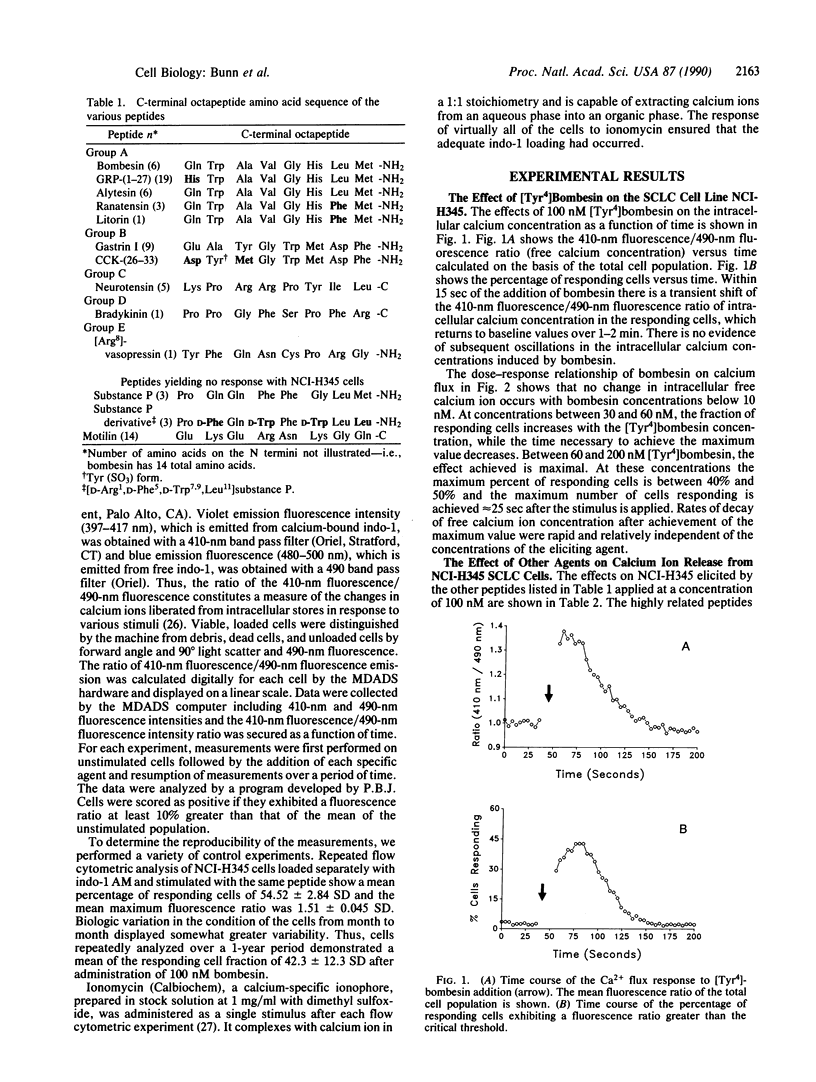
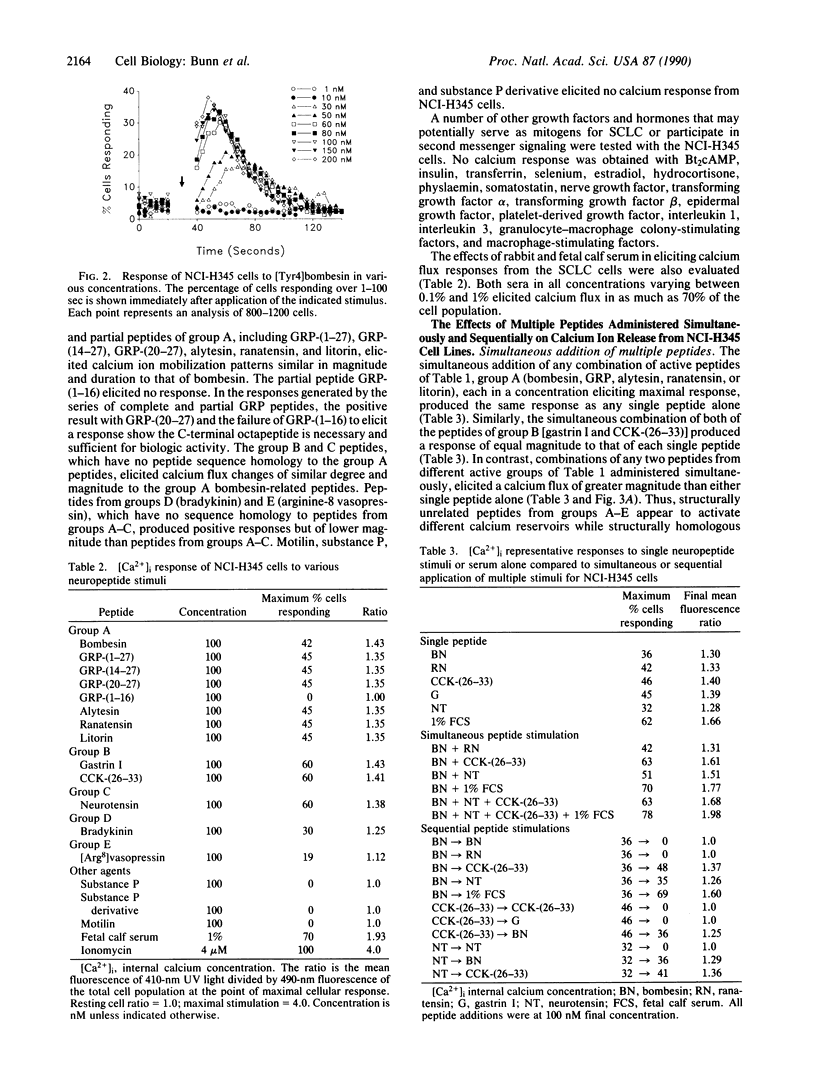
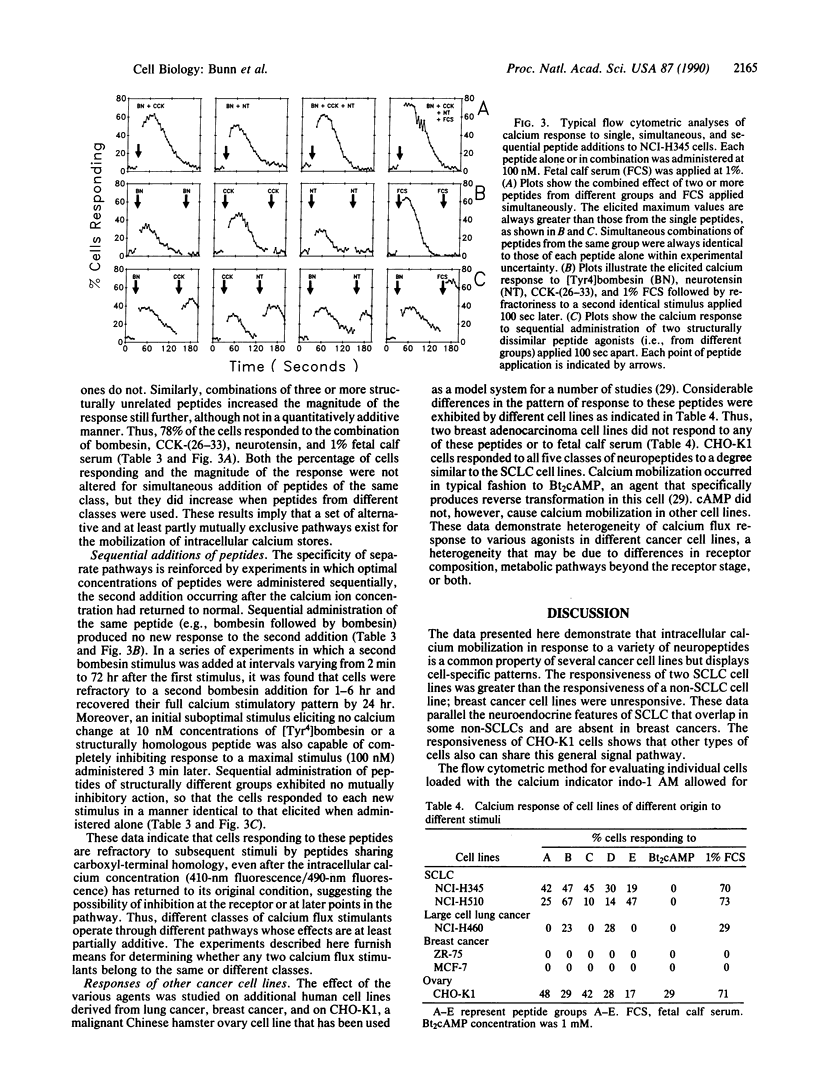
Calcium ion flux following the administration of a series of neuropeptides, N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate, and serum was monitored by flow cytometry in selected lung and breast cancer cell lines and Chinese hamster ovary cell line CHO-K1. Calcium ion flux was monitored in individual cells by flow cytometry using the indicator indo-1 AM. Five groups of neuropeptides produced calcium flux changes in lung cancer cell lines and CHO-K1 cells but not in breast cancer cells. The peak increase in free calcium was reached within 10 sec of peptide administration and declined to resting levels in 70-120 sec. When two or more members of the same group were administered simultaneously, calcium flux changes were identical to that produced by each single peptide. When two or more members of different groups were administered simultaneously, an increased calcium release occurred. When identical peptides or peptides from the same group were administered sequentially after the return of calcium concentrations to resting values, no calcium flux resulted from the second peptide. When peptides from different active groups were administered sequentially, a new calcium flux occurred after each peptide. These data are interpreted to mean that members of each active group of peptides trigger a different calcium flux pathway. Thus, many such pathways and different metabolic states exist within the cell. Elucidation of calcium flux pathways in normal and cancer cells may lead to greater understanding of the nature of the malignant defect.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo S. M., Kane M. A., King T. E., Jr, Schwarz M. I., Grauer L., Miller Y. E. Increased levels of bombesin-like peptides in the lower respiratory tract of asymptomatic cigarette smokers. J Clin Invest. 1989 Oct;84(4):1105–1113. doi: 10.1172/JCI114273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashall F., Puck T. T. Cytoskeletal involvement in cAMP-induced sensitization of chromatin to nuclease digestion in transformed Chinese hamster ovary K1 cells. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5145–5149. doi: 10.1073/pnas.81.16.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Cambier J., Chen Z. Z., Pasternak J., Ransom J., Sandoval V., Pickles H. Ligand-induced desensitization of B-cell membrane immunoglobulin-mediated Ca2+ mobilization and protein kinase C translocation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6493–6497. doi: 10.1073/pnas.85.17.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Bunn P. A., Jr, Gazdar A. F., Pagan J. A., Minna J. D. Selective growth in serum-free hormone-supplemented medium of tumor cells obtained by biopsy from patients with small cell carcinoma of the lung. Proc Natl Acad Sci U S A. 1981 May;78(5):3185–3189. doi: 10.1073/pnas.78.5.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Cuttitta F., Moody T. W., Minna J. D. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987 Feb 1;47(3):821–825. [PubMed] [Google Scholar]
- Corps A. N., Cheek T. R., Moreton R. B., Berridge M. J., Brown K. D. Single-cell analysis of the mitogen-induced calcium responses of normal and protein kinase C-depleted Swiss 3T3 cells. Cell Regul. 1989 Nov;1(1):75–86. doi: 10.1091/mbc.1.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Linnoila R. I., Hernandez O., DiAugustine R. P., Lazarus L. H. Human lung small-cell carcinoma contains bombesin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2379–2383. doi: 10.1073/pnas.79.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heikkila R., Trepel J. B., Cuttitta F., Neckers L. M., Sausville E. A. Bombesin-related peptides induce calcium mobilization in a subset of human small cell lung cancer cell lines. J Biol Chem. 1987 Dec 5;262(34):16456–16460. [PubMed] [Google Scholar]
- Irvine R. F., Brown K. D., Berridge M. J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J. 1984 Aug 15;222(1):269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E., Daniel T. O. Interrelationship between growth factor-induced pH changes and intracellular Ca2+. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1950–1954. doi: 10.1073/pnas.84.7.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton J. E., Scanlon D. B., Soveny C., Morstyn G. Effects of bombesin antagonists on the growth of small cell lung cancer cells in vitro. Cancer Res. 1988 Sep 1;48(17):4783–4789. [PubMed] [Google Scholar]
- Letterio J. J., Coughlin S. R., Williams L. T. Pertussis toxin-sensitive pathway in the stimulation of c-myc expression and DNA synthesis by bombesin. Science. 1986 Nov 28;234(4780):1117–1119. doi: 10.1126/science.3465038. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Mendoza S. A., Nånberg E., Sinnett-Smith J., Rozengurt E. Ca2+-mobilizing actions of platelet-derived growth factor differ from those of bombesin and vasopressin in Swiss 3T3 mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5768–5772. doi: 10.1073/pnas.84.16.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuoka K., Fukami K., Nakanishi O., Kawai S., Takenawa T. Mitogenesis in response to PDGF and bombesin abolished by microinjection of antibody to PIP2. Science. 1988 Feb 5;239(4840):640–643. doi: 10.1126/science.2829356. [DOI] [PubMed] [Google Scholar]
- McCaffrey P., Ran W., Campisi J., Rosner M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J Biol Chem. 1987 Feb 5;262(4):1442–1445. [PubMed] [Google Scholar]
- Mendoza S. A., Schneider J. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. II. Changes in Na+ and Ca2+ fluxes, Na+/K+ pump activity, and intracellular pH. J Cell Biol. 1986 Jun;102(6):2223–2233. doi: 10.1083/jcb.102.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Ransom J. T., DiGiusto D. L., Cambier J. Flow cytometric analysis of intracellular calcium mobilization. Methods Enzymol. 1987;141:53–63. doi: 10.1016/0076-6879(87)41055-0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Signal transduction pathways in mitogenesis. Br Med Bull. 1989 Apr;45(2):515–528. doi: 10.1093/oxfordjournals.bmb.a072339. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Bollag W. E., Rasmussen H. The effects of bombesin on polyphosphoinositide and calcium metabolism in Swiss 3T3 cells. J Biol Chem. 1987 Jan 5;262(1):182–188. [PubMed] [Google Scholar]
- Trepel J. B., Moyer J. D., Heikkila R., Sausville E. A. Modulation of bombesin-induced phosphatidylinositol hydrolysis in a small-cell lung-cancer cell line. Biochem J. 1988 Oct 15;255(2):403–410. doi: 10.1042/bj2550403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S., Zuckerman J. E., Bostwick D. G., Bensch K. G., Sikic B. I., Raffin T. A. Gastrin releasing peptide is a selective mitogen for small cell lung carcinoma in vitro. J Clin Invest. 1985 Jan;75(1):306–309. doi: 10.1172/JCI111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. A substance P antagonist also inhibits specific binding and mitogenic effects of vasopressin and bombesin-related peptides in Swiss 3T3 cells. Biochem Biophys Res Commun. 1986 May 29;137(1):135–141. doi: 10.1016/0006-291x(86)91186-1. [DOI] [PubMed] [Google Scholar]


