Abstract
Wip1 is deregulated in numerous human malignancies. However, its roles in non-small cell lung cancer (NSCLC) remain unclear. In the current study, the expression of Wip1 was investigated in NSCLC and its clinical significance was detected. Immunohistochemical staining was used to measure the expression of (wild-type p53 induced phosphatase 1) Wip1, p38 mitogen-activated protein kinase (MAPK), p53, p16 protein in a group of 60 NSCLC and 20 normal lung tissues. In addition, western blotting was performed to detect the Wip1 protein in fresh tissues. The correlations between clinical characteristics and Wip1 expression were analyzed using SPSS, version 16.0 software. The expression of Wip1 was positive in 63.3% (38/60) of NSCLC tissues, and in none of the normal lung tissues (0/20; P<0.01). In addition, Wip1 overexpression was significantly associated with tumor length and differentiation (P=0.008 and 0.03, respectively). The expression of Wip1 was negatively correlated with that of p38MAPK, p53 and p16 (r=−0.284, −0.352 and −0.348, respectively). The results of the current study demonstrated that Wip1 was frequently overexpressed in NSCLC, which may serve an essential role in the p38MAPK/p53/p16 signaling pathway.
Keywords: Wip1, NSCLC, p53, p16, p38MAPK
Introduction
Lung cancer is one of the most common malignancies worldwide, and approximately 85% new cases are non-small cell lung cancer (NSCLC). Despite therapeutic advances, the majority of patients with lung cancer undergo locally advanced and distant metastasis, with an overall five-year survival rate less than 20% (1). Thus, it is imperative to further investigate the molecular mechanisms underlying the progression of NSCLC.
The protein phosphatase wild-type p53 induced phosphatase 1 (Wip1) is a major serine/threonine phosphatase of the protein phosphatase 2C family encoded by the protein phosphatase, Mg2+/Mn2+ dependent 1D gene (2). The overexpression and amplification of Wip1 had been previously identified in numerous types of human cancer including medulloblastomas (3), clear cell renal cell carcinoma (4), colorectal cancer (5) and breast cancer (6). In addition, Bulavin et al (7) demonstrated that Wip1 functioned as a proto-oncogene in breast cancer. ATM serine/threonine kinase is one of the master regulators of the DNA damage-induced response signaling pathway (8), which was previously identified to be inactivated by Wip1 expression and potentially reduced the rate of tumor evolution (9). In addition, p38 mitogen-activated protein kinase (MAPK) was considered as a downstream target of Wip1, which regulated the cell cycle by inhibiting cyclin D1 under stress (10) and directly suppressed p53 activities through Ser33 and Ser46 dephosphorylation by controlling cellular apoptosis (8). An additional study further confirmed that Wip1 overexpression in mesenchymal stem cells diminished the p38MAPK activity and reduced the levels of p16 (11). Accordingly, Wip1 overexpression was identified to suppress checkpoint kinase 1 (CHK1) activity, whereas downregulation of Wip1 elevated CHK1 to activate the G2/M checkpoint following DNA damage (12).
However, the expression and functions of Wip1 in NSCLC remain unclear. In the current study, the expression of Wip1 was identified in NSCLC, and its clinical significance was determined.
Materials and methods
Tissue collection
Between April and September 2014, a total of 60 NSCLC tissues were obtained from patients who underwent tumor resection at the First Affiliated Hospital of China Medical University (Heping, China). The patients were comprised of 34 males and 26 females with a mean age of 58.6 years (range, 42–72 years). None of the 60 patients received any chemotherapy or radiotherapy prior to the operation. All cases were pathologically diagnosed and staged according to the TNM classification system (13). The 60 fresh specimens of tumor tissue and 20 adjacent normal lung tissues (5 cm distance from the cancer) were immediately obtained during the surgery: One part was stored in 4% paraformaldehyde solution and then embedded in 10% paraffin for immunohistochemistry, and the other part was preserved in liquid nitrogen for western blot analysis.
In the cancer group, 34 cases were identified with lymph node involvement (N+).
As for TNM stage, 44 cases were at stage I~II and 16 were at stage III~IV. In addition, 16 cases were highly differentiated and 44 cases exhibited with moderate or low differentiation. There were 40 cases, which presented with a tumor length larger than 3 cm. In addition, there were 6 cases who presented with distant metastasis. The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of the First Affiliated Hospital of China Medical University. Written informed consent was obtained from individual participants.
Main reagents and immunohistochemistry
The rabbit anti-human Wip1 monoclonal antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA; cat. no. sc-130655). The monoclonal mouse antibodies against p16 (cat. no. 6598), p53 (cat. no. 9284), phosphorylation of p38 (cat. no. 9215) and GAPDH (cat. no. 2118) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Immunohistochemistry kit and Bicinchoninic Acid (BCA) Protein Extraction kit were obtained from OriGene Technologies, Inc. (Beijing, China). RIPA buffer was purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
A 4-µm section was prepared from the paraffin-embedded block and dehydrated, and incubated in 3% hydrogen peroxide for 12 min to block endogenous peroxidase, followed by trypsin treatment for 18 min. A 10% goat serum was introduced at room temperature for 20 min, and the Wip1 antibody (1:100) was added to the tissues and incubated at 4°C overnight. As for the negative control, the primary antibody was replaced with PBS. The secondary antibodies in a ready to use kit (SP-9000; ZSGB-BIO, Beijing, China) were added as appropriate and 3,3′-diaminobenzidine staining was visualized using the hematoxylin stain. Two pathologists scored the slides respectively. For Wip1 staining assessment, samples with more than 10% cells that were yellow/brown-stained were classified as positive.
Western blot analysis
Western blot was performed to detect the expression of Wip1 in fresh tissues. The RIPA buffer was used to extract the total protein. Next, the protein concentrations were quantified by the BCA Protein Assay kit. A total of 50 µg total protein of each sample was loaded onto the 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA). Following blocking for 1 h in 5% non-fat milk, the membranes were incubated with primary antibodies overnight at 4°C (Wip1, 1:200; GAPDH, 1:1,000). On the second day, the membranes were incubated with the secondary antibodies followed by ECL chemiluminescence (Qihai Biotec, Shanghai, China). The results were analyzed using Gel-Pro-Analyzer software version 6.3 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All statistical analyses were performed using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Fisher's exact test was used to determine the differences of the Wip1 protein in cancer tissues and normal tissues. The χ2 test was used to assess the association between the clinicopathological parameters. Spearman's rank correlation coefficient was applied to see the correlation between Wip1 and p53, Wip1 and p16, and Wip1 and p38MAPK, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
Wip1 protein expression in NSCLC and normal tissues
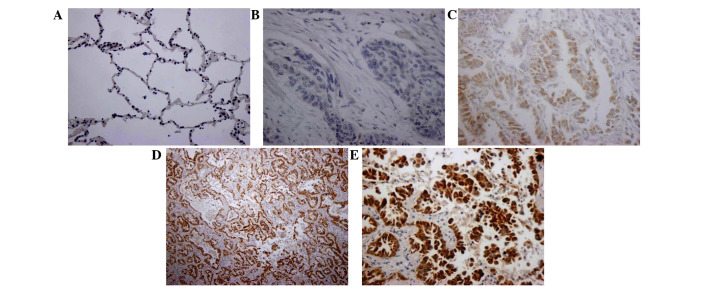
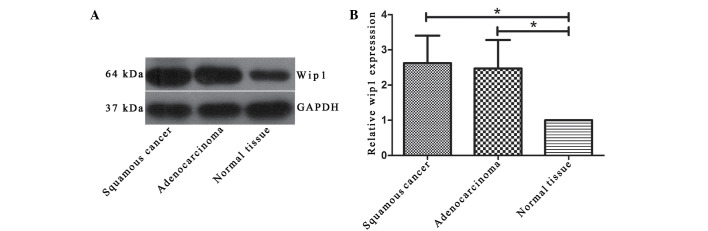
According to the immunohistochemistry data, the Wip1 expression was not detected in normal tissues (Fig. 1A). In NSCLC tissues, Wip1 was stained negative (Fig. 1B), light yellow (Fig. 1C) or yellowish-brown (Fig. 1D and E). Collectively, Wip1 was expressed in 63.3% (38/60) of NSCLC tissue, which was significantly greater than in normal tissues (0%; P<0.01; Table I). Furthermore, the expression of Wip1 was investigated using western blot analysis. The data indicated that there was a significant increase of Wip1 expression in the NSCLC tissues compared with the normal tissues (P<0.05; Fig. 2).
Figure 1.
Expression of Wip1 in NSCLC tissues and matched normal tissues. (A) Negative staining of Wip1 in matched normal tissues, ×100. (B and C) Negative staining of Wip1 in NSCLC tissues, ×400. (D and E) Positive staining of Wip1 in NSCLC tissues, ×100 and ×400, respectively. Wip1, wild-type p53 induced phosphatase 1; NSCLC, non-small cell lung cancer.
Table I.
The expression of Wip1 in cancer tissues and matched normal tissues.
| Group | Total | Wip1 +ve | Wip1 -ve | P-value |
|---|---|---|---|---|
| Cancer tissue | 60 | 38 | 22 | <0.001 |
| Normal tissue | 20 | 0 | 20 |
Wip1, wild-type p53 induced phosphatase 1.
Figure 2.
Wip1 protein levels in squamous cancer adenocarcinoma tissuse were significantly upregulated. (A) Representative images western blot analysis results. (B) The quantification of Wip1 protein in different tissue types; GAPDH was set as the endogenous control. *P<0.05. Wip1, wild-type p53 induced phosphatase 1.
Association between Wip1 expression and clinicopathological factors of patients
The associations between Wip1 expression and clinicopathological factors are summarized in Table II. The increased expression of Wip1 indicated a significant association with tumor length (P<0.01) and degree of differentiation (P<0.05), which may serve as a necessary prognostic marker for patients. However, no significant correlation was detected between Wip1 and other clinicopathological features including gender, lymph node metastasis, smoking history, age, pathological type, TNM stage and distant metastasis. In addition, western blot analysis demonstrated that were was no correlation between Wip1 and pathological types (data not shown; Fig. 2).
Table II.
Association between Wip1 expression and clinicopathological factors in 60 patients with non-small cell lung cancer.
| Characteristics | Total | Wip1 positive (n=38) | Wip1 negative (n=22) | P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 34 | 20 | 14 | |
| Female | 26 | 18 | 8 | 0.407 |
| Lymph node metastasis | ||||
| NO | 26 | 16 | 10 | |
| N+ | 34 | 22 | 12 | 0.801 |
| Smoking history | ||||
| Positive | 28 | 18 | 10 | |
| Negative | 32 | 20 | 12 | 0.886 |
| Age (years) | ||||
| ≥60 | 30 | 18 | 12 | |
| <60 | 30 | 20 | 10 | 0.592 |
| Pathological type | ||||
| Squamous cancer | 22 | 12 | 10 | |
| Adenocarcinoma | 38 | 26 | 12 | 0.282 |
| Tumor length (cm) | ||||
| >3 cm | 40 | 30 | 10 | |
| ≤3 cm | 20 | 8 | 12 | 0.008 |
| TNM stage | ||||
| I~II | 44 | 26 | 18 | |
| III~IV | 16 | 12 | 4 | 0.408 |
| Differentiation | ||||
| High | 16 | 6 | 9 | |
| Moderate + low | 44 | 32 | 13 | 0.03 |
| Distant metastasis | ||||
| Present | 6 | 4 | 2 | |
| Absent | 54 | 34 | 20 | 1 |
Wip1, wild-type p53 induced phosphatase 1; smoking history, one pack of cigarettes lasts for more than ten years.
Association between Wip1, p53, p16 and p38MAPK
According to the immunohistochemistry data, the expression of p53 was inversely correlated with Wip1 (r=−0.352). A total of 16/25 (64%) tumors with negative Wip1 exhibited strong staining for p53. In contrast, 25/35 (71.4%) cases with positive Wip1 exhibited weak staining for p53.
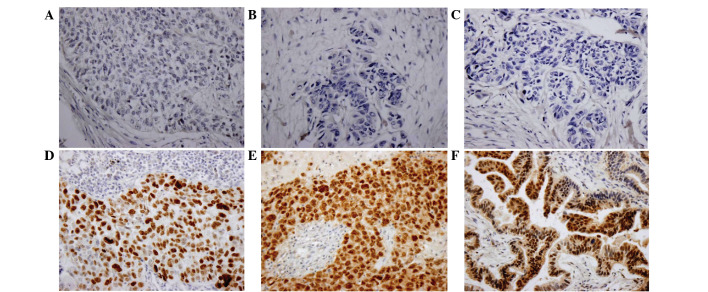
Subsequently, whether Wip1 was correlated with the levels of p38MAPK in NSCLC was investigated. Out of 29 Wip1-positive tumors, 21 (72.4%) did not stain for p38MAPK. On the contrary, only 61.3% (19/31) of tumors expressing negative Wip1 displayed strong staining for p38MAPK (Table III; r=−0.284). In addition, 15 cases (15/26, 57.7%) of negative Wip1 exhibited high p16 expression (r=−0.348; Fig. 3).
Table III.
Associations between Wip1, p53, p16 and p38 mitogen-activated protein kinase.
| p53 | p16 | p38 | ||||
|---|---|---|---|---|---|---|
| Wip1 | (+) | (−) | (+) | (−) | (+) | (−) |
| (+) | 10 | 25 | 8 | 26 | 8 | 21 |
| (−) | 16 | 9 | 15 | 11 | 19 | 12 |
| r | −0.352 | −0.348 | −0.284 | |||
Wip1, wild-type p53 induced phosphatase 1.
Figure 3.
Immunohistochemical staining for p53, p16 and p38MAPK in NSCLC tissues. Negative expression of (A) p53, (B) p16 and (C) p38MAPK in NSCLC tissues. Positive expressions of (D) p53, (E) p16 and (F) p38MAPK in NSCLC tissues. Magnification, 400x. MAPK, mitogen-activated protein kinase; NSCLC, non-small cell lung cancer.
Discussion
Oncogene activation and cancer suppressor gene inactivation are the key factors of tumor initiation and progression. As previously reported, the Wip1 gene has been identified as one of the p53 target genes induced by ionizing radiation (2), which inhibits the activity of p53 (7), downregulated the expression of p38 mitogen activated protein kinase (14) and reduced the level of p16 protein levels (15). Increased evidence suggests that Wip1 serves a vital role in human cancer.
In the current study, it was identified that Wip1 was significantly increased in NSCLC tissues when compared with normal tissues. Statistical analysis indicated that the overexpression of Wip1 was notably associated with tumor length and histological differentiation. By contrast, a previous study suggested that Wip1 expression was not associated with tumor size and pathological staging (16). It was identified that Wip1 expression was increased in the low and moderate differentiation groups when compared with the high differentiation group, which indicates that Wip1 may be a novel prognostic predictor for NSCLC.
Currently, the common treatment for NSCLC is surgical resection, combined with chemotherapy and/or radiotherapy prior and subsequent to surgery. However, the survival rate with this strategy is not satisfactory (17). Therefore, inhibiting positive regulators of cell proliferation, activating tumor suppressors and inducing apoptosis are primary interventional strategies in modern cancer therapy. A previous study indicated that Wip1 overexpression in transgenic mice promoted cell transformation and accelerated tumor progression (18). Consistently, another study demonstrated that small interfering RNA knockdown of Wip1 in medulloblastoma cells increased the p53 expression and induced apoptosis (19,20).
Further studies demonstrated that Wip1 overexpression disrupted the homeostasis maintained by the p38MAPK-p53-Wip1 pathway, caused the inactivation of Wnt-p53 through p38MAPK dephosphorylation (15). In the present study, the correlation between the expression of Wip1 and p53, p38MAPK and p16 was investigated in NSCLC tissues. A clear correlation between Wip1 and p38MAPK was observed. In addition, Wip1 expression was observed to be concomitant with reduced p53 levels. These data suggest that Wip1 functionally inactivates p53 and regulates the p38MAPK-p53-Wip1 signal transduction pathway. Previously, Bulavin et al (21) reported that Wip1-null mouse embryonic fibroblasts exhibited significant p16 accumulation and reduced cyclin-dependent kinase 4 activity through p38MAPK signaling in a p53-independent manner.
In summary, the current study demonstrated that Wip1 was frequently overexpressed and associated with the progression of NSCLC, which may serve an essential role in the p38MAPK/p53/p16 signaling pathway.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 2.Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer WE, Van De Woude GF, O'Connor PM, Appella E. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci USA. 1997;94:6048–6053. doi: 10.1073/pnas.94.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buss MC, Remke M, Lee J, Gandhi K, Schniederjan MJ, Kool M, Northcott PA, Pfister SM, Taylor MD, Castellino RC. The WIP1 oncogene promotes progression and invasion of aggressive medulloblastoma variants. Oncogene. 2015;34:1126–1140. doi: 10.1038/onc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Qi L, Han W, Wan X, Jiang S, Li Y, Xie Y, Liu L, Zeng F, Liu Z, Zu X. Overexpression of wip1 is associated with biologic behavior in human clear cell renal cell carcinoma. PLoS One. 2014;9:e110218. doi: 10.1371/journal.pone.0110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li ZT, Zhang L, Gao XZ, Jiang XH, Sun LQ. Expression and significance of the Wip1 proto-oncogene in colorectal cancer. Asian Pac J Cancer Prev. 2013;14:1975–1979. doi: 10.7314/APJCP.2013.14.3.1975. [DOI] [PubMed] [Google Scholar]
- 6.Ruark E, Snape K, Humburg P, Loveday C, Bajrami I, Brough R, Rodrigues DN, Renwick A, Seal S, Ramsay E, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493:406–410. doi: 10.1038/nature11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G, Nebreda AR, Anderson CW, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 8.Dudgeon C, Shreeram S, Tanoue K, Mazur SJ, Sayadi A, Robinson RC, Appella E, Bulavin DV. Genetic variants and mutations of PPM1D control the response to DNA damage. Cell Cycle. 2013;12:2656–2664. doi: 10.4161/cc.25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipponi D, Muller J, Emelyanov A, Bulavin DV. Wip1 controls global heterochromatin silencing via ATM/BRCA1-dependent DNA methylation. Cancer Cell. 2013;24:528–541. doi: 10.1016/j.ccr.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Casanovas O, Miró F, Estanyol JM, Itarte E, Agell N, Bachs O. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J Biol Chem. 2000;275:35091–35097. doi: 10.1074/jbc.M006324200. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Lee MO, Moon BH, Shim SH, Fornace AJ, Jr, Cha HJ. Senescent growth arrest in mesenchymal stem cells is bypassed by Wip1-mediated downregulation of intrinsic stress signaling pathways. Stem Cells. 2009;27:1963–1975. doi: 10.1002/stem.121. [DOI] [PubMed] [Google Scholar]
- 12.Liao Q, Guo X, Li X, Xiong W, Li X, Yang J, Chen P, Zhang W, Yu H, Tang H, et al. Prohibitin is an important biomarker for nasopharyngeal carcinoma progression and prognosis. Eur J Cancer Prev. 2013;22:68–76. doi: 10.1097/CEJ.0b013e328354d351. [DOI] [PubMed] [Google Scholar]
- 13.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. Internation Association for the Study of Lung Cancer: The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 14.Koom WS, Park SY, Kim W, Kim M, Kim JS, Kim H, Choi IK, Yun CO, Seong J. Combination of radiotherapy and adenovirus-mediated p53 gene therapy for MDM2-overexpressing hepatocellular carcinoma. J Radiat Res. 2012;53:202–210. doi: 10.1269/jrr.11110. [DOI] [PubMed] [Google Scholar]
- 15.Saito-Ohara F, Imoto I, Inoue J, Hosoi H, Nakagawara A, Sugimoto T, Inazawa J. PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res. 2003;63:1876–1883. [PubMed] [Google Scholar]
- 16.Yang DH, He JA, Li J, Ma WF, Hu XH, Xin SJ, Duan ZQ. Expression of proto-oncogene Wip1 in breast cancer and its clinical significance. Zhonghua Yi Xue Za Zhi. 2010;90:519–522. (In Chinese) [PubMed] [Google Scholar]
- 17.Sculier JP. Nonsmall cell lung cancer. Eur Respir Rev. 2013;22:33–36. doi: 10.1183/09059180.00007012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarulli GA, De Silva D, Ho V, Kunasegaran K, Ghosh K, Tan BC, Bulavin DV, Pietersen AM. Hormone-sensing cells require Wip1 for paracrine stimulation in normal and premalignant mammary epithelium. Breast Cancer Res. 2013;15:R10. doi: 10.1186/bcr3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Liu C, Zhao Z, Gao N, Chen G, Wang Y, Cui J. Clinical implications of GRHL3 protein expression in breast cancer. Tumour Biol. 2014;35:1827–1831. doi: 10.1007/s13277-013-1244-7. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Zhang H, Hu X, Xin S, Duan Z. Abnormality of pl6/p38MAPK/p53/Wipl pathway in papillary thyroid cancer. Gland Surg. 2012;1:33–38. doi: 10.3978/j.issn.2227-684X.2012.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, Appella E, Fornace AJ., Jr Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16 (Ink4a)-p19 (Arf) pathway. Nat Genet. 2004;36:343–350. doi: 10.1038/ng1317. [DOI] [PubMed] [Google Scholar]