Abstract
Postharvest physiological deterioration (PPD) is a global challenge in the improvement of cassava value chain. However, how to reduce cassava spoilage and reveal the mechanism of injured cassava storage roots in response to PPD were poorly understood. In the present study, we investigated the activities of antioxidant enzymes of cassava injured storage roots in PPD-susceptible (SC9) and PPD-tolerant (QZ1) genotypes at the time-points from 0h to 120h, and further analyzed their proteomic changes using two-dimensional electrophoresis (2-DE) in combination with MALDI-TOF-MS/MS. Ninety-nine differentially expressed proteins were identified from SC9 and QZ1 genotypes in the pairwise comparison of 24h/0h, 48h/0h, 72h/0h and 96h/0h. Of those proteins were associated with 13 biological functions, in which carbohydrate and energy metabolism related proteins were the biggest amount differential proteins in both genotypes, followed by chaperones, DNA and RNA metabolism, and defense system. We speculated that SOD in combination with CAT activities would be the first line of defense against PPD to support PPD-tolerant cassava varieties. The four hub proteins including CPN60B, LOS2, HSC70-1 and CPN20B, produced from the network of protein-protein interaction, will be the candidate key proteins linked with PPD. This study provides a new clue to improve cassava PPD-tolerant varieties and would be helpful to much better understand the molecular mechanism of PPD of cassava injured storage roots.
Introduction
Cassava (Manihot esculenta Crantz) is a staple food crop in Africa, Latin America and Asia [1, 2]. However, the rapid post-harvest physiological deterioration (PPD), a unique phenomenon in harvesting storage roots, has become a major constraint for extending cassava shelf-life compared with other root crops [3]. PPD is an active process involving in the changes of gene expression, novel protein synthesis and secondary metabolite accumulation [4–6]. Undesirable vascular streaking developed quickly was observed in the harvest storage roots and then caused deterioration within 2-3d. Because of PPD phenomenon, cassava storage roots have to be consumed soon after harvest [7].
The previous studies have been carried out to understand the biochemical and molecular mechanism of PPD [8–10]. PPD in cassava storage roots has been shown to be associated with an oxidative burst [11–16]. Iyer et al. (2010) showed that superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) in storage roots were more highly expressed in the regions closer to the site injured by machine [17]. This oxidative burst was reported to associate with cyanide production, which is rapid response to the mechanical injured in cassava storage roots [18]. Overexpressing CAT and SOD genes in cassava storage roots could availably decrease the accumulation of reactive oxygen species (ROS) independently from the ascorbate pool, and then reduce the PPD onset [19].
PPD is a major challenge for increasing cassava value chain. This phenomenon significantly reduces shelf-life of cassava storage roots for fresh consumption and decreases the income of smallholders. The previous reports indicated that extending the shelf-life of cassava to several weeks would reduce financial losses by $2.9 billion in Nigeria alone over a 20 years period [20]. Many scientists have made a lot of efforts to prevent or reduce the occurrence of PPD. Sánchez et al. (2006) reported that yellow-root cassava cultivars with higher β-carotene content have a delayed onset of PPD by 1 to 2 d [21]. In addition, Morante et al. (2010) surveyed different cassava germplasm resources and found three genotypes with high total carotenoid contents could delay PPD for up to 40 d after cassava harvesting [22], suggesting carotenoid may act as the roles of antioxidants. Pruning cassava plant could delays PPD before harvesting for a few days, but it reduced the dry matter content of the storage root [7].
Initial studies of PPD were focused on the changes of gene expression [14]. However, it was difficult to understand the PPD trigger mechanism and global regulation without the assistance of proteomic dataset. With the release and annotation of the cassava genome [23–25], it is possible to expand cassava proteome coverage and better characterize its modulation during PPD. Owiti et al. (2011) used the isobaric tags for relative and absolute quantification (iTRAQ) to analyze the proteomic changes during the early and late PPD. A total of 2600 proteins in cassava storage root were identified. Their functions included ROS scavenging, programmed cell death, defense response, signaling and cell-wall metabolism [26]. Vanderschuren et al. (2014) reported that about 300 proteins with significant abundance regulation during PPD were identified. They involved in oxidative stress, phenylpropanoid biosynthesis (including scopoletin), glutathione cycle, fatty acid α-oxidation, folate transformation and reduction II of sulfate. Among the identified proteins, the glutathione peroxidase (GPX) used glutathione to detoxify hydrogen peroxide (H2O2) and was considered a candidate for reducing PPD. Over-expressing GPX in Arabidopsis thaliana showed that PPD delay was probably associated with the reduction of lipid peroxidation and H2O2 accumulation [27]. Those results will provide a strategy to delay PPD in cassava storage roots through reducing ethene biosynthesis and increasing enzymes involved in suberization and lignifications. Ansari et al. (2014) reported that a burst of endogenous ethylene under stress revealed the fruits stayed at a high risk of pathogen infection, and caused various physiological disorders, then became deteriorations [28].
Through the previous studies had showed the differences linked with PPD in cassava as described above, proteomic changes of cassava injured storage roots in response to PPD were poorly reported. In the present study, we measured H2O2 and antioxidant activities in injured storage roots under PPD between PPD-susceptive (SC9) and PPD-tolerant (QZ1) genotypes stored at room temperature for 120h. In order to further solve cassava spoilage from understanding PPD molecular mechanism, we used comparative proteomics to explore the globally differential proteins and construct their interaction to speculate their potential relationship associated with PPD. These results will give insights into the molecular mechanism in response to PPD for the improvement of cassava breeding.
Materials and methods
Plant material preparation
Cassava genotypes QZ1 and SC9 were planted at Cassava Germplasm Bank of Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agricultural Sciences (CATAS-Danzhou campus, China), and carefully harvested after 10 months. The halved storage roots with uniform size were randomly stored at 26°C to 28°C and 70% to 80% relative humidity. After 0, 24, 48, 72, 96, and 120 h, the injured storage roots were taken out and frozen in liquid nitrogen subsequently, and then stored at -80°C for use. Three storage roots, taken from three cassava plants, respectively, were used as one replicate, and three biological replicates were conducted in the present study.
Determination of dry matter content
Cassava storage roots were carefully harvested, and cut into pieces. The dry matter content, expressed as the percentage of dry weight relative to fresh weight, was determined according to the method GB/T12087-2008 [29].
Measurement of β-carotene content using high performance liquid chromatography (HPLC)
β-carotene content was measured using HPLC according to the method described from Yang et al. (2015) [30]. Cassava storage root was ground to a fine powder using the liquid nitrogen with mortar and pestle, and 1.0 g of sample powder was fully mixed with 2 ml cooled acetone extraction, and then added 2 ml cooled petroleum ether, centrifuged at 3000 rpm for 5 min at 4°C. The suspension was transferred to a new tube, and 1 ml of cooled petroleum ether was added to the residue. This procedure was repeated three times until the residue became colorless. The all suspensions were dried using nitrogen using Termovap Sample Concentrator. The dried extract was re-dissolved in 500 ml acetone, and collected the dissolved fraction using a disposable syringe. After filtered, the dissolved fractions were collected to a brown bottle for HPLC (Agilent 1260 Infinity LC) analysis. Samples were separated on a Waters YCM Carotenoids S-3 column (4.6 mm × 250 mm), and isocratic eluted with solvent system (methanol: tert-butyl methyl ether = 7:3). The samples were read at a wavelength of 450 nm. β-carotene content was calculated using the following formula: β-carotene contents (μg/g) = Ax ×Cs (μg/ml) × V (ml)/As × W (g); where Ax is the peak area of sample β-carotene absorbance, Cs (μg/ml) is the concentration of standard sample, V (ml) is the total extract volume, As is the area of standard absorbance, W (g) is the sample weight.
Visual PPD evaluation
PPD evaluation of cassava storage roots stored at incubated rooms at 0, 24, 48, 72, 96, and 120 h was conducted. Quantitation of vascular discoloration was done on captured images using Image J software (http://rsb.info.nih.gov/ij/, NIH, MD, USA) [19].
Determination of H2O2 content and antioxidant capacity
The content of H2O2 and the activities of SOD, CAT, and POD were determined using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) according to the manufacturer’s instruction Hu et al. (2016) [31]. Ascorbic peroxidase (APX) activity was measured following the commercial assay kit (Beijing Solarbio Technology Company, China) according to the supplier’s protocol.
Protein extraction and 2-DE separation
Proteins from storage roots of QZ1 and SC9 genotypes during PPD programme were extracted with phenol extraction according to Chen et al. (2009) [32]. Two-dimensional electrophoresis (2-DE) was carried out according to the method of An et al. (2014) [33]. Immobilized linear pH gradient strips (pH 4–7, 13 cm, GE Healthcare, UK) were loaded with 312 μl rehydration buffer containing 300 μg sample proteins at room temperature in tray for 12–16 h. Isoelectric focusing (IEF) was carried out using GE Healthcare Isoelectric Focusing System (GE Healthcare, UK) under the following conditions: 300 V for 0.05 h in gradient mode, 300 V for 0.10 h in step and hold, 3500 V for 1.30 h in gradient mode, 3500 V for 4.20 h in step and hold, and 300 V for 20 min in gradient mode at 20°C. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with 12% acrylamide gels. The resultant 2-DE gels were stained with Colloidal Coomassie Blue G-250 for 2–3 days. The stained gels were scanned by Image Scanner III (GE healthcare) and analyzed with Delta2D (DECODON GmbH, Greifswald, Germany) software. Relative comparison of the intensity abundance between control and treatments (three replicate samples for each group) was performed using the Scheffe’s test (P≤0.05). The protein spots with at least 2-fold change were considered to be differentially expressed proteins (DEPs).
Tryptic in-gel digestion and MALDI-TOF/TOF MS analysis
Tryptic in-gel digestion and protein identification were performed by the methods reported in An et al. (2016) [34]. Differential proteins were identified using MALDI-TOF-TOF-MS/MS at Analysis and Testing Center, Jiangsu University and Beijing Genome Institute (Shenzhen). The mass spectra were acquired on a Matrix-assisted Laser Desorption ionization tandem time of flight mass spectrometer (Ultraflex-TOF-TOF, German). The MS spectra were searched against the NCB (http://www.ncbi.nlm.nih.gov) and cassava databases (http://phytozome.jgi.doe.gov/pz/portal.html#!search) using the MASCOT version 2.2.03 (http://www.matrixscience.com). Peptide mass tolerance was set as 0.3 Da and MS/MS ion mass tolerance was set at 0.15 Da, one missed cleavage was allowed, carbamidomethylation of cysteine as a fixed modification, and oxidation of methionine as a variable modification. Routine protein identification required sequence-confirmed data for a minimum of two peptides with recognition as the top ranking match in the Mascot Standard scoring system [33]. The proteins ID in NCBI database was changed according to cassava database in Phytozome. The classification analysis of differential proteins was according to the gene ontology (http://phytozome.jgi.doe.gov/pz/portal.html#!search).
Generation of protein-protein interaction (PPI) networks
DEPs of cassava SC9 and QZ1 associated with PPD were submitted to search tool for the retrieval of their corresponding interaction genes (STRING). All interactions in STRING were provided with a probabilistic combined score 0.4. PPI network was constructed at String online software (http://string-db.org/newstring_cgi/show_input_page.pl) and metabolically function at PMN (http://pmn.plantcyc.org/CASSAVA/NEW-IMAGE?type=GENE&object=G2Z-8222&redirect=T). The proteins in the PPI network considered as nodes and the degree of a node corresponded to the number of interaction with other proteins. The proteins with high degrees were serves as hub nodes.
Detection of quantitative real-time PCR (qRT-PCR)
The expressions of PPD responsive genes were validated by qRT-PCR with RNA samples extracted with a RNAprep Pure Plant Plus Kit according to the supplier’s protocol (TIANGEN, Code: DP441). The RNA quality was determined by running an agarose gel with GelStain (TransGen, Biotech, Code: GS101-01) staining. The RNA concentration was determined with NanoVue™ Plus ultramicro spectrophotometer (GE Healthcare). Reverse transcription was performed according to the manufacturer’s protocol (TransGen, Biotech, Code: AT311-02). Each cDNA sample was diluted 10 times in sterile ddH2O, and 1μl of this dilution was used as a template for real-time RT-PCR. The primers were listed in S1 Table. The real-time RT-PCR reactions were performed in a 10 μl volume containing 5 μl of 2 × SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) (TaKaRa, Code: RR820A), 1 μl (100 ng/μl) cDNA, and 0.8 μl (10 μM of each primer) primers in a Thermo Scientific PikoREAL thermocycler. Quantification was performed by sample of target genes to beta-actin gene using the comparative Ct method. The ΔCt was calculated by subtracting the average Ct of each treatment stage from the average Ct of beta-actin. The ΔΔCt was calculated by subtracting the ΔCt of each treatment stage from the ΔCt of the 0 h stage. The formula 2-ΔΔCt was used to calculate the relative fold change between the treatment stages [35]. All of the samples were measured in triplicate, and the experiments were performed on three biological replicates.
Statistical analysis
All the data were represented by an average of at least three biological replicates, with error bars representing standard deviations. Data were analyzed using ANOVA, which was performed by using SPSS Statistics V20 software to Duncan’s multiple comparison tests. Significance was determined at the 0.05 level.
Results
Changes in dry matter, starch and β-carotene contents in cassava injured storage roots
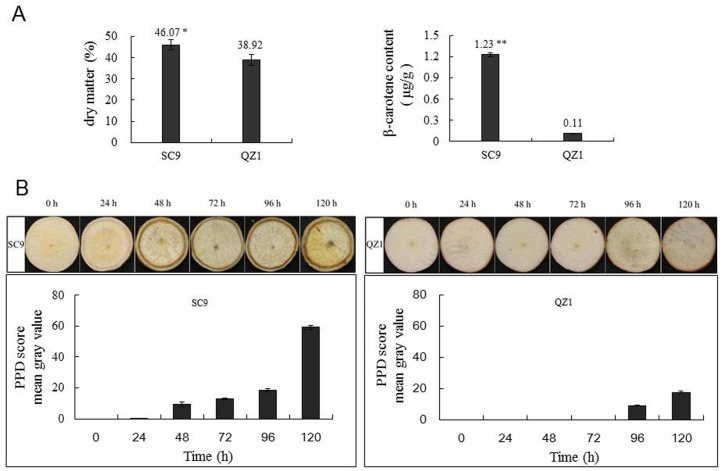
As showed in Fig 1 A, the dry matter and beta-carotene of QZ1 genotype were 38.92% and 0.11 μg/g, respectively, They were significantly lower than those in SC9 genotype (46.07%, 1.23 μg/g), especially, the differences in β-carotene content reached a significant level (p<0.01) between SC9 and QZ1 genotypes. In order to visually evaluate the PPD phenomenon, the visions of injured cassava storage roots were observed in the present study. Fig 1B showed that the PPD symptom score in the injured storage root of QZ1 genotype was 0–17.5% during 120h, while in SC9 was 0–59.2%, suggesting that QZ1 could have significantly PPD tolerance higher than that of SC9.
Fig 1.
(A) The analysis of dry matter and β-carotene contents of cassava injured storage roots in both genotypes SC9 and QZ1. Values are the means ± SE from three biological replicates. * and ** indicate significant differences at P < 0.05 and 0.01, respectively, by SPSS Duncan’s multiple comparison tests. (B) PPD score analysis of injured storage root in SC9 and QZ1 genotypes after postharvest. Visual examination of SC9 and QZ1 storage root slices at the time-points from 0h to 120h. PPD scores at different time-points were obtained using ImageJ image processing software based on the mean gray values percentages of vascular discoloration. The mean gray value at 0h was set to 0.
Analysis of H2O2 content and enzyme activities during PPD
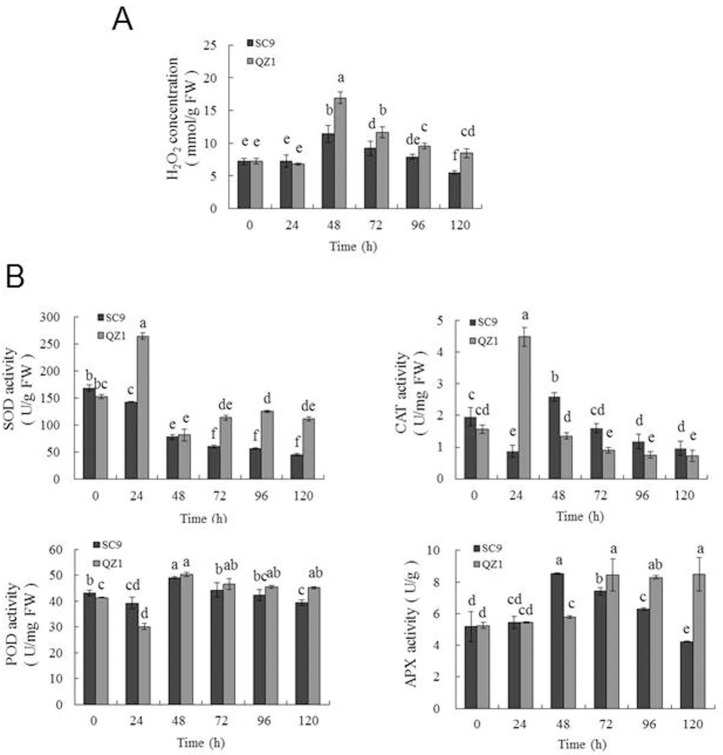
Antioxidant enzymes play important roles in ROS scavenging and minimizing oxidative damage under stress conditions [36]. In plant systems, the major initial sources of ROS during normal metabolism are the production of superoxide (O2•− and H2O2, which can modulate ROS in the PPD response [13, 37, 38]. In the present study, the concentrations of H2O2 (Fig 2A), which is considered as the major ROS in plants, were detected to be highest in the time-point of 48h in both genotypes (SC9, 11.4 mmol/g FW; QZ1, 16.92 mmol/g FW) under 120h-injured storage roots, and then gradually dropped to a low amount, while the H2O2 content in QZ1 was higher than that in SC9 at the time-points from 48h to 120h (Fig 2A). The activities of SOD, POD and APX (Fig 2B) in QZ1 genotype were higher than those in SC9 genotype, which was consistent with the changes of H2O2 concentration. These results indicated that QZ1 genotype produced H2O2 concentration more than that in SC9 genotype during injured storage roots, but the ability of ROS scavenging was also higher than that in SC9 genotype.
Fig 2. Changes in H2O2 contents and enzymatic activities at the time-points from 0h to 120h in two cassava genotypes.
(A) H2O2 contents (mmol/g of fresh weight); (B) SOD activities (U/g of fresh weight), CAT activities (U/mg of fresh weight), POD activities (U/mg of fresh weight), and APX activities (U/g). Each bar represents the mean of three independent replicates with standard error. Different letters on the columns indicate the statistical difference at p< 0.05 by SPSS to Duncan’s multiple comparison tests.
Protein identification and statistical analysis
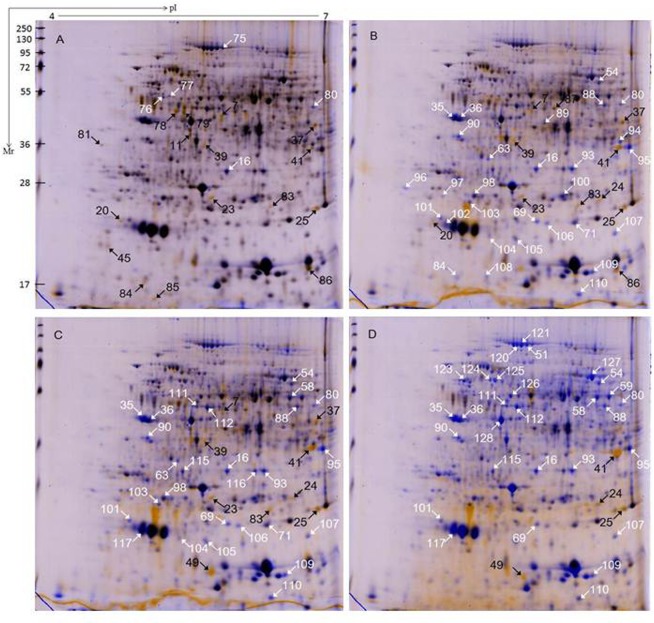
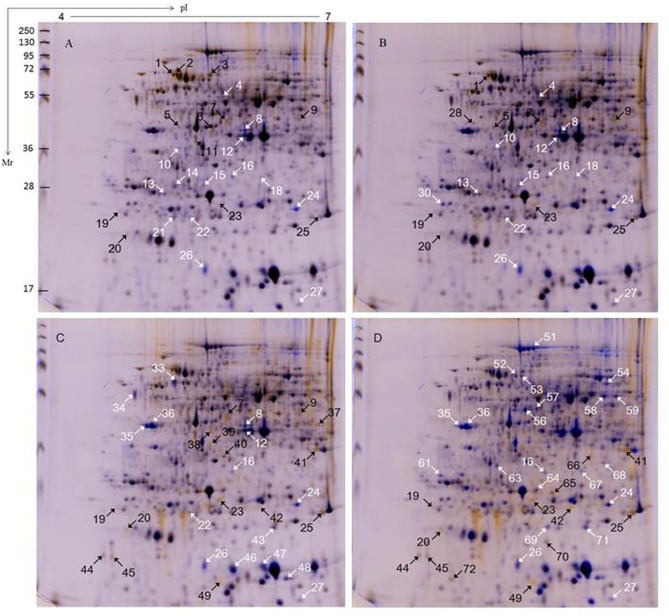
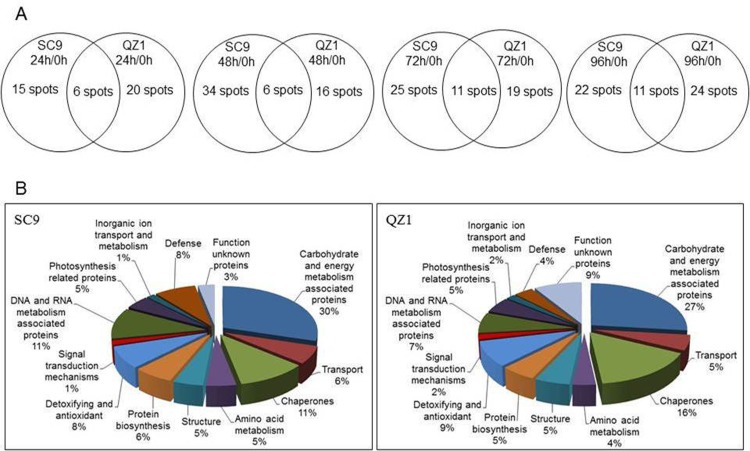
Proteins extracted from injured storage roots of two genotypes SC9 and QZ1 in response to PPD were separated by 2-DE. 2-DE images of proteins extracted from 0h time-point in the injured storage roots of SC9 and QZ1 genotypes were used as control, respectively. A total of 108 differential protein spots with greater than 2-fold altered intensity in the pairwise comparison of 24h/0h, 48h/0h, 72h/0h and 96h/0h were detected in both genotypes, in which 99 proteins were identified using MALDI-TOF-MS/MS. These identified proteins were involved in 13 biological functions including carbohydrate and energy metabolism (29), chaperones (15), DNA and RNA metabolism (9), detoxifying and antioxidant (8), defense (7), structure (6), transport (5), protein biosynthesis (5), amino acid metabolism (4), photosynthesis related proteins (4), signal transduction mechanisms (2), inorganic ion transport and metabolism (1) and function unknown proteins (6) (Table 1, S2 Table and S3 Table). Of these, 62 differential proteins were from SC9 genotype (Fig 3) and 56 differentially expressed proteins were identified from QZ1 genotype (Fig 4) against cassava database (https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Mesculenta) [25]. In both genotypes, there were 20 common proteins (CPs) (spots 7, 11, 16, 23–25, 35–37, 39, 41, 45, 49, 51, 54, 58, 59, 63, 69 and 71) listed in Table 1. Of these identified proteins, they were annotated via the survey of gene banks as shown in Table 1. There were 2 CPs (spots 16, 24) existed at the time-points of 24h, 48h, 72h and 96h; 2 CPs (spots 7, 23) at the former three time-points (24h, 48h and 72h); 1 CP (spot 25) at the latter three time-points (48h, 72h and 96h); 4 CPs (spots 35, 36, 41, 49) at the latter two time-points (72h and 96h) (Fig 5A). Carbohydrate and energy metabolism related proteins were the biggest amount in both genotypes (SC9, 30%; QZ1, 27%) (Fig 5B), followed by chaperones (SC9, 11%; QZ1, 16%), DNA and RNA metabolism associated proteins (SC9, 11%; QZ1, 7%), and defense (SC9, 8%; QZ1, 4%) (Fig 5B).
Table 1. Identification of differential proteins in cassava genotypes SC9 and QZ1 at the time-points from 0h to 96h.
The spots showing differential expression (> 2.0-fold of the normalized volume) were counted after gel analysis and manual editing with Delta2D software.
| Spot numbera | Description | Protein IDb | Theoretical pI/Mw (kDa) | Scorec/No. of unique peptides matchedd | Fold changes in the pairwise comparison of 24/0h, 48/0h, 72/0h and 96/0h in QZ1/SC9 | Differential proteins in the pairwise comparison in QZ1 | Differential proteins in the pairwise comparison in SC9 | Protein ID in the Database of Arabidopsis thaliana |
|---|---|---|---|---|---|---|---|---|
| Carbohydrate and energy metabolism associated proteins (29) | ||||||||
| 3 | V-type proton ATPase catalytic subunit | cassava4.1_003676m | 5.01/27.32 | 265/5 | 3.589±0.012(+) | 24h/0h | VHA-A | |
| 10 | Phosphoglycerate mutase(2,3-diphosphoglycerate-dependent) | cassava4.1_004733m | 6.05/65.11 | 90/3 | ∞(-) | 24h/0h, 48h/0h | iPGAM2 | |
| 11* | UDP-glycosyltransferase 78D1-related | cassava4.1_007209m | 6.05/45.30 | 104/4 | 2.399±0.083(+)/2.088±0.074(+) | 24h/0h, | 24h/0h | UGT78D2 |
| 21 | triosephosphate isomerase, chloroplastic | cassava4.1_012016m | 7.26/39.89 | 85/2 | 2.625±0.036(-) | 24h/0h | TIM | |
| 27 | N-acetyltransferase 9 | cassava4.1_034206m | 7.38/16.53 | 88/2 | 4.003±0.148(-) | 24h/0h, 48h/0h, 72h/0h,96h/0h | AT2G32030 | |
| 28 | ADP ribosylation factor-related | cassava4.1_020559m | 4.39/8.75 | 136/5 | 3.120±0.102(+) | 48h/0h | TTN5 | |
| 33 | ATP synthase | cassava4.1_006420m | 5.05/51.24 | 78/2 | 2.684±0.120(-) | 72h/0h | VAB2 | |
| 36* | pyruvate dehydrogenase E1 component subunit beta, mitochondrial | cassava4.1_010116m | 6.04/40.22 | 306/2 | 2.586±0.017(-)/2.682±0.015(-) | 72h/0h, 96h/0h | 48h/0h, 72h/0h, 96h/0h | MAB1 |
| 39* | pyruvate dehydrogenase E1 component subunit beta, mitochondrial | cassava4.1_010116m | 6.08/34.46 | 55/2 | 4.413±0.203(+)/∞(+) | 72h/0h | 24h/0h, 48h/0h, 72h/0h | MAB1 |
| 40 | Uroporphyrinogen decarboxylase/Uroporphyrinogen-III carboxy-lyase | cassava4.1_009378m | 6.71/40.63 | 66/2 | 2.134±0.018(+) | 72h/0h | HEME2 | |
| 43 | Triose-phosphate isomerase/Triosephosphate mutase | cassava4.1_014432m | 5.87/27.23 | 88/3 | 2.020±0.016(-) | 72h/0h | TPI | |
| 51* | Glycogen phosphorylase/Polyphosphorylase | cassava4.1_002466m | 5.09/109.63 | 83/3 | 3.101±0.171(-)/12.799±0.453(-) | 96h/0h | 96h/0h | AT3G29320 |
| 54* | ATP synthase subunit beta-1, mitochondrial-related | cassava4.1_004726m | 5.95/59.86 | 430/3 | 6.296±0.110(-)/3.756±0.124(-) | 96h/0h | 48h/0h, 72h/0h, 96h/0h | AT5G08680 |
| 56 | glucose-1-phosphate adenylyltransferase small subunit, chloroplastic | cassava4.1_005518m | 5.16/57.58 | 117/2 | 5.752±0.135(-) | 96h/0h | ADG1 | |
| 57 | ATP synthase subunit beta-1, mitochondrial-related | cassava4.1_004726m | 5.95/59.86 | 387/3 | ∞(-) | 96h/0h | AT5G08680 | |
| 75 | Glycogen phosphorylase/Polyphosphorylase | cassava4.1_002466m | 5.26/108.52 | 56/3 | 2.563±0.116(-) | 24h/0h | AT3G29320 | |
| 77 | enolase | cassava4.1_007673m | 6.05/49.74 | 174/5 | 8.288±0.314(-) | 24h/0h | LOS2 | |
| 78 | Transaldolase/Glycerone transferase | cassava4.1_007758m | 4.76/42.25 | 83/5 | 4.060±0.107(+) | 24h/0h | TRA2 | |
| 81 | methionine sulfoxide reductase | cassava4.1_016963m | 6.05/23.02 | 86/5 | 2.286±0.025(+) | 24h/0h | PMSR1 | |
| 83 | Triose-phosphate isomerase/Triosephosphate mutase | cassava4.1_014432m | 5.35/28.42 | 254/3 | 6.983±0.216(+) | 24h/0h, 48h/0h,72h/0h | TPI | |
| 87 | ATP synthase subunit beta-1, mitochondrial-related | cassava4.1_004726m | 5.95/59.86 | 643/3 | ∞(+) | 48h/0h | AT5G08680 | |
| 88 | cinnamyl alcohol dehydrogenase 6-related | cassava4.1_010153m | 6.05/38.30 | 63/2 | 3.984±0.115(-) | 48h/0h, 72h/0h, 96h/0h | CAD6 | |
| 89 | ATP synthase subunit beta-1, mitochondrial-related | cassava4.1_004726m | 5.95/59.86 | 342/3 | 2.713±0.126(-) | 48h/0h | AT5G08680 | |
| 102 | pheophorbide a oxygenase, chloroplastic | cassava4.1_005194m | 6.68/53.93 | 53/5 | 3.349±0.164(-) | 48h/0h | ACD1 | |
| 111 | ATP synthase subunit beta-1, mitochondrial-related | cassava4.1_004726m | 6.00/59.93 | 103/3 | ∞(-) | 72h/0h, 96h/0h | AT5G08680 | |
| 115 | Malate dehydrogenase (oxaloacetate-decarboxylating) (NADP(+))/Pyruvic-malic carboxylase | cassava4.1_004164m | 7.92/26.71 | 97/2 | 2.421±0.117(-) | 72h/0h, 96h/0h | NADP-ME2 | |
| 120 | Glycogen phosphorylase/Polyphosphorylase | cassava4.1_002466m | 5.26/108.52 | 65/3 | 8.946±0.314(-) | 96h/0h | AT3G29320 | |
| 121 | Glycogen phosphorylase/Polyphosphorylase | cassava4.1_002466m | 5.26/108.52 | 70/3 | 13.243±0.463(-) | 96h/0h | AT3G29320 | |
| 126 | ATP synthase subunit beta-1, mitochondrial-related | cassava4.1_004726m | 6.00/ 59.12 | 88/3 | 4.503±0.135(-) | 96h/0h | AT5G08680 | |
| Transport (5) | ||||||||
| 25* | abscisic acid 8'-hydroxylase/ABA 8'-hydroxylase | cassava4.1_022491m | 9.38/51.66 | 28/2 | 32.747±0.538(+)/5.369±0.146(+) | 24h/0h, 48h/0h, 72h/0h, 96h/0h | 24h/0h, 48h/0h, 72h/0h, 96h/0h | CYP707A4 |
| 46 | Ethanolamine-phosphate cytidylyltransferase/Phosphorylethanolamine transferase | cassava4.1_008241m | 6.68/53.93 | 67/3 | 2.049±0.113(-) | 72h/0h | AT1G77060 | |
| 71* | myosin | cassava4.1_000217m | 4.79/200.17 | 44/3 | 2.624±0.096(-)/2.461±0.108(-) | 96h/0h | 48h/0h, 72h/0h | CIP1 |
| 94 | Mitochondrial outer membrane translocase complex, subunit Tom7 | cassava4.1_020644m | 10.62/8.20 | 122/5 | ∞(-) | 48h/0h | AT5G41685 | |
| 116 | dynactin sununit P25 | cassava4.1_013839m | 5.78/29.50 | 331/3 | 5.501±0.148(-) | 72h/0h | GAMMACA1 | |
| Chaperones (15) | ||||||||
| 1 | heat chock protein 70KDa | cassava4.1_001607m | 5.53/72.35 | 86/2 | 2.113±0.087(+) | 24h/0h, 48h/0h | Hsp70b | |
| 2 | heat shock 70 KDa protein 10, mitochondrial | cassava4.1_002964m | 4.88/69.39 | 83/5 | 2.827±0.069(+) | 24h/0h | HSC70-1 | |
| 4 | chaperonin | cassava4.1_004458m | 5.50/60.52 | 107/5 | ∞(-) | 24h/0h, 48h/0h | HSP60 | |
| 45* | regulator of chromosome condensatio(RCC1) family with fyve zinc finger domain-related | cassava4.1_000603m | 4.37/34.27 | 236/2 | 3.400±0.118(+)/4.112±0.136(+) | 72h/0h, 96h/0h | 24h/0h | BRX |
| 47 | 17.6 KDa class I heat shock protein 1-related | cassava4.1_017974m | 6.00/17.96 | 86/3 | 2.388±0.085(-) | 72h/0h | AT1G53540 | |
| 52 | heat shock 70 Kda protein 10, mitochondrial | cassava4.1_002955m | 5.35/73.40 | 117/2 | 2.738±0.097(-) | 96h/0h | MTHSC70-2 | |
| 53 | heat shock 70 Kda protein 10, mitochondrial | cassava4.1_002964m | 6.34/73.40 | 95/5 | 2.244±0.082(-) | 96h/0h | MTHSC70-2 | |
| 70 | 22.0 KDa heat chock protein | cassava4.1_033525m | 6.05/21.51 | 142/3 | 2.471±0.079(+) | 96h/0h | ATHSP22.0 | |
| 72 | annexin D1-related | cassava4.1_021183m | 6.81/36.20 | 83/2 | 3.736±0.117(+) | 96h/0h | ANNAT1 | |
| 100 | 20 KDa chaperonin, chloroplastic | cassava4.1_014410m | 8.87/27.76 | 341/5 | 2.120±0.068(-) | 48h/0h | CPN20 | |
| 104 | 25.3 KDa heat shock protein, chloroplastic | cassava4.1_015256m | 7.26/25.96 | 89/3 | 7.025±0.264(-) | 48h/0h,72h/0h | HSP21 | |
| 105 | 22.0 KDa heat shock protein | cassava4.1_033525m | 6.05/28.05 | 249/3 | 4.240±0.131(-) | 48h/0h | ATHSP22.0 | |
| 110 | 17.6 KDa class I heat shock protein 1-related | cassava4.1_018134m | 5.13/1796 | 132/5 | 44.884±0.597(-) | 48h/0h, 72h/0h, 96h/0h | AT1G07400 | |
| 117 | 25.3 KDa heat shock protein, chlorplastic | cassava4.1_015256m | 8.39/26.26 | 92/5 | 36.764±0.543(-) | 72h/0h, 96h/0h | HSP21 | |
| 124 | chaperonin 60 subunit beta 1, chloroplastic-related | cassava4.1_003907m | 5.42/11.40 | 215/5 | ∞(-) | 96h/0h | CPN60B | |
| Amino acid metabolism (4) | ||||||||
| 13 | aspartic proteinase A1-related | cassava4.1_005735m | 4.73/56.41 | 88/5 | 2.874±0.122(-) | 24h/0h, 48h/0h | APA1 | |
| 23* | Phosphatidylserine decarboxylase/PS decarboxylase | cassava4.1_025763m | 6.11/73.51 | 74/2 | 10.605±0.267(+)/∞(+) | 24h/0h, 48h/0h, 72h/0h, 96h/0h | 24h/0h, 48h/0h, 72h/0h | PSD2 |
| 85 | ubiquitin-conjugating enzyme E2 1-related | cassava4.1_018317m | 9.59/23.04 | 85/2 | 10.822±0.301(+) | 24h/0h | UBC2 | |
| 90 | diaminopimelate epimerase,chloroplastic | cassava4.1_009997m | 8.71/45.83 | 97/3 | ∞(-) | 48h/0h, 72h/0h, 96h/0h | AT3G53580 | |
| Structure(6) | ||||||||
| 5 | actin | cassava4.1_033108m | 5.52/39.83 | 81/2 | 2.633±0.085(+) | 24h/0h, 48h/0h | ACT7 | |
| 8 | actin family protein | cassava4.1_009807m | 5.16/41.89 | 37/4 | 3.621±0.106(-) | 24h/0h, 48h/0h, 72h/0h | ACT7 | |
| 12 | ACT7; actin 7 | cassava4.1_009934m | 5.05/41.25 | 34.8/4 | 2.134±0.105(-) | 24h/0h, 48h/0h, 72h/0h, 96h/0h | ACT7 | |
| 76 | tubulin beta-4 chain-related | cassava4.1_007713m | 5.12/50.11 | 153/4 | 2.289±0.076(-) | 24h/0h | TUB8 | |
| 79 | actin | cassava4.1_033108m | 5.31/41.70 | 78/2 | 3.199±0.142(+) | 24h/0h | ACT7 | |
| 128 | actin | cassava4.1_033108m | 5.16/41.88 | 131/5 | 12.864±0.257(-) | 96h/0h | ACT7 | |
| Protein biosynthesis (5) | ||||||||
| 7* | elongation factor family protein | cassava4.1_003058m | 6.12/46.93 | 135/4 | 8.593±0.215(+)/16.389±0.266(+) | 24h/0h, 48h/0h, 72h/0h | 24h/0h, 48h/0h, 72h/0h | RABE1b |
| 15 | CCAAT-binding transcription factor-related | cassava4.1_026597m | 7.03/20.10 | 90/4 | 2.119±0.105(-) | 24h/0h, 48h/0h | NF-YB5 | |
| 49* | translation initiation factor 5A-related | cassava4.1_018059m | 5.87/21.99 | 56/5 | ∞(+)/15.243±0.364(+) | 72h/0h, 96h/0h | 72h/0h, 96h/0h | ELF5A-1 |
| 98 | asparagine synthetase | cassava4.1_014428m | 5.30/22.97 | 73/4 | 2.431±0.086(-) | 48h/0h, 72h/0h | AILP1 | |
| 123 | protein disulfide isomerase | cassava4.1_008355m | 5.16/56.38 | 93/5 | 13.184±0.249(-) | 96h/0h | PDIL1-1 | |
| Detoxifying and antioxidant (8) | ||||||||
| 16* | L-ascorbate peroxidases, chloroplastic/mitochondrial-related | cassava4.1_009867m | 9.59/42.17 | 67/5 | 4.850±0.147(-)/18.851±0.328(-) | 24h/0h, 48h/0h, 72h/0h,96h/0h | 24h/0h, 48h/0h, 72h/0h, 96h/0h | TAPX |
| 22 | proteasome subunit beta type | cassava4.1_011091m | 7.06/31.53 | 116/4 | ∞(-) | 24h/0h, 48h/0h, 72h/0h | AT3G26340 | |
| 24* | L-ascorbate peroxidase 2, cytosolic | cassava4.1_013461m | 6.96/26.70 | 143/3 | 9.216±0.251(-)/6.096±0.157(+) | 24h/0h, 48h/0h, 72h/0h, 96h/0h | 48h/0h, 72h/0h, 96h/0h | APX2 |
| 61 | Dehydrin (Dehydrin) | cassava4.1_015875m | 5.28/24.37 | 109/2 | 2.653±0.098(-) | 96h/0h | ERD10 | |
| 64 | L-ascorbate peroxidase 2, cytosolic | cassava4.1_013461m | 5.31/27.67 | 173/3 | 2.306±0.102(-) | 96h/0h | APX1 | |
| 103 | ferritin | cassava4.1_013978m | 5.48/26.06 | 75/4 | ∞(-) | 48h/0h, 72h/0h | FER1 | |
| 112 | monodehydroascorbate reductase, cytoplasmic isoform 1-related | cassava4.1_007980m | 6.53/54.02 | 92/5 | 5.837±0.176(-) | 72h/0h, 96h/0h | MDAR1 | |
| 127 | NADP-dependent malic enzyme 2-related | cassava4.1_004170m | 8.39/27.65 | 173/5 | 28.623±0.428(-) | 96h/0h | NADP-ME2 | |
| Signal transduction mechanisms (2) | ||||||||
| 30 | 14-3-3-like protein GF14 lambda | cassava4.1_014519m | 4.82/29.44 | 169/2 | 2.138±0.084(-) | 48h/0h | AT5G65430.3 | |
| 86 | ADP-ribosylation factor A1F | cassava4.1_016194m | 9.68/16.69 | 74/3 | 6.225±0.208(+) | 24h/0h, 48h/0h | ATARF | |
| DNA and RNA metabolism associated proteins (8) | ||||||||
| 26 | Oxaloacetate decarboxylase/Oxaloacetate carboxy-lyase | cassava4.1_017832m | 5.35/18.06 | 212/2 | 26.759±0.364(-) | 24h/0h, 48h/0h, 72h/0h, 96h/0h | AT5G16450 | |
| 37* | RNA-binding KH domain-containing protein | cassava4.1_002529m | 7.53/112.26 | 89/5 | 11.450±0.266(+)/6.094±0.219(+) | 72h/0h | 24h/0h, 48h/0h, 72h/0h | HEN4 |
| 41* | Adenosylhomocysteine nucleosidase/S-adenosylhomocysteine/5'-methylthioadenosine nucleosidase | cassava4.1_021711m | 6.52/36.25 | 71/5 | ∞(+)/14.019±0.241(+) | 72h/0h, 96h/0h | 24h/0h, 48h/0h, 72h/0h, 96h/0h | AT4G24350 |
| 48 | Nucleolar RNA-binding protein NIFK | cassava4.1_018378m | 7.85/16.85 | 135/2 | 2.090±0.096(-) | 72h/0h | GRP7 | |
| 80 | 60S ribosomal protein L4 | cassava4.1_008812m | 10.77/44.64 | 45/4 | 13.966±0.258(-) | 24h/0h, 48h/0h, 72h/0h, 96h/0h | AT3G09630 | |
| 84 | leucine-rich repeat containing protein | cassava4.1_025603m | 6.57/42.25 | 90/4 | 2.202±0.104(+) | 24h/0h, 48h/0h | AT3G14470 | |
| 96 | zinc-finger of the FCS-type, C2-C2 (zf-FLZ) | cassava4.1_018370m | 9.59/13.05 | 81/5 | ∞(-) | 48h/0h | AT1G78020 | |
| 107 | ATDCL4,DCL4;dicer-like 4 | cassava4.1_017435m | 8.38/20.24 | 225/8 | 36.480±0.567(-) | 48h/0h, 72h/0h, 96h/0h | DCL4 | |
| Photosynthesis related proteins (3) | ||||||||
| 58* | ribulose bisphosphate carboxylase large chain-related | Manes.S113700 | 6.60/51.81 | 267/2 | 3.344±0.130(-)/10.700±0.241(-) | 96h/0h | 96h/0h | RBCL |
| 59* | ribulose bisphosphate carboxylase large chain-related | Manes.S113700 | 6.60/51.81 | 436/3 | 3.304±0.149(-) | 96h/0h | RBCL | |
| 63 * | Carboxyvinyl-carboxyphosphonate phosphorylmutase/CPEP phosphonomutase | cassava4.1_011697m | 8.25/50.32 | 120/5 | 2.014±0.106(-)/2.023±0.111(-) | 96h/0h | 48h/0h | AT2G43180 |
| Inorganic ion transport and metabolism (1) | ||||||||
| 69* | calcium-binding protein CML15-related (CaM) | cassava4.1_034063m | 4.22/17.53 | 78/3 | 2.732±0.077(-)/∞(-) | 96h/0h | 48h/0h, 72h/0h, 96h/0h | AT3G25600 |
| Defense (7) | ||||||||
| 18 | thiamine thiazole synthase | cassava4.1_010620m | 5.30/39.74 | 78/5 | ∞(-) | 24h/0h | THI1 | |
| 67 | zinc finger protein ZAT2-related | cassava4.1_028217m | 6.10/45.39 | 93/3 | ∞(-) | 96h/0h | ZAT11 | |
| 93 | thiamine thiazole synthase | cassava4.1_010620m | 5.30/34.37 | 94/5 | 23.471±0.420(-) | 48h/0h, 72h/0h, 96h/0h | THI1 | |
| 95 | annexin D1-related | cassava4.1_021183m | 6.81/36.20 | 59/2 | 14.957±0.364(-) | 48h/0h, 72h/0h, 96h/0h | ANNAT1 | |
| 97 | ATFER2,FER2;ferritin 2 | cassava4.1_014185m | 5.1/29.47 | 134/5 | ∞(-) | 48h/0h | FER2 | |
| 101 | Cysteine-rich TM module stress tolerance (CYSTM) | cassava4.1_020864m | 9.27/27.56 | 146/5 | 4.182±0.146(-) | 48h/0h, 72h/0h, 96h/0h | AT5G04080 | |
| 109 | RING/U-box superfamily protein | cassava4.1_017114m | 8.17/20.92 | 270/12 | ∞(-) | 48h/0h, 72h/0h, 96h/0h | AT3G10910 | |
| Function unknown proteins (6) | ||||||||
| 34 | RHO GDP-dissociation inhibitor | cassava4.1_015415m | 5.16/27.29 | 82/2 | 2.042±0.076(-) | 72h/0h | SCN1 | |
| 35* | Plant protein of unknown function (DUF247) | cassava4.1_007729m | 7.13/55.95 | 125/2 | 2.839±0.104(-)/8.282±0.259(-) | 72h/0h, 96h/0h | 48h/0h, 72h/0h, 96h/0h | AT3G50120 |
| 44 | unnamed protein | cassava4.1_020024m | 10.33/10.83 | 63/2 | 2.321±0.116(+) | 72h/0h, 96h/0h | ||
| 66 | Uncharacterized conserved protein | cassava4.1_020840m | 9.86/7.84 | 89/2 | 44.827±0.572(+) | 96h/0h | ||
| 68 | Arabidopsis protein of unknown function (DUF241) | cassava4.1_028170m | 8.27/33.01 | 147/3 | 2.009±0.083(-) | 96h/0h | AT2G17080 | |
| 106 | Domain of unknown function (DUF4283) (DUF4283)//Zinc knuckle (zf-CCHC_4) | cassava4.1_025719m | 7.26/13.05 | 146/3 | ∞(-) | 48h/0h, 72h/0h | AT3G31430 | |
b, NCBI accession number.
c, Probability-based MOWSE (molecular weight search) scores.
d, The number of unique peptides identified by MALDI-TOF-MS/MS, and individual ions scores are all identity or extensive homology (p<0.05).
* indicates CP spots between SC9 and QZ1 genotypes. (+) means up-regulated compare with 0h, while (-) means down-regulated compare with 0h.
Fig 3. 2-DE analysis of SC9 genotype at the time-points from 0h to 96h.
Total of proteins (300 μg) were loaded on a 13 cm IPG strip with linear gradient (pH 4−7) and SDS-PAGE was performed on a 12% gel. Proteins were stained with CBB G-250. The wrapped 2-DE maps showed the pairwise comparison at the time-points of 24h/0 h (A), 48 h/0h (B), 72h/0h (C) and 96h/0h (D). The white and black arrows indicated proteins that showed detectable changes (>2.0-fold of the normalized volume) in abundance compared with those observed in the control of 0 h; white indicated a down-regulated match, and black indicated an up-regulated match.
Fig 4. 2-DE analysis of QZ1 genotype at the time-points from 0h to 96h.
Total of proteins (300 μg) were loaded on a 13 cm IPG strip with linear gradient (pH 4−7) and SDS-PAGE was performed on a 12% gel. Proteins were stained with CBB G-250. The wrapped 2-DE maps showed the pairwise comparison at the time-points of 24h/0 h (A), 48 h/0h (B), 72h/0h (C) and 96h/0h (D). The white and black arrows indicated proteins that showed detectable changes (>2.0-fold of the normalized volume) in abundance compared with those observed in the control of 0 h; white indicated a down-regulated match, and black indicated an up-regulated match.
Fig 5. Venn diagrams and functional categories of differential proteins identified in cassava storage roots at the time-points from 0h to 96h.
Unknown proteins included those whose functions had not been described. (A), venn diagrams of 24h/0h, 48h/0h, 72h/0h and 96h/0h in both genotypes (B), functional categories of 64 and 56 differential proteins identified in SC9 and QZ1 genotypes roots.
PPI networks
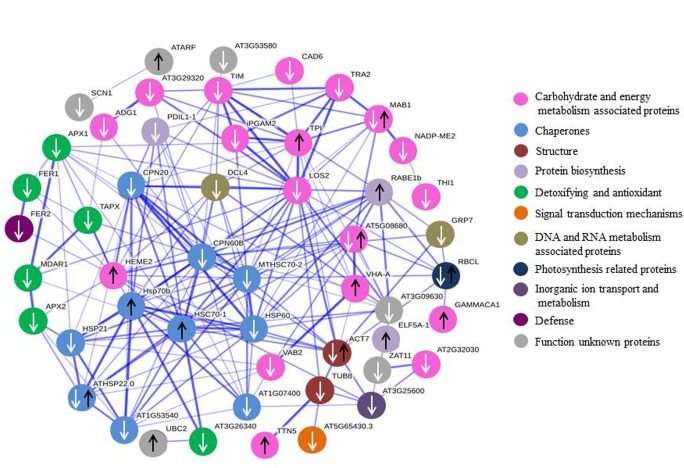
The total proteins identified in each genotype were used to construct a PPI network by employing the STRING interface using the model plant Arabidopsis thaliana database [39]. The PPI network was constructed with 76 nodes and 196 edges (Fig 6), in which the proteins such as CPN60B (spot 124, chaperonin 60 subunit beta 1), LOS2 (spot 77, enolase), HSC70-1 (spot 2, heat shock 70 KDa protein 10) and CPN20B (spot 100, 20 KDa chaperonin) were viewed as hub proteins with a degree of 24, 21, 19 and 18 edges, respectively. In the PPI network, the proteins associated with carbohydrate and energy metabolism constituted the major nodes, followed by chaperones, and detoxifying and antioxidant.
Fig 6. PPI network of differential proteins by string online software according to the database of A. thaliana PPI.
White arrows indicate down-regulated proteins, and black arrows indicated up-regulated proteins. The different colour circle indicates the proteins with different biological functions. The network nodes are proteins, whereas the edges represent the predicted or known functional association.
qRT-PCR analysis of gene expression
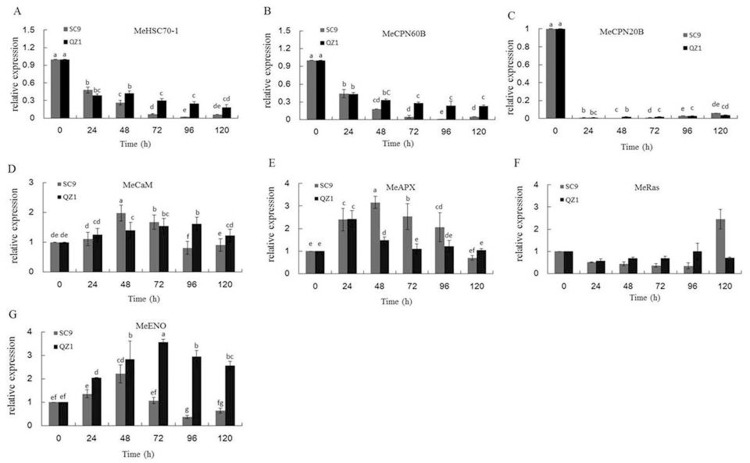
Fig 7 showed the transcription levels of MeHSC70-1, MeCPN60B, MeCPN20B, MeCaM, MeAPX, MeRas and MeENO of injured storage roots in response to PPD using qRT-PCR. MeHSC70-1, MeCPN60B, MeCPN20B expression levels was down-regulated in SC9 and QZ1 genotypes (Fig 7A–7C). MeCaM expression levels in SC9 were higher than that of QZ1 at the time-points of 48h and 72h, and then decreased more than that in QZ1 (Fig 7D). The expression of MeAPX gene was higher than that of QZ1 after the time-point of 48h (Fig 7E). However, from the time-points of 24h to 96h, the expression of MeRas gene in QZ1 was higher than that in SC9 (Fig 7F). The expression level of gene MeENO in QZ1 genotype was higher than in SC9 at all time-points, in addition, the highest expressed level was observed at the time-points of 48h and 72h, respectively (Fig 7G).
Fig 7.
The expressed levels of genes including MeHSC70-1 (A), MeCPN60B (B), MeCPN20B (C), MeCaM (D), MeAPX (E), MeRas (F) and MeENO (G) in cassava injured storage roots using qRT-PCR. Each bar represents the mean of three independent replicates with standard error. Different letters on the columns indicate the statistical difference at p< 0.05 by SPSS to Duncan’s multiple comparison tests.
Discussion
PPD causes cassava storage root to be spoilage, and reduce the shelf-life in the cassava value chain. This phenomenon is a truly global challenge. Many breeders tried to develop solutions that increase the time it takes for cassava to become inedible or unfit processing following post-harvest, such as releasing PPD-tolerant cassava varieties to benefit smallholders and cassava-production manufactures. It was reported that PPD was negatively correlated with dry matter content in the roots [21, 22]. In addition, Chavez et al. (2000) reported 0.5μg/g β-carotene concentration was a threshold for cassava PPD tolerant varieties. When β-carotene concentration in cassava storage roots was higher than 0.5μg/g, the root PPD was always lower than 30% [40]. However, Morante et al (2010) mentioned that cassava MCol 2436 had the lowest levels of carotenoid (9.1 μg/g total carotenoid content) and considerable PPD damage. Perhaps there is a threshold effect and a minimum concentration of carotenoids (>9.1 μg/g) is required for their antioxidant properties to be effective [22]. It was reported that over 90% of the total carotenoids present in sweet yellow cassava were β-carotene [41]. In the present study, β-carotene contents were showed significant differences in SC9 (1.23μg/g) and QZ1 (0.11μg/g), but those data were much lower than 9.1 μg/g. QZ1 had higher PPD tolerance than that of SC9, suggesting β-carotene antioxidant property in SC9 was not effective. The result was consistent with the report from Morante et al (2010).
As previous reports indicated that PPD was associated with ROS production [13, 18, 19, 42]. PPD onset was mostly regulated by the balance between ROS and changes in the activities of antioxidant enzymes [38]. H2O2 is moderately reactive. It has a relatively long half-life and high permeability across membranes [16]. In the present study, H2O2 content increased apparently to the highest value in the injured storage roots of SC9 and QZ1 at the time-point of 48h (Fig 2A). SOD and CAT activities increased to the highest values in PPD-tolerant genotype QZ1 at the time-point of 24h; however, CAT and APX activities in PPD-susceptive genotype SC9 reached the higher values at the time-point of 48h (Fig 2B and 2C). SOD has been reported to work in collaboration with CAT which acted in tandem to remove H2O2 [43, 44]. It seems to show that the high activities of SOD and CAT antioxidant in QZ1 may be used to remove the increased H2O2. It means SOD in combination with CAT activities would be the first line of defense against PPD for the PPD-tolerant cassava variety, and could be used as a signaling to detect PPD phenomenon ahead of H2O2. The first line of defense against PPD in SC9 was weak and resulted in the production of PPD phenomenon at the time-point of 24h (Fig 1). POD was likely to participate with PPD onset because its activity was increased for the injured storage roots in response to PPD, whilst high tolerant cultivars exhibited lower level of POD activity during the post-harvest period [13]. This result was in accord with the data showed in the time-point of 24h of QZ1 in the present study. Xu et al. (2014) reported APX was used as simultaneously activated antioxidant to participate the defense mechanisms via cyclic ROS scavenging [45]. APX increased in the PPD-susceptible cultivars SAN and IAC to storage for 3d to produce PPD phenomenon, however, in the PPD-tolerant BRA cultivar for 5d to find PPD [46], suggesting APX may participate in the construction of the second line of defense in order to maintain the low levels of ROS produced from PPD. The second line of defense against PPD is the presence of endogenous antioxidant chemicals, such as other antioxidant enzymes [46–48].
Functional classification of the identified proteins from 2-DE images showed that the differential proteins in response to PPD were related to chaperones, DNA and RNA metabolism and defense in both genotypes. The identified proteins were also involved in ROS detoxification including APX1 (spots, 16, 24, 64), monodehydroascorbate reductase, cytoplasmic isoform 1-related (spot 112) and NADP-dependent malic enzyme 2-related (spot 127). APX was an important enzyme for detoxification of H2O2 in plants [49, 50]. Its expression was in response to diverse abiotic stress conditions. Overexpressing APX in chloroplasts in plants produced tolerant ability to salinity stress and drought conditions [51, 52]. However, in the present study, qRT-PCR data showed that APX expressions in SC9 were higher than that in QZ1 between the time-points of 48h and 96h (Fig 7D), which were consistent with the 2-DE data (Table 1). It may indicate that PPD-susceptible/tolerant (SC9/QZ1) genotypes all booted up the second line of defense against PPD at the time-point of 24h. APX expression in SC9 increased to a highest value at the time-point of 48h, but QZ1 decreased at the same time-point. These data showed that the first line of defense, consisting of SOD in combination with CAT activities, may play an important role against PPD in the PPD-tolerant genotype QZ1. This defense system could support the PPD tolerance in QZ1. The second line of defense, consisting of APX, and CaM, may work together against PPD in the PPD-susceptible SC9. This result was in accord with Owiti et al. (2011) reported [26].
In the previous reports, Ca2+- CaM may be linked with regulating PPD onset [53]. Followed the storage-root wounding, calcium changes preceded a burst in ROS [54, 55]. The Ca2+- CaM complex bound and activated a collection of target proteins leading to a physiological response [26]. Owiti et al. (2011) showed the expression of CaM played an important role in signal transduction under heat stress, and the expressions of several heat shock proteins (HSPs) were correlated with accumulation of CaM transcripts and proteins in plants [26]. In the present study, CaM expression in QZ1 was up-regulated at the time-points from 24h to 120h, but it was down-regulated in SC9 at the time-points of 96h and 120h. The homology of protein CaM in the present study compared with reported by Owiti reached 71.19% using DANMAN software. HSPs were down-regulated in SC9 genotype (spots, 100, 104, 105, 110, 117). In QZ1 genotype there were two up-regulated HSPs (spots, 1 and 2), four down-regulated HSPs (spots, 47, 52, 53, 70). Of these HSPs in PPI network, heat shock 70 KDa protein (spot 2) was recognized as hub protein (Fig 6). It was reported that the HSPs were involved in response to environmental stress such as heat, cold, drought and salinity stress [56, 57]. In plants, the HSPs have been speculated to act as an antioxidant under oxidative stress [58] or as an important tool for genetic manipulation of protein content in cassava storage root contributing to a natural sink for protein and carotenoid accumulation in intense yellow roots [59]. Further elucidation of the roles of HSPs in binding specifically with PPD would provide our understanding of the molecular machinery controlling PPD onset.
Ras GTPase binding protein is the essential negative regulator of the Ras signaling pathway [60] and induced by salt stress in smooth cordgrass [61]. ADP-ribosylation factor A1F (spot 86), a member of the ARF family of GTP-binding proteins of the Ras superfamily, involved in signal transduction. It is known that transcript accumulation is not always correlated with protein A1F synthesis. The differences between transcript and protein levels may be due to the mechanisms of control gene expression responsible by modulation of genes coding for proteins involving in PPD.
Enolase (ENO, spot 77) was found to have significantly increased oxidation. It is a metalloenzyme involved in the catalysis of 2-phosphoglycerate to phosphoenolpyruvate in the penultimate step of glycolysis [62]. Manaa et al. (2011) [63] reported that the enolase abundance was increased in both wild-type and stress tolerant the roots of tomato under salt stress. Enolase was increased in response to heat stress in rice [64] and flooding stress in soybean roots [65, 66]. These results indicated that enolase was an abiotic stress-responsive protein. In the present study, enolase, used as a hub protein, was highly decreased in abundance in the roots of the PPD-susceptible SC9 genotype during storage stages under injured stress. In addition, the gene MeENO transcriptional level in SC9 genotype was lower than in QZ1 (Fig 7G). These results suggest that balance of glycolysis metabolism might be involved in injured tolerance in cassava during storage stages.
Conclusions
In the present study, QZ1 was resistant to PPD in storage roots compared to SC9, mainly due to the following two defense lines. SOD in combination with CAT activities would be the first line of defense against PPD for the PPD-tolerant cassava variety, The second line of defense, consisting of APX and CaM, may work together against PPD in the PPD-susceptible SC9. The 108 differential protein spots on the 2-DE gel image were detected in the injured storage roots stored at room temperature for 120h. Of these, 99 differential proteins were identified by MALDI-TOF-MS/MS. These identified proteins were involved in 13 biological functions. All differential proteins were used to generate the PPI network and 4 hub proteins including CPN60B, LOS2, HSC70-1 and CPN20B were speculated to be the candidate key proteins associated with PPD, which will provide insights into the improvement of cassava PPD-tolerant varieties and further analyze the PPD-tolerant mechanisms of cassava storage roots.
Supporting information
(DOCX)
(XLSX)
(XLSX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by NSFC-CGIAR International (Regional) Cooperation and Exchange Programs (31361140366), National Science and Technology Infrastructure Program (2015BAD15B01), National Natural Science Foundation of China (31271776), National Nonprofit Institute Research Grant of CATAS-TCGRI (1630032014051), and the Initial Fund of High-level Creative Talents in Hainan Province (2012-Chen Songbi). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu J, Zheng Q, Ma Q, Gadidasu KK, Zhang P (2011) Cassava genetic transformation and its application in breeding. J Integr Plant Biol 53(7): 552–569. 10.1111/j.1744-7909.2011.01048.x [DOI] [PubMed] [Google Scholar]
- 2.Sayre R, Beeching JR, Cahoon EB, Egesi C, Fauquet C, Fellman J, et al. (2011) The BioCassava plus program: biofortification of cassava for sub-Saharan Africa. Annu Rev Plant Biol 62: 251–272. 10.1146/annurev-arplant-042110-103751 [DOI] [PubMed] [Google Scholar]
- 3.Sánchez T, Dufour D, Moreno JL, Pizarro M, Aragón IJ, Domínguez M, et al. (2013) Changes in extended shelf life of cassava roots during storage in ambient conditions. Postharvest Biol Technol 86: 520–528. [Google Scholar]
- 4.Bayoumi SA, Rowan MG, Beeching JR, Blagbrough IS (2010) Constituents and secondary metabolite natural products in fresh and deteriorated cassava roots. Phytochem 71(5–6): 598–604. [DOI] [PubMed] [Google Scholar]
- 5.Blagbrough IS, Bayoumi SA, Rowan MG, Beeching JR (2010) Cassava: an appraisal of its phytochemistry and its biotechnological prospects. Phytochem 71(17–18): 1940–1951. [DOI] [PubMed] [Google Scholar]
- 6.Gnonlonfin GJ, Sanni A, Brimer L (2012) Review scopoletin—a coumarin phytoalexin with medicinal properties. Crit Rev Plant Sci 31(1): 47–56. [Google Scholar]
- 7.van Oirschot QE, O’ Brien GM, Dufour D, EI-Sharkawy MA, Mesa E (2000) The effect of pre-harvest pruning of cassava upon root deterioration and quality characteristics. J Sci Food Agric 80: 1866–1873. [Google Scholar]
- 8.Buschmann H, Reilly K, Rodriguez MX, Tohme J, Beeching JR (2000) Hydrogen peroxide and flavan-3-ols in storage roots of cassava (Manihot esculenta Crantz) during postharvest deterioration. J Agric Food Chem 48(11): 5522–5529. [DOI] [PubMed] [Google Scholar]
- 9.Han Y, Gómez-Vásquez R, Reilly K, Li H, Tohme RM, Cooper RM, et al. (2001) Hydroxyproline-rich glycoproteins expressed during stress responses in cassava. Euphytica 120: 59–70. [Google Scholar]
- 10.Huang J, Bachem C, Jacobsen E, Visser RG (2001) Molecular analysis of differentially expressed genes during postharvest deterioration in cassava (Manihot esculenta Crantz) tuberous roots. Euphytica 120: 85–93. [Google Scholar]
- 11.Reilly K, Han Y, Tohme J, Beeching JR (2001) Isolation and characterisation of a cassava catalase expressed during post-harvest physiological deterioration. Biochimi Biophys Acta 1518(3): 317–323. [DOI] [PubMed] [Google Scholar]
- 12.Reilly K, Gómez-Vásquez R, Buschmann H, Tohme J, Beeching JR (2003) Oxidative stress responses during cassava post-harvest physiological deterioration. Plant Mol Biol 53: 669–685. [DOI] [PubMed] [Google Scholar]
- 13.Reilly K, Gómez-Vásquez R, Buschmann H, Tohme J, Beeching JR (2004) Oxidative stress responses during cassava post-harvest physiological deterioration. Plant Mol Biol 56(4): 625–641. [DOI] [PubMed] [Google Scholar]
- 14.Reilly K, Bernal D, Cortés DF, Gómez-Vásquez R, Tohme J, Beeching JR (2007) Towards identifying the full set of genes expressed during cassava post-harvest physiological deterioration. Plant Mol Biol 64(1–2): 187–203. 10.1007/s11103-007-9144-0 [DOI] [PubMed] [Google Scholar]
- 15.Sakurai T, Plata G, Rodríguez-Zapata F, Seki M, Salcedo A, Toyoda A, et al. (2007) Sequencing analysis of 20,000 full-length cDNA clones from cassava reveals lineage specific expansions in gene families related to stress response. BMC Plant Biol 7: 66 10.1186/1471-2229-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uarrota VG, Moresco R, Coelho B, Nunes Eda C, Peruch LA, Neubert Ede O, et al. (2014) Metabolomics combined with chemometric tools (PCA, HCA, PLS-DA and SVM) for screening cassava (Manihot esculenta Crantz) roots during postharvest physiological deterioration. Food Chem 161: 67–78. 10.1016/j.foodchem.2014.03.110 [DOI] [PubMed] [Google Scholar]
- 17.Iyer S, Mattinson DS, Fellman JK (2010) Study of the early events leading to cassava root postharvest deterioration. Tropical Plant Biol 3: 151–165. [Google Scholar]
- 18.Zidenga T, Leyva-Guerrero E, Moon H, Siritunga D, Sayre R (2012) Extending cassava root shelf life via reduction of reactive oxygen species production. Plant Physiol 159(4): 1396–1407. 10.1104/pp.112.200345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Duan X, Yang J, Beeching JR, Zhang P (2013) Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol 161(3): 1517–1528. 10.1104/pp.112.212803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudi N, Norton G, Alwang J, Asumugha G (2010) Economic impact analysis of marker-assisted breeding for resistance to pests and post-harvest deterioration in cassava. Af J Agr Resour Econ 4(2): 110–122. [Google Scholar]
- 21.Sánchez T, Chávez AL, Ceballos H, Rodriguez-Amaya DB, Nestel P, Ishitani M (2006) Reduction or delay of post-harvest physiological deterioration in cassava roots with higher carotenoid content. J Sci Food Agric 86: 634–639. [Google Scholar]
- 22.Morante N, Sánchez T, Ceballos H, Calle F, Pérez JC, Egesi C, et al. (2010) Tolerance to postharvest physiological deterioration in cassava roots. Crop Sci 50: 1333–1338. [Google Scholar]
- 23.Prochnik S, Marri PR, Desany B, Rabinowicz PD, Kodira C, Mohiuddin M, et al. (2012) The cassava genome: Current progress, future directions. Trop Plant Biol 5(1): 88–94. 10.1007/s12042-011-9088-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Feng B, Xiao J, Xia Z, Zhou X, Li P, et al. (2014) Cassava genome from a wild ancestor to cultivated varieties. Nat Commun 5: 5110 10.1038/ncomms6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bredeson JV, Lyons JB, Prochnik SE, Wu GA, Ha CM, Edsinger-Gonzales E, et al. (2016) Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat Biotechnol 34(5): 562–570. 10.1038/nbt.3535 [DOI] [PubMed] [Google Scholar]
- 26.Owiti J, Grossmann J, Gehrig P, Dessimoz C, Laloi C, Hansen MB, et al. (2011) iTRAQ-based analysis of changes in the cassava root proteome reveals pathways associated with post-harvest physiological deterioration. Plant J 67(1): 145–156. 10.1111/j.1365-313X.2011.04582.x [DOI] [PubMed] [Google Scholar]
- 27.Vanderschuren H, Nyaboga E, Poon JS, Baerenfaller K, Grossmann J, Hirsch-Hohhmann M, et al. (2014) Large-scale proteomics of the cassava storage root and identification of a target gene to reduce postharvest deterioration. Plant Cell 26(5): 1913–1924. 10.1105/tpc.114.123927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansari MW, Tuteja N (2015) Post-harvest quality risks by stress/ethylene: management to mitigate. Protoplasma 252(1): 21–32. 10.1007/s00709-014-0678-0 [DOI] [PubMed] [Google Scholar]
- 29.Gu B, Yao Q, Li K, Chen S (2013) Change in physicochemical traits of cassava roots and starches associated with genotypes and environmental factors. Starch/Stärke 65: 253–263. [Google Scholar]
- 30.Yang L, Zhou K, An F, Li K, Chen S (2015). Optimization of HPLC determination method for β-carotene in cassava tuberous roots. Chin J Trop Agri 35(12): 67–72. [Google Scholar]
- 31.Hu W, Kong H, Guo Y, Zhang Y, Ding Z, Tie W, et al. (2016) Comparative physiological and transcriptomic analyses reveal the actions of melatonin in the delay of postharvest physiological deterioration of cassava. Front Plant Sci 7: 736 10.3389/fpls.2016.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Gollop N, Heuer B (2009) Proteomic analysis of salt-stressed tomato (Solanum lycopersicum) seedlings: effect of genotype and exogenous application of glycinebetaine. J Exp Bot 60(7): 2005–2019. 10.1093/jxb/erp075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An F, Fan J, Li J, Li QX, Li K, Zhu W, et al. (2014) Comparison of leaf proteomes of cassava (Manihot esculenta Crantz) cultivar NZ199 diploid and autotetraploid genotypes. Plos one 9(4): e85991 10.1371/journal.pone.0085991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An F, Chen T, Stéphanie DM, Li K, Li QX, Carvalho LJ, et al. (2016) Domestication syndrome is investigated by proteomic analysis between cultivated cassava (Manihot esculenta Crantz) and its wild relatives. Plos One 11(3): e0152154 10.1371/journal.pone.0152154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 36.An D, Yang J, Zhang P (2012) Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genomics 13: 64 10.1186/1471-2164-13-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beeching JR, Han YH, Gómez-Vásquez R, Day RC, Cooper RM (1998) Wound and defense responses in cassava as related to post-harvest physiological deterioration. Recent Adv Phytochem 32: 231–248. [Google Scholar]
- 38.Ma Q, Zhang T, Zhang P, Wang ZY (2016) Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J Pineal Res 60(4): 424–434. 10.1111/jpi.12325 [DOI] [PubMed] [Google Scholar]
- 39.Szklarczyk D, Jensen LJ (2015) Protein-protein interaction databases. Methods Mol Biol 1278: 39–56. 10.1007/978-1-4939-2425-7_3 [DOI] [PubMed] [Google Scholar]
- 40.Chavez AL, Bedoya JM, Sánchez T, Iglesias C, Ceballos H, Roca W (2000) Iron, carotene, and ascorbic acid in cassava roots and leaves. Food Nutr Bull 21(4): 410–413. [Google Scholar]
- 41.Carvalho LM, Oliveira AR, Godoy RL, Pacheco S, Nutti MR, de Carvalho JL, et al. (2012) Retention of total carotenoid and β-carotene in yellow sweet cassava (Manihot esculenta Crantz) after domestic cooking. Food Nutr Res 56: 15788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uarrota VG, Maraschin M (2015) Metabolomic, enzymatic, and histochemical analyzes of cassava roots during postharvest physiological deterioration. BMC Res Notes 8: 648 10.1186/s13104-015-1580-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isamah GK, Asagba SO, Ekakitie AO (2003) Lipid peroxidation, activities of superoxide dismutase and catalase during post-harvest deterioration of cassava (Manihot esculenta Crantz) root tubers. Int Biodeter Biodegr 52: 129–133. [Google Scholar]
- 45.Xu J, Yang J, Duan X, Jiang Y, Zhang P (2014) Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol 14: 208 10.1186/s12870-014-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uarrota VG, Moresco R, Schmidt EC, Bouzon ZL, Nunes Eda C, Neubert Ede O, et al. (2016) The role of ascorbate peroxidase, guaiacol peroxidase, and polysaccharides in cassava (Manihot esculenta Crantz) roots under postharvest physiological deterioration. Food Chem 197(Pt A): 737–746. 10.1016/j.foodchem.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 47.Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- 48.van Doorn WG, Ketsa S (2014) Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharvest Biol Tech 95: 64–69. [Google Scholar]
- 49.Rasool S, Ahmad A, Siddiqi TO, Ahmad P (2013) Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physio Plant 35: 1039–1050. [Google Scholar]
- 50.Ahmad P, Ashraf M, Hakeem KR, Azooz MM, Rasool S, Chandna R, et al. (2014) Potassium starvation-induced oxidative stress and antioxidant defense responses in Brassica juncea. J Plant Interact 9(1): 1–9. [Google Scholar]
- 51.Zare S, Paknivat H (2012) Changes in activities of antioxidant enzymes in oilseed rape in response to salinity stress. Intl J Agri Crop Sci 4(7): 398–403. [Google Scholar]
- 52.Liang W, Wang L, Shi J, Lei X, Yang J, Wu S, et al. (2014) Differential expression of antioxidant proteins in the drought-tolerant cyanobacterium Nostoc flagelliforme under desiccation. Plant Omics J 7(4): 205–212. [Google Scholar]
- 53.Jian C. Storage ability and proteomic analysis of post-harvest tuberous roots of cassava. Thesis, Hainan University. 2013.
- 54.Beneloujaephajri E, Costa A, L’Haridon F, Métraux JP, Binda M (2013) Production of reactive oxygen species and wound-induced resistance in Arabidopsis thaliana against Botrytis cinerea are preceded and depend on a burst of calcium. BMC Plant Biol 13: 160 10.1186/1471-2229-13-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D (2011) Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J 68: 100–113. 10.1111/j.1365-313X.2011.04671.x [DOI] [PubMed] [Google Scholar]
- 56.Sun W, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577: 1–9. [DOI] [PubMed] [Google Scholar]
- 57.Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in abiotic stress response. Trends Plant Sci 9: 1360–1385. [DOI] [PubMed] [Google Scholar]
- 58.Levine RL, Mosoni L, Berlett BS, Stadtman ER (1996) Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci USA 93: 15036–15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carvalho LJCB, Lippolis J, Chen S, de Souza CRB, Vieira EA, Anderson JV (2012) Characterization of carotenoid-protein complexes and gene expression analysis associated with carotenoid sequestration in pigmented cassava (Manihot esculenta Crantz) Storage Root. Open Biochem J 2012, 6: 116–130. 10.2174/1874091X01206010116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pamonsinlapatham P, Hadj-Slimane R, Lepelletier Y, Allain B, Toccafondi M, Garbay C, et al. (2009) P120-Ras GTPase activating protein (RasGAP): a multi-interacting protein in downstream signaling. Biochimie 91(3): 320–328. 10.1016/j.biochi.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 61.Baisakh N, Subudhi P, Varadwaj P (2008) Primary responses to salt stress in a halophyte, smooth cordgrass (Spartina alterniflora Loisel.). Funct Integr Genomics 8(3): 287–300. 10.1007/s10142-008-0075-x [DOI] [PubMed] [Google Scholar]
- 62.Boone CH, Grove RA, Adamcova D, Braga CP, Adamec J (2016) Revealing oxidative damage to enzymes of carbohydrate metabolism in yeast: An integration of 2D DIGE, quantitative proteomics, and bioinformatics. Proteomics 16(13): 1889–1903. 10.1002/pmic.201500546 [DOI] [PubMed] [Google Scholar]
- 63.Manaa A, Ben Ahmed H, Valot B, Bouchet JP, Aschi-Smiti S, Causse M, et al. (2011) Salt and genotype impact on plant physiology and root proteome variations in tomato. J Exp Bot 62(8): 2797–2813. 10.1093/jxb/erq460 [DOI] [PubMed] [Google Scholar]
- 64.Timabud T, Yin X, Pongdontri P, Komatsu S (2016) Gel-free/label-free proteomic analysis of developing rice grains under heat stress. J Proteomics 133: 1–19. 10.1016/j.jprot.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 65.Nanjo Y, Maruyama K, Yasue H, Yamaguchi-Shinozaki K, Shinozaki K, Komatsu S (2011) Transcriptional responses to flooding stress in roots including hypocotyl of soybean seedlings. Plant Mol Biol 77(1–2): 129–144. 10.1007/s11103-011-9799-4 [DOI] [PubMed] [Google Scholar]
- 66.Yin X, Nishimura M, Hajika M, Komatsu S (2016) Quantitative proteomics reveals the flooding-tolerance mechanism in mutant and abscisic acid-treated soybean. J Proteome Res 15(6): 2008–2025. 10.1021/acs.jproteome.6b00196 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
(XLSX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.