Abstract
Peptide transporter 2 (PEPT2) is a high-affinity low-capacity transporter belonging to the proton-coupled oligopeptide transporter family. Although many aspects of PEPT2 structure-function are known, including its localization in choroid plexus and neurons, its regional activity in brain, especially extracellular fluid (ECF), is uncertain. In this study, the pharmacokinetics and regional brain distribution of cefadroxil, a β-lactam antibiotic and PEPT2 substrate, were investigated in wildtype and Pept2 null mice using in vivo intracerebral microdialysis. Cefadroxil was infused intravenously over 4 hours at 0.15 mg/min/kg, and samples obtained from plasma, brain ECF, cerebrospinal fluid (CSF) and brain tissue. A permeability-surface area experiment was also performed in which 0.15 mg/min/kg cefadroxil was infused intravenously for 10 min, and samples obtained from plasma and brain tissues. Our results showed that PEPT2 ablation significantly increased the brain ECF and CSF levels of cefadroxil (2- to 2.5-fold). In contrast, there were no significant differences between wildtype and Pept2 null mice in the amount of cefadroxil in brain cells. The unbound volume of distribution of cefadroxil in brain was 60% lower in Pept2 null mice indicating an uptake function for PEPT2 in brain cells. Finally, PEPT2 did not affect the influx clearance of cefadroxil, thereby, ruling out differences between the two genotypes in drug entry across the blood-brain barriers. These findings demonstrate, for the first time, the impact of PEPT2 on brain ECF as well as the known role of PEPT2 in removing peptide-like drugs, such as cefadroxil, from the CSF to blood.
Keywords: microdialysis, peptide transporter 2, blood-cerebrospinal fluid barrier, cefadroxil, brain extracellular fluid
Graphical Abstract
1. Introduction
Proton-coupled oligopeptide transporters (POTs) move di-/tripeptides and peptidomimetics across biologic membranes down an electrochemical membrane gradient, thereby playing an important role in the absorption, distribution and elimination of substrates in the body [1]. Among the four mammalian POTs, peptide transporter 2 (PEPT2, also known as SLC15A2) is a high-affinity and low-capacity transporter. It is widely expressed in brain, kidney, lung, eye and mammary gland [2,3]. The functional activity of PEPT2 has been studied using a variety of substrates including the synthetic dipeptide glycylsarcosine (GlySar) [4–6], endogenous peptidomimetics (e.g., 5-aminolevulinic acid) [7,8], neuropeptides (e.g., carnosine and kyotorphin) [9–11], as well as peptide-like drugs (e.g., cefadroxil) [12,13]. In kidney, PEPT2 is expressed at the apical membrane of proximal tubule epithelial cells where it plays an important role in the reabsorption of substrates from urine, thereby limiting renal clearance [14].
PEPT2 is also expressed at the apical membrane of choroid plexus epithelial cells (CSF-facing), the site of the blood-cerebrospinal fluid barrier (BCSFB), where it facilitates substrate efflux from CSF to blood, thus reducing substrate distribution in CSF [15]. According to an immunolocalization study in rat brain, PEPT2 is distributed in brain parenchyma, particularly at the plasma membrane of neural cells (neonates and adults) and astrocytes (neonates only) [16]. As an uptake transporter in brain cells, PEPT2 plays a role in the homeostasis of neuropeptides and the distribution of peptide-like therapeutics within brain parenchyma. Furthermore, our previous studies in wildtype and Pept2 null mice indicate that PEPT2 has a profound influence on the neurological effects of its substrates in the central nervous system (CNS). For instance, PEPT2 reduces the neurotoxicity of 5-aminolevulinic acid [7] and the anti-nociceptive effect of kyotorphin [10]. However, in these two studies, the effect of PEPT2 in ECF is inferred since our study design did not allow direct measurement of this biological fluid.
In addition to small peptides, PEPT2 is able to transport peptide-like drugs that have similar structures to the backbones of di- or tripeptides (e.g., cephalosporins, angiotensin-converting enzyme inhibitors) as well as antiviral nucleoside prodrugs [17]. Among such drugs, cefadroxil is a first-generation cephalosporin with a broad spectrum antibacterial activity, high PEPT2 affinity, and favorable biological stability [18,19]. Thus, cefadroxil serves as a good model compound to study the role and relevance of PEPT2 in the disposition of peptide-like drugs. Comparing wildtype and Pept2 null mice, Shen et al. [12] found that PEPT2 was almost entirely responsible for the renal reabsorption of cefadroxil. Moreover, the CSF/blood concentration ratio was higher (6- to 7-fold) in the Pept2 null mice, indicating the CSF-to-blood efflux function of PEPT2 at the BCSFB.
Intracerebral microdialysis is the only method in vivo that allows for the direct measurement of drug concentrations in ECF [20,21]. Microdialysis also has the advantage of enabling repeated sampling without fluid loss on freely moving animals. In our previous microdialysis study in rats [22], an attempt was made to investigate the role of PEPT2 on cefadroxil disposition in ECF and CSF by functional ablation of PEPT2 via competitive inhibition of cefadroxil transport using intraventricular infusion of the dipeptide Ala-Ala. Unfortunately, the results were negative, probably because of the biological instability of Ala-Ala in vivo [23]. Compared to inhibition studies, Pept2 null mice may be a better tool to specifically study the effects of PEPT2 on cefadroxil brain distribution.
We hypothesize that PEPT2 ablation will impact the disposition of cefadroxil in the brain extracellular fluid (ECF), the site of action of many neuroactive agents. With this in mind, the primary objective of this study was to determine, using in vivo intracerebral microdialysis, the pharmacokinetics and regional brain distribution of cefadroxil in wildtype and Pept2 null mice following a 4-hr intravenous infusion. The secondary objective was to calculate the permeability-surface area product of cefadroxil in mice, as a measure of the impact of PEPT2 on drug transport from plasma to brain (i.e., influx clearance), following a 10-min intravenous infusion.
2. Material and methods
2.1. Chemicals
Cefadroxil and cefadroxil-D4 were purchased from Sigma-Aldrich (St. Louis, MO). [3H]Cefadroxil (0.57 Ci/mmol; 97.6% purity) was obtained from Moravek Inc (Brea, CA) and [14C]dextran 70,000 (1.4 mCi/g) was obtained from American Radiolabeled Chemicals (ARC, St. Louis, MD). Methanol and acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals and solvents were of analytical grade or better. Ultrapure water was obtained using the Milli-Q Reference Water Purification System (Millipore, Billerica, MA). Perfusion fluid for microdialysis consisted of Ringer’s solution, which contained 145 mM NaCl, 0.6 mM KCl, 1.0 mM MgCl2, and 1.2 mM CaCl2 in 2 mM phosphate buffer, pH 7.4.
2.2. Animals
Pept2 null mice (Pept2−/−) with >99% C57BL/6 genetic background were developed previously in our laboratory [24]. Male wildtype (Pept2+/+) and Pept2 null mice (12–16 weeks) were bred in-house and maintained in a temperature- and humidity-controlled environment with 12-hour light/dark cycles and unlimited access to food and water (Unit for Laboratory Animal Medicine, University of Michigan, Ann Arbor, MI). All procedures in this study were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health and were approved by the University of Michigan Committee on Use and Care of Animals.
2.3. Animal surgery
Mice were placed on a heating pad to maintain body temperature and anesthetized by 2% isoflurane inhalation together with 0.5 L/min oxygen. Once fully anesthetized, a 3-fr polyurethane cannula (fused with a 2-fr polyurethane tip) was inserted into the right jugular vein for cefadroxil infusion and a 2-fr polyurethane cannula (fused with a 1-fr polyurethane tip) was inserted into the left carotid artery for blood sampling. To avoid clotting, a locking solution of 500 IU/mL heparin and 50% glycerol was used to fill the arterial catheter. The two vessel catheters were passed subcutaneously to the upper back of mice and then fixed to a silicone cup sutured to the skin.
Following catheter insertion, the mouse was placed on a stereotaxic frame equipped with an anesthesia mask (Stoelting, Wood Dale, IL). A guide cannula was implanted into the right brain striatum (coordinates: 0.6 mm anteroposterior, −1.8 mm lateral and −2.0 mm dorsoventral) and then fixed to the skull with two anchor screws and dental cement. A pre-emptive dose of buprenorphine (0.08 mg/kg) was administered subcutaneously and additional doses were given every eight hours for one day after surgery. The mouse was allowed to recover for 5–6 days prior to experimentation. One day before microdialysis, the mouse was moved to an infusion cage (Harvard Apparatus, MA, US) where it could move freely, and have access to food and water. At the same time, the dummy in the guide cannula was replaced by a CMA7 microdialysis probe containing a 2-mm cuprophane membrane and 6000 Dalton cut-off (CMA, Stockholm, Sweden).
2.4. Microdialysis study design
Microdialysis was initiated 1.5 hour prior to drug infusion (i.e., stabilization period) and maintained during the infusion of cefadroxil. During this time, Ringer’s solution was perfused through the microdialysis probe at 0.5 μL/min using a CMA 402 pump (Stockholm, Sweden). Cefadroxil (6 mg/mL in saline) was then infused intravenously at a constant rate of 0.15 mg/min/kg for 4 hours using a Harvard Apparatus 22 pump (Holliston, MA, US). During the infusion, microdialysis samples were collected every 20 min using a CMA 142 microfraction collector (Stockholm, Sweden) and kept on ice at 4°C. Blood samples (≈ 0 μL each) were harvested from the carotid artery at 5, 10, 20, 40, 60, 80, 100, 120, 180, and 240 min after initiating the intravenous drug infusion. Plasma was obtained by centrifuging the blood at 10,600 g for 5 min. Before terminating the cefadroxil infusion, a combination of ketamine (120 mg/kg) and xylazine (10 mg/kg) was administered intraperitoneally to anesthetize the mouse so that CSF could be collected quickly from the cisterna magna. The mouse was then decapitated and the brain divided into left and right cortex (including hippocampus), left and right basal ganglia (including striatum), and cerebellum. Samples were weighed and stored at −80°C until analysis (as were the microdialysis and plasma samples). If blood was observed in CSF or a hemorrhage was found in brain tissue, then those samples were discarded.
The CMA7 microdialysis probes were calibrated by determining the relative recovery of [3H]cefadroxil in three mice using in vivo retrodialysis. Briefly, three microdialysis samples were collected from each mouse following the 1.5-hr stabilization period, after which their radioactivities were measured in a dual-channel liquid scintillation counter (Beckman Coulter LS 6000SC, Fullerton, CA, USA).
2.5. Permeability-surface area product study design
This study was performed in order to determine whether or not PEPT2 affected the transport of cefadroxil from plasma to brain. The permeability-surface area (PS) product was measured by giving a 10-min intravenous infusion of cefadroxil to wildtype and Pept2 null mice, during which time it was assumed that there was no efflux of cefadroxil from the brain. Briefly, following 2% isoflurane anesthesia, catheters were implanted in the right jugular vein and left carotid artery for drug administration and blood sampling, respectively. Immediately after surgery, and under isoflurane maintenance, cefadroxil (0.6 mg/mL in saline) was infused at a dose of 0.15 mg/min/kg for 10 min. Blood samples (≈ 10 μL each) were obtained at 1, 2.5, 5, 7.5 and 10 min after initiating the cefadroxil infusion, and then centrifuged to collect the plasma. Upon termination of the infusion, mice were decapitated and select brain tissues of five regions were harvested and weighed, as described as in the microdialysis study. All samples were stored at −80°C until analysis.
2.6. Vascular Space Measurement
The amount of cefadroxil in brain was corrected for drug in the cerebrovascular space by measuring vascular volume in different regions of the brain. Under sodium pentobarbital anesthesia (50 mg/kg, intraperitoneal), catheters were implanted in the right jugular vein and left carotid artery of mice. The animals then received an intravenous infusion of 0.15 mg/min/kg [3H]cefadroxil for 10 min, with [14C]dextran 70,000 (0.8 μCi/mouse), a vascular marker, being administered via the arterial catheter 2 min prior to terminating the drug infusion. Mice were decapitated at the end of infusion and the blood instantly collected from the neck for drug measurements in the blood and plasma. Brain tissues were collected, as described previously in the microdialysis study. Samples were weighed and solubilized with 0.33 mL of 1 M hyamine hydroxide for two days at 37°C. A 30-μL aliquot of 30% hydrogen peroxide was then added to bleach the sample, which was followed by the addition of 6 mL Cytoscint liquid scintillation cocktail (MP Biomedicals, Solon, OH, US) and vortex mixing. Radioactivity was measured in each sample using a dual-channel liquid scintillation counter (Beckman Coulter LS 6000SC).
2.7. Liquid chromatography-tandem mass spectrometry assay
Quantification of cefadroxil samples from the microdialysis and permeability-surface area studies was performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Specifically, after diluting 8 μL of microdialysis sample with 20 μL of methanol (which contained deuterated cefadroxil, cefadroxil-D4, as an internal standard (IS)), a 5-μL aliquot was injected into the LC-MS/MS. CSF samples (1–2 μL) were diluted 5- to 10-fold in Ringer’s solution, and then treated and analyzed exactly the same way as described for the microdialysis samples. For plasma, 5-μL samples were diluted 40-fold in methanol (containing IS) in order to precipitate the proteins. After shaking for 5 min and centrifuging at 17,000 g for 10 min, a 5-μL aliquot of the resultant supernatant was injected into the LC-MS/MS. To obtain brain homogenate, water was added to each brain sample (1:4 ratio, W/V), which was ground using a plastic pestle and then further homogenized finely with a sonicator (QSONICA, Newtown, CT). For brain samples from the microdialysis study, protein was precipitated from 20 μL of homogenate by adding methanol (containing IS) at a ratio of 1:5, followed by 5 min of shaking and 10 min of centrifuging at 17,000 g. A 5-μL of the resultant supernatant was then injected into the LC-MS/MS. Brain samples from the permeability-surface area study had lower levels of cefadroxil and, as a result, protein from 100 μL of homogenate was precipitated by 500 μL of acetonitrile (containing IS), and the supernatant dried using a SpeedVac Concentrator (Thermo Scientific, Waltham, MA) at room temperature for 2 hours. The sample was reconstituted in 100 μL of methanol to concentrate the drug 5-fold, and a 5-μL aliquot was then injected into the LC-MS/MS. For each biological matrix, standard curves were generated (i.e., 0.5–200 ng/mL for dialysate and CSF; 0.1–15.0 μg/mL for plasma; 25–1000 ng/g brain for microdialysis study; 5–100 ng/g brain for PS study) and quality control samples (at low, medium and high concentrations) analyzed along with the samples. The coefficient of determination (r2) was ≥ 0.999 for all standard curves.
The LC-MS/MS system consisted of a Prominence HPLC system (Shimadzu Corporation, Kyoto, Japan) and a triple quadrupole mass spectrometer (API 4000 QTRAP®, AB SCIEX, Concord, ON, Canada). Sample analytes were separated using an Atlantis C18 column (2.1 mm×150 mm, particle size 5 μm; Waters, Milford, MA) and gradient elution delivered by two Shimadzu pumps for 10 min at 0.2 mL/min. The linear gradient consisted of mobile phases A (0.02% formic acid) and B (100% methanol), where mobile phases flowed at 0.5% B from 0–0.5 min, at 5–40% B from 0.5–3 min, at 40–95% B from 3–4 min, at 95% B from 4–6 min, at 95–5% B from 6–7 min, and at 5% B from 7–10 min. Under these conditions, cefadroxil and cefadroxil-D4 had retention times of 6.7 min. The mass spectrometer was set in positive electrospray mode using a Turbo V™ ion source. The transitions were monitored in a Multiple Reaction Monitoring (MRM) mode with m/z 363.9→208.1 for cefadroxil and m/z 368.0→212.0 for cefadroxil-D4. Analysts 1.0 (AB SCIEX, Concord, ON, Canada) was used to acquire and process all LC-MS/MS data.
2.8. Data Analysis
Cefadroxil clearance was calculated at steady-state using the plasma concentration obtained at the end of the 4-hour infusion (Cp,240) in which:
| #(1) |
Relative recovery of cefadroxil from the CMA7 microdialysis probes was estimated during in vivo retrodialysis of [3H]cefadroxil and calculated as:
| #(2) |
where DPMin is the radioactivity of perfusate and DPMout is the radioactivity of dialysate, measured in disintegrations per minute (DPM). Accordingly, the unbound concentration of cefadroxil in brain ECF (Cu,ECF) was determined during the 20-min microdialysis collection period using the midpoint time of drug in dialysate (Cout) in which:
| #(3) |
To obtain the ratio of Cu,ECF to Cp, plasma concentrations of cefadroxil were estimated at the midpoint time (Cp,mid) using drug levels at two adjacent time points (Cp,1 and Cp,2) such that:
| #(4) |
The radiolabeled compounds, [14C]dextran and [3H]cefadroxil, were used to obtain the vascular volume (Vbl in mL/g brain) for each brain region and the concentration ratio of cefadroxil in blood to that in plasma (Rbl-p). The vascular volume-corrected amount of cefadroxil in brain (Abrain in ng/g brain) was calculated as:
| #(5) |
where Ameasured is the amount of drug actually measured in brain samples.
The amount of cefadroxil in brain cells (Acell) was calculated by subtracting the drug content in brain ECF from Abrain in which:
| #(6) |
where VECF is the volume of extracellular space in brain (0.18 mL/g brain) [25] and Cu,ECF,220-240 is the unbound concentration of cefadroxil in brain ECF, as determined over the 220–240 min time interval.
A useful measure of drug distribution in brain parenchyma is the unbound volume of distribution in brain (Vu,brain in mL/g brain), which was calculated as:
| #(7) |
The PS product, a measure of drug transport from plasma to brain (i.e., influx clearance), was calculated as:
| #(8) |
where Abrain is the amount of cefadroxil in each brain region at the 10-min infusion time and AUC0-10 is the area under the plasma concentration-time curve of cefadroxil, from 0–10 min, calculated using the trapezoidal method.
2.9. Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). A two-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons was performed to examine the influence of two factors on a variable (i.e., time and genotype or brain region and genotype). Student’s t-test was used to compare a variable between wildtype and PEPT2 null groups if they had equal variance, whereas Welch’s t-test was used for unequal variance. Paired t-tests were used to compare two matched variables in the same animals (e.g., Cu,ECF and Ccsf). A value of p ≤ 0.05 was considered statistically significant. All statistical analyses were carried out using GraphPad Prism v7.0 (GraphPad Software Inc., San Diego, CA).
3. Results
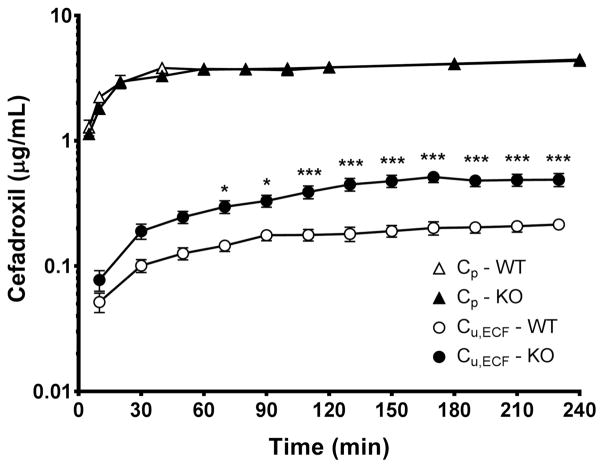
Steady-state plasma concentrations of cefadroxil were reached within one hour of the 4-hr intravenous infusion of drug at 0.15 mg/min/kg (Fig. 1). As shown, there was no significant difference in plasma concentrations of cefadroxil between wildtype and Pept2 null mice at any of the times sampled and clearance was virtually the same (34.8 ± 1.2 vs. 36.5 ± 3.4 mL/min/kg, respectively). At the last time point, the plasma concentration of cefadroxil was 4.35±0.14 μg/mL in wildtype mice and 4.45±0.35 μg/mL in Pept2 null animals (p=0.795). Compared to plasma, the unbound concentrations of cefadroxil in brain ECF showed a slower approach to steady-state. Throughout the 4-hr constant-rate infusion period, Pept2 null mice exhibited higher brain ECF drug levels than wild-type mice and achieved significant differences during the last 2 hours of cefadroxil infusion. As shown in Table 1 and Fig. 2, the concentrations of cefadroxil in brain ECF were about 2- to 2.5-fold greater in Pept2 null mice than in wildtype animals during this time period.
Fig. 1.
Concentration-time profiles of cefadroxil in the plasma (total drug, Cp) and brain extracellular fluid (unbound drug, Cu,ECF) during a 4-hr intravenous infusion of 0.15 mg/min/kg cefadroxil in wildtype and Pept2 null mice. Data are expressed as mean ± SEM (n=10–12). ***p<0.001 when comparing Cp or Cu,ECF between the two genotypes, as indicated by two-way ANOVA with Bonferroni correction for multiple comparisons.
Table 1.
Distribution of cefadroxil in plasma and brain following a 4-hr intravenous infusion of drug at 0.15 mg/min/kg in wildtype (WT) and Pept2 null (KO) mice
| Parameters | Unit | Wildtype
|
Pept2 null
|
KO/WT | t-test | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | n | CV% | Mean ± SEM | n | CV% | p value | |||
| CL | mL/min/kg | 34.8 ± 1.2 | 10 | 11.0 | 36.5 ± 3.4 | 12 | 32.6 | 1.05 | 0.6504 † |
| Cp,240 | μg/mL | 4.35 ± 0.14 | 10 | 10.4 | 4.45 ± 0.35 | 12 | 27.4 | 1.02 | 0.7953 † |
| Cu,ECF,220-240 | μg/mL | 0.215 ± 0.019 | 10 | 28.0 | 0.489 ± 0.059 | 12 | 41.7 | 2.28 | 0.0006 † |
| Ccsf | μg/mL | 0.268 ± 0.033 | 6 | 30.4 | 0.533 ± 0.090 | 9 | 50.6 | 1.99 | 0.0198 † |
| Cu,ECF,210/Cp,210‡ | 0.050 ± 0.006 | 10 | 36.0 | 0.121 ± 0.016 | 12 | 45.9 | 2.39 | 0.0010 † | |
| Abrain | μg/g brain | 0.374 ± 0.065 | 12 | 60.1 | 0.331 ± 0.058 | 11 | 58.1 | 0.88 | 0.6282 |
| Acell | μg/g brain | 0.339 ± 0.065 | 12 | 66.9 | 0.244 ± 0.058 | 11 | 78.3 | 0.72 | 0.2961 |
| Vu,brain | mL/g brain | 2.17 ± 0.43 | 12 | 68.1 | 0.83 ± 0.17 | 11 | 68.7 | 0.38 | 0.0109 † |
CL is the plasma clearance; Cp,240 the plasma concentration at the end of infusion (i.e., 240 min); Cu,ECF,220-240, the unbound concentration in brain ECF during the 220–240 min microdialysis period; Ccsf, the cefadroxil concentration in cerebrospinal fluid; Cu,ECF,210/Cp,210, the concentration ratio of unbound drug in brain ECF to total drug in plasma at 210 min after initiating the infusion; Abrain, the total amount of cefadroxil in brain parenchyma, corrected for vascular volume; Acell, the amount of cefadroxil in brain cells, corrected for ECF; and Vu,brain, the unbound volume of distribution in brain.
Welch’s t-test was performed to compare wildtype and Pept2 null mice in the case of unequal variance. For equal variance, a student’s t-test was performed.
Cu,ECF,210/Cp,210 is equivalent to Cu,ECF/Cu,plasma (i.e., Kp,uu) since the total plasma concentration of cefadroxil equals the unbound plasma concentration of drug (Cu,plasma), given that cefadroxil is essentially unbound in plasma (i.e., fu ≈ 1) [28].
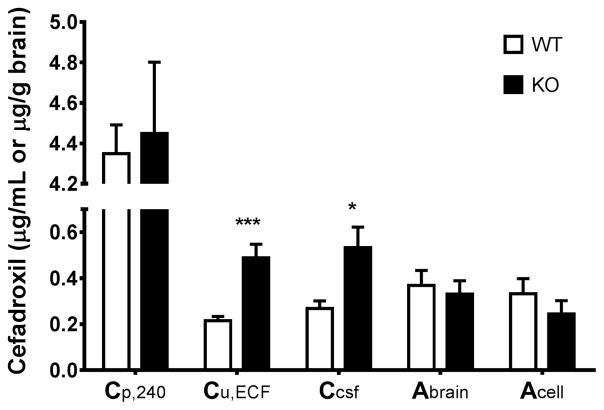
Fig. 2.
Concentrations of cefadroxil in the plasma (total drug, Cp,240), brain extracellular fluid (unbound drug, Cu,ECF or Cu,ECF,220-240) and cerebrospinal fluid (Ccsf), as well as amount of cefadroxil in the brain parenchyma (Abrain) and brain cells (Acell) of wildtype and Pept2 null mice at the end of the 4-hr intravenous infusion of 0.15 mg/min/kg cefadroxil. Data are expressed as mean ± SEM (n=6–12). *p<0.05 and **p<0.001 when comparing a parameter between two genotypes, as indicated by Welch’s t-test (for unequal variance) and by student’s t-test (for equal variance).
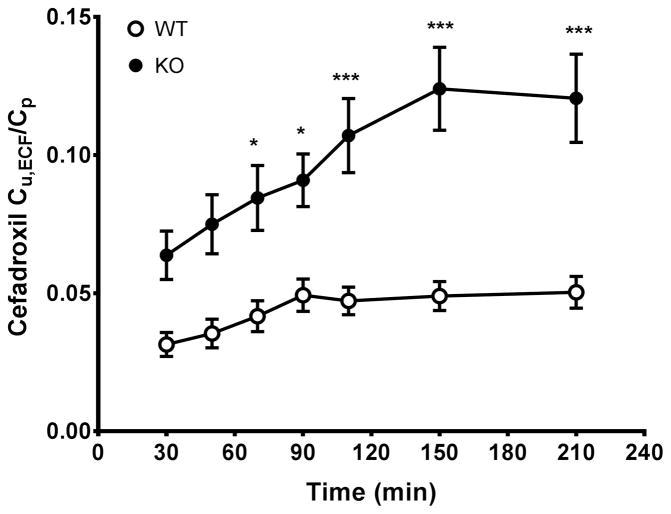
To confirm the effect of PEPT2 on the penetration of cefadroxil into brain, the concentration ratio of unbound drug in brain ECF to total plasma (Cu,ECF/Cp) was calculated as a function of time. As shown in Fig. 3, the Cu,ECF/Cp values of cefadroxil increased slowly in wildtype mice until 1.5 hour, whereas the Cu,ECF/Cp values in Pept2 null mice became stable at around 2.5 hour. The Cu,ECF/Cp ratios of cefadroxil were significantly greater in Pept2 null mice over the last 2–3 hours of infusion and, at steady-state, was almost 2.5-fold higher than wildtype mice at the last sampling point (i.e., 0.050±0.006 in wildtype mice vs. 0.121±0.016 in PEPT2 null animals). Relative recovery of cefadroxil in the CMA7 (2-mm) microdialysis probe, as determined during in vivo retrodialysis of radiolabeled drug, was 4.7±0.1%.
Fig. 3.
Ratio of unbound concentration in brain ECF to total plasma concentration (Cu,ECF/Cp) as a function of time during a 4-hr constant intravenous infusion of 0.15 mg/min/kg cefadroxil in wildtype and Pept2 null mice. Data are expressed as mean ± SEM (n=10–12). *p<0.05 and ***p<0.001 when comparing a ratio between two genotypes, as indicated by two-way ANOVA with Bonferroni correction for multiple comparisons.
Cisterna magna and brain were also sampled at the end of the cefadroxil infusion to evaluate drug distribution in CSF and brain parenchyma at steady-state. Cefadroxil levels in the CSF of Pept2 null mice were two times that of wildtype mice (Table 1 and Fig. 2). PEPT2 ablation, however, had no significant effect on the concentrations of cefadroxil in brain parenchyma or brain cells (Abrain or Acell). Because brain regions were not an influential factor for either Abrain or Acell, according to two-way ANOVA, average Abrain and Acell values were determined from the five brain regions for each mouse. Finally, no significant differences were observed between brain ECF and CSF for each genotype.
In the experiments using radiolabeled cefadroxil and dextran, the blood-to-plasma partitioning (or concentration ratio) of cefadroxil was not significantly different between the two genotypes (n=5 per group), and was calculated as 0.65±0.01 when including all animals. The vascular volume was comparable between wildtype and Pept2 null mice for each brain region (n=5 per genotype), ranging from 8.4–8.7 μL/g brain. The cerebellum, however, had a higher vascular volume (13.3±0.3 μL/g brain) than the other regions.
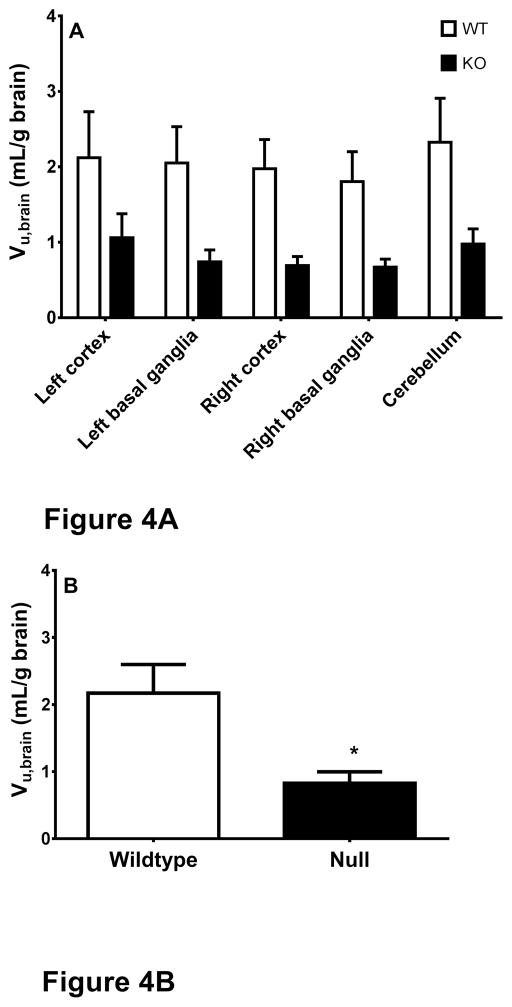
Vu,brain, a measure of intra-brain distribution, describes the relationship between the total amount of drug in brain parenchyma and the unbound concentration of drug in brain ECF. In the present study, Vu,brain values were determined from brain tissue and microdialysis samples at steady-state. As shown in Fig. 4A, wildtype mice had a higher Vu,brain for cefadroxil than Pept2 null mice, indicating that relatively more drug accumulated inside the brain cells of wildtype animals. Two-way ANOVA showed that genotype was a significant influential factor that explained 20.5% of the variation. In contrast, brain region did not have a significant influence on Vu,brain and there was no interaction between genotype and brain region. Because the Vu,brain was independent of brain region, an average Vu,brain was calculated from the five regions of each mouse. As shown in Fig. 4B and Table 1, PEPT2 ablation significantly decreased the average value of Vu,brain from 2.13±0.43 mL/g brain in wildtype mice to 0.83 ± 0.17 mL/g brain in Pept2 null animals.
Fig. 4.
Unbound volume of distribution (Vu,brain) of cefadroxil in different brain regions (A) and whole brain (averaged from the five regions) (B) of wildtype and Pept2 null mice at the end of a 4-hr intravenous infusion of 0.15 mg/min/kg cefadroxil. Data are expressed as mean ± SEM (n=11–12). Two-way ANOVA indicated that genotype and not brain region was an influencing factor for Vu,brain. Welch’s t-test indicated a significant differences in Vu,brain between the two genotypes.
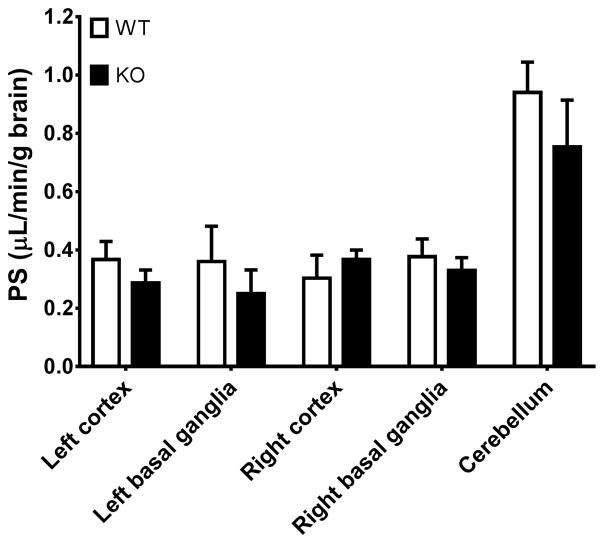
The cefadroxil PS product was measured in both genotypes to examine the effect of PEPT2 on cefadroxil influx from plasma to brain. As shown in Fig. 5, the PS product did not differ significantly between wildtype and Pept2 null mice, regardless of brain region. Still, in cerebellum, the PS product was about 2-fold higher than compared to the other regions. Based on two-way ANOVA, it was shown that although genotype did not have an effect on the PS product, brain region was an influential factor. This finding was essentially due to the disparate value in cerebellum PS product as compared to either side of the cortex or basal ganglia.
Fig. 5.
Permeability-surface area (PS) product of cefadroxil in different brain regions based on the study design using a 10-min intravenous infusion of 0.15 mg/min/kg cefadroxil in wildtype and Pept2 null mice. Data are expressed as mean ± SEM (n=3). Two-way ANOVA indicated that brain region but not genotype was an influencing factor for PS product (i.e., specific to cerebellum as compared to the other brain regions).
4. Discussion
The present study used intracerebral microdialysis as a tool to study the role of PEPT2 in the transport and distribution of cefadroxil in brain of wildtype and Pept2 null mice. In this regard, the major findings of this study were that: 1) PEPT2 ablation had a significant effect on the accumulation of cefadroxil in brain, where ECF and CSF concentrations were increased 2- to 2.5-fold in Pept2 null mice as compared to wildtype animals; 2) the unbound volume of distribution in brain (Vu,brain) was 60% of and significantly lower in Pept2 null mice than in wildtype animals, indicating an uptake function of PEPT2 in brain cells; and 3) PEPT2 ablation did not affect the influx clearance of cefadroxil from plasma to brain, as suggested by comparable PS product values between the genotypes in all five brain regions.
The rate of drug transport across barriers of the CNS can be described by two pharmacokinetic parameters [26,27]: 1) the rate of drug entering the brain from blood that is usually characterized by an influx clearance (CLin) and 2) the rate of drug being removed from the brain and back to blood that is usually characterized by an efflux clearance (CLout). Although CLin and CLout characterize the rate of transport across blood-brain barriers, the unbound partition coefficient (Kp,uu), determined by the net influx and efflux clearances such that Kp,uu=CLin/CLout, describes the extent of drug concentration between the brain and blood under steady-state conditions. The Kp,uu is equal to unity in the case of equal CLin and CLout values, for instance, for drugs that move across membranes via passive diffusion only (e.g., by transcellular or paracellular pathways). Although passive diffusion may be determined by the physicochemical properties of drugs, the influx and efflux clearances may be affected by the influx/efflux transporters expressed at the CNS barriers and by drug elimination via metabolism in brain. In practice, Kp,uu can be calculated as the concentration ratio of unbound drug in brain ECF (or CSF) to unbound drug in plasma at steady-state such that: Kp,uu = Cu,brain,ss/Cu,plasma,ss = CLin/CLout.
In the present study, only total plasma concentrations (Cp) of cefadroxil were measured and not unbound concentrations in plasma (Cu,plasma). However, the Cp of cefadroxil is expected to be comparable to Cu,plasma since essentially all of the drug is unbound in plasma (i.e., fu ≈ 1) [28]. As a result, the Kp,uu of cefadroxil in brain ECF is also equal to 0.050±0.006 in wildtype mice which increased to 0.121±0.016 in Pept2 null mice (Table 1). The fact that Kp,uu is much lower than unity for both genotypes suggests that, in addition to PEPT2, other efflux transporters are involved in removing cefadroxil from the brain. Cefadroxil is a substrate of several CNS barrier transporters including the organic anion transporters (OATs) [29], multidrug resistance-associated proteins (MRPs) [30,31], and organic anion transporting polypeptides (OATPs) [32]. Moreover, our previous study showed that co-administration of probenecid (an inhibitor of OATs, MRPs, and OATPs) caused a 2.5-fold increase in the Kp,uu of cefadroxil in rat brain ECF during microdialysis [22]. This finding supports our current study results, suggesting that cefadroxil is, indeed, effluxed from brain to plasma by PEPT2 along with the OATs, MRPs and/or OATPs. Given the 2.4-fold increase in the Kp,uu of cefadroxil in Pept2 null mice (i.e., from 0.050 to 0.121), we examined whether this change was due to an increase in the CLin of cefadroxil or a decrease in the drug’s CLout. In this regard, permeability-surface area studies were performed and, as shown in Fig. 5, no difference was observed between genotypes in the PS product. Thus, we concluded that PEPT2 had no effect on the CLin of cefadroxil and that, by default, PEPT2 reduced the penetration of drug into brain ECF (and CSF) by increasing CLout.
Immunolocalization demonstrates that PEPT2 is expressed on the apical membrane (CSF-facing) of choroid plexus epithelia at the BCSFB [16]. In contrast, there is no evidence that PEPT2 is expressed at the BBB [16,33]. Thus, a likely explanation is that PEPT2 affects the CLout of cefadroxil by removing drug from the CSF, which then provides the “driving force” to reduce drug levels in brain ECF. There are no barriers (i.e., tight junctions) at the brain ECF-CSF interfaces, including the interior ependymal wall of ventricles and the exterior pial-glial surfaces of the subarachnoid space [34]. As a result, water and solutes are able to move (relatively) freely between the ECF and CSF via diffusion and bulk flow, depending upon the direction of the concentration gradient and hydrostatic pressure. Moreover, recent findings revealed perivascular pathways as another mechanism for the exchange of fluids between brain compartments [35]. Thus, subarachnoid CSF moves into brain parenchyma along paravascular spaces surrounding the penetrating arteries while paravenous drainage pathways facilitate the drainage of interstitial fluid (or ECF) into CSF. The above mechanism makes for efficient fluid exchange even for species with large brain volumes (e.g., humans). The BBB is widely believed to be the most important barrier in the CNS due to its proximity to brain cells [36,37]. In contrast, the choroid plexus (i.e., BCSFB) is viewed as a “kidney” for the brain because of its ability to remove metabolites and toxins from the CNS and maintain brain homeostasis [15,34]. However, it is difficult to evaluate the impact of BCSFB transporters alone on substrate distribution in the brain parenchyma because many transporters are expressed only at the BBB or situated at both the BBB and BCSFB [38,39]. PEPT2, as a transporter at the BCSFB and not BBB, provides a unique opportunity to evaluate the significance of choroid plexus transporters on the distribution of drugs in brain ECF. The current study demonstrated that PEPT2 ablation resulted in the approximate 2-fold increase of cefadroxil in both CSF and brain ECF. This important finding confirms the sink function of the CSF circulation as well as the contribution of BCSFB-located transporters on brain ECF levels.
Whereas Kp,uu describes the extent of drug distribution between brain ECF and blood at steady-state, Vu,brain describes drug distribution inside the brain parenchyma [26,40]. For a drug that distributes evenly throughout the whole brain tissue, Vu,brain is expected to be around 0.8 mL/g brain [41], the water volume in brain parenchyma of which brain ECF accounts for ~ 0.18 mL/g brain [25]. Given its low lipophilicity, cefadroxil has negligible nonspecific binding to brain tissue components, which was confirmed using equilibrium dialysis in rat brain homogenate (data not shown). As a result, the decrease in the Vu,brain of all brain regions by PEPT2 ablation is likely due to the absence of uptake function by PEPT2 at the membrane of neural cells.
It should be appreciated that, in this study, PEPT2 ablation did not significantly change the plasma clearance of cefadroxil after intravenous infusion. This finding differed from a study by Shen et al [12] who reported that Pept2 null mice, compared to wildtype animals, exhibited 3-fold greater total clearances and 3-fold lower systemic concentrations following a 1 nmol/g intravenous bolus dose of cefadroxil. However, these same authors [12] were not able to discern differences between the two genotypes when the highest dose level of cefadroxil was administered (i.e., 100 nmol/g intravenous bolus injection). Moreover, the differential plasma concentration-time profiles of cefadroxil were more apparent once the drug levels fell to about 10 μM or lower. Cefadroxil has a Km of 10–40 μM for PEPT2, which is highly dependent upon the experimental study design [13,43–45]. During the 4-hr intravenous infusion of cefadroxil, steady-state plasma concentrations of drug were about 4.4 μg/mL (or 12.1 μM), numbers that were close to the lower end of Km values reported in the literature. Thus, it is possible that renal PEPT2 was saturated during the present intravenous infusion study, thereby, reducing the tubular reabsorption component of renal clearance which is the major route of cefadroxil elimination [42]. In contrast, concentrations of cefadroxil in brain ECF and CSF were in the range of 0.2–0.5 μg/mL (or 0.5–1.5 μM), values that were much lower than the Km of cefadroxil for PEPT2. Thus, our ability to elucidate the significance of PEPT2 function in brain was more favorable.
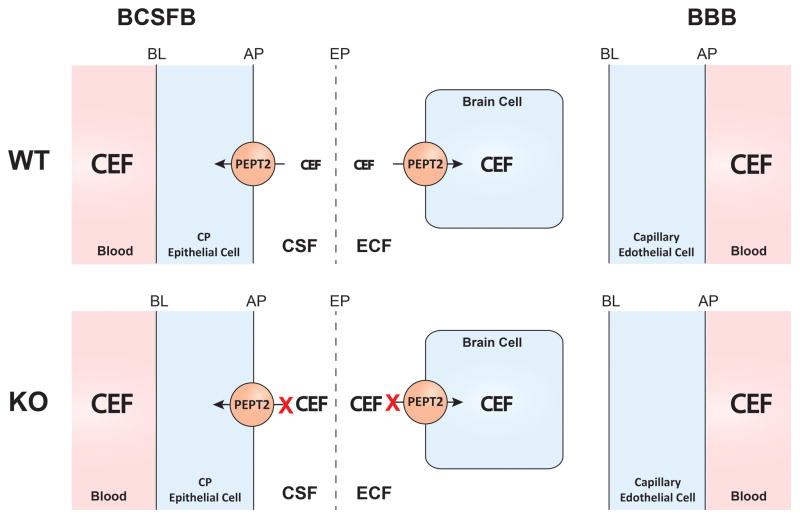
Using intracerebral microdialysis, this study examined the role of PEPT2 on the distribution of cefadroxil in various brain compartments. As shown in the schematic (Fig. 6), PEPT2 in wildtype mice functions as an efflux transporter at the blood-CSF interface and as an uptake transporter at the ECF-brain cell interface. In the absence of PEPT2 function (i.e., null mice), cefadroxil concentrations increased significantly in the CSF and brain ECF. However, the amount of drug in brain cells did not change significantly because, although brain ECF levels of cefadroxil increased, the drug had a reduced uptake into neurons during PEPT2 ablation. Thus, the PEPT2 transporter plays an important role in reducing cefadroxil concentrations in brain ECF and CSF, but not on drug levels in brain cells, due to its dual function in brain.
Fig. 6.
A schematic illustration showing the dual role of PEPT2 in affecting the disposition of cefadroxil (CEF) in brain. Apical membrane (AP), basolateral membrane (BL), blood-brain barrier (BBB), blood-cerebrospinal fluid barrier (BCSFB), cerebrospinal fluid (CSF), choroid plexus (CP), ependyma (EP), extracellular fluid (ECF), wildtype (WT) and Pept2 null (KO) mice.
Results from this study set a good example in demonstrating that total amounts of drug in brain do not necessarily reflect the distribution of drug in subcompartments (i.e., locally), especially in brain ECF. Although CSF concentrations of cefadroxil in this study were comparable to that in brain ECF, other investigators have reported differences in drug distribution between these two brain fluids in general, suggesting that CSF is not always a good surrogate for drug levels in brain ECF [46]. For drugs whose pharmacological response depends upon ECF concentrations, it may be better to determine their distribution in brain ECF and not depend upon sampling whole brain or CSF. Microdialysis serves as an exquisite, although complicated, tool to directly measure the regional distribution kinetics of drug, including ECF, in brain [47].
Due to the presence of multiple barriers, such as endothelial tight junctions and efflux transporters, it is difficult to deliver β-lactam antibiotics into brain for the treatment of the CNS diseases (e.g., meningitis, cerebritis and glioma). At present, cephalosporin antibiotics (e.g., ceftriaxone and cefotaxime) used to treat CNS infections [48] are not PEPT2 substrates [49]. Cefadroxil, as a substrate of multiple efflux transporters including PEPT2, has very low levels in CSF and brain ECF, thereby, excluding its use for CNS infections [22]. Thus, caution should be exercised in developing drug candidates having the general structural features of PEPT2 substrates [3,50] for diseases of the brain.
A better understanding of PEPT2 structure-function is also important because of the presence of genetic variants of PEPT2. In this regard, a study of PEPT2 polymorphisms in human showed that two main variants existed, namely hPEPT2*1 and hPEPT2*2 [51]. Whereas allelic frequencies of the two main haplotypes were even among Caucasians and Africans, different variant frequencies were found among other ethnic groups [52]. Moreover, when hPEPT2*1 and hPEPT2*2 were expressed in Chinese hamster ovary cells, a 3-fold difference in Km was observed for GlySar uptake [51]. It has been reported that the hPEPT2*2 haplotype, along with the 5-aminolevulinic acid dehydratase single nucleotide polymorphism 2, can increase the levels of 5-aminolevulinic acid (5-ALA) in brain and increase the risk of lead-induced neurotoxicity [53–55]. Thus, in addition to affecting the neurological effects of endogenous peptides/mimetics such as 5-ALA, PEPT2 polymorphisms may alter the pharmacological effects of drugs targeting CNS diseases, though more evidence is needed to confirm this premise.
In conclusion, this is the first study to determine the in vivo significance of PEPT2 on the distribution of cefadroxil (an important β-lactam antibiotic) in brain parenchyma, brain ECF and CSF. By applying intracerebral microdialysis to wildtype and Pept2 null mice, the ECF and CSF levels of cefadroxil were similar in each genotype, and increased about 2- to 2.5-fold during PEPT2 ablation. These findings convincingly demonstrate the impact of PEPT2 on brain ECF as well as the known role of PEPT2 in removing peptide/mimetic drugs from the CSF to plasma. Moreover, this study establishes that PEPT2 is involved in the uptake of peptide/mimetic drugs from brain ECF into the brain cells. Finally, our results suggest that PEPT2 plays an important role in modulating the physiological, pharmacological and toxicological activities of CNS-relevant endogenous substrates and drugs.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of General Medical Sciences grant R01-GM115481 (to D.E.S.) and by a Rackham Predoctoral Fellowship (to X.C.) provided by the University of Michigan.
Footnotes
Note: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith DE, Clemencon B, Hediger MA. Proton-coupled oligopeptide transporter family slc15: Physiological, pharmacological and pathological implications. Mol Aspects Med. 2013;34:323–336. doi: 10.1016/j.mam.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W, Liang R, Ramamoorthy S, Fei YJ, Ganapathy ME, Hediger MA, Ganapathy V, Leibach FH. Molecular cloning of PEPT 2, a new member of the H+/peptide cotransporter family, from human kidney. Biochim Biophys Acta. 1995;1235(2):461–6. doi: 10.1016/0005-2736(95)80036-f. [DOI] [PubMed] [Google Scholar]
- 3.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447(5):610–8. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- 4.Ocheltree SM, Shen H, Hu Y, Keep RF, Smith DE. Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther. 2005;315(1):240–7. doi: 10.1124/jpet.105.089359. [DOI] [PubMed] [Google Scholar]
- 5.Shu C, Shen H, Teuscher NS, Lorenzi PJ, Keep RF, Smith DE. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther. 2002;301(3):820–9. doi: 10.1124/jpet.301.3.820. [DOI] [PubMed] [Google Scholar]
- 6.Xiang JM, Chiang PP, Hu YJ, Smith DE, Keep RF. Role of PEPT2 in glycylsarcosine transport in astrocyte and glioma cultures. Neurosci Lett. 2006;396(3):225–229. doi: 10.1016/j.neulet.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Shen H, Keep RF, Smith DE. Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J Neurochem. 2007;103(5):2058–65. doi: 10.1111/j.1471-4159.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- 8.Doring F, Walter J, Will J, Focking M, Boll M, Amasheh S, Clauss W, Daniel H. Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin Invest. 1998;101(12):2761–7. doi: 10.1172/JCI1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang J, Jiang H, Hu Y, Smith DE, Keep RF. Kyotorphin transport and metabolism in rat and mouse neonatal astrocytes. Brain Res. 2010;1347:11–8. doi: 10.1016/j.brainres.2010.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, Hu Y, Keep RF, Smith DE. Enhanced antinociceptive response to intracerebroventricular kyotorphin in Pept2 null mice. J Neurochem. 2009;109(5):1536–43. doi: 10.1111/j.1471-4159.2009.06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamal MA, Jiang HD, Hu YJ, Keep RF, Smith DE. Influence of genetic knockout of Pept2 on the in vivo disposition of endogenous and exogenous carnosine in wild-type and Pept2 null mice. Am J Physiol-Regul Integr Comp Physiol. 2009;296(4):R986–R991. doi: 10.1152/ajpregu.90744.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab Dispos. 2007;35(7):1209–16. doi: 10.1124/dmd.107.015263. [DOI] [PubMed] [Google Scholar]
- 13.Ocheltree SM, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. Mechanisms of cefadroxil uptake in the choroid plexus: studies in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther. 2004;308(2):462–7. doi: 10.1124/jpet.103.060400. [DOI] [PubMed] [Google Scholar]
- 14.Daniel H, Rubio-Aliaga I. An update on renal peptide transporters. Am J Physiol Renal Physiol. 2003;284(5):F885–92. doi: 10.1152/ajprenal.00123.2002. [DOI] [PubMed] [Google Scholar]
- 15.Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56(12):1765–91. doi: 10.1016/j.addr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Shen H, Smith DE, Keep RF, Brosius FC., 3rd Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol Pharm. 2004;1(4):248–56. doi: 10.1021/mp049944b. [DOI] [PubMed] [Google Scholar]
- 17.Brandsch M. Transport of drugs by proton-coupled peptide transporters: pearls and pitfalls. Expert Opin Drug Metab Toxicol. 2009;5(8):887–905. doi: 10.1517/17425250903042292. [DOI] [PubMed] [Google Scholar]
- 18.Ganapathy ME, Brandsch M, Prasad PD, Ganapathy V, Leibach FH. Differential recognition of beta-lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J Biol Chem. 1995;270(43):25672–7. doi: 10.1074/jbc.270.43.25672. [DOI] [PubMed] [Google Scholar]
- 19.Buck RE, Price KE. Cefadroxil a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1977;11(2):324–30. doi: 10.1128/aac.11.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elmquist WF, Sawchuk RJ. Application of microdialysis in pharmacokinetic studies. Pharm Res. 1997;14(3):267–88. doi: 10.1023/a:1012081501464. [DOI] [PubMed] [Google Scholar]
- 21.Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange ECM, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL, Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BWY, Shah VP, Stahle L, Ungerstedt U, Welty DF, Yeo H. AAPS-FDA workshop white paper: Microdialysis principles, application and regulatory perspectives. Pharm Res. 2007;24(5):1014–1025. doi: 10.1007/s11095-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Loryan I, Payan M, Keep RF, Smith DE, Hammarlund-Udenaes M. Effect of transporter inhibition on the distribution of cefadroxil in rat brain. Fluids Barriers CNS. 2014;11(1):25. doi: 10.1186/2045-8118-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourne A, Barnes K, Taylor BA, Turner AJ, Kenny AJ. Membrane peptidases in the pig choroid plexus and on other cell surfaces in contact with the cerebrospinal fluid. Biochem J. 1989;259(1):69–80. doi: 10.1042/bj2590069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Smith DE, Keep RF, Xiang JM, Brosius FC. Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in choroid plexus. J Biol Chem. 2003;278(7):4786–4791. doi: 10.1074/jbc.M207397200. [DOI] [PubMed] [Google Scholar]
- 25.Levin VA, Fenstermacher JD, Patlak CS. Sucrose and inulin space measurements of cerebral cortex in four mammalian species. Am J Physiol. 1970;219(5):1528–33. doi: 10.1152/ajplegacy.1970.219.5.1528. [DOI] [PubMed] [Google Scholar]
- 26.Hammarlund-Udenaes M, Friden M, Syvanen S, Gupta A. On the rate and extent of drug delivery to the brain. Pharm Res. 2008;25(8):1737–50. doi: 10.1007/s11095-007-9502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bostrom E, Simonsson US, Hammarlund-Udenaes M. In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab Dispos. 2006;34(9):1624–31. doi: 10.1124/dmd.106.009746. [DOI] [PubMed] [Google Scholar]
- 28.Huh Y, Keep RF, Smith DE. Impact of lipopolysaccharide-induced inflammation on the disposition of the aminocephalosporin cefadroxil. Antimicrob Agents Chemother. 2013;57(12):6171–8. doi: 10.1128/AAC.01497-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khamdang S, Takeda M, Babu E, Noshiro R, Onozato ML, Tojo A, Enomoto A, Huang XL, Narikawa S, Anzai N, Piyachaturawat P, Endou H. Interaction of human and rat organic anion transporter 2 with various cephalosporin antibiotics. Eur J Pharmacol. 2003;465(1–2):1–7. doi: 10.1016/s0014-2999(03)01381-5. [DOI] [PubMed] [Google Scholar]
- 30.Akanuma S, Uchida Y, Ohtsuki S, Kamiie J, Tachikawa M, Terasaki T, Hosoya K. Molecular-weight-dependent, anionic-substrate-preferential transport of beta-lactam antibiotics via multidrug resistance-associated protein 4. Drug Metab Pharmacokinet. 2011;26(6):602–11. doi: 10.2133/dmpk.DMPK-11-RG-063. [DOI] [PubMed] [Google Scholar]
- 31.de Waart DR, van de Wetering K, Kunne C, Duijst S, Paulusma CC, Oude Elferink RP. Oral availability of cefadroxil depends on ABCC3 and ABCC4. Drug Metab Dispos. 2012;40(3):515–21. doi: 10.1124/dmd.111.041731. [DOI] [PubMed] [Google Scholar]
- 32.Nakakariya M, Shimada T, Irokawa M, Maeda T, Tamai I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics. Drug Metab Pharmacokinet. 2008;23(5):347–55. doi: 10.2133/dmpk.23.347. [DOI] [PubMed] [Google Scholar]
- 33.Berger UV, Hediger MA. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat Embryol (Berl) 1999;199(5):439–49. doi: 10.1007/s004290050242. [DOI] [PubMed] [Google Scholar]
- 34.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 37.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Kusuhara H, Sugiyama Y. Efflux transport systems for organic anions and cations at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56(12):1741–1763. doi: 10.1016/j.addr.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Westerhout J, Danhof M, De Lange ECM. Preclinical Prediction of Human Brain Target Site Concentrations: Considerations in Extrapolating to the Clinical Setting. J Pharm Sci. 2011;100(9):3577–3593. doi: 10.1002/jps.22604. [DOI] [PubMed] [Google Scholar]
- 40.Loryan I, Sinha V, Mackie C, Van Peer A, Drinkenburg WH, Vermeulen A, Heald D, Hammarlund-Udenaes M, Wassvik CM. Molecular properties determining unbound intracellular and extracellular brain exposure of CNS drug candidates. Mol Pharm. 2015;12(2):520–32. doi: 10.1021/mp5005965. [DOI] [PubMed] [Google Scholar]
- 41.Reinoso RF, Telfer BA, Rowland M. Tissue water content in rats measured by desiccation. J Pharmacol Toxicol Methods. 1997;38(2):87–92. doi: 10.1016/s1056-8719(97)00053-1. [DOI] [PubMed] [Google Scholar]
- 42.Hartstein AI, Patrick KE, Jones SR, Miller MJ, Bryant RE. Comparison of pharmacological and antimicrobial properties of cefadroxil and cephalexin. Antimicrob Agents Chemother. 1977;12(1):93–7. doi: 10.1128/aac.12.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ries M, Wenzel U, Daniel H. Transport of cefadroxil in rat-kidney brush-border membranes is mediated by 2 electrogenic H+-coupled systems. J Pharmacol Exp Ther. 1994;271(3):1327–1333. [PubMed] [Google Scholar]
- 44.Boll M, Herget, Wagener M, Weber WM, Markovich D, Biber J, Clauss W, Murer H, Daniel H. Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proc Natl Acad Sci USA. 1996;93:284–289. doi: 10.1073/pnas.93.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen H, Keep RF, Hu Y, Smith DE. PEPT2 (Slc15a2)-mediated unidirectional transport of cefadroxil from cerebrospinal fluid into choroid plexus. J Pharmacol Exp Ther. 2005;315:1101–1108. doi: 10.1124/jpet.105.090654. [DOI] [PubMed] [Google Scholar]
- 46.de Lange ECM, Danhof M. Considerations in the use of cerebrospinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting - Implications of the barriers between blood and brain. Clin Pharmacokinet. 2002;41(10):691–703. doi: 10.2165/00003088-200241100-00001. [DOI] [PubMed] [Google Scholar]
- 47.de Lange EC, Bouw MR, Mandema JW, Danhof M, de Boer AG, Breimer DD. Application of intracerebral microdialysis to study regional distribution kinetics of drugs in rat brain. Br J Pharmacol. 1995;116(5):2538–44. doi: 10.1111/j.1476-5381.1995.tb15107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–84. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 49.Luckner P, Brandsch M. Interaction of 31 beta-lactam antibiotics with the H+/peptide symporter PEPT2: analysis of affinity constants and comparison with PEPT1. Eur J Pharm Biopharm. 2005;59(1):17–24. doi: 10.1016/j.ejpb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Rubio-Aliaga I, Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci. 2002;23(9):434–40. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- 51.Pinsonneault J, Nielsen CU, Sadee W. Genetic variants of the human H+/dipeptide transporter PEPT2: analysis of haplotype functions. J Pharmacol Exp Ther. 2004;311(3):1088–96. doi: 10.1124/jpet.104.073098. [DOI] [PubMed] [Google Scholar]
- 52.Liu R, Tang AMY, Tan YL, Limenta LMG, Lee EJD. Interethnic differences of PEPT2 (SLC15A2) polymorphism distribution and associations with cephalexin pharmacokinetics in healthy Asian subjects. Eur J Clin Pharmacol. 2009;65(1):65–70. doi: 10.1007/s00228-008-0488-4. [DOI] [PubMed] [Google Scholar]
- 53.Sobin C, Flores-Montoya MG, Gutierrez M, Parisi N, Schaub T. delta-Aminolevulinic acid dehydratase single nucleotide polymorphism 2 (ALAD(2)) and peptide transporter 2*2 haplotype (hPEPT2*2) differently influence neurobehavior in low-level lead exposed children. Neurotoxicol Teratol. 2015;47:137–145. doi: 10.1016/j.ntt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobin C, Gutierrez M, Alterio H. Polymorphisms of delta-aminolevulinic acid dehydratase (ALAD) and peptide transporter 2 (PEPT2) genes in children with low-level lead exposure. Neurotoxicology. 2009;30(6):881–887. doi: 10.1016/j.neuro.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobin C, Parisi N, Schaub T, Gutierrez M, Ortega AX. delta-Aminolevulinic Acid Dehydratase Single Nucleotide Polymorphism 2 and Peptide Transporter 2*2 Haplotype May Differentially Mediate Lead Exposure in Male Children. Arch Environ Contam Toxicol. 2011;61(3):521–529. doi: 10.1007/s00244-011-9645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]