ABSTRACT
Available chemotherapeutic options are very limited against Mycobacterium abscessus, which imparts a particular challenge in the treatment of cystic fibrosis (CF) patients infected with this rapidly growing mycobacterium. New drugs are urgently needed against this emerging pathogen, but the discovery of active chemotypes has not been performed intensively. Interestingly, however, the repurposing of thiacetazone (TAC), a drug once used to treat tuberculosis, has increased following the deciphering of its mechanism of action and the detection of significantly more potent analogues. We therefore report studies performed on a library of 38 TAC-related derivatives previously evaluated for their antitubercular activity. Several compounds, including D6, D15, and D17, were found to exhibit potent activity in vitro against M. abscessus, Mycobacterium massiliense, and Mycobacterium bolletii clinical isolates from CF and non-CF patients. Similar to TAC in Mycobacterium tuberculosis, the three analogues act as prodrugs in M. abscessus, requiring bioactivation by the EthA enzyme, MAB_0985. Importantly, mutations in the transcriptional TetR repressor MAB_4384, with concomitant upregulation of the divergently oriented adjacent genes encoding an MmpS5/MmpL5 efflux pump system, accounted for high cross-resistance levels among all three compounds. Overall, this study uncovered a new mechanism of drug resistance in M. abscessus and demonstrated that simple structural optimization of the TAC scaffold can lead to the development of new drug candidates against M. abscessus infections.
KEYWORDS: Mycobacterium abscessus, thiacetazone, TetR regulator, MmpL, therapeutic activity, drug resistance mechanism
INTRODUCTION
Mycobacterium abscessus is the most pathogenic and chemotherapy-resistant rapidly growing mycobacterium. It is commonly associated with contaminated traumatic skin wounds and with postsurgical soft-tissue infections (1). Among the nontuberculous mycobacteria (NTM), M. abscessus represents the most frequent mycobacterial species isolated from cystic fibrosis (CF) patients with pulmonary infections, with a prevalence of 3 to 6% in this population (2). Patients with chronic M. abscessus infection had greater rates of lung function decline than those with no NTM infections (3). Therefore, infections due to M. abscessus represent a major threat in many CF centers worldwide (4). These infections remain extremely difficult to treat, as this mycobacterium is intrinsically resistant to a broad range of antibiotics, including most antitubercular drugs (5). The prognosis of pulmonary infections is poor, particularly in the context of CF, with a cure rate of 30 to 50% in spite of lengthy courses of antibiotics often complemented by surgery (6).
The American Thoracic Society has recommended a treatment regimen for M. abscessus infections consisting of a combination of a macrolide (clarithromycin or azithromycin), an aminoglycoside (amikacin), and a β-lactam (cefoxitin or imipenem) for a period of 1 year (4). The M. abscessus abscessus and M. abscessus bolletii subspecies possess an erm(41) RNA methylase gene that confers inducible resistance to macrolides (7). In contrast, the M. abscessus massiliense subspecies lacks inducible resistance to macrolides due to the presence of a truncated erm(41) gene. Consequently, treatment regimens for M. abscessus are dependent on the infecting subspecies. For these reasons, new chemical entities are urgently needed to further improve M. abscessus treatment outcomes. The fact that the M. abscessus genome sequence shares a high similarity with that of M. tuberculosis (8) suggests the presence of shared biochemical pathways between these mycobacteria. Therefore, the existing data acquired during previous tuberculosis drug discovery campaigns could represent a valuable source of information to rapidly identify new chemotypes with strong activity against M. abscessus, thus alleviating the necessity to initiate new chemical screens de novo. Indeed, we have recently validated the usefulness of this cross-screen approach by identifying and characterizing the mode of action of a highly efficient piperidinol-based compound, from a previously known set of potent nontoxic antitubercular hits, against M. abscessus (9). Such compounds could, therefore, be further exploited as new pharmacophores for the preparation of analogues with activity against M. abscessus.
Thiacetazone (TAC) has formerly been used in combination with isoniazid to treat patients infected with multidrug-resistant M. tuberculosis strains (10) but was removed from the antitubercular chemotherapeutic regimen due to its secondary toxic effects, especially in HIV-positive patients (11, 12). TAC is a prodrug that requires S-oxidation of its thiocarbonyl moiety by the flavin-containing monooxygenase EthA to exert its antimycobacterial activity (13), and mutations in ethA are associated with TAC resistance in M. tuberculosis (14). Upon activation, TAC has recently been shown to bind to the HadA component of the HadABC dehydratase complex, leading to inhibition of mycolic acid biosynthesis (15). Interestingly, we and others have reported that NTM such as Mycobacterium avium (16), Mycobacterium smegmatis (17), and M. abscessus (18) are naturally resistant to TAC. However, a TAC analogue, SRI-224, was previously tested against M. avium and found to be more effective than TAC in vitro as well as in mice (16) and subsequently shown to be very active against M. tuberculosis (19). This emphasizes that TAC analogues can be synthesized with improved potency not only against M. tuberculosis but also against NTM. Since a simple modification of the parental TAC scaffold to create SRI-224 led to greater potency, the effect of further simple structural modifications was investigated, leading to a second generation of TAC analogues (20). Among these compounds, two exhibited MICs 10-fold lower than the parental molecule and inhibited mycolic acid biosynthesis in M. tuberculosis (20). This prompted us to investigate the potential antimicrobial efficacy of these TAC derivatives against M. abscessus.
In this study, we present the activity of a wide panel of TAC derivatives against the smooth (S) and rough (R) variants of M. abscessus and demonstrate that simple modifications of the parental molecule represents a useful approach to develop active compounds against M. abscessus. We also provide evidence that these derivatives are activated by EthA in M. abscessus and identify mutations in the putative TetR regulator MAB_4384 that were found to be associated with high resistance levels to these compounds.
RESULTS
Identification of new thiacetazone derivatives active against M. abscessus.
Since previous TAC analogues were found to be more efficient against M. avium (16) and M. tuberculosis (19), we were prompted to address whether TAC analogues also exhibit potent activity against M. abscessus, notorious for being the most drug-resistant mycobacterial species (21), despite being refractory to TAC inhibition in liquid cultures (MIC of >100 μg/ml) (18). As shown in Table 1, the compounds exhibited a broad range of activity against the rough (R) variant of M. abscessus CIP104536, from active compounds (<10 μg/ml) to derivatives exhibiting only modest (<50 μg/ml) or very poor activity (≥100 μg/ml). In particular, compounds R2, R3, D6, D15, and D17 were found to be very active compared to the parental TAC molecule, with MIC values ranging from 1.6 to 6.2 μg/ml. All of these compounds were also very active against M. tuberculosis (Table 1). This indicates that TAC analogues can be effective against M. abscessus. The experiments described below involve the D6, D15, and D17 compounds, but larger numbers of analogues will be required to develop a robust structure-activity relationship for the thiosemicarbazone class of anti-M. abscessus agents. Nevertheless, structural activity requirements seem to be apparent for activity against M. abscessus that are similar to those for activity against M. tuberculosis (20). Modifications at the para position of the benzene ring (R3 position [Table 1]) suggest that a good balance between size, lipophilicity, and flexibility is required for activity with active compounds D6, D15, and D17 (Fig. 1), exhibiting calculated logarithm of the partition coefficient (clogP) values of around 3.3 with isopropyl, propyl, and phenoxy groups, respectively. Conversely, compounds D25, D8, and KKM4 are significantly less active while possessing lipophilic yet inflexible substituents at the para position, with clogP values of 4.9, 2.3, and 2.9, respectively. Similarly, compounds D31 and D5, for example, which contain H or the smaller flexible methoxy group at the para position, with a clogP value of 1.7, are also significantly less active. However, the activity of D6, D15, and D17 compounds against M. abscessus compared to their activity against M. tuberculosis appears to be significantly reduced.
TABLE 1.
Activity of 38 TAC-related analogues against M. abscessusa
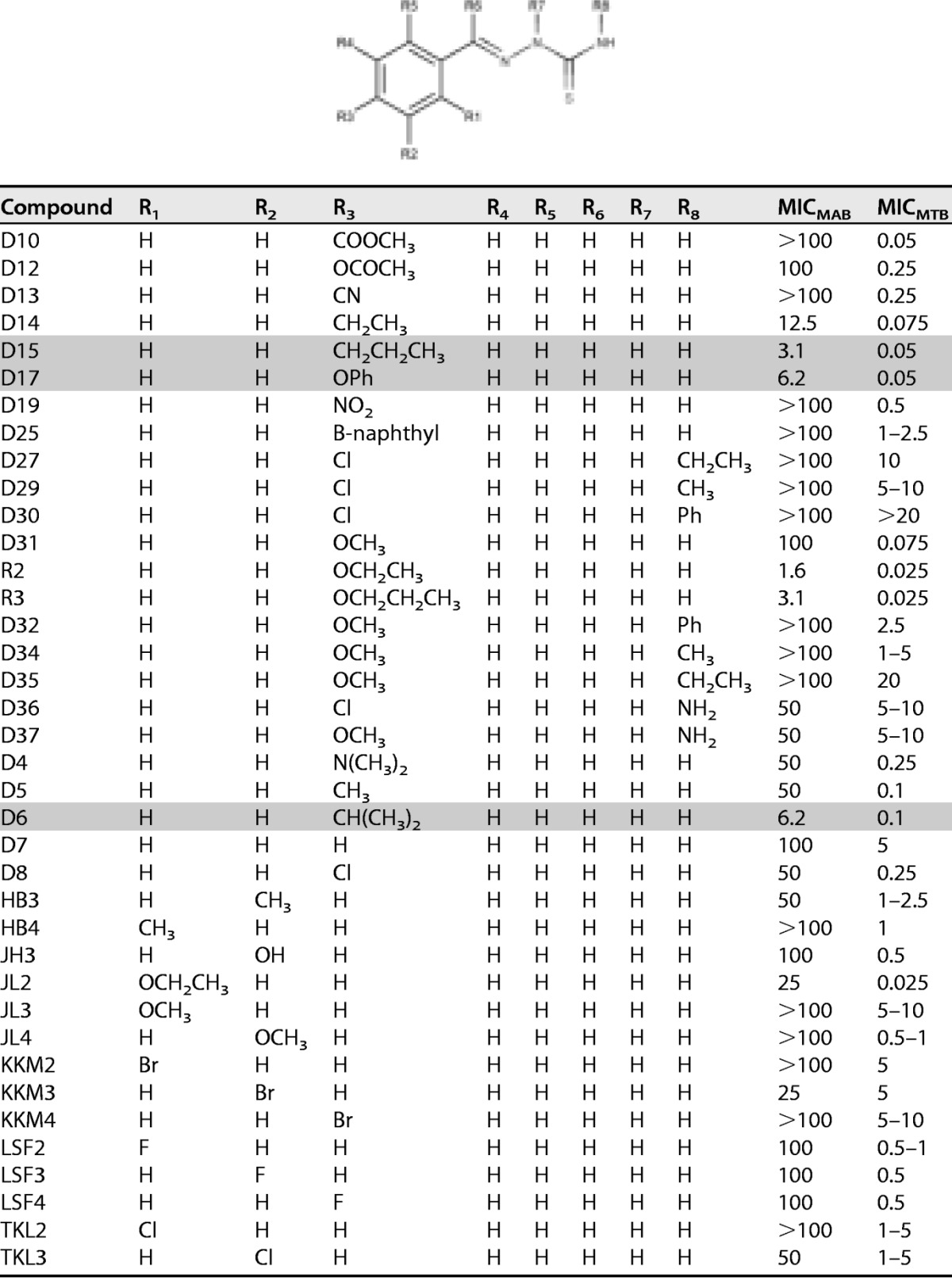
The MIC (μg/ml) against M. abscessus (MICMAB) was determined in cation-adjusted Mueller-Hinton broth for the rough M. abscessus CIP104536 reference strain. Shaded lines highlight the more active compounds. The MICs against M. tuberculosis mc27000 (MICMTB) were determined on Middlebrook 7H10 with OADC, some of which were reported previously (20).
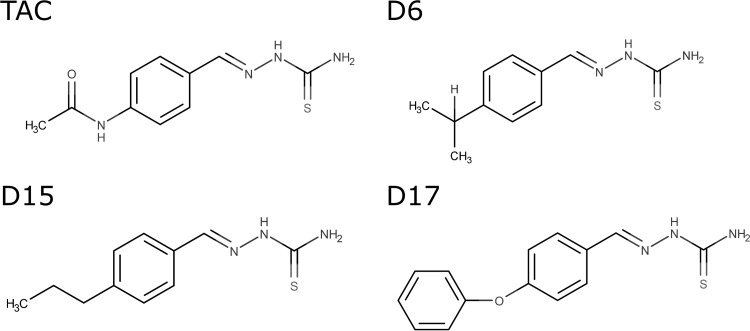
FIG 1.
Chemical structures of TAC and the D6, D15, and D17 analogues. All 4 compounds differ in the substitutions at the para position.
D6, D15, and D17 are bacteriostatic inhibitors of M. abscessus growth.
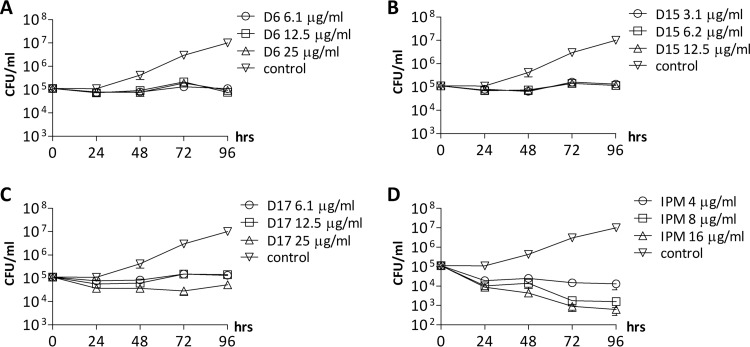
Exposure of M. abscessus R in log-phase growth to increasing concentrations of D6 (Fig. 2A), D15 (Fig. 2B), or D17 (Fig. 2C), corresponding to 1×, 2×, and 4× the MIC of each compound, resulted in a 2-log difference in viable CFU after 96 h compared to the nontreated cultures. However, since the CFU numbers after treatment were similar to those of the inoculum, the results suggest that these compounds are bacteriostatic in vitro. In contrast, exposure to imipenem, an active β-lactam drug against M. abscessus (22), was associated with a killing effect that was time and concentration dependent (Fig. 2D).
FIG 2.
Bacteriostatic activity of TAC analogues against M. abscessus. (A to D) Concentration- and time-dependent activity of the three effective TAC analogues, as well as imipenem, was monitored. The number of CFU was determined after 0, 24, 48, 72, and 96 h of incubation with different concentrations of each drug. Values are means from two independent experiments. IPM, imipenem.
Activity of TAC analogues against clinical isolates.
The lack of activity of TAC was first confirmed using a broad panel of clinical isolates of the M. abscessus complex (MIC of ≥100 μg/ml), subclassified into three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense (23, 24). The distinction between these subspecies is clinically relevant because they respond differently to antibiotics (23). In contrast to TAC, D6, D15, and D17 exhibited potent activity against the different strains isolated from either CF patients or non-CF patients, with MICs ranging from 3.1 to 25 μg/ml for D6, 0.4 to 6.2 μg/ml for D15, and 0.8 to 25 μg/ml for D17 (Table 2). These same strains also exhibited various susceptibility profiles for amikacin, one of the most widely used drugs in clinical settings. The absence of cross-resistance for the two M. massiliense and M. bolletii strains resistant to amikacin (MIC of >100 μg/ml) with D6, D15, or D17 (Table 2) suggests that D6, D15, and D17 target a biological function that is not targeted by amikacin. In addition, smooth (S) and R variants of each subspecies were usually equally sensitive to the analogues. These data suggest that further structural optimization and study of these TAC derivatives will lead to improved activity, providing potential drugs for future development of new treatment options.
TABLE 2.
Comparison of the activity of TAC, D6, D15, and D17 against clinical isolates from CF and non-CF patients
| Strain | Morphotype | Source (reference) | MIC (μg/ml) fora: |
||||
|---|---|---|---|---|---|---|---|
| D6 | D15 | D17 | TAC | AMK | |||
| M. abscessus | |||||||
| CIP104536 | R | Non-CFb (25) | 12.5 | 3.1 | 6.2 | 200 | 12.5 |
| 2524 | R | CF | 6.2 | 3.1 | 6.2 | 100 | 25 |
| 2648 | R | CF | 6.2 | 1.6 | 3.1 | 100 | 12.5 |
| 3022 | R | Non-CF | 3.1 | 0.8 | 1.6 | 100 | 12.5 |
| CF | R | CF | 3.1 | 1.6 | 3.1 | 100 | 12.5 |
| CIP104536 | S | Non-CFb (25) | 25 | 6.2 | 25 | >200 | 25 |
| 3321 | S | Non-CF | 6.2 | 1.6 | 6.25 | 200 | 25 |
| 1298 | S | CF | 25 | 6.2 | 25 | >200 | 12.5 |
| 2587 | S | CF | 12.5 | 3.1 | 12.5 | 200 | 25 |
| 2069 | S | Non-CF | 12.5 | 6.2 | 12.5 | 100 | 25 |
| CF | S | CF | 12.5 | 3.1 | 6.2 | 200 | 12.5 |
| M. massiliense | |||||||
| CIP108297 | R | Addison disease (26) | 6.2 | 3.1 | 3.1 | 100 | 12.5 |
| 210 | R | CF | 3.1 | 0.4 | 1.6 | 100 | 12.5 |
| CIP108297 | S | Addison disease (26) | 12.5 | 1.6 | 3.1 | >200 | 25 |
| 111 | S | CF | 6.2 | 3.1 | 6.2 | 200 | 25 |
| 212 | S | CF | 12.5 | 3.1 | 12.5 | >200 | 25 |
| 185 | S | CF | 12.5 | 3.1 | 6.2 | 200 | 25 |
| 140 | S | CF | 6.2 | 3.1 | 6.2 | 200 | 25 |
| 100 | S | CF | 6.2 | 1.6 | 6.2 | 200 | >100 |
| 107 | S | CF | 12.5 | 3.1 | 6.2 | >200 | 12.5 |
| 122 | S | CF | 3.1 | 1.6 | 1.6 | 100 | 12.5 |
| 120 | S | CF | 12.5 | 3.1 | 6.2 | >200 | 12.5 |
| M. bolletii | |||||||
| 19 | R | Non-CF | 6.2 | 1.6 | 3.1 | 100 | 50 |
| 108 | R | CF | 3.1 | 0.4 | 0.8 | 100 | 12.8 |
| 112 | R | CF | 3.1 | 6.2 | 12.5 | 200 | >100 |
| CIP108541 | S | Not reportedb | 12.5 | 3.1 | 6.2 | 100 | 25 |
| 17 | S | Non-CF | 12.5 | 1.6 | 6.2 | 200 | 12.5 |
| 97 | S | CF | 6.2 | 0.8 | 3.1 | 200 | 12.5 |
| 114 | S | CF | 12.5 | 6.2 | 12.5 | 200 | 12.5 |
| 116 | S | CF | 3.1 | 0.4 | 1.6 | 200 | 12.5 |
The MIC was determined in cation-adjusted Mueller-Hinton broth for different subspecies belonging to the M. abscessus complex. AMK, amikacin.
Seed stock of ATCC 19977T, isolated from a knee abscess.
EthA-dependent activity of TAC analogues in M. abscessus.
Many of the current antimycobacterial agents require some form of cellular activation unmasking reactive groups, which in turn will bind to their specific targets (27). TAC, like ethionamide, requires activation by the flavin-containing monooxygenase EthA (13). EthR, whose gene is adjacent to ethA in M. tuberculosis and in M. smegmatis, represses the transcription of ethA, subsequently preventing the conversion of the prodrugs to active molecules (28). EthR belongs to the TetR/CamR family of transcriptional regulators that negatively regulates the expression of EthA (29). Either overexpression of ethA or deletion of ethR shows increased susceptibility of M. bovis BCG to TAC due to higher production of active metabolites (13). In contrast, mutations in ethA are linked to TAC resistance in M. tuberculosis (14). However, whether M. abscessus possesses a functional EthA-like enzyme has never been established. One possible explanation is that relevant investigations were impeded by the fact that this species is resistant to most drugs requiring EthA activation. We therefore took advantage of the potential involvement of a putative EthA-like-dependent mechanism in the activity of the TAC analogues. Due to the presence of multiple putative monooxygenases in M. abscessus, we searched for ethA gene candidates that were genetically linked to putative TetR repressor-encoding genes. This led to the identification of MAB_0985-MAB_0984c as the most likely homologues of the ethA-ethR couple in M. abscessus. The MAB_0985 and MAB_0984c coding sequences were then cloned individually in pMV261 under the control of the constitutive hsp60 promoter (30), and the resulting constructs, pMV261_ethAMAB and pMV261_ethRMAB, were introduced in M. abscessus. Overexpression of M. abscessus EthR (EthRMAB) in M. abscessus was first confirmed by Western blotting using an antibody raised against the M. tuberculosis EthR (EthRMTB) protein (see Fig. S1 in the supplemental material). The MICs for the strain overexpressing EthAMAB were compared to those of the wild-type (WT) strain, and a 4-fold increase in sensitivity to TAC, D6, D15, and D17 was noticed (Table 3). This indicates that MAB_0985 encodes a functional EthA homologue in M. abscessus which participates in the activation process of TAC and its related analogues. Conversely, the EthRMAB-overexpressing strain exhibited a 2- to 4-fold-increased level of resistance to the TAC analogues, which was confirmed in both S and R variants of M. abscessus (Table 3). Similar results were also obtained when overexpressing EthAMTB and EthRMTB in M. abscessus (Table 3). Overall, these drug susceptibility profiles predict a direct correlation between the level of EthA-EthR expression and resistance to the TAC analogues. Therefore, the activity of D6, D15, and D17 in M. abscessus is dependent on activation by EthAMAB.
TABLE 3.
MICs of TAC and its related analogues D6, D15, and D17 against recombinant M. abscessus strains
| Straina | MIC (μg/ml) forb: |
|||
|---|---|---|---|---|
| D6 | D15 | D17 | TAC | |
| CIP104536 S | 25 | 6.2 | 25 | >200 |
| pMV261_ethAMAB | 6.25 | 1.65 | 6.25 | 50 |
| pMV261_ethAMTB | 12.5 | 3.1 | 12.5 | 100 |
| pMV261_ethRMAB | 100 | 25 | 50 | >200 |
| pMV261_ethRMTB | 50 | 12.5 | 25 | >200 |
| CIP104536 R | 12.5 | 3.1 | 6.2 | 100–200 |
| pMV261_ethAMAB | 3.1 | 1.6 | 1.6 | 50 |
| pMV261_ethAMTB | 3.1 | 1.6 | 3.1 | 50 |
| pMV261_ethRMAB | 50 | 12.5 | 50 | >200 |
| pMV261_ethRMTB | 50 | 12.5 | 25 | >200 |
M. abscessus was transformed with different constructs to overexpress either the EthAMAB (MAB_0985) or EthRMAB (MAB_0984c) protein from M. abscessus or from M. tuberculosis, as indicated.
Data are representative of three independent experiments. The MIC was determined in cation-adjusted Mueller-Hinton broth.
Mutations in MAB_4384 confer resistance to TAC analogues.
To search for the mechanism of resistance and as an indication of the potential molecular target of the inhibitor, a comprehensive approach was used involving the selection of spontaneous D15-resistant mutants of M. abscessus. Spontaneous resistant strains selected with 90 μg/ml D15 arose at a frequency of 7 × 10−7. In an initial experiment, 5 resistors were isolated using the reference CIP104536 S strain, exhibiting extremely high resistance levels (MIC of >200 μg/ml) compared to the parental strain (MIC of 6.2 μg/ml) (Table 4). In an independent round of selection, another set of six D15-resistant strains were derived from the parental R strain, designated D15_R1 to D15_R6, all exhibiting high resistance to D15 (MIC of >200 μg/ml) (Table 4). Since resistance to TAC in M. tuberculosis has been associated with mutations either in the EthA activator (14) or in the HadABC drug target (20, 31), PCR amplification and sequencing of the ethAMAB and hadABCMAB loci were performed in all 11 mutants. These sequences failed to reveal mutations, thus ruling out the involvement of these genes in the drug resistance phenotype. We then undertook whole-genome sequencing of the D15_S1, D15_S3, and D15_S7 resistant strains, followed by a comparative analysis of the sequences with the parental strain. This approach identified single-nucleotide polymorphisms (SNP) in MAB_2093, MAB_2540c, and MAB_3114 as well as a single-nucleotide insertion in MAB_4384 in D15_S1, a single SNP in MAB_4622c in D15_S3, and two SNPs in MAB_1921 and MAB_4384 in D15_S7 (see Table S2 in the supplemental material). The presence of two distinct mutations in MAB_4384 in two separate mutants (D15_S1 and D15_S7) prompted us to PCR amplify and sequence this gene in all 11 mutant strains. Unexpectedly, MAB_4384-associated mutations were discovered in all mutants (Table 4). Overall, these mutations accounted for amino acid replacements (M1A in two mutants, D14N and F57L). The remaining mutations consisted of single-nucleotide insertions at position c32 in three mutants or deletions at position a304 or a334, resulting in frameshifts affecting most of the C terminus and eventual premature stop codons (Table 4 and Fig. 3A). Mutants D15_R3 and D15_R6 harbored an additional SNP at position 305, just after the a304 deletion. All 11 D15-resistant mutants were also coresistant to D6 and D17 (MIC of >200 μg/ml) but remained susceptible to clofazimine, amikacin, imipenem, clarithromycin, cefoxitin, and tigecycline (Table 4), pointing out a unique mechanism of resistance.
TABLE 4.
Characteristics and drug susceptibility profiles of 11 spontaneous D15-resistant M. abscessus mutants against various drugs and TAC analogues
| Straina | MIC (μg/ml) forb: |
Mutation in MAB_4384c |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D6 | D15 | D17 | CFZ | AMK | IPM | CLR | FOX | TGC | SNP/indel | aa change | |
| CIP104536 S | 25 | 6.2 | 25 | 1 | 25 | 4 | 2 | 16 | 4 | ||
| D15_S1 | >200 | >200 | >200 | 1 | 25 | 4 | 2 | 16 | 4 | c32 insert | Stop |
| D15_S3 | >200 | >200 | >200 | 1 | 25 | 4 | 2 | 16 | 4 | g40a | D14N |
| D15_S4 | >200 | >200 | >200 | 1 | 25 | 4 | 2 | 16 | 4 | c32 insert | Stop |
| D15_S6 | >200 | >200 | >200 | 1 | 25 | 4 | 2 | 16 | 4 | g40a | D14N |
| D15_S7 | >200 | >200 | >200 | 1 | 25 | 4 | 2 | 16 | 4 | t169c | F57L |
| CIP104536 R | 12.5 | 3.1 | 6.2 | 1 | 12.5 | 4 | 4 | 16 | 8 | ||
| D15_R1 | >200 | >200 | >200 | 1 | 12.5 | 4 | 4 | 16 | 8 | t2c | M1A |
| D15_R2 | >200 | >200 | >200 | 1 | 12.5 | 4 | 4 | 16 | 8 | t2c | M1A |
| D15_R3 | >200 | >200 | >200 | 1 | 12.5 | 4 | 4 | 16 | 8 | a304del, t305c | Stop |
| D15_R4 | >200 | >200 | >200 | 1 | 12.5 | 4 | 4 | 16 | 8 | c32 insert | Stop |
| D15_R5 | >200 | >200 | >200 | 1 | 12.5 | 4 | 4 | 16 | 8 | a334del | Stop |
| D15_R6 | >200 | >200 | >200 | 1 | 12.5 | 4 | 4 | 16 | 8 | a304del, t305c | Stop |
The mutants were derived from either the CIP104536 S or R parental strain and selected in the presence of 90 μg/ml D15.
MICs were determined in cation-adjusted Mueller-Hinton broth by visually scanning for growth. CFZ, clofazimine; AMK, amikacin; IPM, imipenem; CLR, clarithromycin; FOX, cefoxitin; TGC, tigecycline.
Single-nucleotide polymorphisms and/or indels were identified in MAB_4384, and corresponding amino acid (aa) changes are also indicated.
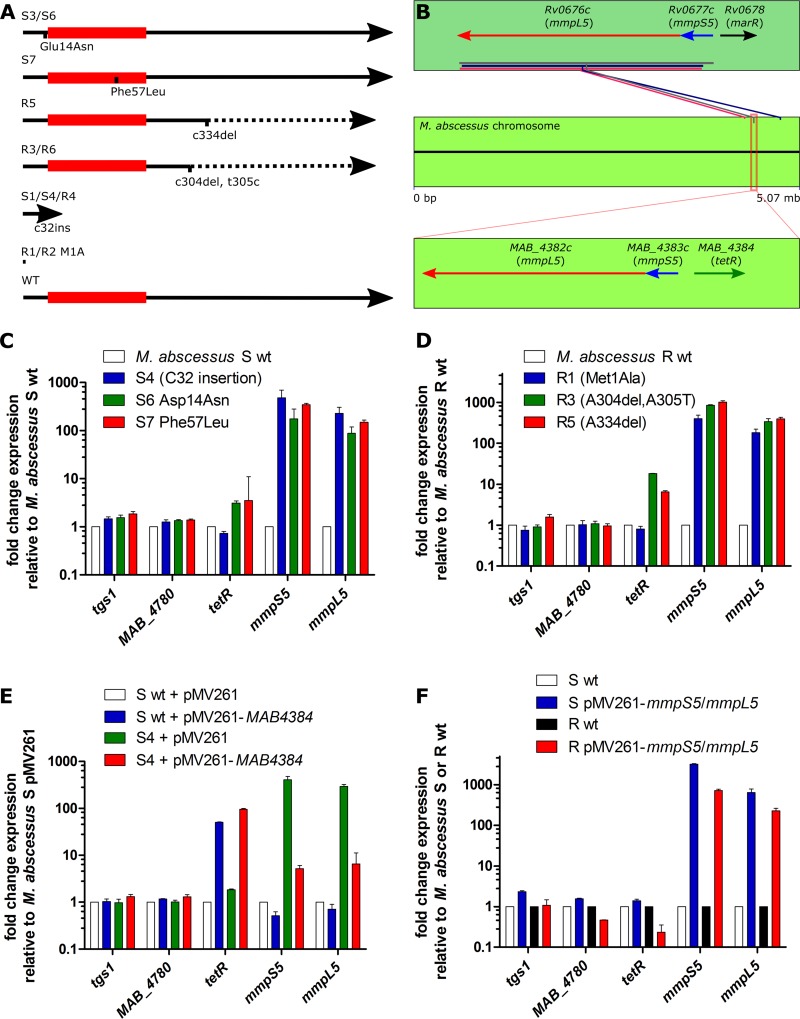
FIG 3.
Mutations in MAB_4384, encoding a TetR repressor, results in upregulation of MAB_4383c (mmpS5) and MAB_4382c (mmpL5) genes. (A) Mutations in MAB_4384 resulting in resistance to TAC analogues. The red box indicates the putative HTH DNA-binding domain. Broken lines indicate alterations in the primary amino acid sequence as a result of the frameshifts caused by indel mutations. The M1A mutations in R1 and R2 result in no translation. (B) Three putative mmpS5-mmpL5 operons are found in M. abscessus. Boxed in red is the MAB_4383c-MAB_4382c operon, which bears the highest homology to the mmpS5-mmpL5 locus from M. tuberculosis, as well as the upstream regulator MAB_4384. While both the M. tuberculosis and M. abscessus operons are preceded by putative regulators, these regulators share very little amino acid sequence identity. (C) Exponential-growth-phase cultures in Mueller-Hinton broth were used for total RNA isolation in order to determine relative gene expression by qRT-PCR and the ΔΔCT method. sigA-normalized gene expression in several spontaneous D15-resistant mutants reared in the S background relative to WT M. abscessus S. (D) sigA-normalized gene expression in several spontaneous D15-resistant mutants reared in the R background relative to WT M. abscessus R. (E) sigA-normalized gene expression in the WT and the D15_S4 mutant overexpressing MAB_4384 (pMV261-MAB_4384) relative to M. abscessus carrying the empty pMV261 vector. (F) sigA-normalized gene expression in the MmpS5-MmpL5-overexpressing (pMV261-mmpS5/mmpL5) strains relative to M. abscessus carrying the empty pMV261 vector. Histograms and error bars shown in panels C to F depict median fold change and the interquartile range, respectively, and were calculated from at least three independent qRT-PCR experiments, each in which fresh RNA was reverse transcribed prior to the qRT-PCR. Data are representative of two independently repeated RNA extractions.
MAB_4384 encodes a TetR repressor of an MmpS/MmpL efflux pump system.
BLAST analysis indicated that MAB_4384 encodes a putative transcriptional regulator of the TetR family. TetR-like regulators, with a conserved helix-turn-helix (HTH) DNA-binding domain and a C-terminal ligand regulatory domain, are widespread among bacteria. They are often associated with antibiotic resistance and the regulation of genes encoding small-molecule exporters (32). Among their wide range of cellular activities, including osmotic stress, homeostasis, biosynthesis of antibiotics, virulence, and pathogenicity of bacteria, TetR regulators are also known to modulate the expression level of multidrug resistance efflux pumps. They bind to the promoters of efflux pumps and are regulated by a plethora of ligands, leading to the conformational changes of the protein and abolishment of the protein dimerization and DNA binding, thus relieving the repression (33). Interestingly, the two genes adjacent to MAB_4384 transcribed in the opposite direction are MAB_4383c and MAB_4382c, which encode the mycobacterial membrane proteins MmpS and MmpL, respectively (Fig. 3B). MAB_4383c and MAB_4382c proteins share 56% and 65% identity with MmpS5 and MmpL5 from M. tuberculosis, respectively. The latter, which belongs to the superfamily of RND (resistance, nodulation, and division) transporters, have been documented recently to function in the efflux of azoles, clofazimine, and bedaquiline (34, 35).
To investigate whether MAB_4384 regulates the neighboring mmpS5-mmpL5 genes, quantitative PCR was first performed in both the parental M. abscessus S strain and in its D15-resistant derivatives harboring various MAB_4384 alleles (strains D15_S4, D15_S6, and D15_S7). The results clearly show a marked increase in the expression level of both the mmpS5 and mmpL5 transcripts in all three mutants (Fig. 3C). The expression levels of MAB_4780, encoding a dehydratase required for M. abscessus pathogenicity (18), or tgs1, encoding the primary triacylglycerol synthase involved in the synthesis and accumulation of triglycerides in M. abscessus (36), were included as unrelated gene controls. As anticipated, expression of MAB_4780 or tgs1 remained unchanged in the various strains tested (Fig. 3C). Similar results were obtained when measuring the expression levels of mmpS5 and mmpL5 transcripts in the D15_R1, D15_R3, and D_R5 resistant mutants carrying the mutated MAB_4384 alleles in the R background (Fig. 3D). To genetically confirm that MAB_4384 represses the mmpS5-mmpL5 operon, MAB_4384 was cloned into the multicopy plasmid under the control of the hsp60 promoter and introduced in the WT M. abscessus S background. Results shown in Fig. 3E clearly indicate that the expression level of MAB_4384 was strongly induced, and this correlated with the concomitant downregulation of the mmpS5 and mmpL5 transcript levels. Similar results were obtained when introducing the pMV261-MAB_4384 construct in D15_S4, although repression of mmpS5-mmpL5 was not reduced to levels seen in the WT strain (Fig. 3E). Overall, these results indicate that MAB_4384 acts as a repressor of the mmpS5-mmpL5 locus and that absence of this regulator (presumably in strains D15_S4 and D15_R1) or expression of a nonfunctional regulator (presumably strains D15_S6, D15_S7, D15_R3, and D15_R5) leads to gene derepression and to high levels of resistance to D6, D15, and D17.
Overexpression of MmpS5/MmpL5 in M. abscessus increases MICs to TAC analogues.
To confirm that resistance to D6, D15, and D17 is mediated by an MmpS5/MmpL5-dependent efflux mechanism, M. abscessus CIP104536 S and R were transformed with pMV261_mmpS5/mmpL5 in which the mmpS5-mmpL5 locus was placed under the control of the hsp60 promoter. As shown in Fig. 3F, transcription of both mmpS5 and mmpL5 genes was strongly increased in the strains carrying pMV261_mmpS5/mmpL5 compared to the corresponding control strains harboring the empty plasmid. Importantly, the MIC of D6, D15, and D17 in the MmpS5/MmpL5 overexpression S and R strains was highly increased, reaching the levels of the corresponding TetR-mutated strains (MIC of >200 μg/ml) (Table 5). However, the strains remained sensitive to amikacin. Overall, these results suggest that the TAC analogues are substrates of the MmpL5-driven efflux pump and that the sole overexpression of MmpS5/MmpL5 is sufficient to generate high levels of drug resistance to these compounds.
TABLE 5.
MICs of D6, D15, D17, and amikacin against MmpS5/MmpL5-overexpressing strains
| Straina | MIC (μg/ml) forb: |
|||
|---|---|---|---|---|
| D6 | D15 | D17 | AMK | |
| CIP104536 S | ||||
| Wild type | 25 | 12.5 | 12.5 | 25 |
| pMV261_mmpS5/mmpL5 | >200 | >200 | >200 | 25 |
| CIP104536 R | ||||
| Wild type | 12.5 | 3.1 | 6.25 | 25 |
| pMV261_mmpS5/mmpL5 | >200 | >200 | >200 | 12.5 |
M. abscessus S and R strains were transformed with pMV261_mmpS5/mmpL5.
The MICs were determined in cation-adjusted Mueller-Hinton broth. Data are representative of two independent experiments. AMK, amikacin.
DISCUSSION
M. abscessus is an organism increasingly recognized as a causative agent of chronic lung disease, often in patients with altered host defenses or disrupted airway clearance mechanisms, such as in CF. It represents a threat to infect the airway in patients with CF, with reports suggesting increased prevalence in recent years (37, 38). It can behave as an invasive pathogen leading to progressive pulmonary decline, which can preclude safe lung transplantation (3). Moreover, treatment against M. abscessus is often unsuccessful or poorly tolerated. M. abscessus is notorious for being one of the most drug-resistant mycobacterial species for which treatment options remain highly challenging (21). Given the lack of new active molecules, recent studies have explored the possible synergy of already existing drug combinations against M. abscessus (39) or of repurposing old drugs (40). Recently, a phenotypic screen on whole M. abscessus with a validated chemical series highly active against M. tuberculosis (41) allowed the description a new compound exhibiting promising activity against M. abscessus (9). This validated the usefulness of implementing data obtained during previous tuberculosis drug discovery screens to directly identify new chemotypes with strong activity against M. abscessus without initiating chemical screens de novo. Here, a similar strategy was applied by screening a previously characterized library of TAC-based analogues that included very active compounds against M. tuberculosis (20). Although TAC is inactive against M. abscessus, several analogues of TAC demonstrated potent activity against a broad panel of clinical isolates exhibiting different susceptibility profiles for other drugs, regardless of whether they were from CF or non-CF origin. Their efficacy was dependent on neither the bacterial morphotype nor the M. abscessus subspecies. This is of particular interest since M. abscessus, M. massiliense, and M. bolletii infections are known to exhibit different drug sensitivity test results, responses to antibiotics, and clinical symptoms (42, 43).
Whole-genome sequencing of in vitro-selected mutants resistant to D15 that were also coresistant to D6 and D17 identified mutations in MAB_4384. Subsequent PCR/sequencing unraveled mutations in this gene in all 11 D15-resistant strains. MAB_4384 belongs to the family of TetR regulators, which represents the most abundant family of HTH regulators in mycobacteria, making up 26% to 48% of the HTH DNA-binding capacity in mycobacterial species with large numbers found essentially in the soil-dwelling species (44). In agreement with the general assumption that approximately 60% of the TetR regulators are divergently oriented with their neighbor, MAB_4384 was found in the opposite orientation relative to its mmpS5-mmpL5 target genes. That the different point mutations (M1A, D14N, and F57L) were associated with comparable levels of resistance to D15, as well as similar fold increases in upregulation of mmpS5-mmpL5 in the mutants harboring premature stop codons, suggests that they lost their activity, resulting in derepression of mmpS5-mmpL5 gene expression. All three nonsynonymous replacements are located in the N-terminal domain, suggesting that some of these mutations affect the DNA-binding capacity of the repressor. Mutations in the start codon (M1A) may prevent translation of the MAB_4384 transcript. Similar mutations in the start codon were reported earlier in Rv0678, the repressor of MmpS5-MmpL5 in M. tuberculosis, and account for resistance to clofazimine in strains selected in vitro for clofazimine resistance (45). A recent report also highlighted the presence of the same start codon mutation (replacement of N-formylmethionine by alanine) in Rv0678 in a clinical isolate from a multidrug-resistant TB patient following bedaquiline treatment (46).
Although highly reminiscent of the mechanisms accounting for resistance to azoles, clofazimine, and bedaquiline in M. tuberculosis that together implicate a wide panel of mutations in the regulator, causing overexpression of the MmpS5-MmpL5 efflux pump, a major difference resides in the nature of the regulators involved (34, 35, 45–47). Whereas Rv0678 is a member of the MarR family of regulators (48), MAB_4384 belongs to the TetR family, and the change in the induction level of mmpS5-mmpL5 in the M. abscessus mutants was found to be much more pronounced than those found in M. tuberculosis (34, 35). While electrophoretic mobility shift assays indicated a direct binding of Rv0678 with the intergenic regions between mmpS5 and Rv0678, consistent with a previous finding reporting altered mmpS5-mmpL5 gene expression in Mycobacterium bovis strains carrying mutations in the orthologous gene of Rv0678 (34), shifts were also shown using the promoter regions of mmpS2-mmpL2, mmpS4-mmpL4, and Rv0991-Rv0992 (48). Since in mycobacteria the number of mmpL genes varies considerably from one species to another and since M. abscessus has at least 31 mmpL paralogues, including three mmpS5-mmpL5 paralogues, it remains possible that the MAB_4384 TetR repressor also regulates additional mmpS-mmpL couples. Interestingly, another TetR-encoding gene, MAB_4312, is found upstream and divergently oriented to the mmpS5-mmpL5 paralogue MAB_4311c-MAB_4310c, which shares surprisingly low protein identity of only 17% with MAB_4384 when aligned using ClustalW, although it displays a similar size. Thus, it is tempting to speculate that this TetR regulates MAB_4311c-MAB_4310c expression in response to stimuli different from those of MAB_4384 and that its mmpS5-mmpL5 pair is involved in the extrusion of yet-undiscovered and unrelated compounds. This is also of particular interest, as by facilitating lipid egress, the MmpL transporters contribute largely to the assembly of the cell wall and therefore can participate in the interactions between mycobacteria and their eukaryotic hosts (49). As most studies have focused on MmpLs in M. tuberculosis, very little is known regarding their role in NTM. In this context, we have recently demonstrated the role of MmpL4a in the transport of glycopeptidolipids (GPL) in smooth M. bolletii and identified a point mutation in MmpL4a which inactivates the activity of the GPL transporter and which was associated with a rough morphotype and increased virulence of the mutant strain in infected zebrafish (50, 51). Of note, inactivation of mmpL5 in M. tuberculosis severely compromised the ability of the mutant to multiply in mouse lungs (52), and more recently, Wells et al. established that MmpS4/L4 and MmpS5/L5 form independent and nonredundant siderophore transporter systems (53). These transport systems not only are required for the export of de novo synthesized mycobactins but also are involved in the recycling of exogenous siderophores (54). Whether the MmpS5-MmpL5 couple identified in this study contributes to the export of siderophores and pathogenicity of M. abscessus remains to be addressed.
Another striking difference is that, in M. tuberculosis, MmpS5-MmpL5 act as multisubstrate efflux pumps, responsible for low resistance levels to antitubercular drugs, such as azole, clofazimine, and bedaquiline. Although the M. abscessus mutants are highly resistant to the TAC analogues, they failed to show any cross-resistance against most antibiotics used for the treatment of M. abscessus infection. This suggests that despite their high identity level, the M. tuberculosis and M. abscessus orthologues do not share the same substrate specificity.
In conclusion, the present study shows that mutations in the MmpL cognate transcriptional regulators represent an important drug resistance mechanism and that high resistance levels to drugs can be achieved through efflux mechanisms involving the MmpS5-MmpL5 system in M. abscessus.
MATERIALS AND METHODS
Synthesis of TAC analogues.
A library of TAC analogues was evaluated against M. tuberculosis following synthesis using the general method reported previously (20). All compounds were dissolved in dimethyl sulfoxide (DMSO).
Bacterial strains.
M. abscessus CIP104536T, M. bolletii CIP108541T, and M. massiliense CIP108297T reference strains were used along with a series of clinical isolates, some of which were reported previously (40). Strains were routinely grown and maintained at 30°C in Middlebrook 7H9 broth (BD Difco) supplemented with 0.05% Tween 80 (Sigma-Aldrich) and 10% oleic acid, albumin, dextrose, catalase (OADC enrichment; BD Difco) (7H9T/OADC) or on Middlebrook 7H10 agar (BD Difco) containing 10% OADC enrichment (7H10OADC) and in the presence of antibiotics, when required. For drug susceptibility testing, bacteria were grown in cation-adjusted Mueller-Hinton broth (CaMHB; Sigma-Aldrich).
Drug susceptibility testing.
The MICs were determined according to the CLSI guidelines (55). The broth microdilution method was used in CaMHB with an inoculum of 5 × 106 CFU/ml in the exponential growth phase. Briefly, the bacterial suspension was seeded in 100-μl volumes in all of the wells of a 96-well plate, except for the first column, to which 198 μl of the bacterial suspension was added to each well. In the first column, 2 μl of compound at its highest concentration was added in six wells (the solvent used to dissolve the compound was added to the two outermost wells as a control). Twofold serial dilutions were then carried out by transferring 100 μl from the wells in the first column to the next column and repeating this for each successive column. Plates were subsequently incubated at 30°C for 3 to 5 days. MICs were recorded by visual inspection and by absorbance at 560 nm to confirm visual recording. Experiments were done in triplicates on three independent occasions.
Time-kill assay.
Microtiter plates were set up as described for MIC determination. Serial dilutions of the bacterial suspension were plated after 0, 24, 48, 72, and 96 h of exposure to different drug concentrations. CFU were enumerated after 4 days of incubation at 30°C.
Selection of spontaneous resistant mutants.
Exponentially growing M. abscessus cultures were plated on 7H10OADC containing 90 μg/ml D15. After 1 week of incubation at 30°C, single colonies were selected and grown in liquid medium, individually subjected to MIC determination, and scored for resistance to D15.
Whole-genome sequencing and target identification.
DNA libraries were prepared for the Illumina HiSeq 2500 (Illumina, San Diego, CA, USA) platform using the Nextera DNA library preparation kit (Illumina) by following the manufacturer's recommendations. Index was introduced to allow multiplexed sequencing. Quality and quantity for each library were checked with the Fragment Analyzer (Advanced Analytical Technologies, Inc., Ames, IA). A 125-bp single-read run was performed, and a coverage of around 100× for each genome was obtained. Single-nucleotide polymorphism (SNP) identification was performed using an in-house sequence analysis pipeline, as previously described (56), and additional bioinformatic analyses were available at the C3BI analysis platform. Confirmation of SNPs in MAB_4384 of the resistant strains was done by PCR amplification and conventional Sanger sequencing.
DNA constructs.
PCR amplification was done using purified genomic DNA of M. abscessus, Phusion DNA polymerase (Finnzymes, Finland), and the primers listed in Table S1 in the supplemental material. The amplicons were digested with MscI and HindIII (ethA or MAB_4384), HindIII alone (mmpS5-mmpL5 cluster), or BamHI and HindIII (ethR or hadABC) and then ligated to linearized pMV261 (30), allowing the expression of each protein under the control of the constitutive hsp60 promoter. All constructs were verified by sequencing.
RNA isolation, reverse transcription, and quantitative real-time PCR.
Log-phase Mueller-Hinton M. abscessus cultures were collected by centrifugation, and the bacterial pellets were resuspended in 800 μl of buffer RA1 from the NucleoSpin RNA kit (Macherey-Nagel, Germany), which was then transferred to a lysing matrix B tube (MP Biomedicals, USA), and lysed using a Mixer Mill MM301 (Retsch, Germany) at a frequency of 30 Hz for 1 min four times with intermittent cooling on ice. From this point on, the NucleoSpin RNA kit manufacturer's protocol was followed. Isolated RNA was checked for purity using spectrophotometry and by automated capillary electrophoresis using the Agilent Bioanalyzer (Agilent, USA). Using the above-mentioned protocol for RNA extraction, A260/A280 values exceeding 2, A260/A230 values exceeding 1.8, and RNA integrity number (RIN) values exceeding 7.5 were routinely obtained. Prior to reverse transcription, a genomic DNA wipeout was performed using amplification-grade DNase I (Invitrogen, USA). A total of 100 ng RNA was reverse transcribed into cDNA using SuperScript II reverse transcriptase (Invitrogen, USA). A dilution series of cDNA was tested to obtain optimal quantitative PCR data for each gene assayed, and it was determined that a 5-fold dilution of all cDNA samples resulted in threshold cycle (CT) values within the efficient PCR range. PCR efficiency was determined for each primer set using a dilution series prepared from purified M. abscessus genomic DNA. Quantitative reverse transcription-PCR (qRT-PCR) was performed using the LightCycler 480 SYBR green I master and the manufacturer's recommendations, including standard cycling conditions. The IDT Scitools Primer Quest tool (https://eu.idtdna.com/Primerquest/Home/Index) was used to design primers for intercalating dye chemistry. The reference gene used for qRT-PCR relative quantitation was sigA, and the ΔΔCT method was used to calculate fold differences in expression between strains.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Heym, A.-L. Roux, and J.-L. Gaillard for clinical isolates of the M. abscessus complex, L. Ma and A. Pawlik for help with whole-genome sequence analysis, and F. Roquet-Baneres for help in MIC determination.
LabEx EpiGenMed is acknowledged for funding I.H. L.K. and R.B. acknowledge support by the Fondation pour la Recherche Médicale (FRM) (DEQ20150331719 and DEQ20130326471, respectively).
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02509-16.
REFERENCES
- 1.Singh M, Dugdale CM, Solomon IH, Huang A, Montgomery MW, Pomahac B, Yawetz S, Maguire JH, Talbot SG. 2016. Rapid-growing mycobacteria infections in medical tourists: our experience and literature review. Aesthet Surg J 36:NP246–NP253. doi: 10.1093/asj/sjw047. [DOI] [PubMed] [Google Scholar]
- 2.Roux A-L, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, Bellis G, Vibet M-A, Le Roux E, Lemonnier L, Gutierrez C, Vincent V, Fauroux B, Rottman M, Guillemot D, Gaillard J-L, Herrmann J-L, OMA Group. 2009. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol 4 7:4124–4128. doi: 10.1128/JCM.01257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esther CR, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71:88–90. doi: 10.1136/thoraxjnl-2015-207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown-Elliott BA, Nash KA, Wallace RJ. 2012. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 25:545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 7.Nash KA, Brown-Elliott AB, Wallace RJ. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, Rottman M, Macheras E, Heym B, Herrmann J-L, Daffé M, Brosch R, Risler J-L, Gaillard J-L. 2009. Nonmycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One 4:e5660. doi: 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupont C, Viljoen A, Dubar F, Blaise M, Bernut A, Pawlik A, Bouchier C, Brosch R, Guérardel Y, Lelièvre J, Ballell L, Herrmann J-L, Biot C, Kremer L. 2016. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol Microbiol 101:515–529. doi: 10.1111/mmi.13406. [DOI] [PubMed] [Google Scholar]
- 10.Davidson PT, Hanh Le Q. 1992. Drug treatment of tuberculosis–1992. Drugs 43:651–673. doi: 10.2165/00003495-199243050-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nunn P, Porter J, Winstanley P. 1993. Thiacetazone—avoid like poison or use with care? Trans R Soc Trop Med Hyg 87:578–582. doi: 10.1016/0035-9203(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 12.Watkins WM, Mungai M, Muhia DK, Mberu EK, Gathua S, Winstanley PA, Gilks CF, Nunn P. 1996. Cutaneous hypersensitivity reactions to thiacetazone, HIV infection and thiacetazone concentrations in plasma. Br J Clin Pharmacol 41:160–162. doi: 10.1111/j.1365-2125.1996.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 13.Dover LG, Alahari A, Gratraud P, Gomes JM, Bhowruth V, Reynolds RC, Besra GS, Kremer L. 2007. EthA, a common activator of thiocarbamide-containing drugs acting on different mycobacterial targets. Antimicrob Agents Chemother 51:1055–1063. doi: 10.1128/AAC.01063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBarber AE, Mdluli K, Bosman M, Bekker LG, Barry CE. 2000. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 97:9677–9682. doi: 10.1073/pnas.97.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grzegorzewicz AE, Eynard N, Quémard A, North EJ, Margolis A, Lindenberger JJ, Jones V, Korduláková J, Brennan PJ, Lee RE, Ronning DR, McNeil MR, Jackson M. 2015. Covalent modification of the Mycobacterium tuberculosis FAS-II dehydratase by isoxyl and thiacetazone. ACS Infect Dis 1:91–97. doi: 10.1021/id500032q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermudez LE, Reynolds R, Kolonoski P, Aralar P, Inderlied CB, Young LS. 2003. Thiosemicarbazole (thiacetazone-like) compound with activity against Mycobacterium avium in mice. Antimicrob Agents Chemother 47:2685–2687. doi: 10.1128/AAC.47.8.2685-2687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrère-Kremer S, Blaise M, Singh VK, Alibaud L, Tuaillon E, Halloum I, van de Weerd R, Guérardel Y, Drancourt M, Takiff H, Geurtsen J, Kremer L. 2015. A new dehydratase conferring innate resistance to thiacetazone and intra-amoebal survival of Mycobacterium smegmatis. Mol Microbiol 96:1085–1102. doi: 10.1111/mmi.12992. [DOI] [PubMed] [Google Scholar]
- 18.Halloum I, Carrère-Kremer S, Blaise M, Viljoen A, Bernut A, Le Moigne V, Vilchèze C, Guérardel Y, Lutfalla G, Herrmann J-L, Jacobs WR, Kremer L. 2016. Deletion of a dehydratase important for intracellular growth and cording renders rough Mycobacterium abscessus avirulent. Proc Natl Acad Sci U S A 113:E4228–E4237. doi: 10.1073/pnas.1605477113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alahari A, Trivelli X, Guérardel Y, Dover LG, Besra GS, Sacchettini JC, Reynolds RC, Coxon GD, Kremer L. 2007. Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria. PLoS One 2:e1343. doi: 10.1371/journal.pone.0001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coxon GD, Craig D, Corrales RM, Vialla E, Gannoun-Zaki L, Kremer L. 2013. Synthesis, antitubercular activity and mechanism of resistance of highly effective thiacetazone analogues. PLoS One 8:e53162. doi: 10.1371/journal.pone.0053162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre A-L, Dubée V, Cortes M, Dorchêne D, Arthur M, Mainardi J-L. 2016. Bactericidal and intracellular activity of β-lactams against Mycobacterium abscessus. J Antimicrob Chemother 71:1556–1563. doi: 10.1093/jac/dkw022. [DOI] [PubMed] [Google Scholar]
- 23.Bastian S, Veziris N, Roux A-L, Brossier F, Gaillard J-L, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adékambi T, Reynaud-Gaubert M, Greub G, Gevaudan M-J, La Scola B, Raoult D, Drancourt M. 2004. Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J Clin Microbiol 42:5493–5501. doi: 10.1128/JCM.42.12.5493-5501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlik A, Garnier G, Orgeur M, Tong P, Lohan A, Le Chevalier F, Sapriel G, Roux A-L, Conlon K, Honoré N, Dillies M-A, Ma L, Bouchier C, Coppée J-Y, Gaillard J-L, Gordon SV, Loftus B, Brosch R, Herrmann JL. 2013. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol Microbiol 90:612–629. doi: 10.1111/mmi.12387. [DOI] [PubMed] [Google Scholar]
- 26.Macheras E, Konjek J, Roux A-L, Thiberge J-M, Bastian S, Leão SC, Palaci M, Sivadon-Tardy V, Gutierrez C, Richter E, Rüsch-Gerdes S, Pfyffer GE, Bodmer T, Jarlier V, Cambau E, Brisse S, Caro V, Rastogi N, Gaillard J-L, Heym B. 2014. Multilocus sequence typing scheme for the Mycobacterium abscessus complex. Res Microbiol 165:82–90. doi: 10.1016/j.resmic.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Mori G, Chiarelli LR, Riccardi G, Pasca MR. 2016. New prodrugs against tuberculosis. Drug Discov Today. doi: 10.1016/j.drudis.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Baulard AR, Betts JC, Engohang-Ndong J, Quan S, McAdam RA, Brennan PJ, Locht C, Besra GS. 2000. Activation of the pro-drug ethionamide is regulated in mycobacteria. J Biol Chem 275:28326–28331. doi: 10.1074/jbc.M003744200. [DOI] [PubMed] [Google Scholar]
- 29.Engohang-Ndong J, Baillat D, Aumercier M, Bellefontaine F, Besra GS, Locht C, Baulard AR. 2004. EthR, a repressor of the TetR/CamR family implicated in ethionamide resistance in mycobacteria, octamerizes cooperatively on its operator. Mol Microbiol 51:175–188. [DOI] [PubMed] [Google Scholar]
- 30.Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 31.Grzegorzewicz AE, Korduláková J, Jones V, Born SEM, Belardinelli JM, Vaquié A, Gundi VAKB, Madacki J, Slama N, Laval F, Vaubourgeix J, Crew RM, Gicquel B, Daffé M, Morbidoni HR, Brennan PJ, Quémard A, McNeil MR, Jackson M. 2012. A common mechanism of inhibition of the Mycobacterium tuberculosis mycolic acid biosynthetic pathway by isoxyl and thiacetazone. J Biol Chem 287:38434–38441. doi: 10.1074/jbc.M112.400994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuthbertson L, Nodwell JR. 2013. The TetR family of regulators. Microbiol Mol Biol Rev 77:440–475. doi: 10.1128/MMBR.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos JL, Martínez-Bueno M, Molina-Henares AJ, Terán W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. 2005. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev 69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milano A, Pasca MR, Provvedi R, Lucarelli AP, Manina G, Ribeiro AL, Manganelli R, Riccardi G. 2009. Azole resistance in Mycobacterium tuberculosis is mediated by the MmpS5-MmpL5 efflux system. Tuberculosis (Edinb) 89:84–90. doi: 10.1016/j.tube.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viljoen A, Blaise M, de Chastellier C, Kremer L. 2016. MAB_3551c encodes the primary triacylglycerol synthase involved in lipid accumulation in Mycobacterium abscessus. Mol Microbiol 102:611–627. doi: 10.1111/mmi.13482. [DOI] [PubMed] [Google Scholar]
- 37.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN. 2010. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martiniano SL, Nick JA, Daley CL. 2016. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med 37:83–96. doi: 10.1016/j.ccm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Maurer FP, Bruderer VL, Ritter C, Castelberg C, Bloemberg GV, Böttger EC. 2014. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother 58:3828–3836. doi: 10.1128/AAC.02448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S, Bouzinbi N, Chaturvedi V, Godreuil S, Kremer L. 2014. In vitro evaluation of a new drug combination against clinical isolates belonging to the Mycobacterium abscessus complex. Clin Microbiol Infect 20:O1124–O1127. doi: 10.1111/1469-0691.12780. [DOI] [PubMed] [Google Scholar]
- 41.Ballell L, Bates RH, Young RJ, Alvarez-Gomez D, Alvarez-Ruiz E, Barroso V, Blanco D, Crespo B, Escribano J, González R, Lozano S, Huss S, Santos-Villarejo A, Martín-Plaza JJ, Mendoza A, Rebollo-Lopez MJ, Remuiñan-Blanco M, Lavandera JL, Pérez-Herran E, Gamo-Benito FJ, García-Bustos JF, Barros D, Castro JP, Cammack N. 2013. Fueling open-source drug discovery: 177 small-molecule leads against tuberculosis. ChemMedChem 8:313–321. doi: 10.1002/cmdc.201200428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh W-J, Jeon K, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 43.Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, Yano S, Shigeto E, Kuraoka T, Kajiki A, Kobashi Y, Kokubu F, Sato A, Yoshida S, Iwamoto T, Saito H. 2012. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J Clin Microbiol 50:3556–3561. doi: 10.1128/JCM.01175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balhana RJC, Singla A, Sikder MH, Withers M, Kendall SL. 2015. Global analyses of TetR family transcriptional regulators in mycobacteria indicates conservation across species and diversity in regulated functions. BMC Genomics 16:479. doi: 10.1186/s12864-015-1696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Chen J, Cui P, Shi W, Zhang W, Zhang Y. 2015. Identification of novel mutations associated with clofazimine resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 70:2507–2510. doi: 10.1093/jac/dkv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Somoskovi A, Bruderer V, Hömke R, Bloemberg GV, Böttger EC. 2015. A mutation associated with clofazimine and bedaquiline cross-resistance in MDR-TB following bedaquiline treatment. Eur Respir J 45:554–557. doi: 10.1183/09031936.00142914. [DOI] [PubMed] [Google Scholar]
- 47.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radhakrishnan A, Kumar N, Wright CC, Chou T-H, Tringides ML, Bolla JR, Lei H-T, Rajashankar KR, Su C-C, Purdy GE, Yu EW. 2014. Crystal structure of the transcriptional regulator Rv0678 of Mycobacterium tuberculosis. J Biol Chem 289:16526–16540. doi: 10.1074/jbc.M113.538959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalut C. 2016. MmpL transporter-mediated export of cell-wall associated lipids and siderophores in mycobacteria. Tuberculosis (Edinb) 100:32–45. doi: 10.1016/j.tube.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Bernut A, Viljoen A, Dupont C, Sapriel G, Blaise M, Bouchier C, Brosch R, de Chastellier C, Herrmann J-L, Kremer L. 2016. Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue conserved in all mycobacterial MmpL family members. Mol Microbiol 99:866–883. doi: 10.1111/mmi.13283. [DOI] [PubMed] [Google Scholar]
- 51.Székely R, Cole ST. 2016. Mechanistic insight into mycobacterial MmpL protein function. Mol Microbiol 99:831–834. doi: 10.1111/mmi.13306. [DOI] [PubMed] [Google Scholar]
- 52.Lamichhane G, Tyagi S, Bishai WR. 2005. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infect Immun 73:2533–2540. doi: 10.1128/IAI.73.4.2533-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells RM, Jones CM, Xi Z, Speer A, Danilchanka O, Doornbos KS, Sun P, Wu F, Tian C, Niederweis M. 2013. Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis. PLoS Pathog 9:e1003120. doi: 10.1371/journal.ppat.1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones CM, Wells RM, Madduri AVR, Renfrow MB, Ratledge C, Moody DB, Niederweis M. 2014. Self-poisoning of Mycobacterium tuberculosis by interrupting siderophore recycling. Proc Natl Acad Sci U S A 111:1945–1950. doi: 10.1073/pnas.1311402111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of Mycobacteria, Nocardiae and other aerobic actinomycetes: approved standard, 2nd ed M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 56.Boritsch EC, Frigui W, Cascioferro A, Malaga W, Etienne G, Laval F, Pawlik A, Le Chevalier F, Orgeur M, Ma L, Bouchier C, Stinear TP, Supply P, Majlessi L, Daffé M, Guilhot C, Brosch R. 2016. pks5-recombination-mediated surface remodelling in Mycobacterium tuberculosis emergence. Nat Microbiol 1:15019. doi: 10.1038/nmicrobiol.2015.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.