Abstract
Background: Calorie restriction (CR) retards aging and increases longevity in many animal models. However, it is unclear whether CR can be implemented in humans without adverse effects on body composition.
Objective: We evaluated the effect of a 2-y CR regimen on body composition including the influence of sex and body mass index (BMI; in kg/m2) among participants enrolled in CALERIE-2 (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy), a multicenter, randomized controlled trial.
Design: Participants were 218 nonobese (BMI: 21.9–28.0) adults aged 21–51 y who were randomly assigned to 25% CR (CR, n = 143) or ad libitum control (AL, n = 75) in a 2:1 ratio. Measures at baseline and 12 and 24 mo included body weight, waist circumference, fat mass (FM), fat-free mass (FFM), and appendicular mass by dual-energy X-ray absorptiometry; activity-related energy expenditure (AREE) by doubly labeled water; and dietary protein intake by self-report. Values are expressed as means ± SDs.
Results: The CR group achieved 11.9% ± 0.7% CR over 2-y and had significant decreases in weight (−7.6 ± 0.3 compared with 0.4 ± 0.5 kg), waist circumference (−6.2 ± 0.4 compared with 0.9 ± 0.5 cm), FM (−5.4 ± 0.3 compared with 0.5 ± 0.4 kg), and FFM (−2.0 ± 0.2 compared with −0.0 ± 0.2 kg) at 24 mo relative to the AL group (all between-group P < 0.001). Moreover, FFM as a percentage of body weight at 24 mo was higher, and percentage of FM was lower in the CR group than in the AL. AREE, but not protein intake, predicted preservation of FFM during CR (P < 0.01). Men in the CR group lost significantly more trunk fat (P = 0.03) and FFM expressed as a percentage of weight loss (P < 0.001) than women in the CR group.
Conclusions: Two years of CR had broadly favorable effects on both whole-body and regional adiposity that could facilitate health span in humans. The decrements in FFM were commensurate with the reduced body mass; although men in the CR group lost more FFM than the women did, the percentage of FFM in the men in the CR group was higher than at baseline. CALERIE was registered at clinicaltrials.gov as NCT00427193.
Keywords: body composition, calorie restriction, humans, long-term, nonobese
INTRODUCTION
Aging is associated with a decline in both the quantity and quality of fat-free mass (FFM)14 in parallel with increases in body weight and adiposity (1). These age-associated changes in body composition negatively affect physical function and heighten the risk of metabolic disorders, including insulin resistance, type 2 diabetes, hypertension, atherosclerosis, and cancer (2). Central adiposity, in particular, increases with advancing age (3, 4) and increases the risk of metabolic syndrome, highlighting the importance of adipose tissue distribution in deleterious age-associated diseases. Interventions that attenuate age-associated changes in body composition could delay or even prevent the onset of metabolic disease and as a result improve quality of life and health span (5).
Calorie restriction (CR) is a dietary intervention that involves a relative deficit in energy intake while preserving adequate intake of protein and essential micronutrients. CR is the only dietary intervention that has shown promise regarding a reduction in the rate of biological aging in many nonhuman species (6). However, it is unclear whether long-term CR can be implemented in nonobese humans without unfavorable effects on body composition, for example, decreased FFM relative to body fat or lack of a reduction in central adiposity.
The CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy)-2 study is a multisite, randomized controlled trial (RCT) to test the effect of long-term CR in humans (7). Phase 1, or CALERIE-1, was a pilot phase consisting of site-specific studies to determine the best approach to CR (8–10). The primary objective of CALERIE-2 was to test the hypothesis that 2 y of CR, involving a 25% reduction in energy intake from baseline in nonobese men and women aged 21–50 y, would result in the same adaptive changes that occur in rodents subjected to CR, namely a slowing of metabolic aging and protection against age-related disease processes. In separate reports of CALERIE-2 findings we have shown that sustained CR over the 2-y period is feasible, safe, and well tolerated in nonobese humans (11). No changes were observed in the 2 primary outcomes (resting metabolic rate and core body temperature) (12), but improvements in cardiometabolic risk factors, adaptive decreases in daily energy expenditure, and decreases in the 2 secondary outcomes (circulating T3 and TNF-α) were observed that were consistent with potential antiaging effects (12). Additional relevant changes in exploratory outcomes have included small decreases in bone mineral density in the lumbar spine and femoral neck (13), persistent increases in serum insulin-like growth factor (IGF) binding protein-1, and no change in IGF-1 or biologically relevant increases in cortisol (14). Herein, we describe a preplanned secondary analysis that examined the short- and long-term effects of CR on body composition, including whole-body and regional changes. Because of the increased interest in examining sex-specific differences in response to interventions, we also examined differential effects of CR on men and women and in normal-weight compared with overweight individuals.
METHODS
Overview
CALERIE-2 (NCT00427193) was a 2-y, multicenter, parallel-group, RCT that implemented a single protocol across the sites. With a 2:1 allocation in favor of CR, participants were randomly assigned either to the CR group, which aimed to reduce energy intake by 25%, or to the ad libitum control (AL) group, which aimed to maintain habitual energy intake on an ad libitum basis (7, 12, 15). The study protocol was approved by the institutional review boards at Tufts University (Boston, Massachusetts), Pennington Biomedical Research Center (Baton Rouge, Louisiana), Washington University (St. Louis, Missouri), and Duke University (Durham, North Carolina). Study oversight was provided by the NIH-appointed data and safety monitoring board, and all participants provided written, informed consent.
Participants
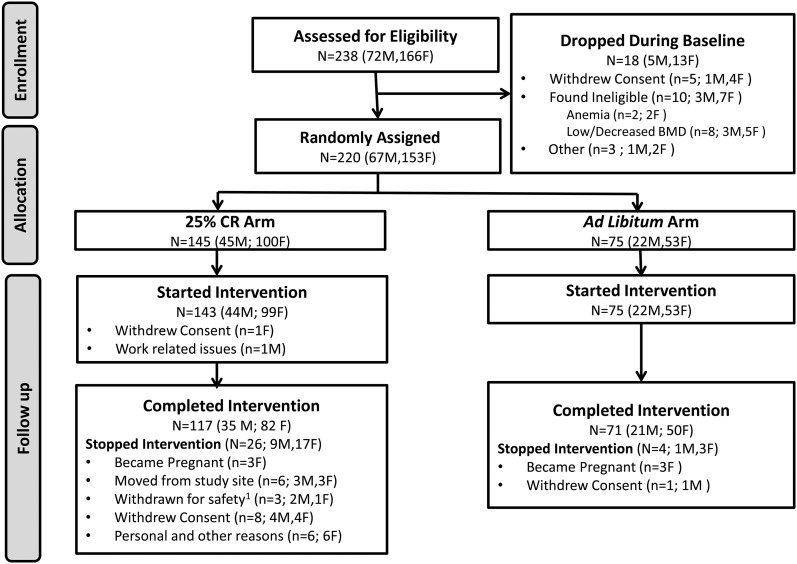
Healthy individuals from both sexes and all races were eligible to participate. Men were required to be between 21 and 50 y of age (inclusive) and women 21–47 y of age (inclusive) to avoid menopause. All participants were required to be normal weight or slightly overweight, with a BMI (in kg/m2) of 22.0–28.0 at screening. Eligibility was assessed during 3 comprehensive screening visits to identify subjects who were physically and psychologically healthy to participate in a 2-y study of CR and willing and able to adhere to the rigors of the study. Details on the study recruitment and screening process and exclusion criteria can be found elsewhere (7, 16) and are summarized in the Consolidated Standards of Reporting Trials diagram in Figure 1.
FIGURE 1.
Participant enrollment and retention, breakdown by sex. Study participant flow and reasons for attrition broken down by men and women within the ad libitum control group and CR. 1Reasons why the participants were withdrawn for safety: A BMD deficit in 1 participant and anemia in 2 other participants did not resolve, and they were permanently withdrawn from the intervention. Following intention-to-treat principles, their data were nevertheless included in the analysis dataset. BMD, bone mineral density; CR, calorie restriction.
Study design
Baseline testing was conducted over a 6-wk period and included a detailed set of evaluations to determine health status of the study participants before random assignment and 2 back-to-back measurements of energy expenditure with doubly labeled water (DLW) to determine individual energy requirements from which the 25% CR prescription was derived (7, 12). After baseline testing, participants were randomly assigned to either the CR or the AL group. Specifically, random assignment was stratified by sex and BMI within each clinical site with BMI dichotomized into normal weight (22.0 ≤ BMI < 25.0) or overweight (25.0 ≤ BMI < 28.0). Within each stratum, individuals were allocated in a 2:1 ratio in favor of the 25% CR intervention. Randomization sequences within each stratum were generated a priori by the coordinating center by using a permuted block randomization technique. The actual treatment assignment was carried out centrally by using a telephone-based, interactive voice-response system.
The CR intervention targeted an immediate and sustained 25% restriction of energy intake from individual baseline DLW-measured requirements. Details about the intervention have been reported previously (7, 15, 17). The CR intervention was driven by a mathematical model (18), with the anticipated trajectory of predicted weekly changes in body weight for each participant for 1 y of 25% CR (15). The weekly weight-loss graph showing the targeted weight-loss trajectory served as a guide for providing intensive nutritional and behavioral guidance in individual sessions in addition to structured curriculum and counseling delivered in a group format and was also used as the primary tool to promote adherence during the intervention (15). The AL group was advised to continue their current diet on a completely ad libitum basis. The AL group did not receive any specific dietary intervention or counseling and had quarterly contact with study investigators and participated in outcome assessments at roughly the same schedule as the CR participants. No specific level of physical activity was prescribed to participants in either group. A multivitamin and mineral supplement (Nature Made Multi Complete; Pharmavite LLC) and an additional calcium supplement (1000 mg/d; Douglas Laboratories) were provided to all participants to ensure that current recommendations for micronutrients were met.
Outcome assessments
All body-composition and related measurements described in this section were obtained during baseline testing and at months 12 (±14 d) and 24 (±14 d) after random assignment.
Anthropometric measurements
Participants were weighed by using a calibrated scale standardized across study sites (Scale Tronix 5200; Welch Allyn) in the morning after an overnight fast of ≥8 h while subjects were wearing only a preweighed hospital gown. Height was measured twice by using a calibrated wall-mounted stadiometer. BMI was calculated from clinic weight at baseline and at each time point and by using height measured at screening. Waist circumference was measured at the natural waist and at the umbilicus.
Body fat mass, lean body mass, total body protein, and appendicular mass
Body composition [fat mass (FM), lean body mass (LBM), and bone mass] was measured by dual-energy X-ray absorptiometry (DXA) by using the Hologic 4500A, Delphi W, or Discovery A scanners according to a standardized protocol. FM and FFM were determined for the whole body as well as the trunk and appendicular (arms and legs) by using Hologic software version Apex 3.3. LBM, also known as lean soft-tissue mass, was calculated as the difference between FFM and bone mass. A measure of total body protein was calculated as the difference between body weight and the sum of fat mass from DXA, total body water (TBW; from 18O dilution described in the next paragraph), and bone mineral content (BMC) obtained from the DXA output. All scans were analyzed at a centralized reading center (University of California, San Francisco), which was also responsible for centralized quality control across study sites. Machine performance was monitored with spine and whole-body phantoms, including baseline cross-calibration and longitudinal scans. Precision of DXA was assessed by the chief DXA operator at each site on the site-specific scanner. The CV for the precision of FM ranged from 0.95% to 1.23%, for FFM from 0.43% to 0.58%, for LBM and lean soft-tissue mass from 0.44% to 0.63%, and for BMC from 0.74% to 0.90%.
TBW by 18O dilution
TBW was determined by using the isotope dilution technique according to the procedures detailed by Wong et al. (19). Two predose spot urine samples were collected from each participant. Each participant then ingested 1.38 g of 10 atom percent 18O labeled water (Sigma-Aldrich Corp) per kilogram body weight. Six more spot urine samples were collected from each participant at 3 and 4 h postdose and again on days 7 and 14 (12). The 18O content of the urine and plasma samples was measured by gas-isotope-ratio mass spectrometry (20). The 18O dilution space was calculated from the zero-time intercept of the 18O turnover rate by using the back extrapolation method. The 18O dilution space was converted to TBW after correcting for the 1% overestimation of TBW due to isotope exchange with nonaqueous exchangeable oxygen in the body (21). By using the ratio of TBW data from the 18O dilution method and the FFM data from the DXA, the hydration of FFM was calculated (22).
Nutrient intake
Six-day food records were collected by using paper logs, entered into the University of Minnesota Nutrient Data System program, and analyzed at a central reading center (University of Cincinnati) for calories, macronutrient composition (percentage of energy), fiber, and variety. Protein intake was expressed both as absolute (g/d) and relative (g · kg body mass−1 · d−1) quantities. The percentage of difference (mean ± SD) in the reported compared with the DLW-measured energy intake at 12 mo was −13.34% ± 16.57% in the CR group and −16.74% ± 18.33% in the AL group and at 24 mo was −13.89% ± 17.08% in the CR group and −18.34% ± 18.59% in the AL group.
Percentage of CR
The average percentage of CR (%CR) over 6-mo intervals was calculated retrospectively by the intake-balance method and with the 6-, 12-, 18-, and 24-mo measures of total daily energy expenditure (TDEE) from DLW and changes in body composition between the time periods and relative to baseline (17–19, 23).
Activity-related energy expenditure
Activity-related energy expenditure (AREE) was estimated from measurements of TDEE and resting metabolic rate (RMR) with an estimate for the thermic effect of food by using the formula AREE = (TDEE × 0.9) − RMR. TDEE was determined by using the DLW method. RMR was assessed by indirect calorimetry with a calibrated Vista-MX metabolic cart (Vacumed). Thermic effect of food was estimated to be TDEE × 0.1. At baseline, energy intake was assumed to equal TDEE because participants were weight stable; at the 1- and 2-y time points, energy intake was computed from TDEE and changes in body energy stores based on DXA measurements (12, 23).
Statistical methods
The same statistical methodologies used in the parent RCT were applied (12). Briefly, the analysis was performed under the intention-to-treat (ITT) criterion. Repeated-measures ANCOVA (24, 25) was applied with change from baseline as the dependent variable and treatment, time, and the treatment × time interaction as independent variables. Design variables, site, sex, baseline BMI stratum, and baseline value of the outcome were included as covariates to increase precision. To avoid arbitrary assumptions of linearity in time, time was treated as a categorical variable; similarly, an unstructured model was assumed for the covariance matrix among the repeated observations. All hypotheses, e.g., main effects, interactions, within-group changes over time, and between-group differences at the individual time points, were tested by defining appropriate contrasts among the associated regression parameters. The predicted mean changes ± SEs are the adjusted values from these contrasts. For the change from month 12 to 24, the within-treatment arm P values test the null hypothesis that there was zero change in the interval. For variables with a P < 0.05, the null hypothesis was rejected with the conclusion that there was a decrease (or increase) during the interval as indicated by the change value. On the other hand, if P ≥ 0.05, we could not conclude that the change was zero, but we could state that the test failed to reject the null hypothesis of no change. The estimates of the change in the interval of 12–24 mo were incorporated into our testing structure and were redundant information contained in the 0- to 12- and 0- to 24-mo estimated effects of change. They are presented for clarity, but because they were not part of our prespecified overall analytic structure and were not part of the structure for the overall type I error, we made no adjustments for P values either between or within outcomes for these 12- to 24-mo effects. Lack of consistency between sexes (male, female) and baseline BMI groups (normal weight, overweight) was evaluated by adding and testing interaction terms of these variables with CR group and time in this model.
For any particular outcome, type I error was controlled by using a hierarchical gatekeeping strategy (26). The treatment × visit interaction term was tested first. If significant, between-group differences at each time point were then tested at α = 0.05. If not, the treatment main effect was tested next. If significant, then between-group differences at each time point were tested at α = 0.05. Otherwise a Bonferroni correction was applied at each time point, with the P values adjusted by multiplying the nominal P value by the number of tests (truncated at 1.0) (27). Within-group changes from baseline to the follow-up visits, however, fell outside of this hierarchy and were always protected by a Bonferroni correction. Although we adopted a rigorous approach for controlling type I error within a specific outcome, we did not apply any adjustment (such as Bonferroni corrections) to control for the type I error inherent in the multiplicity of tests performed across the broad list of body-composition outcomes. The residuals were visually checked for approximate normality. In the presence of extreme values, appropriate transformations (e.g., log-transform) were performed; when no transformation was deemed adequate, group differences were tested by using Wilcoxon’s rank-sum test. By using a median split on %CR achieved at 24 mo, supplemental analyses were conducted to examine the variability in body-composition changes in high-and-low CR groups. There is a loss of power when one halves the sample size. Inferential statistics on the results from these groups are presented, but statistical comparisons are qualified because %CR was not randomized. Rather, participants achieved their level of %CR over the 2-y period, and thus the subjects in these groups might vary on a range of confounders not included in the model.
Pearson’s correlation was used to examine relations between changes in body weight and change in regional body-composition variables and to examine the association between changes in FM and hormonal measures. A stepwise multiple-regression model with candidate prespecified predictors [age, sex, baseline BMI, AREE, protein intake per kilogram of body weight (28), and IGF] (14) was used to determine which variables were associated with the change in FFM with CR in men and women. All analyses were performed by using Statistical Analysis Software version 9.3. Results are reported as means ± SEs except when otherwise noted.
RESULTS
As shown in the Consolidated Standards of Reporting Trials diagram (Figure 1), 238 participants were deemed eligible and completed screening assessments; 10 participants were subsequently determined to be ineligible, and 8 withdrew during baseline leading to 220 individuals (67 men, 153 women) being randomly assigned. Two individuals in the CR group dropped out before starting the intervention, resulting in an ITT cohort of 218 participants [75 in the AL group (22 men, 53 women) and 143 in the CR group (44 men, 99 women)]. Thirty participants (4 in the AL group, 26 in the CR group) dropped out of the study for personal and other reasons and, following ITT principles, were included in the primary analyses [details are published elsewhere (12, 16)].
Baseline
Study participants were predominantly female (69.7%) and Caucasian (77.1%) with ages ranging from 20.7 to 50.8 y. The AL and CR groups did not differ in baseline demographic and body-composition characteristics (Table 1). Further, no significant differences were observed between the AL and CR groups for these variables within each sex except for age (P < 0.05) and BMI subgroup (data not shown).
TABLE 1.
Demographic and anthropometric characteristics at baseline for the 218 participants who started the 2-y study1
| Men (n = 66) |
Women (n = 152) |
Overall (n = 218) |
||||
| AL (n = 22) | CR (n = 44) | AL (n = 53) | CR (n = 99) | AL (n = 75) | CR (n = 143) | |
| Demographics | ||||||
| Age, y | 37.8 ± 1.512 | 40.5 ± 1.083 | 37.9 ± 0.95 | 36.8 ± 0.723 | 37.9 ± 0.80 | 38.0 ± 0.61 |
| Sex | 22 (29.3) | 44 (30.8) | 53 (70.7) | 99 (69.2) | — | — |
| Race, n (%) | ||||||
| White | 18 (81.8) | 37 (84.1) | 39 (73.6) | 74 (74.7) | 57 (76.0) | 111 (77.6) |
| African American | 1 (4.6) | 2 (4.6) | 10 (18.9) | 13 (13.1) | 11 (14.7) | 15 (10.5) |
| Other | 3 (13.6) | 5 (11.4) | 4 (7.6) | 12 (12.1) | 7 (9.3) | 17 (11.9) |
| Anthropometry | ||||||
| Height, m | 176.7 ± 1.13 | 177.1 ± 1.08 | 165.0 ± 0.93 | 165.2 ± 0.64 | 168.4 ± 0.96 | 168.9 ± 0.72 |
| Weight, kg | 79.8 ± 1.41 | 81.6 ± 1.25 | 68.0 ± 0.95 | 67.7 ± 0.64 | 71.5 ± 1.00 | 72.0 ± 0.79 |
| BMI, kg/m2 | 25.6 ± 0.36 | 26.0 ± 0.24 | 24.9 ± 0.22 | 24.8 ± 0.17 | 25.1 ± 0.19 | 25.2 ± 0.15 |
| Body composition | ||||||
| Body fat, % | 25.7 ± 0.85 | 26.1 ± 0.46 | 36.8 ± 0.58 | 36.0 ± 0.44 | 33.6 ± 0.76 | 32.9 ± 0.51 |
P values (data not shown) as calculated by Wilcoxon’s test for continuous and ordinal values and Fisher’s exact test for categorical values were not statistically significant for between-group (AL compared with CR) differences for men, women, and overall participants for all listed variables. AL, ad libitum control; CR, calorie restriction.
Mean ± SE (all such values).
Age at baseline was significantly different between men and women in the CR group (P < 0.05).
Body-composition changes
The 2-y intervention yielded 11.9% CR and resulted in a sustained 10.4% weight loss. The effects of CR on the change in body composition were remarkably consistent at both 0–12 and 0–24 mo and for the 12- to 24-mo time points across most body-composition measures. In general, there were large, significant changes from baseline in the CR group that persisted over the follow-up period at 24 mo, smaller nonsignificant changes in the AL group, and highly significant between-group differences at both 12- and 24-mo time points (Tables 2 and 3). Except for a few that are noted, no significant differences were observed for changes between 12 and 24 mo between or within the AL and CR groups for most major body-composition variables. An example of this general pattern (i.e., for body weight) is discussed below in detail.
TABLE 2.
A comparison of whole-body composition at baseline and after 12 and 24 mo between the AL control and CR groups1
| Men (n = 66) |
Women (n = 152) |
Overall (n = 218) |
|||||||
| AL (n = 22) | CR (n = 44) | AL (n = 53) | CR (n = 99) | AL (n = 75) | P-AL3 | CR (n = 143) | P-CR3 | Overall P: AL-CR2 | |
| Weight, kg | |||||||||
| Baseline | 79.8 ± 1.41 | 81.6 ± 1.25 | 68.0 ± 0.95 | 67.7 ± 0.64 | 71.5 ± 1.00 | — | 72.0 ± 0.79 | — | 0.978 |
| Δ Month 12 | −0.9 ± 0.72 | −9.5 ± 0.47 | −0.2 ± 0.40 | −7.8 ± 0.32 | −0.4 ± 0.35 | 0.157 | −8.3 ± 0.27 | <0.001 | <0.001 |
| Δ Month 24 | −0.2 ± 0.76 | −8.9 ± 0.57 | 0.6 ± 0.63 | −7.0 ± 0.37 | 0.4 ± 0.50 | 1.000 | −7.6 ± 0.32 | <0.001 | <0.001 |
| Δ Month 12–24 | 0.7 ± 0.45 | 0.8 ± 0.31 | 0.8 ± 0.58 | 1.0 ± 0.18 | 0.8 ± 0.42 | 0.021 | 0.9 ± 0.16 | <0.001 | 0.893 |
| Body fat, % | |||||||||
| Baseline | 25.7 ± 0.85 | 26.1 ± 0.46 | 36.8 ± 0.58 | 36.0 ± 0.44 | 33.6 ± 0.76 | — | 32.9 ± 0.51 | — | 0.336 |
| Δ Month 12 | −0.6 ± 0.52 | −5.4 ± 0.44 | −0.2 ± 0.29 | −5.4 ± 0.25 | −0.3 ± 0.26 | 0.381 | −5.4 ± 0.22 | <0.001 | <0.001 |
| Δ Month 24 | 0.4 ± 0.39 | −4.8 ± 0.45 | 0.3 ± 0.46 | −4.5 ± 0.29 | 0.3 ± 0.34 | 1.000 | −4.6 ± 0.25 | <0.001 | <0.001 |
| Δ Month 12–24 | 1.0 ± 0.48 | 0.8 ± 0.23 | 0.4 ± 0.41 | 1.0 ± 0.17 | 0.6 ± 0.32 | 0.038 | 1.0 ± 0.13 | <0.001 | 0.293 |
| Fat mass, kg | |||||||||
| Baseline | 20.5 ± 0.83 | 21.3 ± 0.56 | 25.2 ± 0.66 | 24.4 ± 0.43 | 23.8 ± 0.58 | — | 23.5 ± 0.36 | — | 0.611 |
| Δ Month 12 | −0.5 ± 0.55 | −6.3 ± 0.43 | −0.1 ± 0.33 | −6.0 ± 0.25 | −0.2 ± 0.28 | 0.777 | −6.1 ± 0.22 | <0.001 | <0.001 |
| Δ Month 24 | 0.4 ± 0.50 | −5.8 ± 0.45 | 0.6 ± 0.53 | −5.2 ± 0.29 | 0.5 ± 0.40 | 0.846 | −5.4 ± 0.25 | <0.001 | <0.001 |
| Δ Month 12–24 | 0.9 ± 0.45 | 0.7 ± 0.23 | 0.6 ± 0.48 | 0.9 ± 0.14 | 0.7 ± 0.36 | 0.014 | 0.9 ± 0.12 | <0.001 | 0.761 |
| FFM, kg | |||||||||
| Baseline | 59.3 ± 1.12 | 60.3 ± 0.90 | 42.8 ± 0.50 | 43.2 ± 0.41 | 47.6 ± 0.99 | — | 48.5 ± 0.77 | — | 0.475 |
| Δ Month 12 | −0.3 ± 0.25 | −3.0 ± 0.20 | 0.0 ± 0.16 | −1.6 ± 0.13 | −0.1 ± 0.13 | 0.196 | −2.0 ± 0.12 | <0.001 | <0.001 |
| Δ Month 24 | −0.6 ± 0.38 | −3.0 ± 0.32 | 0.3 ± 0.22 | −1.6 ± 0.17 | 0.0 ± 0.20 | 1.000 | −2.0 ± 0.16 | <0.001 | <0.001 |
| Δ Month 12–24 | −0.3 ± 0.25 | 0.1 ± 0.20 | 0.3 ± 0.19 | 0.0 ± 0.13 | 0.1 ± 0.16 | 1.000 | 0.0 ± 0.11 | 1.000 | 0.596 |
| Total lean body mass, kg | |||||||||
| Baseline | 56.5 ± 1.07 | 57.5 ± 0.87 | 40.5 ± 0.47 | 40.9 ± 0.39 | 45.2 ± 0.96 | — | 46.0 ± 0.75 | — | 0.479 |
| Δ Month 12 | −0.3 ± 0.26 | −3.0 ± 0.20 | −0.0 ± 0.15 | −1.6 ± 0.13 | −0.1 ± 0.13 | 0.147 | −2.0 ± 0.13 | <0.001 | <0.001 |
| Δ Month 24 | −0.6 ± 0.38 | −3.0 ± 0.32 | 0.2 ± 0.22 | −1.6 ± 0.17 | −0.0 ± 0.20 | 0.823 | −2.0 ± 0.16 | <0.001 | <0.001 |
| Δ Month 12–24 | −0.3 ± 0.25 | 0.1 ± 0.20 | 0.2 ± 0.19 | 0.0 ± 0.13 | 0.1 ± 0.15 | 1.000 | 0.0 ± 0.11 | 1.000 | 0.793 |
| Trunk FFM, kg | |||||||||
| Baseline | 27.6 ± 0.55 | 27.8 ± 0.40 | 20.8 ± 0.28 | 20.7 ± 0.21 | 22.8 ± 0.44 | — | 22.9 ± 0.34 | — | 0.935 |
| Δ Month 12 | 0.0 ± 0.16 | −1.3 ± 0.12 | 0.1 ± 0.09 | −0.5 ± 0.08 | 0.1 ± 0.08 | 1.000 | −0.8 ± 0.07 | <0.001 | <0.001 |
| Δ Month 24 | 0.0 ± 0.21 | −1.2 ± 0.17 | 0.1 ± 0.13 | −0.6 ± 0.10 | 0.1 ± 0.11 | 1.000 | −0.8 ± 0.09 | <0.001 | <0.001 |
| Δ Month 12–24 | −0.0 ± 0.20 | 0.2 ± 0.13 | 0.0 ± 0.12 | −0.0 ± 0.09 | 0.0 ± 0.10 | 1.000 | 0.0 ± 0.07 | 1.000 | 0.807 |
| Hydration of FFM (TBW:FFM ratio) | |||||||||
| Baseline | 70.7 ± 0.39 | 70.7 ± 0.26 | 72.6 ± 0.32 | 72.9 ± 0.22 | 72.0 ± 0.27 | — | 72.2 ± 0.19 | — | 0.924 |
| Δ Month 12 | 0.5 ± 0.58 | 2.6 ± 0.26 | 0.7 ± 0.28 | 1.7 ± 0.22 | 0.6 ± 0.26 | 0.180 | 1.9 ± 0.18 | <0.001 | <0.001 |
| Δ Month 24 | 0.4 ± 0.44 | 1.5 ± 0.23 | 0.7 ± 0.28 | 0.9 ± 0.27 | 0.6 ± 0.23 | 0.314 | 1.1 ± 0.21 | <0.001 | 0.030 |
| Δ Month 12–24 | −0.1 ± 0.45 | −1.3 ± 0.25 | −0.0 ± 0.32 | −0.8 ± 0.27 | −0.0 ± 0.26 | 1.000 | −0.9 ± 0.20 | <0.001 | 0.011 |
| TBW, kg | |||||||||
| Baseline | 41.9 ± 0.79 | 42.7 ± 0.62 | 31.0 ± 0.38 | 31.5 ± 0.29 | 34.2 ± 0.68 | — | 34.9 ± 0.52 | — | 0.419 |
| Δ Month 12 | 0.0 ± 0.39 | −0.6 ± 0.22 | 0.3 ± 0.15 | −0.4 ± 0.12 | 0.2 ± 0.16 | 0.675 | −0.5 ± 0.11 | 0.001 | <0.001 |
| Δ Month 24 | −0.2 ± 0.35 | −1.2 ± 0.28 | 0.5 ± 0.21 | −0.9 ± 0.16 | 0.3 ± 0.18 | 0.456 | −0.9 ± 0.14 | <0.001 | <0.001 |
| Δ Month 12–24 | −0.2 ± 0.31 | −0.7 ± 0.21 | 0.2 ± 0.21 | −0.3 ± 0.13 | 0.1 ± 0.17 | 1.000 | −0.4 ± 0.11 | <0.001 | 0.010 |
| TBW 18O, % | |||||||||
| Baseline | 52.5 ± 0.72 | 52.3 ± 0.38 | 45.8 ± 0.49 | 46.6 ± 0.34 | 47.7 ± 0.54 | — | 48.3 ± 0.35 | — | 0.306 |
| Δ Month 12 | 0.9 ± 0.62 | 5.9 ± 0.44 | 0.8 ± 0.37 | 5.2 ± 0.25 | 0.8 ± 0.31 | 0.002 | 5.4 ± 0.22 | <0.001 | <0.001 |
| Δ Month 24 | 0.1 ± 0.48 | 4.7 ± 0.41 | 0.5 ± 0.39 | 4.1 ±0.31 | 0.4 ± 0.30 | 0.133 | 4.3 ± 0.25 | <0.001 | <0.001 |
| Δ Month 12–24 | −0.8 ± 0.41 | −1.5 ± 0.25 | −0.2 ± 0.43 | −1.3 ± 0.19 | −0.4 ± 0.32 | 0.237 | −1.4 ± 0.15 | <0.001 | 0.010 |
| Total-body protein, kg | |||||||||
| Baseline | 14.6 ± 0.39 | 14.9 ± 0.32 | 9.5 ± 0.18 | 9.5 ± 0.14 | 11.0 ± 0.32 | — | 11.2 ± 0.25 | — | 0.713 |
| Δ Month 12 | −0.5 ± 0.37 | −2.5 ± 0.16 | −0.4 ± 0.14 | −1.4 ± 0.11 | −0.4 ± 0.15 | 0.015 | −1.7 ± 0.10 | <0.001 | <0.001 |
| Δ Month 24 | −0.4 ± 0.30 | −1.9 ± 0.20 | −0.5 ± 0.13 | −0.9 ± 0.14 | −0.5 ± 0.13 | 0.017 | −1.2 ± 0.12 | <0.001 | <0.001 |
| Δ Month 12–24 | 0.0 ± 0.26 | 0.7 ± 0.16 | −0.0 ± 0.18 | 0.4 ± 0.13 | −0.0 ± 0.15 | 1.000 | 0.5 ± 0.11 | <0.001 | 0.005 |
| Bone mineral content, kg | |||||||||
| Baseline | 2.78 ± 0.070 | 2.78 ± 0.055 | 2.25 ± 0.039 | 2.28 ± 0.030 | 2.40 ± 0.044 | — | 2.44 ± 0.033 | — | 0.528 |
| Δ Month 12 | 0.04 ± 0.016 | 0.02 ± 0.009 | 0.02 ± 0.009 | 0.01 ± 0.007 | 0.03 ± 0.008 | <0.001 | 0.01 ± 0.005 | 0.012 | 0.181 |
| Δ Month 24 | 0.04 ± 0.017 | −0.00 ± 0.011 | 0.03 ± 0.011 | 0.01 ± 0.007 | 0.03 ± 0.009 | 0.001 | 0.00 ± 0.006 | 1.000 | 0.011 |
| Δ Month 12–24 | −0.01 ± 0.013 | −0.02 ± 0.006 | 0.00 ± 0.007 | −0.01 ± 0.004 | 0.00 ± 0.006 | 1.000 | −0.01 ± 0.004 | 0.010 | 0.048 |
Values are means ± SEs for observed values at baseline and observed changes from baseline to months 12 and 24 and from month 12 to 24 shown for AL and CR groups for men, women, and all participants. P values for changes from baseline to months 12 and 24 and from month 12 to 24 are based on intention-to-treat statistical analysis of the adjusted mean change from the repeated-measures analysis adjusted for baseline covariates. AL, ad libitum control group; CR, calorie restriction group; FFM, fat-free mass; TBW, total body water.
Between-group P values test for a significant between-group difference in the change score at the time point. All P values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see Methods).
Within-group P values test for a significant change from baseline to the follow-up time point and months 12–24 in that group.
TABLE 3.
A comparison of regional body composition and fat distribution between AL control and CR groups at 12 and 24 mo1
| Men (n = 66) |
Women (n = 152) |
Overall (n = 218) |
|||||||
| AL (n = 22) | CR (n = 44) | AL (n = 53) | CR (n = 99) | AL (n = 75) | P-AL3 | CR (n = 143) | P-CR3 | Overall P2: AL-CR | |
| Natural waist circumference, cm | |||||||||
| Baseline | 88.5 ± 1.18 | 89.0 ± 0.82 | 78.3 ± 0.76 | 77.0 ± 0.55 | 81.3 ± 0.83 | — | 80.7 ± 0.65 | — | 0.542 |
| Δ Month 12 | −0.9 ± 0.87 | −8.5 ± 0.57 | 0.3 ± 0.47 | −5.4 ± 0.41 | −0.0 ± 0.42 | 1.000 | −6.3 ± 0.35 | <0.001 | <0.001 |
| Δ Month 24 | 0.6 ± 0.82 | −8.0 ± 0.62 | 1.1 ± 0.68 | −5.3 ± 0.43 | 0.9 ± 0.54 | 0.223 | −6.2 ± 0.37 | <0.001 | <0.001 |
| Δ Month 12–24 | 1.4 ± 0.61 | 0.7 ± 0.45 | 0.6 ± 0.61 | 0.2 ± 0.33 | 0.9 ± 0.46 | 0.067 | 0.3 ± 0.26 | 1.000 | 0.208 |
| Trunk fat, % | |||||||||
| Baseline | 25.9 ± 1.05 | 26.9 ± 0.64 | 34.0 ± 0.65 | 33.2 ± 0.53 | 31.6 ± 0.70 | — | 31.2 ± 0.48 | — | 0.510 |
| Δ Month 12 | −0.5 ± 0.66 | −6.8 ± 0.57 | −0.3 ± 0.35 | −6.6 ± 0.32 | −0.4 ± 0.31 | 0.429 | −6.7 ± 0.28 | <0.001 | <0.001 |
| Δ Month 24 | 0.7 ± 0.49 | −6.0 ± 0.59 | 0.4 ± 0.55 | −5.6 ± 0.37 | 0.5 ± 0.41 | 1.000 | −5.7 ± 0.31 | <0.001 | <0.001 |
| Δ Month 12–24 | 1.2 ± 0.62 | 1.0 ± 0.29 | 0.6 ± 0.50 | 1.1 ± 0.22 | 0.8 ± 0.39 | 0.022 | 1.1 ± 0.18 | <0.001 | 0.524 |
| Trunk fat mass, kg | |||||||||
| Baseline | 9.8 ± 0.51 | 10.3 ± 0.34 | 10.9 ± 0.34 | 10.4 ± 0.24 | 10.5 ± 0.29 | — | 10.4 ± 0.20 | — | 0.606 |
| Δ Month 12 | −0.1 ± 0.33 | −3.6 ± 0.27 | −0.0 ± 0.17 | −3.0 ± 0.15 | −0.0 ± 0.15 | 1.000 | −3.2 ± 0.13 | <0.001 | <0.001 |
| Δ Month 24 | 0.5 ± 0.30 | −3.2 ± 0.29 | 0.3 ± 0.28 | −2.7 ± 0.17 | 0.4 ± 0.21 | 0.507 | −2.8 ± 0.15 | <0.001 | <0.001 |
| Δ Month 12–24 | 0.6 ± 0.28 | 0.5 ± 0.14 | 0.3 ± 0.26 | 0.5 ± 0.09 | 0.4 ± 0.20 | 0.017 | 0.5 ± 0.07 | <0.001 | 0.810 |
| Appendicular fat mass, kg | |||||||||
| Baseline | 9.3 ± 0.35 | 9.4 ± 0.30 | 13.4 ± 0.37 | 13.1 ± 0.25 | 12.2 ± 0.36 | — | 12.0 ± 0.24 | — | 0.773 |
| Δ Month 12 | −0.3 ± 0.25 | −2.6 ± 0.20 | −0.0 ± 0.17 | −2.9 ± 0.12 | −0.1 ± 0.14 | 0.851 | −2.8 ± 0.10 | <0.001 | <0.001 |
| Δ Month 24 | −0.0 ± 0.22 | −2.4 ± 0.20 | 0.2 ± 0.26 | −2.5 ± 0.14 | 0.1 ± 0.19 | 1.000 | −2.4 ± 0.11 | <0.001 | <0.001 |
| Δ Month 12–24 | 0.2 ± 0.19 | 0.3 ± 0.10 | 0.2 ± 0.23 | 0.5 ± 0.07 | 0.2 ± 0.17 | 0.214 | 0.4 ± 0.06 | <0.001 | 0.261 |
Values are means ± SEs for observed values at baseline and observed changes from baseline to months 12 and 24 and from month 12 to 24 shown for AL and CR groups for men, women, and all participants. P values for changes from baseline to months 12 and 24 and from month 12 to 24 are based on intention-to-treat statistical analysis of the adjusted mean change from the repeated-measures analysis adjusted for baseline covariates. AL, ad libitum control; CR, calorie restriction.
Between-group P values test for a significant between-group difference in the change score at the time point. All P values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see Methods).
Within-group P values test for a significant change from baseline to the follow-up time point and months 12–24 in that group.
Body weight
As shown in Table 2, weight change was significantly different between the CR and AL groups at both time points (P < 0.001). The CR group achieved significant weight loss after 12 mo (−8.3 ± 0.27 kg; P < 0.001; representing an 11.6% change in weight from baseline) and 24 mo (−7.6 ± 0.32 kg; P < 0.001; representing a 10.4% change in weight from baseline), whereas no significant changes in weight were observed in the AL group at either time point (P > 0.10 at months 12 and 24). There was a significant gain in weight between months 12 and 24 within the CR (P < 0.001) and AL groups (P = 0.02), but the group difference in the change was nonsignificant (P = 0.89).
Whole-body and regional changes
The pattern of significant changes within the CR group, no significant changes within the AL group, and highly significant between-group differences were observed with respect to changes in FM and FFM measured across the whole body (Supplemental Table 1, Tables 2 and 3) and specific regions including the trunk and appendicular regions (Supplemental Table 1, Figure 2, Tables 2 and 3). Total body proteins were significantly decreased within both the CR (P < 0.001 at months 12 and 24) and AL (P = 0.02 at months 12 and 24) groups. There was a significant gain in FM between months 12 and 24 within the CR (P < 0.001) and AL (P = 0.01) groups; however, FM change was not significantly different between groups (P = 0.76). Between-group changes in BMC were not significant at 12 mo (P = 0.18) but were significant at 24 mo (P = 0.01) and between 12 and 24 mo (P = 0.05) because of a decrease in BMC in the CR group (P = 0.01), although the AL group had a very small nonsignificant increase over this interval (Table 2). Notable exceptions to this general pattern of significant change were 1) TBW for which the CR group lost more but had a higher percentage of TBW than the AL group at 12 and 24 mo (P < 0.001 for both time points and for both TBW and percentage of TBW) and a decrease within the CR group (P < 0.001) but not in the AL group (P = 1.0) between 12 and 24 mo, resulting in a significant between group difference (P = 0.01), and 2) changes in total-body protein between months 12 and 24 (Table 2) when a significant increase was observed within the CR group (P < 0.001) but not within the AL group (P = 1.0), resulting in a significant between-group difference (P = 0.01).
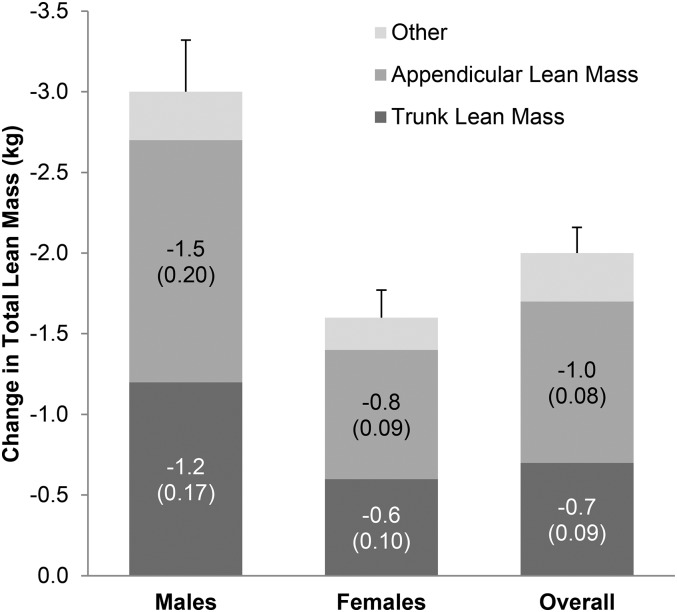
FIGURE 2.
Change in total, trunk and appendicular lean body mass at 24 mo in the CR group. Lean body mass, also the same as lean soft tissue mass, was calculated as fat-free mass minus bone mineral content. Absolute means (SEs in parentheses) are shown for the CR group only for trunk and appendicular (arms and legs) lean mass changes within men (n = 44) and women (n = 99) and the overall CR group (n = 143). CR, calorie restriction.
Weight change at 12 and 24 mo was highly correlated to change in FM, FFM, LBM, trunk fat, TBW, and appendicular lean mass (P < 0.05 for both 12 and 24 mo time points) in men and women except for TBW (P = 0.54) and appendicular lean mass (P = 0.06) at 12 mo in men (Supplemental Table 2). Total BMC was not correlated to weight change.
Change in body composition between low- and high-CR subgroups (based on a median of 12.15% split on %CR achieved)
Body composition changes in the low- and high-CR groups based on the median split of %CR achieved at 24 mo showed significant differences in absolute body-composition changes between the low- and high-CR groups including sex-specific differences at the various time points as shown in Table 4. However, when FM and FFM changes were expressed as a percentage of weight lost, no statistically significant differences were observed within the male or female group or between the low- and high-CR group except for the change in BMC, for which men in the high-CR group lost significantly less BMC as a percentage of weight at 12 mo (P = 0.03) and significantly more BMC at 24 mo (P = 0.02) than men in the low-CR group (Table 4).
TABLE 4.
A comparison of key body-composition variables between low- and high-CR groups at 12 and 24 mo and between 12 and 24 mo1
| Men |
Women |
All |
|||||||
| Low CR(n = 18) | High CR(n = 17) | P2 | Low CR(n = 39) | High CR(n = 41) | P3 | Low CR(n = 57) | High CR(n = 58) | P4 | |
| Weight, kg | |||||||||
| Baseline | 82.33 ± 1.88 | 82.55 ± 2.32 | 0.987 | 67.28 ± 1.10 | 68.97 ± 0.88 | 0.277 | 72.03 ± 1.33 | 72.95 ± 1.22 | 0.731 |
| Δ Month 12 | −9.03 ± 0.76 | −10.48 ± 0.48 | 0.058 | −6.77 ± 0.46 | −9.14 ± 0.42 | <0.001 | −7.48 ± 0.42 | −9.53 ± 0.34 | <0.001 |
| Δ Month 24 | −8.16 ± 0.88 | −9.73 ± 0.79 | 0.046 | −5.75 ± 0.51 | −8.26 ± 0.47 | <0.001 | −6.51 ± 0.46 | −8.69 ± 0.41 | <0.001 |
| Δ Month 12–24 | 0.87 ± 0.46 | 0.75 ± 0.47 | 0.586 | 1.02 ± 0.22 | 0.88 ± 0.29 | 0.788 | 0.97 ± 0.21 | 0.84 ± 0.24 | 0.570 |
| Body fat, % | |||||||||
| Baseline | 26.06 ± 0.60 | 25.76 ± 0.71 | 0.632 | 34.88 ± 0.75 | 37.23 ± 0.58 | 0.015 | 32.10 ± 0.77 | 33.87 ± 0.83 | 0.090 |
| Δ Month 12 | −5.25 ± 0.80 | −6.10 ± 0.38 | 0.192 | −5.13 ± 0.42 | −5.96 ± 0.32 | 0.057 | −5.17 ± 0.38 | −6.00 ± 0.25 | 0.020 |
| Δ Month 24 | −4.28 ± 0.76 | −5.53 ± 0.52 | 0.299 | −4.48 ± 0.50 | −4.60 ± 0.34 | 0.436 | −4.42 ± 0.41 | −4.87 ± 0.29 | 0.244 |
| Δ Month 12–24 | 0.97 ± 0.38 | 0.57 ± 0.31 | 0.478 | 0.65 ± 0.25 | 1.36 ± 0.22 | 0.049 | 0.75 ± 0.21 | 1.13 ± 0.19 | 0.171 |
| FFM, kg | |||||||||
| Baseline | 60.86 ± 1.19 | 61.14 ± 1.67 | 0.908 | 43.68 ± 0.75 | 43.18 ± 0.57 | 0.459 | 49.10 ± 1.24 | 48.44 ± 1.25 | 0.515 |
| Δ Month 12 | −2.90 ± 0.34 | −3.18 ± 0.27 | 0.564 | −1.23 ± 0.18 | −1.96 ± 0.18 | 0.010 | −1.76 ± 0.19 | −2.32 ± 0.16 | 0.027 |
| Δ Month 24 | −2.78 ± 0.49 | −3.04 ± 0.45 | 0.564 | −0.82 ± 0.19 | −2.32 ± 0.23 | <0.001 | −1.44 ± 0.23 | −2.53 ± 0.21 | <0.001 |
| Δ Month 12–24 | 0.13 ± 0.32 | 0.14 ± 0.25 | 0.779 | 0.41 ± 0.18 | −0.36 ± 0.16 | 0.006 | 0.32 ± 0.16 | −0.21 ± 0.14 | 0.033 |
| Total lean body mass, kg | |||||||||
| Baseline | 58.04 ± 1.17 | 58.39 ± 1.61 | 0.908 | 41.32 ± 0.72 | 40.93 ± 0.53 | 0.488 | 46.60 ± 1.20 | 46.05 ± 1.21 | 0.533 |
| Δ Month 12 | −2.91 ± 0.34 | −3.21 ± 0.27 | 0.520 | −1.24 ± 0.18 | −1.97 ± 0.18 | 0.011 | −1.76 ± 0.19 | −2.34 ± 0.16 | 0.028 |
| Δ Month 24 | −2.75 ± 0.49 | −3.05 ± 0.45 | 0.458 | −0.82 ± 0.19 | −2.32 ± 0.23 | <0.001 | −1.43 ± 0.23 | −2.54 ± 0.21 | <0.001 |
| Δ Month 12–24 | 0.16 ± 0.32 | 0.16 ± 0.26 | 0.779 | 0.41 ± 0.19 | −0.35 ± 0.16 | 0.006 | 0.33 ± 0.16 | −0.20 ± 0.14 | 0.031 |
| BMC, kg | |||||||||
| Baseline | 2.824 ± 0.065 | 2.758 ± 0.090 | 0.438 | 2.359 ± 0.047 | 2.246 ± 0.048 | 0.055 | 2.506 ± 0.048 | 2.396 ± 0.053 | 0.067 |
| Δ Month 12 | 0.002 ± 0.013 | 0.032 ± 0.011 | 0.020 | 0.008 ± 0.010 | 0.013 ± 0.010 | 0.962 | 0.006 ± 0.008 | 0.019 ± 0.008 | 0.279 |
| Δ Month 24 | −0.026 ± 0.016 | 0.014 ± 0.016 | 0.083 | 0.003 ± 0.010 | 0.007 ± 0.010 | 0.557 | −0.006 ± 0.008 | 0.009 ± 0.008 | 0.129 |
| Δ Month 12–24 | −0.029 ± 0.008 | −0.019 ± 0.011 | 0.330 | −0.005 ± 0.005 | −0.007 ± 0.007 | 0.931 | −0.012 ± 0.005 | −0.010 ± 0.006 | 0.665 |
| Total-body water, kg | |||||||||
| Baseline | 43.24 ± 0.87 | 42.90 ± 1.12 | 0.987 | 31.76 ± 0.55 | 31.55 ± 0.41 | 0.564 | 35.38 ± 0.85 | 34.88 ± 0.81 | 0.537 |
| Δ Month 12 | −0.49 ± 0.30 | −0.49 ± 0.33 | 0.564 | −0.06 ± 0.17 | −0.86 ± 0.15 | 0.002 | −0.19 ± 0.15 | −0.75 ± 0.15 | 0.006 |
| Δ Month 24 | −1.08 ± 0.39 | −1.23 ± 0.42 | 0.632 | −0.31 ± 0.20 | −1.30 ± 0.23 | <0.001 | −0.55 ± 0.19 | −1.28 ± 0.20 | 0.004 |
| Δ Month 12–24 | −0.60 ± 0.37 | −0.74 ± 0.21 | 0.729 | −0.25 ± 0.19 | −0.43 ± 0.20 | 0.419 | −0.36 ± 0.17 | −0.52 ± 0.15 | 0.363 |
| Trunk FM, kg | |||||||||
| Baseline | 10.25 ± 0.51 | 10.47 ± 0.58 | 0.805 | 9.96 ± 0.41 | 11.08 ± 0.35 | 0.037 | 10.05 ± 0.32 | 10.90 ± 0.30 | 0.049 |
| Δ Month 12 | −3.38 ± 0.44 | −4.05 ± 0.30 | 0.161 | −2.74 ± 0.25 | −3.51 ± 0.20 | 0.005 | −2.95 ± 0.22 | −3.67 ± 0.17 | 0.002 |
| Δ Month 24 | −2.80 ± 0.45 | −3.70 ± 0.39 | 0.171 | −2.36 ± 0.28 | −3.00 ± 0.21 | 0.021 | −2.50 ± 0.24 | −3.21 ± 0.19 | 0.013 |
| Δ Month 12–24 | 0.58 ± 0.21 | 0.36 ± 0.20 | 0.419 | 0.38 ± 0.13 | 0.51 ± 0.12 | 0.301 | 0.45 ± 0.11 | 0.46 ± 0.10 | 0.807 |
| Trunk FFM, kg | |||||||||
| Baseline | 27.98 ± 0.52 | 28.13 ± 0.74 | 0.882 | 20.82 ± 0.38 | 20.68 ± 0.30 | 0.481 | 23.12 ± 0.54 | 22.90 ± 0.55 | 0.518 |
| Δ Month 12 | −1.36 ± 0.20 | −1.32 ± 0.17 | 0.754 | −0.36 ± 0.12 | −0.73 ± 0.11 | 0.058 | −0.68 ± 0.12 | −0.91 ± 0.10 | 0.176 |
| Δ Month 24 | −1.02 ± 0.25 | −1.25 ± 0.25 | 0.347 | −0.21 ± 0.11 | −0.89 ± 0.14 | <0.001 | −0.47 ± 0.12 | −1.00 ± 0.13 | 0.001 |
| Δ Month 12–24 | 0.34 ± 0.17 | 0.07 ± 0.19 | 0.382 | 0.15 ± 0.12 | −0.15 ± 0.13 | 0.118 | 0.21 ± 0.10 | −0.09 ± 0.11 | 0.061 |
| Appendicular FM, kg | |||||||||
| Baseline | 9.64 ± 0.47 | 9.20 ± 0.52 | 0.441 | 12.61 ± 0.40 | 13.67 ± 0.34 | 0.026 | 11.75 ± 0.36 | 12.48 ± 0.39 | 0.099 |
| Δ Month 12 | −2.60 ± 0.38 | −2.83 ± 0.17 | 0.567 | −2.59 ± 0.18 | −3.32 ± 0.16 | 0.002 | −2.60 ± 0.17 | −3.19 ± 0.13 | 0.002 |
| Δ Month 24 | −2.21 ± 0.37 | −2.58 ± 0.19 | 0.418 | −2.22 ± 0.21 | −2.76 ± 0.17 | 0.021 | −2.22 ± 0.18 | −2.71 ± 0.14 | 0.016 |
| Δ Month 12–24 | 0.39 ± 0.17 | 0.16 ± 0.12 | 0.314 | 0.37 ± 0.10 | 0.56 ± 0.11 | 0.194 | 0.38 ± 0.09 | 0.45 ± 0.09 | 0.561 |
| Appendicular lean mass, kg | |||||||||
| Baseline | 26.59 ± 0.65 | 26.23 ± 0.74 | 0.737 | 18.02 ± 0.38 | 17.73 ± 0.28 | 0.489 | 20.51 ± 0.62 | 20.05 ± 0.59 | 0.511 |
| Δ Month 12 | −1.25 ± 0.16 | −1.50 ± 0.14 | 0.277 | −0.59 ± 0.09 | −0.96 ± 0.10 | 0.009 | −0.78 ± 0.09 | −1.11 ± 0.09 | 0.009 |
| Δ Month 24 | −1.30 ± 0.27 | −1.57 ± 0.19 | 0.633 | −0.40 ± 0.10 | −1.12 ± 0.12 | <0.001 | −0.66 ± 0.12 | −1.24 ± 0.10 | <0.001 |
| Δ Month 12–24 | −0.05 ± 0.17 | −0.07 ± 0.15 | 0.621 | 0.19 ± 0.09 | −0.16 ± 0.08 | 0.006 | 0.12 ± 0.08 | −0.14 ± 0.07 | 0.036 |
| Δ FM as % of Δ weight5 | |||||||||
| Δ Month 12 | −2.30 ± 71.98 | 69.40 ± 2.66 | 0.438 | 87.51 ± 5.78 | 78.77 ± 1.70 | 0.521 | 59.15 ± 23.31 | 75.97 ± 1.53 | 0.742 |
| Δ Month 24 | 75.45 ± 8.78 | 40.50 ± 25.51 | 0.632 | 80.78 ± 6.24 | 72.64 ± 2.49 | 0.098 | 79.10 ± 5.06 | 63.22 ± 7.77 | 0.123 |
| Δ FFM as % of Δ weight5 | |||||||||
| Δ Month 12 | 102.30 ± 71.98 | 30.60 ± 2.66 | 0.438 | 12.46 ± 5.78 | 21.29 ± 1.70 | 0.496 | 40.83 ± 23.31 | 24.07 ± 1.53 | 0.721 |
| Δ Month 24 | 24.55 ± 8.78 | 59.50 ± 25.51 | 0.632 | 19.18 ± 6.24 | 27.50 ± 2.51 | 0.092 | 20.88 ± 5.06 | 36.88 ± 7.77 | 0.117 |
| ΔBMC as % of Δ weight5 | |||||||||
| Δ Month 12 | −0.67 ± 0.71 | −0.35 ± 0.11 | 0.022 | −0.24 ± 0.25 | −0.16 ± 0.15 | 0.879 | −0.37 ± 0.28 | −0.21 ± 0.11 | 0.411 |
| Δ Month 24 | 0.32 ± 0.22 | −0.87 ± 0.65 | 0.024 | 0.28 ± 0.40 | −0.17 ± 0.15 | 0.832 | 0.30 ± 0.28 | −0.38 ± 0.22 | 0.144 |
Values are means ± SEs for observed values at baseline and observed changes from baseline to months 12 and 24 and from month 12 to 24 shown for men, women, and all participants. Low- and high-CR groups are calorie based on median split (12.15%) on percentage of CR achieved. P values from Wilcoxon’s rank-sum test for changes from baseline to months 12 and 24 and from month 12 to 24. CR, calorie restriction; FFM, fat-free mass; FM, fat mass.
Between-group P values test for a significant change from baseline to the follow-up time point and months 12–24, in men.
Between-group P values test for a significant change from baseline to the follow-up time point and months 12–24, in women.
Between-group P values test for a significant between-group difference in the change of score at the time point, all CR participants.
For the interval from month 12 to 24, the weight changes were small, so the denominator of Δ FM/Δ weight was small, causing the ratio to be large and the numbers too unwieldy to be shown.
Body-composition change differences between men and women in the CR group
Supplemental analyses were performed to determine whether between-group differences were consistent for men and women. The sex × treatment and sex × treatment × time interaction terms were added to the repeated-measures model. If the 3-factor interaction was not significant, it was removed; if the 2-factor interaction was not significant, it was removed, thereby leaving a model with only the sex main effect. Table 5 shows the estimated treatment effect for men and women in the CR group and the model from which statistical significance was determined. Weight loss in the CR group was significantly affected by sex, with men in the CR group losing more weight than the women in the CR group (P = 0.04). The percentage of weight loss at 24 mo, however, was not significantly different between men and women (P = 0.51; data not shown). Sex did not have a significant effect on the change in waist circumference, BMI, or total fat (expressed as FM or percentage of fat) or in the appendicular fat (data not shown). However, men in the CR group lost significantly more trunk FM than the women in the CR group at 24 mo (P = 0.03). For the change in the percentage of TBW (%TBW), women in the CR group had a significantly smaller increase in %TBW than the men in the CR group (P = 0.002). There was a significant sex × treatment interaction effect for the change in FFM and LBM (P < 0.01 for both). Men in the CR group lost significantly more FFM and LBM than the women in the CR group for the whole-body, trunk, and appendicular regions. This sex effect, but not the sex × treatment effect, was also significant when the change in FFM and LBM were expressed as a percentage of change in weight.
TABLE 5.
Significant differences over time (baseline to 24 mo) between the men and women in the CR group1
| Men |
Women |
Male-female difference2 |
||||
| Outcome | Mean | P | Mean | P | Difference | P |
| Body compartment | ||||||
| Clinic weight,3 kg | −8.33 ± 0.42 | <0.001 | −7.32 ± 0.29 | <0.001 | −1.01 ± 0.49 | 0.04 |
| FFM,4 kg | −3.25 ± 0.32 | <0.001 | −1.61 ± 0.16 | <0.001 | −1.64 ± 0.36 | <0.001 |
| LBM,4 kg | −3.24 ± 0.33 | <0.001 | −1.63 ± 0.16 | <0.001 | −1.61 ± 0.36 | <0.001 |
| Trunk fat mass,3 kg | −3.24 ± 0.18 | <0.001 | −2.81 ± 0.13 | <0.001 | −0.44 ± 0.20 | 0.03 |
| Trunk FFM,4 kg | −1.24 ± 0.17 | <0.001 | −0.62 ± 0.08 | <0.001 | −0.63 ± 0.18 | <0.001 |
| Appendicular FFM,4 kg | −1.34 ± 0.19 | <0.001 | −0.76 ± 0.08 | <0.001 | −0.58 ± 0.20 | 0.004 |
| Body water by 18O dilution,3 % | 6.07 ± 0.42 | <0.001 | 4.45 ± 0.24 | <0.001 | 1.63 ± 0.53 | 0.002 |
| Δ FFM as % of Δ weight5 | 33.97 ± 5.20 | <0.001 | 23.43 ± 4.88 | <0.001 | 10.54 ± 2.79 | <0.001 |
| Δ LBM as % of Δ weight5 | 33.99 ± 5.30 | <0.001 | 23.69 ± 4.98 | <0.001 | 10.30 ± 2.83 | <0.001 |
Values are means ± SEs. Adjusted for baseline covariates, for outcomes for which there is a significant sex main effect or a significant treatment interaction with sex. CR, calorie restriction; FFM, fat-free mass; LBM, lean body mass.
Least-squares adjusted mean of the male-female difference in the CR group from the repeated-measures analysis adjusted for baseline covariates. A positive value implies that the men gained more (or lost less) than the women. P values were derived from the appropriate model (see Methods).
Signifies a sex main effect (only), i.e., the between-group difference is the same for men and women. The P value corresponds to that specific effect in the appropriate model.
Indicates a sex × treatment 2-way interaction, i.e., the between-group differential is different for men and women. The P value corresponds to that specific effect in the appropriate model.
Group difference in percentage of FFM:weight-loss ratio calculated by Wilcoxon’s rank-sum test. The P value corresponds to that specific effect in the appropriate model.
Body-composition change differences between CR normal-weight and CR overweight participants (data not shown)
A similar model was applied to investigate consistency in the treatment effects between the 2 BMI strata. There was no significant effect of baseline BMI (normal weight compared with overweight) on the changes in weight or body composition within the CR group with the exception of the change in %TBW (where normal-weight individuals had a larger increase, P = 0.02) when compared with overweight individuals.
Predictors of change in FFM
A multiple stepwise regression model with candidate predictors [age, sex, baseline BMI, percentage of protein intake per kilogram of body weight, AREE, IGF-1 (data for protein intake, AREE, and IGF-1 shown in Table 6)] for the determinants of change in FFM in CR participants at 12 and 24 mo showed that larger decreases in FFM were associated with older age (P < 0.01), male sex (P < 0.01), lower AREE (P < 0.01), and higher baseline BMI (P = 0.03).
TABLE 6.
Select candidates’ predictors of change in body composition1
| Men (n = 66) |
Women (n = 152) |
Overall (n = 218) |
|||||||
| AL (n = 22) | CR (n = 44) | AL (n = 53) | CR (n = 99) | AL (n = 75) | P-AL3 | CR (n = 143) | P-CR3 | Overall P2:AL-CR | |
| Self-reported total energy intake, kcal/d | |||||||||
| Baseline | 2382 ± 106.9 | 2556 ± 75.9 | 1905 ± 54.8 | 1935 ± 47.3 | 2045 ± 55.5 | — | 2126 ± 46.7 | — | 0.463 |
| Δ Month 12 | 19 ± 90.7 | −443 ± 65.5 | −138 ± 59.9 | −269 ± 42.0 | −91 ± 50.4 | 0.098 | −322 ± 35.9 | <0.001 | <0.001 |
| Δ Month 24 | −133 ± 110.6 | −434 ± 68.7 | −126 ± 49.3 | −194 ± 49.4 | −128 ± 47.8 | 0.015 | −267 ± 41.3 | <0.001 | 0.073 |
| Δ Month 12–24 | −152 ± 92.1 | −6 ± 63.6 | 4 ± 53.5 | 85 ± 36.8 | −46 ± 47.2 | 1.000 | 57 ± 32.3 | 0.168 | 0.065 |
| Protein intake/body weight, g/kg | |||||||||
| Baseline | 1.35 ± 0.057 | 1.29 ± 0.038 | 1.16 ± 0.042 | 1.17 ± 0.028 | 1.21 ± 0.036 | — | 1.20 ± 0.023 | — | 0.951 |
| Δ Month 12 | −0.00 ± 0.062 | 0.10 ± 0.051 | −0.03 ± 0.046 | 0.10 ± 0.030 | −0.02 ± 0.037 | 1.000 | 0.10 ± 0.026 | <0.001 | 0.007 |
| Δ Month 24 | −0.16 ± 0.059 | 0.07 ± 0.056 | −0.08 ± 0.052 | 0.07 ± 0.040 | −0.10 ± 0.040 | 0.083 | 0.07 ± 0.032 | 0.017 | <0.001 |
| Δ Month 12–24 | −0.16 ± 0.057 | −0.04 ± 0.035 | −0.05 ± 0.050 | −0.02 ± 0.035 | −0.08 ± 0.039 | 0.069 | −0.02 ± 0.026 | 1.000 | 0.202 |
| AREE, kcal/d | |||||||||
| Baseline | 906 ± 61.2 | 989 ± 38.4 | 695 ± 23.9 | 729 ± 19.4 | 758 ± 27.1 | — | 808 ± 20.4 | — | 0.183 |
| Δ Month 12 | 33 ± 65.1 | −163 ± 52.6 | −46 ± 33.2 | −78 ± 22.6 | −21 ± 30.6 | 1.000 | −104 ± 22.5 | <0.001 | 0.068 |
| Δ Month 24 | −58 ± 62.7 | −88 ± 49.7 | 4 ± 36.5 | −104 ± 27.6 | −16 ± 31.8 | 1.000 | −99 ± 24.3 | 0.001 | 0.052 |
| Δ Month 1224 | −92 ± 60.2 | 64 ± 44.2 | 58 ± 36.0 | −35 ± 25.8 | 11 ± 32.0 | 1.000 | −4 ± 22.7 | 1.000 | 0.827 |
| IGF-1, ng/mL | |||||||||
| Baseline | 179.6 ± 9.72 | 173.1 ± 6.33 | 184.5 ± 7.11 | 176.6 ± 4.35 | 183.1 ± 5.74 | — | 175.5 ± 3.58 | — | 0.589 |
| Δ Month 12 | −8.5 ± 9.43 | −12.8 ± 6.04 | −23.9 ± 6.14 | 0.5 ± 4.61 | −19.3 ± 5.18 | <0.001 | −3.4 ± 3.73 | 0.163 | 0.109 |
| Δ Month 24 | −22.4 ± 6.13 | −20.7 ± 5.84 | −16.4 ± 6.25 | −7.0 ± 4.22 | −18.2 ± 4.74 | <0.001 | −11.2 ± 3.47 | <0.001 | 1.000 |
| Δ Month 12–24 | −13.9 ± 7.54 | −9.4 ± 6.12 | 8.4 ± 5.06 | −6.1 ± 5.06 | 1.7 ± 4.35 | 1.000 | −7.1 ± 3.96 | 0.102 | 0.467 |
| Long-term calculated energy intake, kcal/d | |||||||||
| Baseline | 2785 ± 78.63 | 2891 ± 54.36 | 2222 ± 33.99 | 2283 ± 26.42 | 2390 ± 44.73 | — | 2467 ± 34.04 | — | 0.148 |
| Δ Month 12 | 18.3 ± 60.92 | −440.3 ± 37.11 | −63.1 ± 28.28 | −350.9 ± 19.34 | −38.3 ± 27.15 | 1.000 | −378.1 ± 17.86 | <0.001 | <0.001 |
| Δ Month 24 | 13.3 ± 54.95 | −341.8 ± 37.85 | −45.6 ± 27.63 | −279.9 ± 19.93 | −26.9 ± 25.68 | 1.000 | −298.7 ± 18.13 | <0.001 | <0.001 |
| Δ Month 12–24 | −5.0 ± 19.14 | 94.4 ± 17.84 | 13.5 ± 11.85 | 82.3 ± 9.37 | 7.6 ± 10.09 | 1.000 | 86.0 ± 8.46 | <0.001 | <0.001 |
| Carbohydrates, % | |||||||||
| Baseline | 44.1 ± 1.50 | 46.1 ± 1.03 | 45.5 ± 0.83 | 47.0 ± 0.64 | 45.1 ± 0.73 | — | 46.8 ± 0.54 | — | 0.078 |
| Δ Month 12 | −0.2 ± 0.96 | 3.6 ± 1.26 | 0.3 ± 1.04 | 3.5 ± 0.64 | 0.2 ± 0.78 | 0.867 | 3.5 ± 0.59 | <0.001 | <0.001 |
| Δ Month 24 | 1.0 ± 1.24 | 1.8 ± 1.35 | −0.2 ± 0.97 | 2.5 ± 0.64 | 0.2 ± 0.77 | 0.723 | 2.3 ± 0.60 | <0.001 | <0.001 |
| Δ Month 12–24 | 1.3 ± 1.06 | −1.6 ± 0.92 | −0.7 ± 1.20 | −0.9 ± 0.66 | −0.1 ± 0.89 | 1.000 | −1.1 ± 0.54 | 0.139 | 0.272 |
| Fat intake, % | |||||||||
| Baseline | 33.5 ± 1.03 | 34.3 ± 0.82 | 35.2 ± 0.71 | 33.1 ± 0.47 | 34.7 ± 0.59 | — | 33.5 ± 0.41 | — | 0.034 |
| Δ Month 12 | 0.3 ± 0.83 | −5.2 ± 1.16 | −1.0 ± 0.78 | −4.0 ± 0.64 | −0.6 ± 0.60 | 1.000 | −4.4 ± 0.57 | <0.001 | <0.001 |
| Δ Month 24 | −0.8 ± 1.27 | −3.5 ± 1.25 | −0.3 ± 0.94 | −3.0 ± 0.59 | −0.4 ± 0.76 | 1.000 | −3.1 ± 0.56 | <0.001 | <0.001 |
| Δ Month 12–24 | −1.1 ± 1.19 | 1.6 ± 0.77 | 0.9 ± 0.91 | 1.2 ± 0.63 | 0.3 ± 0.73 | 1.000 | 1.3 ± 0.50 | 0.028 | 0.221 |
| Protein, % | |||||||||
| Baseline | 18.6 ± 0.81 | 16.6 ± 0.38 | 16.6 ± 0.44 | 16.6 ± 0.33 | 17.2 ± 0.40 | — | 16.6 ± 0.25 | — | 0.252 |
| Δ Month 12 | −0.5 ± 0.61 | 2.2 ± 0.60 | 0.9 ± 0.56 | 1.4 ± 0.38 | 0.5 ± 0.44 | 0.184 | 1.6 ± 0.32 | <0.001 | 0.047 |
| Δ Month 24 | −1.4 ± 0.62 | 1.9 ± 0.61 | 0.3 ± 0.49 | 0.6 ± 0.42 | −0.2 ± 0.40 | 1.000 | 1.0 ± 0.35 | 0.004 | 0.068 |
| Δ Month 12–24 | −0.9 ± 0.66 | −0.2 ± 0.57 | −0.6 ± 0.74 | −0.9 ± 0.35 | −0.7 ± 0.54 | 0.409 | −0.6 ± 0.30 | 0.101 | 0.933 |
Values are means ± SEs for observed values at baseline and observed changes from baseline to months 12 and 24 and from month 12 to 24 shown for AL and CR groups for men, women, and all participants. P values for changes from baseline to months 12 and 24 and from month 12 to 24 are based on intention-to-treat statistical analysis of the adjusted mean change from the repeated-measures analysis adjusted for baseline covariates. AL, ad libitum control group; AREE, activity-related energy expenditure; CR, calorie restricted group; IGF, insulin-like growth factor.
Between-group P values test for a significant between-group difference in the change score at the time point. All P values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see Methods).
Within-group P values test for a significant change from baseline to the follow-up time point and months 12–24 in that group.
DISCUSSION
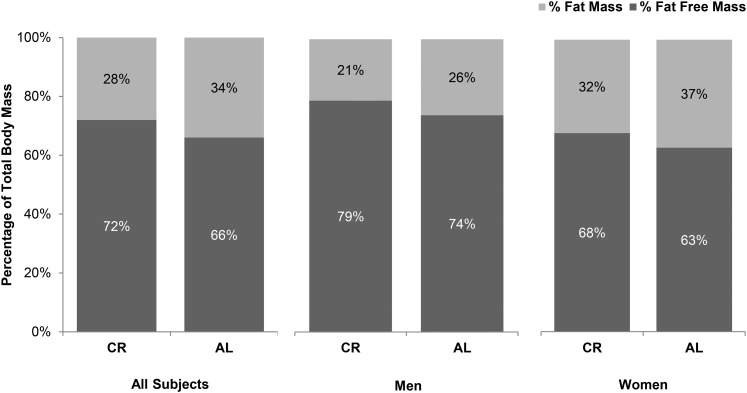
This analysis provides the first information on the effects of 2 y of CR on the quality of body-composition change in nonobese humans. The uniqueness of our study is the 2-y duration, which is the longest RCT of CR to date and allows for the examination of the effects of CR beyond what is attributable to acute weight loss, as well as our focus on body-composition changes in nonobese individuals. Our findings show that at the end of the 2-y CR period, body composition was relatively higher in FFM and lower in FM (72% FFM, 28% FM) (Figure 3) compared with baseline (67% FFM, 33% FM). Additionally, we observed large improvements in indexes of central adiposity, including smaller waist circumference and reductions in percentage of trunk fat in this nonobese population. Furthermore, body composition in our CR participants at 24 mo was higher in FFM and lower in FM than in CALERIE-2 controls (Figure 3). Collectively, these observations suggest that 2 y of CR did not have adverse effects on body composition despite the absence of specific exercise recommendations and a focus on changing weight exclusively through changes in dietary intake. On the contrary, the quality of body composition improved with CR, and the FFM and FM changes in this nonobese population were consistent with expected changes as predicted by both the Forbes and Thomas models (29).
FIGURE 3.
Body composition of the participants in the CR group (n = 143) at 24 mo compared with the AL group participants (n = 75). Participants in the CR group: men, n = 44; women, n = 99. Participants in the AL group: men, n = 22; women, n = 53. Percentage of fat mass and percentage of fat-free mass in the participants in the CR group at 24 mo were not only comparable to those of the men and women in the AL group, but they were significantly higher for percentage of fat-free mass and lower for percentage of fat mass (P < 0.01 for both men and women). AL, ad libitum control; CR, calorie restriction.
It is widely accepted that both the total amount of adipose tissue mass and the storage locations are important factors for health. The relative distribution of fat in the upper body compared with the lower half (30, 31), particularly in central regions (32) and in ectopic tissues such as liver, heart, pancreas, and skeletal muscle, leads to more deleterious metabolic abnormalities and contributes to the development of insulin resistance, impaired glucose tolerance, dyslipidemia, and diabetes in middle-aged persons (33, 34). We observed significant decreases in not only whole-body adiposity but also central adiposity measures, including waist circumference and trunk fat in this healthy, nonobese adult population. At 24 mo, 80% of the CR group (who were overweight at baseline) achieved normal weight compared with a 27% increase in those who became overweight in the control group, strongly suggesting a benefit of CR in chronic disease risk reduction. The significant changes in central adiposity (i.e., substantial and significant decreases in waist circumference and trunk percentage of fat, which were comparable in both men and women) is indicative of potential mobilization of fat stores from depots that have been implicated with greater metabolic risk (35, 36). The collective changes in both overall and regional decreases in FM suggest potential added benefits to cardiometabolic risk reduction with CR and improvements in health span.
The extent to which CR differentially effects body composition in men and women is not fully understood. The men in this study lost a higher percentage of their weight as FFM (38%) relative to the women (28%), and this change remained significant over the 2-y study period. The results are consistent with the findings of a meta-analysis examining the impact of weight-loss interventions on body-composition changes (varying methods were used for body-composition assessment in included studies; the subgroup analysis of only diet is used in this comparison), which showed that men had a 7% greater loss in FFM than women did for the same total (10 kg) weight loss (37). Of possible relevance to our findings, age at baseline was ∼10% higher in the men in the CR group than in the women; however, the inclusion of age in the models did not affect the finding of higher percentage of FFM loss in men than in women or the findings for other changes in body composition. It must be noted that, although the absolute changes in FFM in our men in the CR group are not consistent with attenuation of the age-related declines in FFM, the overall percentage of FFM as a proportion of body mass was much higher at 24 mo than at baseline.
Previous studies examining sex-specific differences in FFM change have differed from ours in numerous ways: some were prospective observations of weight change; others involved exercise; for the most part the populations studied were obese, overweight, or elderly; and most studies were substantially shorter in duration (37–39). Hence, it is difficult to directly compare the results from our 2-y study in normal-weight and slightly overweight men and women or extend findings from these previous reports to those that we observed in this study.
A comparison of body-composition changes as a function of the level of %CR, achieved by using a median split of %CR to define low and high CR, showed no remarkable differences in key body-composition variables when the change was expressed as a percentage of weight loss except for the decrease in BMC in men, which was significantly higher in the high-CR group than in the low-CR group and persisted when the change was expressed as a percentage of weight loss. This BMC finding could be caused by the fact that the men in the high-CR group lost more weight than the men in the low-CR group.
Determinants of the changes in FFM in the CR group overall were also examined with the intent to better inform practitioners of CR and to maximize the benefit of future CR interventions. Consistent with previous reports, higher AREE was the strongest predictor of smaller changes in FFM during CR. Being younger, having a lower initial BMI, and being female were also associated with smaller losses in FFM as a result of CR. Contrary to previous reports (40–42), protein intake (expressed as either g total protein/d or g · kg body weight−1 · d−1) was not a significant predictor of FFM change in our study participants. It is possible that because on average, reported protein intake in our participants was not lower than normal, the impact of dietary protein intake on FFM was not within a range that could affect body composition in this study.
Limitations of the current study design include the inability to directly examine changes in abdominal fat distribution. Although we were able to get a broad picture of the changes in the adipose tissue distribution from the waist circumference and trunk fat measures, further compartmentalization of the depots of fat changes would have enhanced the interpretation of our findings of decreased central adiposity. In addition, our findings between the men and the women in the CR group may have been influenced by the smaller number of men than women who enrolled in the study. We are also unable to specifically address the effects of CR on longevity in this 2-y study design. Finally, although we adopted a rigorous approach for controlling type I error for any specific outcome, we did not apply a similar approach across the broad list of body-composition outcomes as is appropriate for this exploratory aim in CALERIE-2. This implies that our results will need to be confirmed in future studies. Despite the study limitations, as the only 2-y study of human CR, the results are unique and have important implications for the use of CR protocols to enhance human health during aging.
In summary, to our knowledge this is the first study to report detailed body-composition changes throughout a 2-y CR intervention in nonobese men and women, and it showed that body composition is not adversely affected by CR in the absence of prescribed exercise. In addition, the resulting percentage of FM and FFM was similar to CALERIE-2 controls who did not lose weight. Finally, maintaining a sustained level of physical activity during CR may be required to help preserve body-composition profiles commensurate with healthy aging. The sustainability of the CR lifestyle and benefits to health span beyond the 2-y period should be examined in this cohort and will be important for the understanding of longer-term CR in humans.
Acknowledgments
The members of the CALERIE Study Group are shown in the following list of the Principal Investigators (PIs), Co-Investigators (CIs), Site Intervention Leaders (SILs), Intervention Counselors (ICs), Study Managers (SMs), Project Leaders (PLs), Study Coordinators (SCs), and Other Staff (OS). Pennington Biomedical Research Center (Clinical Site)—PI: Eric Ravussin; CIs: Catherine Champagne, Alok Gupta, Corby Martin, Leanne Redman, Steven Smith, Donald Williamson; SIL: Corby Martin; ICs: Michelle Begnaud, Barbara Cerniauskas, Allison Davis, Jeanne Gabrielle, Heather Walden; SMs: Natalie Currier, Mandy Shipp; SCs: Sarah Masters, Melody McNicoll; OS: Shelly Prince, Courtney Brock, Renee Puyau, Conrad Earnest, Jennifer Rood, Tiffany Stewart, Lillian Levitan, Crystal Traylor, Susan Thomas, Valerie Toups, Karen Jones, Stephanie Tatum, Celeste Waguespack, Kimberly Crotwell, Lisa Dalfrey, Amy Braymer, Rhonda Hilliard, Onolee Thomas, Jennifer Arceneaux, Stacie LaPrarie, Allison Strate, Jana Ihrig, Susan Mancuso, Christy Beard, Alicia Hymel, Desti Shepard, John Correa, Denise Jarreau, Brenda Dahmer, Grace Bella, Elizabeth Soroe, Bridget Conner, Paige McCown, Stephanie Anaya, Melissa Lupo. Tufts University (Clinical Site) —PI: Susan B. Roberts; CIs: Sai Krupa Das, Simin Meydani, Roger Fielding, Isaac Greenberg, Anastassios Pittas, Edward Saltzman, Tammy Scott; SIL: Cheryl Gilhooly; ICs: Kimberly Gerber, Isaac Greenberg, Marjory Kaplan, Christy Karabetian, Russell Kennedy, Lisa Robinson; SM: Paul Fuss; OS: Assefa, Senait, Verona Bembridge, Maria Berlis, Scarlett Buer, Robert Carabello, Cherie Campbell, Lauren Collins, Marybeth Doherty, Alicia Freed, Chervonte Hernandez, Gyna Jean-Baptiste, Mary Krasinski, Marie Lim-Lucas, Ekaterina Maslova, Barbara Maxwell, Jean McShea, Ann Muchowski, Margaret Mulkerrin, Kerry Murphy, Carol Nelsen, Megan O’Neill, Helen Rasmussen, Brenda Roche, Eneida Roman, Gregory Sproull, Marie St. Victor, Susan Storer, Katherine Strissel, Stephanie Valliere, Margaret Vilme, Justin Wheeler, Jill Wiley, Fania Yangarber. Washington University (Clinical Site)—PI: John O. Holloszy; CIs: Susan Racette, Dennis Villareal, Luigi Fontana, Sam Klein, Charles Lambert, B Selma Mohammed; SIL: Rick Stein; ICs: Karen Cotton, Margaret Hof, Cherie Massmann, Kathleen Obert, Marni Pearlman, Tina M Reising, Laura Weber; SM: Mary Uhrich; SC: Morgan Schram; OS: Mel Meyer, Chelsea Carlen, Lisa Kee, Barbara Larson, Mary McFerson, Rebecca Sabatino, Bridgett Toennies. Duke Clinical Research Institute (Coordinating Center)—PI: James Rochon; CIs: Connie W. Bales, William E. Kraus, Carl F. Pieper; PL: Katherine M. Galan; OS: Richard Adrian, Eleanor Law Allen, William Blasko, Manjushri Bhapkar, Nikka Brown, Maria Butts, Elaina K. Cossin, Jennifer Curry, Jamie Daniel, Kathleen S. Diemer, Lee Greiner, Darryl Johnson, Cassandra Jones, Lauren Lindblad, Luanne McAdams, Marty Mansfield, Senthil Murugesan, Lucy Piner, Christopher Plummer, Mike Revoir, Pamela Smith, Monica Spaulding, James Topping. Baylor College of Medicine (DLW Laboratory)—PI: William W. Wong; OS: Lucinda L. Clarke, Chun W. Liu, J. Kennard Fraley. University of California at San Francisco (DXA Reading Center)—PI: Ann V. Schwartz; CI: John Shepherd; OS: Lisa Palermo, Susan Ewing, Michaela Rahorst, Caroline Navy. University of Vermont (Biochemistry Laboratory)—PI: Michael Lewis; CI: Russell P. Tracy; OS: Rebekah Boyle, Elaine Cornell, Patrick Daunais, Dean Draayer, Melissa Floersch, Nicole Gagne, Florence Keating, Angela Patnoad. University of Cincinnati (Nutrition Reading Center)—PI: Marcia Schmidt; OS: Marcia Gavin, Frida Wiener, Ashley Hughes, Laura Benken. University of Pittsburgh (Intervention Counseling Curriculum)—PI: Amy Otto. Data and Safety Monitoring Board—Jeffrey Halter (Chair), David M. Buchner, Patricia Elmer, Mark Espeland, Steven B. Heymsfield, Xavier Pi-Sunyer, Thomas Prohaska, Sue Shapses, John Speakman, Richard Weindruch. National Institute on Aging (Primary Funding Agency)—Evan C. Hadley, Judy Hannah, Sergei Romashkan. National Institute of Diabetes and Digestive and Kidney Diseases (Cosponsor)—Mary Evans.
The authors’ responsibilities were as follows—SKD, SB Roberts, CKM, WEK, and ER: designed the research (project conception, development of the overall research plan, and study oversight); SKD, CKM, SB Racette, and PJF: conducted the research (hands-on conduct of the experiments and data collection); SKD, SB Roberts, MVB, WEK, WWW, ES, CFP, AVS, ER, and LMR: provided essential reagents or provided essential materials (applies to authors who contributed by providing animals, constructs, databases, etc., necessary for research); SKD, MVB, WWW, CFP, AVS, and LMR: analyzed the data or performed the statistical analysis; SKD and LMR: wrote the manuscript (only authors who made a major contribution); and all authors: had primary responsibility for the final content, manuscript review, edits, and feedback. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AL, ad libitum control; AREE, activity-related energy expenditure; BMC, bone mineral content; CALERIE, Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy; CR, calorie restriction; DLW, doubly labeled water; DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; FM, fat mass; IGF, insulin-like growth factor; ITT, intention-to-treat; LBM, lean body mass; RCT, randomized controlled trial; RMR, resting metabolic rate; TBW, total body water; TDEE, total daily energy expenditure; %CR, percentage of calorie restriction; %TBW, percentage of total body water.
REFERENCES
- 1.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009;8:339–48. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep 2009;9:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borkan GA, Hults DE, Gerzof SG, Burrows BA, Robbins AH. Relationships between computed tomography tissue areas, thicknesses and total body composition. Ann Hum Biol 1983;10:537–45. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RS, Shuman WP, Bradbury VL, Cain KC, Fellingham GW, Beard JC, Kahn SE, Stratton JR, Cerqueira MD, Abrass IB. Body fat distribution in healthy young and older men. J Gerontol 1990;45:M181–5. [DOI] [PubMed] [Google Scholar]
- 5.Heward CB. An approach to extending human lifespan today. In: The future of aging. New York: Springer; 2010. p. 227–78. [Google Scholar]
- 6.Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science 2010;328:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci 2011;66:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 2007;85:1023–30. [DOI] [PubMed] [Google Scholar]
- 9.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO; Washington University School of Medicine CALERIE Group. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 2006;61:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Meyer DEL, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006;295:1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, Rochon J, Bhapkar M, Kraus W; CALERIE Study Group. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget 2016;7:19124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 2015;70:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res 2016;31:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, Klein S, Bhapkar M, Rochon J, Ravussin E, et al. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell 2016;15:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials 2011;32:874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart TM, Bhapkar M, Das S, Galan K, Martin CK, McAdams L, Pieper C, Redman L, Roberts S, Stein RI, et al. Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE Phase 2) screening and recruitment: methods and results. Contemp Clin Trials 2013;34:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E, et al. Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr 2014;99:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, Martin C, Kraus W, Das S, Williamson D, et al. Development of adherence metrics for caloric restriction interventions. Clin Trials 2011;8:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong WW, Roberts SB, Racette SB, Das SK, Redman LM, Rochon J, Bhapkar MV, Clarke LL, Kraus WE. The doubly labeled water method produces highly reproducible longitudinal results in nutrition studies. J Nutr 2014;144:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong WW, Lee LS, Klein PD. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr 1987;45:905–13. [DOI] [PubMed] [Google Scholar]
- 21.Schoeller DA, Van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 1980;33:2686–93. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr 1999;69:833–41. [DOI] [PubMed] [Google Scholar]
- 23.Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab 2012;302:E441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of longitudinal data. Oxford (UK): Oxford University Press; 2002. [Google Scholar]
- 25.Jennrich RI, Schluchter MD. Unbalanced repeated-measures models with structured covariance matrices. Biometrics 1986;42:805–20. [PubMed] [Google Scholar]
- 26.Dmitrienko A, Tamhane AC. Gatekeeping procedures with clinical trial applications. Pharm Stat 2007;6:171–80. [DOI] [PubMed] [Google Scholar]
- 27.Wright SP. Adjusted P-values for simultaneous inference. Biometrics 1992;48:1005–13. [Google Scholar]
- 28.Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH, et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Intern Med 2016;176:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev 2014;15:310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björntorp P. Abdominal fat distribution and the metabolic syndrome. J Cardiovasc Pharmacol 1992;20 Suppl 8:S26–8. [PubMed] [Google Scholar]
- 31.Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301–13. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93(11 Suppl 1):S57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 1997;20:935–42. [DOI] [PubMed] [Google Scholar]
- 34.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–607. [DOI] [PubMed] [Google Scholar]
- 35.Wiklund P, Toss F, Weinehall L, Hallmans G, Franks PW, Nordstrom A, Nordstrom P. Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J Clin Endocrinol Metab 2008;93:4360–6. [DOI] [PubMed] [Google Scholar]
- 36.Okosun IS, Seale JP, Lyn R. Commingling effect of gynoid and android fat patterns on cardiometabolic dysregulation in normal weight American adults. Nutr Diabetes 2015;5:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr 1995;49:1–10. [PubMed] [Google Scholar]
- 38.Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr 2004;80:475–82. [DOI] [PubMed] [Google Scholar]
- 39.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 2005;82:872–8, quiz 915–6. [DOI] [PubMed] [Google Scholar]
- 40.Antonio J, Ellerbroek A, Silver T, Vargas L, Peacock C. The effects of a high protein diet on indices of health and body composition–a crossover trial in resistance-trained men. J Int Soc Sports Nutr 2016;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression. Am J Clin Nutr 2006;83:260–74. [DOI] [PubMed] [Google Scholar]
- 42.Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord 2004;28:57–64. [DOI] [PubMed] [Google Scholar]