Abstract
Background: Low-calorie sweeteners (LCSs) are found in many foods and beverages, but consumers may not realize their presence, and their role in appetite, weight, and health is controversial. Although consumption limits based on toxicologic safety are well established, the threshold required to exert clinically relevant metabolic effects is unknown.
Objectives: This study aimed to determine whether individuals who do not report consumption of LCSs can be correctly characterized as “unexposed” and to investigate whether instructions to avoid LCSs are effective in minimizing exposure.
Design: Eighteen healthy 18- to 35-y-old “nonconsumers” (<1 food or beverage with LCSs/mo) enrolled in a 2-wk trial designed to evaluate the effects of LCSs on the gut microbiota. The trial consisted of 3 visits. At baseline, participants were counseled extensively about avoiding LCSs. After the run-in, participants were randomly assigned to consume diet soda containing sucralose or carbonated water (control) 3 times/d for 1 wk. Food diaries were maintained throughout the study, and a spot urine sample was collected at each visit.
Results: At baseline, 8 participants had sucralose in their urine (29.9–239.0 ng/mL; mean ± SD: 111.4 ± 91.5 ng/mL). After the run-in, sucralose was found in 8 individuals (2 of whom did not have detectable sucralose at baseline) and ranged from 25.0 to 1062.0 ng/mL (mean ± SD: 191.7 ± 354.2 ng/mL). Only 1 participant reported consumption of an LCS-containing food before her visit. After the intervention, sucralose was detected in 3 individuals randomly assigned to receive carbonated water (26–121 ng/mL; mean ± SD: 60.7 ± 52.4 ng/mL).
Conclusions: Despite the selection of healthy volunteers with minimal reported LCS consumption, more than one-third were exposed to sucralose at baseline and/or before randomization, and nearly half were exposed after assignment to the control. This shows that instructions to avoid LCSs are not effective and that nondietary sources (e.g., personal care products) may be important contributors to overall exposure. This trial was registered at clinicaltrials.gov as NCT02877186.
Keywords: artificial sweeteners, diet, diet soda, sucralose, non-nutritive sweeteners, low-calorie sweeteners
INTRODUCTION
Intervention studies report modest benefits of low-calorie sweetener (LCS) consumption on body weight (1), specifically when used to replace sugar-sweetened beverages and/or when consumed in the context of behavioral weight-loss interventions (1). However, LCS consumption has also been linked to changes in taste preferences and dietary patterns, weight gain, metabolic abnormalities, and chronic diseases in epidemiologic studies (2), and a causal relation between LCSs, increased food intake, and weight gain is consistently shown in rodent models (3). This suggests that, although LCSs may be a promising tool for weight management in those who are cognitively engaged in lowering caloric intake (and in those who have access to the required support and resources to do so), the effects of LCSs on obesity-related chronic disease likely differ based on the context of their use.
Given the ongoing controversy surrounding the role of LCSs in weight management and chronic disease, we recently highlighted several key methodologic issues (4), including the need to correctly categorize LCS exposure of clinical study volunteers and to take this information into account when interpreting study findings. Recent data show that ∼25% of children and 40% of adults in the United States consume foods, beverages, or packets [e.g., Splenda (Heartland Consumer Products) or Equal (Merisant Company), often added to tea and coffee as sugar substitutes] containing LCSs (5). Thus, these data conservatively suggest that more than half of adults and a large majority of children are “nonconsumers” and therefore are not exposed. However, LCSs may be consumed inadvertently in foods and beverages not explicitly labeled as “diet” or “sugar-free” (6, 7), and their consumption results in higher plasma sweetener concentrations in children than in adults (8). They are also present in a variety of personal care products such as toothpaste, mouthwash, dietary supplements, and over-the-counter medications. Nursing infants have also been found to be exposed to LCSs via breast milk (9, 10).
We therefore aimed to investigate whether individuals who do not report consumption of LCSs are correctly characterized as “unexposed” and to evaluate whether providing study participants with strict instructions to avoid dietary LCS sources is effective in ensuring their nonexposure. Herein, we specifically focus on sucralose, because it is widely used in both food and beverage applications and after absorption is excreted in the urine, facilitating its detection.
METHODS
Although the current investigation focuses on measuring sucralose concentrations among reportedly unexposed participants, this study was performed as a subanalysis of a larger study designed to evaluate changes in gut microbiome composition after ingestion of diet soda.
University students were recruited from April 2015 to October 2015, through flyers posted on campus and e-mail advertisements sent through departmental list-serves. After contacting the study team, interested volunteers underwent a brief phone screening, during which a trained research assistant obtained information on demographic characteristics and medical history and administered a brief screener to assess LCS consumption to determine eligibility. Eligible volunteers were 18–35 y of age, weighed ≥50 kg, and did not consume foods or beverages containing LCSs (defined as <1 food or beverage containing LCSs/mo). A minimum body weight of 50 kg was specified to ensure that those participants in the intervention group (assigned to consume 3 diet sodas/d) remained well below the acceptable daily intakes for sucralose and acesulfame potassium. Volunteers who were pregnant or lactating or who reported substantial weight changes (within the past 6 mo), prescription medication or dietary supplement use, excessive alcohol consumption (>3 drinks/d), or any active medical condition were excluded. This trial was registered at clinicaltrials.gov as NCT02877186.
Twenty-two healthy adults were enrolled, of whom 18 attended ≥2 study visits. All of the participants provided written informed consent before undergoing any study procedures, and the study protocol was approved by the Institutional Review Board at The George Washington University (IRB 011512). Given that this analysis was conducted as part of a pilot study of LCS effects on the gut microbiota and because the effects of LCS-induced alterations in gut microbiota have not been systematically evaluated in humans, formal power calculations were not performed.
Participants were scheduled for 3 study visits, each 1 wk apart and after a 10-h fast. All of the visits were scheduled between 0730 and 1000. At each visit, height was measured without shoes with the use of a wall-mounted stadiometer, body weight was measured to the nearest 0.1 kg by using a high-precision digital scale, and a spot urine sample was collected. At the baseline visit, participants (already identified as nonconsumers during the phone screening) were counseled extensively to continue LCS avoidance throughout the study and were instructed on how to maintain a food diary. Each subject also received a detailed verbal explanation together with a written information sheet to help them identify LCS-containing products to avoid dietary LCS consumption. Participants were also given a separate document with written instructions on how to record the type, brand, ingredients, and portion sizes of all foods and beverages consumed in a food record. After the baseline visit, participants began a 1-wk run-in period, in which they were asked to record their food and beverage intake, but did not undergo any intervention. After the run-in (visit 2), participants were randomly assigned to consume either diet soda [Diet Rite Cola (Dr Pepper Snapple Group) containing sucralose and acesulfame potassium] or unsweetened carbonated water 3 times/d for 1 wk. Treatment allocation (diet soda or carbonated water) was randomized 1:1 by using a sex-matched paired design. The presence of sucralose in all reported foods and beverages was assessed by using publicly available ingredient information, ingredient lists on food packaging, and participant report of ingredient and recipe information for homemade items.
Urinary sucralose concentrations were measured by using liquid chromatography–mass spectrometry. Each sample was diluted with water and D6-sucralose internal standard, mixed on a vortex for 2 min, and equilibrated at room temperature for 20 min. The diluted sample was then added to a conditioned and cleaned solid-phase extraction column (Waters Corporation). The solid-phase extraction cartridge was washed with water, and the sucralose was eluted with methanol before injection in duplicate into an Acquity I-Class UPLC (Waters Corporation) and an Acquity UPLC BEH C-18 column (2.1 mm × 50 mm, 1.7 μm) coupled with a Q-Exactive MS with an HESI-II electrospray source (Thermo Scientific). Both the sucralose and D6-sucralose were monitored at 2 different masses, and the calibration curve had an r2 > 0.999. The average relative SD for the samples was 3.6%.
Mean urinary sucralose concentrations at each study visit were calculated for both groups and compared by using independent-samples t tests. Analyses were conducted by using Microsoft Excel.
RESULTS
Characteristics of the study participants by treatment group are shown in Table 1. The majority were non-Hispanic white or Asian and of normal weight. There were no significant differences in age, sex, race, or BMI between the intervention groups.
TABLE 1.
Characteristics of study participants
| Variable | All (n = 18) | Diet soda1 (n = 10) | Control2 (n = 8) |
| Age, y | 21.9 ± 3.03 | 22.1 ± 3.2 | 21.5 ± 2.9 |
| Female sex, n (%) | 11 (61) | 6 (60) | 5 (63) |
| Race, n (%) | |||
| White | 10 (55) | 5 (50) | 5 (63) |
| Black | 1 (6) | 1 (10) | 0 (0) |
| Asian | 7 (39) | 4 (40) | 3 (37) |
| BMI, kg/m2 | 23.9 ± 4.0 | 22.9 ± 2.7 | 25.1 ± 5.1 |
| Weight, n (%) | |||
| Normal weight | 14 (78) | 9 (90) | 5 (63) |
| Overweight | 3 (16) | 1 (10) | 2 (25) |
| Obese | 1 (6) | 0 (0) | 1 (12) |
Two participants dropped out after random assignment to diet soda.
One participant in the control group was unable to provide a urine sample at the third visit.
Mean ± SD (all such values).
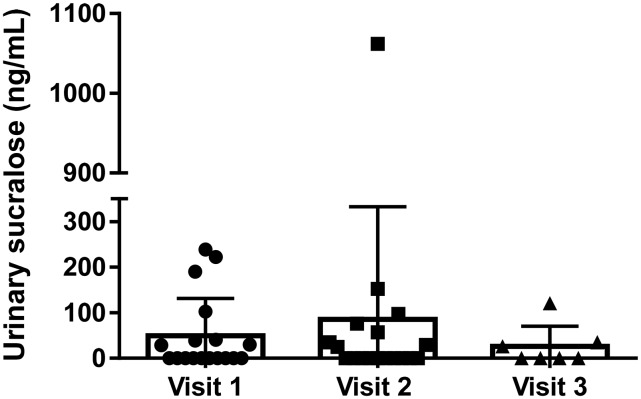
Mean urinary sucralose concentrations at each visit are shown in Figure 1. After eligibility assessment (based on reported consumption of <1 LCS/mo), 8 (44%) participants had measurable sucralose in their urine (29.9–239.0 ng/mL; mean ± SD: 111.4 ± 91.5 ng/mL) at baseline (visit 1).
FIGURE 1.
Urinary sucralose concentrations in reportedly unexposed study participants. Mean ± SD urinary sucralose concentrations in each participant are shown at baseline (indicated by circles; visit 1; n = 18), preintervention (indicated by squares; visit 2; n = 18), and postintervention (indicated by triangles; visit 3, control group only; n = 7).
After the 1-wk run-in period, before and during which participants were counseled extensively to avoid LCSs, sucralose was again detected in 8 (44%) individuals, 2 of whom did not have detectable sucralose at baseline. Concentrations ranged from 25.0 to 1062.0 ng/mL (mean ± SD: 191.7 ± 354.2 ng/mL). However, only 1 of the 8 subjects (97.4 ng sucralose/mL urine) reported consumption of an LCS-containing product (specifically, a sucralose-containing brand of English muffin) during the week before the visit. The source of the sucralose in the other exposed individuals was not identifiable.
As detailed in Supplemental Figure 1, 2 participants dropped out after being randomly assigned to the diet soda (after visit 2). One person was afraid of neurocognitive side effects, and the other reported nausea after the second day of exposure. One participant was unable to provide a urine sample at the third visit. Thus, 15 participants completed the 2-wk study and were included in the analysis at visit 3 (postintervention).
At the postintervention visit, after 3-times-daily consumption of either diet soda or carbonated water for 1 wk, mean urinary sucralose concentrations ranged from 885 to 11,280 ng/mL (mean ± SD: 4086.6 ± 3797.0 ng/mL) in those who were randomly assigned to diet soda to 0–121 ng/mL (mean ± SD: 26.0 ± 44.4 ng/mL) in those randomly assigned to the control (P = 0.01).
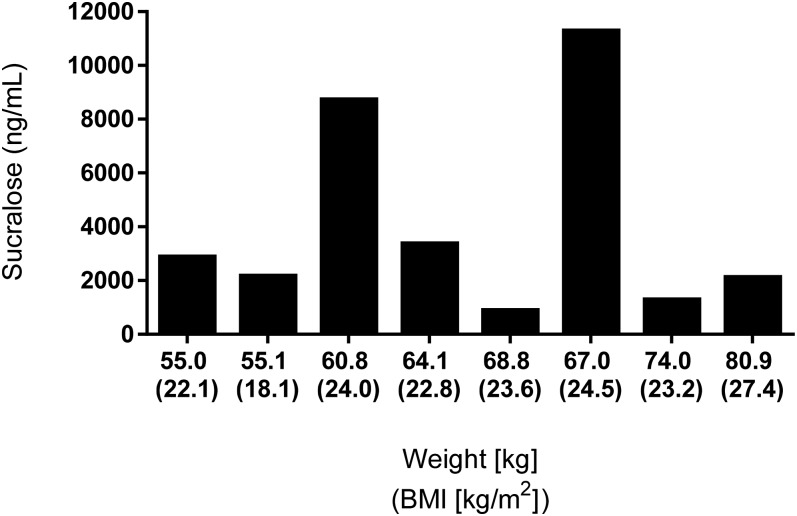
Of the 8 individuals in the diet soda group, all but 1 consumed ≥20 of the 21 cans of soda provided (assessed on the basis of the count of empty cans returned). However, urinary sucralose concentrations varied significantly between individuals, which was not explained by differences in body weight (Figure 2).
FIGURE 2.
Urinary sucralose concentrations in relation to body weight. Day 7 urinary sucralose concentrations obtained after an overnight fast (∼14 h after last consumption of diet soda) are shown for each of the 8 participants randomly assigned to consume diet soda 3 times/d for 1 wk by body weight and BMI.
Of those randomly assigned to receive carbonated water (n = 7), sucralose was detected in the urine of 3 individuals (26.0–121.0 ng/mL; mean ± SD: 60.7 ± 52.4 ng/mL), none of whom reported consumption of a sucralose-containing food or beverage (Figure 1).
DISCUSSION
Despite reported “nonconsumption” of dietary LCSs before enrollment, sucralose was present in the urine of 8 of the 18 (44%) participants at baseline. This finding builds on our previous report that individuals consume LCSs inadvertently in packaged foods and beverages (7) and also highlights the need to investigate nondietary sources of LCSs, such as personal care products, when assessing LCS exposure. Sucralose was also present in the urine of 44% of participants after the 1-wk run-in period, even after the provision of detailed verbal and written instructions to avoid products containing LCSs. Despite careful review of ingredient labels of the foods and beverages reported, the source of sucralose was not identifiable. Although sucralose can be present at trace concentrations in the water supply (11), this source would not explain the measured concentrations in our volunteers, further suggesting that this “unexpected” exposure was in part due to personal care products and nonreported food or beverages. Given that ∼14% of ingested sucralose is absorbed and excreted via the kidneys over 120 h (12), it is unlikely that minimal exposure before study enrollment would explain the presence of sucralose after the 1-wk run-in period.
In addition to highlighting the widespread use of LCSs, even among those apparent “nonconsumers,” our results also show that the provision of detailed information about LCS-containing products is not sufficient to ensure nonexposure. Our findings also show that even 7-d food records do not capture LCS exposure. Although dietary assessment is often subject to error, this omnipresent exposure to LCSs, regardless of whether or not participants were randomly assigned to consume them as part of the intervention, is also critical to consider when randomly assigning participants to a control group with which to compare LCS effects.
Urinary sucralose concentrations measured in the exposed “nonconsumers” in the current study were comparable to concentrations observed 3 h after the ingestion of a beverage containing 55 mg sucralose (representative of a typical diet soda) in a similarly aged, healthy, adult female volunteer (200 ng/mL; AC Sylvetsky, JE Blau, PJ Walter, HM Garraffo, KI Rother, unpublished results, 2017). Thus, concentrations detected in our participants were not trivial and were considerably higher than the residual sucralose expected from occasional consumption (≤1 time/mo) of sucralose-containing products. Furthermore, because the amount of LCS exposure required to exert metabolic effects is unknown, it is important to account for any amount of chronic LCS exposure in the design and interpretation of clinical studies.
Although our results should be interpreted cautiously because of the small sample size, the nonblinded nature of the study, the use of a nonvalidated screener to assess LCS consumption, the lack of data collection on nondietary sources of potential LCS exposure, and the inability to obtain detailed information on dietary intake before study enrollment, our report is strengthened by what we believe is a novel approach in measuring sucralose exposure after specific instructions to avoid dietary LCSs. This is critically important in designing and interpreting clinical studies of LCS effects, particularly when recruiting “LCS-naive” individuals, because both dietary and nondietary sources of sucralose should be considered. The ubiquitous and inadvertent exposure to sucralose further emphasizes the public health significance of understanding the health effects of chronic LCS exposure, because these findings show that diet is not the sole source of LCSs and thus current estimates of dietary LCS consumption likely markedly underestimate LCS exposure in the US population.
Acknowledgments
We thank Elena J Clark for her work in the collection of urine samples and for creating the figures.
The authors’ responsibilities were as follows—ACS, KR, and KIR: designed the research, collected the data, performed the statistical analyses, and wrote the manuscript; PJW and HMG: conducted the laboratory assays and reviewed the manuscript; ACS: had primary responsibility for the final content; and all authors: contributed to the drafting and editing of the manuscript. None of the authors reported a conflict of interest related to the study.
REFERENCES
- 1.Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR, Penfold C, Perry R, Putz P, Yeomans MR, et al. . Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes (Lond) 2016;40:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler SP. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav 2016;164:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab 2013;24:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylvetsky AC, Blau JE, Rother KI. Understanding the metabolic and health effects of low-calorie sweeteners: methodological considerations and implications for future research. Rev Endocr Metab Disord 2016;17:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet 2017. Jan 6 (Epub ahead of print; DOI: 10.1016/j.jand.2016.11.004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sylvetsky AC, Dietz WH. Nutrient-content claims—guidance or cause for confusion? N Engl J Med 2014;371:195–8. [DOI] [PubMed] [Google Scholar]
- 7.Sylvetsky AC, Greenberg M, Zhao X, Rother KI. What parents think about giving nonnutritive sweeteners to their children: a pilot study. Int J Pediatr 2014;2014:819872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sylvetsky AC, Bauman V, Blau JE, Garraffo HM, Walter PJ, Rother KI. Plasma concentrations of sucralose in children and adults. Toxicol Environ Chem 2016;99:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sylvetsky AC, Gardner AL, Bauman V, Blau JE, Garraffo HM, Walter PJ, Rother KI. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health A 2015;78:1029–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rother KI, Sylvetsky AC, Schiffman SS. Non-nutritive sweeteners in breast milk: perspective on potential implications of recent findings. Arch Toxicol 2015;89:2169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange FT, Scheurer M, Brauch HJ. Artificial sweeteners—a recently recognized class of emerging environmental contaminants: a review. Anal Bioanal Chem 2012;403:2503–18. [DOI] [PubMed] [Google Scholar]
- 12.Roberts A, Renwick AG, Sims J, Snodin DJ. Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol 2000;38(Suppl 2):S31–41. [DOI] [PubMed] [Google Scholar]