Abstract
Hyperuricemia is recognized as an important risk factor for gout. High dietary intake of purine-rich foods such as meats and sea foods increases uric acid (UA) levels in the blood. Taxifolin present in Siberian larch and strawberries has been reported to possess health promoting activities including anti-oxidant effect. In this study, we examined anti-hyperuricemic effect of taxifolin in both cultured hepatocytes and hyperuricemic model mice. In cultured AML12 hepatocytes, taxifolin significantly suppressed UA production dose- and time-dependently. In mice with hyperuricemia induced by concurrent administration of guanosine-5′-monophosphate and inosine-5′-monophosphate, oral administration of taxifolin suppressed the increases in plasma and liver UA levels. In addition, it also suppressed hepatic xanthine oxidase (XO) activity. Thus, anti-hyperuricemic effect of taxifolin could be explained, at least partly, by suppressing UA production via inhibition of XO activity in the liver. These results suggest that taxifolin possesses a potent hypouricemic effect and it could be a potential candidate for an anti-hyperuricemic phytochemical.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-016-0061-4) contains supplementary material, which is available to authorized users.
Keywords: Taxifolin, AML12 hepatocyte, Hyperuricemia, Uric acid
Introduction
Hyperuricemia is the high blood uric acid (UA) state and results from overproduction of UA in the body and/or reduced excretion of UA from the body. Hyperuricemia is regarded as an important risk factor for gout. In addition, hyperuricemia is thought to be risk factors for metabolic syndrome (Babio et al. 2015). High dietary intake of purine-rich foods such as meats and sea foods increases UA levels in the blood (Choi et al. 2004). Purine nucleotides such as guanosine-5′-monophosphate (GMP) and inosine-5′-monophosphate (IMP) from foods are metabolized into xanthine via multiple steps of reactions catalyzed by various enzymes like 5′-nucleotidase, purine nucleoside phosphorylase, guanine deaminase and xanthine oxidase (XO) (Ishikawa et al. 2013). In humans, xanthine is finally oxidized to uric acid by XO, which is highly expressed in the liver (Lee 1973). Allopurinol, an XO inhibitor, is prescribed to treat hyperuricemia and gout for decades. However, this drug has adverse effects such as hepatitis, nephropathy and allergy (Kong et al. 2000). Thus, it is anticipated to discover novel substances from foods and natural resources that are capable of suppressing hyperuricemia.
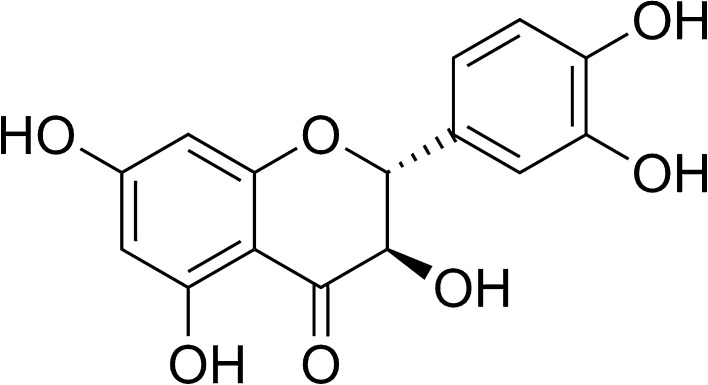
Taxifolin (dihydroquercetin) is flavanonol, a type of flavonoids (Fig. 1), and can be found in Larix sibirica (Diwakar et al. 2012) and Taxus chinensis (Li et al. 2008). Recently, taxifolin is demonstrated to exist in diploid strawberries (Fragaria vesca) (Sun et al. 2014a). This phytochemical has been reported to possess a variety of beneficial effects such as anti-oxidant, anti-cardiomyopathic and anti-inflammatory effects (Sun et al. 2014b; Manigandan et al. 2015; Gupta et al. 1971). Many flavonoids like kaempherol and apigenin are reportedly effective in hyperuricemic model animals (Mo et al. 2007). A recent study has revealed that quercetin lowers plasma UA levels in pre-hyperuricemic humans (Shi and Williamson 2016). However, it remains unclear whether or not taxifolin would be hypouricemic.
Fig. 1.
Structure of taxifolin (dihydroquercetin)
We have recently contrived assay systems in vitro and in vivo in combination for screening anti-hyperuricemic compounds (Adachi et al. 2016). In the present study, we therefore examined the effect of taxifolin on UA production in cultured hepatocytes and mice with purine bodies-induced hyperuricemia, by adopting the new assay systems.
Materials and methods
Materials
AML12 cells were provided by American Type Culture Collection (ATCC® CRL2254) (Manassas, VA, USA). Taxifolin was purchased from Adooq BioScience LLC (Irvine, CA, USA), DMEM/F-12 from Life Technologies (Grand Island, NY, USA), fetal bovine serum (FBS) from Hyclone (Logan, UT, USA), penicillin and streptomycin from Nacalai Tesque, Inc. (Kyoto, Japan), guanosine-5′-monophosphate (GMP) and inosine-5′-monophosphate (IMP) from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and Pierce™ BCA Protein Assay kit from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Selenium, inosine, guanosine and xanthine were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Allopurinol, dexamethasone, carboxymethyl cellulose sodium (CMC-Na), dimethyl sulfoxide (DMSO), recombinant human insulin, transferrin from human blood, uric acid and uric acid assay kit (Uric acid C-test Wako) were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Unless otherwise noted, all other chemicals were purchased from Wako Pure Chemical Industries, Ltd., and they were of guaranteed reagent grade. Twenty-four-well plates were obtained from Sumitomo Bakelite Co., Ltd. (Tokyo, Japan).
Culture of AML12 cells
AML12 cells were cultured in DMEM/F-12 supplemented with 10% FBS, 5 µg/ml recombinant human insulin, 5 µg/ml transferrin from human blood, 3 ng/ml selenium, 40 ng/ml dexamethasone, 100 U/ml penicillin and 100 µg/ml streptomycin (10% FBS/DMEM/F-12) under an atmosphere of 5% CO2/95% humidified air at 37 °C. The cells (1.0 × 105 cells/well) were subcultured into 24-well plates and grown for 72 h in 10% FBS/DMEM/F-12, and then kept for 24 h in serum-free DMEM/F-12.
Effect of taxifolin on uric acid production by AML12 cells
UA production by AML12 hepatocytes was evaluated as described previously (Adachi et al. 2016). In brief, after 24 h culture in serum-free DMEM/F-12, AML12 cells were washed once with phosphate buffered saline without calcium and magnesium [PBS (−)] and then incubated in 200 µl of balanced salt solution (BSS) containing 188 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.8 mM CaCl2, 25 mM NaHCO3, 1 mM NaH2PO4, 10 mM HEPES and 5 mM glucose (Petrie et al. 2013). BSS also contained guanosine and inosine (100 µM each) in combination (GI mixture) as UA precursors in the absence or presence of taxifolin (0, 25, 50 and 100 µM) at the final DMSO concentration of 0.15%. Similarly, the cells were incubated in 200 µl of BSS with GI mixture without or with allopurinol (0, 0.1, 1 and 10 µM) at the final DMSO concentration of 0.15%. On the termination of 2 h incubation, 200 µl of BSS was collected for determination of UA. UA detected in BSS was adopted as an index of its productivity (Adachi et al. 2016). As to taxifolin action, the hepatocytes were incubated for 4 h as well as 2 h. After washed once with PBS (−), the hepatocytes were scraped into 300 µl of buffer containing 50 mM Tris (pH 7.5) and 1 mM sodium phosphate, sonicated, and centrifuged (12,000×g, 5 min, 4 °C). UA levels in the BSS were determined by the uricase method (Uric acid C-test Wako). Protein concentrations in the cell homogenates were determined with a commercial kit (Pierce™ BCA Protein Assay kit). UA production was expressed as nmol per 2 or 4 h per mg cellular protein (nmol/2 or 4 h/mg protein).
Animals
Male ICR mice (Charles River Japan, Inc., Yokohama, Japan) at 4 weeks of age were housed in plastic cages in a room with a 12-h light–dark cycle (dark phase of 18:00–6:00) and constant temperature (22 °C). They were housed in groups of four for 7 days for acclimatization to the environment. The mice were provided with tap water and regular pellet diet (CRF-1, Oriental Yeast Co., Tokyo, Japan) ad libitum. This experiment was carried out in accordance with the guideline for Animal Experiments of Utsunomiya University Animal Research Committee (ethic approval number: A14-0017).
Taxifolin administration to model mice with hyperuricemia
Anti-hyperuricemic effect in hyperuricemic model mice was estimated as described previously (Adachi et al. 2016) with slight modifications. Briefly, after acclimatization to the environment for 1 week, the mice were divided into five groups with similar body weight: normal control group (n = 8), hyperuricemic model control group (n = 8), allopurinol group (n = 8), low-dose of taxifolin group (n = 8) and high-dose of taxifolin group (n = 8). Taxifolin and allopurinol were suspended in 0.5% CMC-Na. After 4 h fasting, allopurinol at 10 mg/kg body weight and taxifolin at 100 mg/kg (low-dose group) and 300 mg/kg body weight (high-dose group) were orally given to the mice once a day for the three consecutive days. Normal control and hyperuricemic model control groups were orally given 0.5% CMC-Na alone for 3 days. On day 3, the mice were intraperitoneally injected with both GMP and IMP (300 mg each/kg body weight) to induce hyperuricemia 1 h after allopurinol or taxifolin administration. GMP and IMP were dissolved in PBS (−). The normal control group was injected with the PBS (−) alone as vehicle. One hour after GMP and IMP injection, the blood was collected under isoflurane anesthesia from the inferior vena cava in the microtube with heparin sodium and then the liver was excised. The blood samples were centrifuged at 5000×g for 10 min at 4 °C to obtain the plasma. The plasma was stored at −80 °C until analyzed. The excised liver was washed with saline, cut into two pieces, frozen in liquid nitrogen, and stored at −80 °C until analyzed.
Liver sample preparation
One piece of the liver sample was homogenized in ice-cold 50 mM potassium phosphate buffer (pH 7.8), sonicated, and centrifuged (12,000×g, 5 min, 4 °C). The supernatant fraction was used for UA determination. Another piece was homogenized by sonication in ice-cold 50 mM potassium phosphate buffer (pH 7.8) containing 1 mM EDTA-2Na and 1 mM PMSF, and centrifuged (12,000×g, 5 min, 4 °C). The supernatant fraction was subjected to the determination of XO activity.
Determination of uric acid level of plasma and liver
Plasma and liver UA levels were determined by the uricase method (Uric acid C-test Wako). Protein concentrations in the liver homogenates were determined with Pierce™ BCA Protein Assay kit. Liver UA levels were expressed as mg per g liver protein.
Liver xanthine oxidase activity assay
Liver XO activity assay was carried out according to the methods previously described (Mo et al. 2007) with slight modifications using 96-well plates. Liver homogenates (40 µl) and ice-cold 50 mM potassium phosphate buffer (pH 7.8) containing 1 mM EDTA-2Na and 1 mM PMSF (30 µl) were applied into 96-well plates. The reaction was initiated by the addition of 180 µl of 150 µM xanthine in the same buffer. Immediately after the addition of the substrate buffer, the absorbance at 295 nm and 37 °C was measured with a Spectramax M5e reader (Molecular Devices, LLC, Sunnyvale, CA, USA) for 30 min. UA production was calculated from the increase of the absorbance for 30 min based on the UA standard curve. Protein concentrations in the liver homogenates were determined with Pierce™ BCA Protein Assay kit. XO activity was expressed as µmol UA produced per min per mg protein.
Statistical analyses
Data are expressed as mean ± SEM. Data on UA production in AML12 cells were analyzed by one-way ANOVA and Tukey’s multiple-comparisons test as a post hoc test. Time-dependent effect of taxifolin on the UA production was examined using an unpaired t test. The results of the animal experiments were analyzed by one-way ANOVA and Dunnett’s multiple-comparisons test as a post hoc test. P values < 0.05 were considered statistically significant. These analyses were conducted by using the Prism 6 software package (GraphPad, San Diego, CA, USA).
Results
Effect of taxifolin on uric acid production in AML12 cells
UA production by AML12 hepatocytes treated with 0.1, 1 and 10 µM allopurinol was significantly and dose-dependently lower than that treated with no allopurinol (0 µM) (Fig. 2a). Similarly, the treatment of hepatocytes with taxifolin for 2 and 4 h significantly and dose-dependently decreased the UA production in the cultured hepatocytes (Fig. 2b, c). Inhibitory effect on UA production was significantly stronger in the hepatocytes treated with taxifolin for 4 h than in those treated for 2 h with the corresponding doses of 25, 50 and 100 µM, respectively (Fig. 2d), indicating that taxifolin time-dependently affected UA production.
Fig. 2.
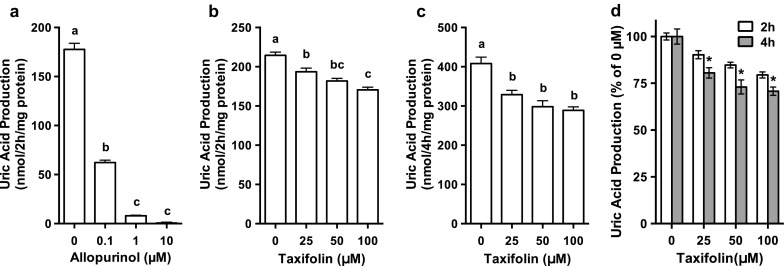
Effects of taxifolin and allopurinol on UA production in AML12 hepatocytes. AML12 cells were treated with 0.1, 1 and 10 µM allopurinol for 2 h (a) or with 25, 50 and 100 µM taxifolin for 2 h (b) or 4 h (c) in balanced salt solution containing guanosine + inosine (100 µM each). d indicates relative UA production by AML12 cells treated with taxifolin for 2 or 4 h, data being expressed as percentage of the 0 µM taxifolin. Each value represents mean ± SEM for six wells (duplicate measurements per well). Values not sharing a common letter are significantly different at P < 0.05 (Tukey’s test). *P < 0.05 compared with the 2 h-treated group with the corresponding concentrations by unpaired t test
Effect of taxifolin on the plasma uric acid level in hyperuricemic model mice
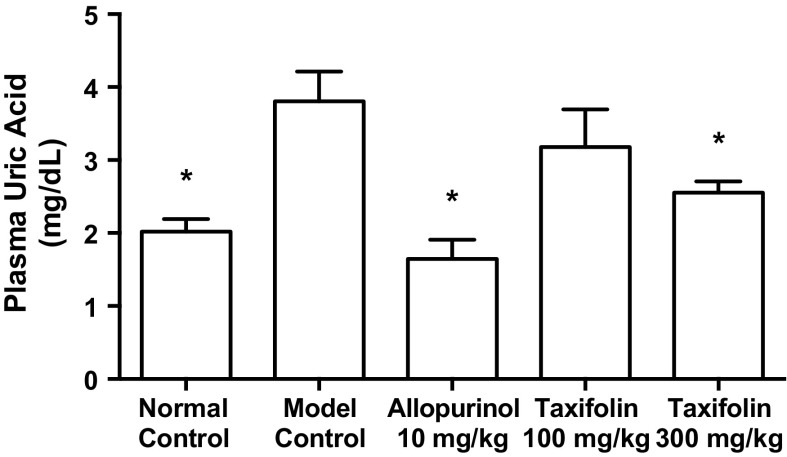
The intraperitoneal injection of GMP and IMP in combination to mice significantly increased the plasma UA concentration (model control group) as compared with that of mice injected with PBS (−) alone (normal control group, Fig. 3). The oral administration of allopurinol at 10 mg/kg body weight and taxifolin at 300 mg/kg body weight significantly suppressed this rise in plasma UA concentration as compared with that of CMC-Na alone (model control group, Fig. 3).
Fig. 3.
Effects of taxifolin and allopurinol on plasma uric acid levels in hyperuricemic mice. The mice were orally administered with taxifolin and allopurinol at the different doses indicated. The mice were then intraperitoneally injected with both GMP and IMP (300 mg each/kg body weight) to induce hyperuricemia. Normal control and model control groups were treated with vehicles instead of test samples and nucleotides. Each value represents mean ± SEM for eight mice (duplicate measurements per mouse). *Significantly different from the model control group at P < 0.05 (Dunnett’s test)
Effect of taxifolin on the liver uric acid level and xanthine oxidase activity in hyperuricemic model mice
As shown in Fig. 4a, the administration of GMP and IMP in combination to mice caused a significant increase in the liver UA level when compared with normal mice (normal control group vs. model control group). The oral administration of allopurinol at 10 mg/kg body weight and taxifolin at 300 mg/kg body weight significantly suppressed the increase in the liver UA level as compared with that of model control group.
Fig. 4.
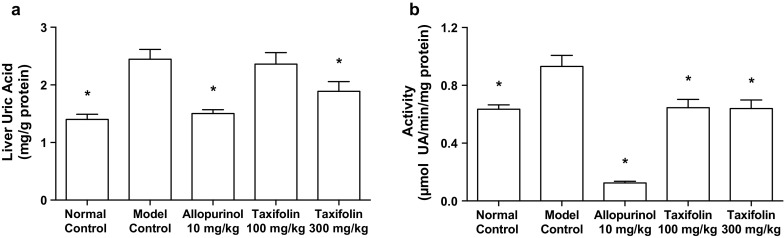
Effects of taxifolin and allopurinol on liver uric acid levels (a) and xanthine oxidase activity (b) in hyperuricemic mice. The mice were orally administered with taxifolin and allopurinol at the different doses indicated. The mice were then intraperitoneally injected with both GMP and IMP (300 mg each/kg body weight) to induce hyperuricemia. Normal control and model control groups were treated with vehicles instead of test samples and nucleotides. Each value represents mean ± SEM for eight mice (duplicate measurements per mouse). *Significantly different from the model control groups at P < 0.05 (Dunnett’s test)
The liver XO activity in model control group was significantly higher than that in normal control group. The XO activities in allopurinol (10 mg/kg body weight), low-dose (100 mg/kg body weight) and high-dose (300 mg/kg body weight) of taxifolin groups were significantly lower than that in model control group (Fig. 4b).
Discussion
In general, in vitro assay systems including cultured cells are suitable for the first screening of candidate compounds that possess certain functions or pharmacological actions, because of small amounts of test samples, rapidity and hence inexpensiveness. The assay systems, especially cultured cells seem to be of use to know detailed mechanisms of effective compounds (Miura et al. 2004; Minakawa et al. 2012; Cheong et al. 2014). Based on the concept, we have recently constructed an assay system for UA production in cultured AML12 hepatocytes (Adachi et al. 2016). Besides the in vitro assay system, we have also contrived in vivo model mice with purine bodies-induced hyperuricemia to which nucleotides GMP and IMP in combination are given as UA precursors, instead of nucleosides guanosine and inosine in combination that are adopted as UA precursors in cultured hepatocytes. This discrimination in use of nucleosides in vitro and nucleotides in vivo arises from differences in UA productivity in cultured hepatocytes and solubility in PBS (−) as vehicle for administration to animals. Despite this discrimination, results obtained in vitro are found to well correlate with those obtained in vivo when allopurinol is employed as the positive control substance, indicating that our in vitro and in vivo assay systems work adequately (Adachi et al. 2016).
Adopting above-mentioned assay systems, the effect of taxifolin on UA production and hyperuricemia was examined in the present study. Results obtained demonstrate that taxifolin is able to reduce UA production by cultured hepatocytes (Fig. 2) and to suppress purine bodies-induced hyperuricemia in mice (Fig. 3), like allopurinol adopted as the positive control drug. Thus, taxifolin is demonstrated for the first time as a potent anti-hyperuricemic substance. So far, we have examined the effects of kaempherol and apigenin (Mo et al. 2007) as well as taxifolin on uric acid productivity in vitro employing AML12 hepatocytes cultured for 2 h (Adachi et al. unpublished observation). Their effects on the UA productivity (expressed as % of control, i.e., 100% at 0 μM of corresponding phytochemicals) at the same concentration of 100 μM are as follows; taxifolin 79.5 ± 1.6%, kaempherol 16.9 ± 4.0%, apigenin 3.3 ± 0.9% (mean ± SEM, n = 6). The inhibitory activity of taxifolin seems to be weaker than those of kaempherol and apigenin. Nonetheless, taxifolin is demonstrated to actually suppress the urine bodies-induced hyperuricemia at the whole body level, demonstrating that assay systems in vitro and in vivo in combination adopted in the present study are forceful for searching overlooked but novel anti-hyperuricemic compounds like taxifolin.
As above-mentioned, taxifolin is found in Larix sibirica (Diwakar et al. 2012), Taxus chinensis (Li et al. 2008) and diploid strawberries (Fragaria vesca) (Sun et al. 2014a). Besides diploid strawberries, we have recently identified taxifolin in octaploid strawberries (Tochiotome; Fragaria × ananassa Duch. cv. Tochiotome, and Skyberry; Fragaria × ananassa Duch. cv. Tochigi i 27 gou) (Supplementary Figs. 1, 2, 3 and Supplementary Scheme). Tochiotome and Skyberry, especially the former, are now popular strawberries on the market in Japan, suggesting they may be hidden suppliers of the potent anti-hyperuricemic phytochemical, taxifolin.
As shown in vivo study, concurrent administration of GMP and IMP caused rises in the plasma and liver UA levels in mice. Taxifolin (300 mg/kg body weight) as well as allopurinol (10 mg/kg body weight) significantly suppressed these rises (Figs. 3, 4a). Administration of GMP and IMP in combination also significantly enhanced hepatic XO activity, while taxifolin (100 and 300 mg/kg body weight) as well as allopurinol (10 mg/kg body weight) significantly canceled the rise in XO activity (Fig. 4b). The results clearly demonstrate that taxifolin exhibits anti-hyperuricemic effect via suppressing XO activity in the liver of hyperuricemic mice. However, it is unclear at present whether taxifolin could inhibit XO directly or indirectly through, for instance, reduced gene expression and/or translation steps. Precise modes of inhibitory action of taxifolin against XO should be clarified in the future.
The rises in the plasma and liver UA levels may be modulated also through other systems, such as UA production from adipose tissues (Tsushima et al. 2013). Additionally, green tea polyphenol and morin have been reported to exhibit anti-hyperuricemic effect by suppressing UA reabsorption and enhancing UA secretion in the kidney of hyperuricemic mice (Chen et al. 2015; Wang et al. 2010). Besides suppressing hepatic UA production via XO inhibition, a possibility that taxifolin may promote UA excretion from the body cannot be ruled out. Further intensive studies are required to elucidate precise mechanisms for anti-hyperuricemic action of taxifolin.
In summary, we have demonstrated that taxifolin, present in Siberian larch and strawberries, suppresses UA production in AML hepatocytes and inhibits the rises in the plasma and hepatic UA levels in purine bodies-induced hyperuricemic model mice. Its inhibitory actions are shown to be modulated, at least partly, by suppressing hepatic UA production via inhibition of XO activities in the liver. Although further studies are needed to clarify precise mechanisms involved, taxifolin may be preventive and remedial for hyperuricemia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported in part by the Regional Innovation Strategy Support Program, MEXT, Japan, and in part by JSPS KAKENHI Grant Number JP16K16273. Authors are grateful to Yuki Takami for her excellent technical assistance.
Abbreviations
- BSS
Balanced salt solution
- GMP
Guanosine-5′-monophosphate
- IMP
Inosine-5′-monophosphate
- UA
Uric acid
- XO
Xanthine oxidase
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Adachi S, Yoshizawa F, Yagasaki K. Assay systems for screening food and natural substances that have anti-hyperuricemic activity: uric acid production in cultured hepatocytes and purine bodies-induced hyperuricemic model mice. Cytotechnology. 2016 doi: 10.1007/s10616-016-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babio N, Martínez-González MA, Estruch R, Wärnberg J, Recondo J, Ortega-Calvo M, Serra-Majem L, Corella D, Fitó M, Ros E, Becerra-Tomás N, Basora J, Salas-Salvadó J. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2015;25:173–180. doi: 10.1016/j.numecd.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Chen G, Tan ML, Li KK, Leung PC, Ko CH. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J Ethnopharmacol. 2015;175:14–20. doi: 10.1016/j.jep.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, Yagasaki K. Antihyperglycemic effect of equol, a daidzein derivative, in cultured L6 myocytes and ob/ob mice. Mol Nutr Food Res. 2014;58:267–277. doi: 10.1002/mnfr.201300272. [DOI] [PubMed] [Google Scholar]
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- Diwakar G, Rana J, Scholten JD. Inhibition of melanin production by a combination of Siberian larch and pomegranate fruit extracts. Fitoterapia. 2012;83:989–995. doi: 10.1016/j.fitote.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Gupta MB, Bhalla TN, Gupta GP, Mitra CR, Bhargava KP. Anti-inflammatory activity of taxifolin. Jpn J Pharmacol. 1971;21:377–382. doi: 10.1254/jjp.21.377. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Aw W, Kaneko K. Metabolic interactions of purine derivatives with human ABC transporter ABCG2: genetic testing to assess gout risk. Pharmaceuticals. 2013;6:1347–1360. doi: 10.3390/ph6111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73:199–207. doi: 10.1016/S0378-8741(00)00305-6. [DOI] [PubMed] [Google Scholar]
- Lee PC. Developmental changes of adenosine deaminase, xanthine oxidase, and uricase in mouse tissues. Dev Biol. 1973;31:227–233. doi: 10.1016/0012-1606(73)90259-5. [DOI] [PubMed] [Google Scholar]
- Li C, Huo C, Zhang M, Shi Q. Chemistry of Chinese yew, Taxus chinensis var. mairei. Biochem Syst Ecol. 2008;36:266–282. doi: 10.1016/j.bse.2007.08.002. [DOI] [Google Scholar]
- Manigandan K, Jayaraj RL, Jagatheesh K, Elangovan N. Taxifolin mitigates oxidative DNA damage in vitro and protects zebrafish (Danio rerio) embryos against cadmium toxicity. Environ Toxicol Pharmacol. 2015;39:1252–1261. doi: 10.1016/j.etap.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Minakawa M, Miura Y, Yagasaki K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem Biophys Res Commun. 2012;422:469–475. doi: 10.1016/j.bbrc.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Miura D, Miura Y, Yagasaki K. Resveratrol inhibits hepatoma cell invasion by suppressing gene expression of hepatocyte growth factor via its reactive oxygen species scavenging property. Clin Exp Metastasis. 2004;21:445–451. doi: 10.1007/s10585-004-2698-1. [DOI] [PubMed] [Google Scholar]
- Mo SF, Zhou F, Lv YZ, Hu QH, Zhang DM, Kong LD. Hypouricemic action of selected flavonoids in mice: structure-activity relationships. Biol Pharm Bull. 2007;30:1551–1556. doi: 10.1248/bpb.30.1551. [DOI] [PubMed] [Google Scholar]
- Petrie JL, Patman GL, Sinha I, Alexander TD, Reeves HL, Agius L. The rate of production of uric acid by hepatocytes is a sensitive index of compromised cell ATP homeostasis. Am J Physiol Endocrinol Metab. 2013;305:E1255–E1265. doi: 10.1152/ajpendo.00214.2013. [DOI] [PubMed] [Google Scholar]
- Shi Y, Williamson G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: a randomised, double-blinded, placebo-controlled, cross-over trial. Br J Nutr. 2016;115:800–806. doi: 10.1017/S0007114515005310. [DOI] [PubMed] [Google Scholar]
- Sun J, Liu X, Yang T, Slovin J, Chen P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMS(n) Food Chem. 2014;146:289–298. doi: 10.1016/j.foodchem.2013.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Chen RC, Yang ZH, Sun GB, Wang M, Ma XJ, Yang LJ, Sun XB. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CP, Wang X, Zhang X, Shi YW, Liu L, Kong LD. Morin improves urate excretion and kidney function through regulation of renal organic ion transporters in hyperuricemic mice. J Pharm Pharm Sci. 2010;13:411–427. doi: 10.18433/J3Q30H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.