Abstract
Antimicrobial proteins and peptides (AMPs) are valuable as leads in the pharmaceutical industry for the development of novel anti-infective drugs. Here we describe the efficient heterologous expression and basic characterization of a Gloverin-family AMP derived from the greater wax moth Galleria mellonella. Highly productive single-cell clones prepared by limiting dilution achieved a 100% increase in productivity compared to the original polyclonal Drosophila melanogaster S2 cell line. Comprehensive screening for suitable expression conditions using statistical experimental designs revealed that optimal induction was achieved using 600 µM CuSO4 at the mid-exponential growth phase. Under these conditions, 25 mg/L of the AMP was expressed at the 1-L bioreactor scale, with optimal induction and harvest times ensured by dielectric spectroscopy and the online measurement of optical density. Gloverin was purified from the supernatant by immobilized metal ion affinity chromatography followed by dialysis. In growth assays, the purified protein showed specific antimicrobial activity against two different strains of Escherichia coli.
Keywords: Antimicrobial protein, Galleria mellonella Gloverin, Stably transformed D. melanogaster S2 cells, Recombinant protein expression, Online process monitoring, Process optimization
Introduction
Antimicrobial proteins and peptides (AMPs) are a diverse group of polypeptide molecules with highly conserved roles in the innate immune systems of bacteria, fungi, plants, insects and vertebrates (Lehrer and Ganz 1999). AMPs often show broad activity against microbes but their mechanisms of action differ from those of conventional antibiotics (Li et al. 2012a). Due to the spread of antibiotic resistance among common pathogens, AMPs therefore offer an alternative route for the development of novel anti-infective drugs (Aoki et al. 2012; Li et al. 2012a). Current research focuses on screening for AMPs and the development of suitable production processes (Müller et al. 2015). The greater wax moth Galleria mellonella is a useful model for the identification and testing of AMPs, and here we investigated the properties of G. mellonella Gloverin (GmGlv), a member of the lepidopteran-specific family of Gloverin-like proteins (Seitz et al. 2003; Brown et al. 2009; Vogel et al. 2011). In order to make such AMPs accessible for further research and commercial development, a satisfactory expression level must be achieved in one of the several host species available for recombinant protein expression, which include bacteria (Escherichia coli), yeasts (such as Pichia pastoris) and insect cells (Müller et al. 2015). These expression systems differ in terms of complexity, space–time-yield and the ability to support protein folding and posttranslational modification (Schmidt 2004). Bacterial and yeast systems are considered the simplest, but because the host cells are microbes they are often susceptible to the effects of AMPs. This is the case for the Gloverin family because the antimicrobial activity of these proteins is based on interference with bacterial lipopolysaccharides (Axén et al. 1997) and fungal membrane components (Xu et al. 2012). Although Bombyx mori Gloverins have been expressed in microbes (Yi et al. 2013), the antimicrobial properties of the product hamper production. As an alternative, Gloverins have been expressed in insect cells using baculovirus vectors (Lundström et al. 2002; Kawaoka et al. 2008). However, this is not suitable for large scale production either because Gloverins can also inactivate baculoviruses (Moreno-Habel et al. 2012).
Insect cells should be ideal for the expression of Gloverins because they closely resemble the natural AMP-producing tissue in terms of protein folding and pro-peptide cleavage, and are unlikely to be susceptible to AMP toxicity. In this context, stable recombinant Drosophila melanogaster S2 cells (rS2 cells) provide an effective system for protein expression (Moraes et al. 2012; de Jongh et al. 2013) and these cells are particularly suitable for the production of biologically active small peptides, such as spider toxins (Escoubas et al. 2003) and insect AMPs. Accordingly Manduca sexta and Plutella xylostella Gloverins have already been expressed successfully in rS2 cells (Xu et al. 2012, 2015). We therefore evaluated rS2 cells for the functional expression of GmGlv and also addressed bottlenecks such as the polyclonal nature of the transformed cell line, which results in an inconsistent expression profile during long-term cultivation (Cherbas and Cherbas 2007). Following the selection of a monoclonal production cell line, we used a statistical experimental design approach to screen for optimal induction conditions. The resulting data were then used to scale the process for the production of GmGlv in a 1-L bioreactor, supported by additional online monitoring techniques. The secreted and functional AMP was recovered using a simple three-step procedure comprising cell removal, chromatography and dialysis, allowing the purified protein to be tested for biological activity against E. coli.
Materials and methods
Maintenance of D. melanogaster S2cells
Unless otherwise stated, D. melanogaster S2 cells (Schneider 1972) were grown at 27 °C in ExCell 420 serum-free medium (Sigma-Aldrich, Taufkirchen, Germany) supplemented with 8 mM l-glutamine (Biochrom, Berlin, Germany). The medium for rS2 cells also contained 10 µg/mL blasticidin S (Invivogen, Toulouse, Germany) to maintain selection pressure. All cell lines were adapted to grow in suspension and were split every 3–4 days to obtain cultures with a density of 1.5 × 106 cells/mL. For long-term preservation, 1.5 × 107 cells were suspended in 1 mL freezing medium (1:1 mixture of spent and fresh ExCell 420 medium plus 7.5% DMSO) for storage in liquid nitrogen.
Construction of the polyclonal expression cell line
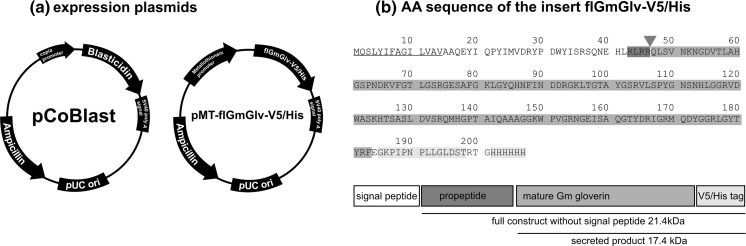
The D. melanogaster expression system (DES®, Thermo Fisher Scientific, Darmstadt, Germany) was set up as previously described (Kollewe 2013). Briefly, wild-type S2 cells in Schneider S2 medium plus 5% FBS (Thermo Fisher Scientific) were co-transfected with the selection plasmid pCoBlast (Thermo Fisher Scientific) and an expression plasmid containing the GmGlv gene (pMT-flGmGlv-V5/His). This was derived from pMT/BiP/V5-His B (Thermo Fisher Scientific) and contained the full-length GmGlv sequence under the control of the copper-inducible D. melanogaster metallothionein promoter. The original BiP signal sequence was removed before inserting the GmGlv sequence because the insert contained its own signal sequence (Fig. 1). To generate a stable polyclonal rS2 cell line, the transfected cells were cultured under selection pressure for 30 days before adapting them to grow in ExCell 420 serum-free medium.
Fig. 1.
a Maps of the vectors pCoBlast and pMT-flGmGlv-V5/His. b Full-length amino acid sequence (AA sequence) of Galleria mellonella Gloverin with its own signal peptide (underlined), putative propeptide convertase recognition sequence (dark gray background) and cleavage site (gray triangle), modified from (Kollewe 2013)
Single-cell cloning and production screening
Monoclonal cell lines for efficient protein production were prepared by limiting dilution as previously described (Uribe et al. 2013; Wang et al. 2012; Scotter et al. 2006). The method is based on the co-cultivation of single transformants with untransfected feeder cells, followed by antibiotic selection of the clones. Ten 96-well plates (Eppendorf, Hamburg, Germany) were prepared by transferring 100 µL antibiotic-free medium to each well and seeding with 5 × 104 untransfected feeder cells and approximately one transformed cell. The cells were allowed to proliferate for 3 days to ensure proper conditioning of the medium before 15 µg/mL blasticidin S was added for selection. The feeder cells decayed over the next 14 days and the resistant colonies became visible. After validation by microscopy, monoclonal colonies were picked and scaled up in 48-, 24-, 12- and 6-well plates. The cells were then induced using 900 µM CuSO4. After 24 h, the supernatant was collected from each well and dot blot analysis was used to identify the most productive cell lines. Cell growth during cultivation was determined by measuring the absorption at OD600 (Bédard et al. 1994). Production clones were tested against the parental polyclonal cell line to confirm their enhanced productivity. The relative amount of His-tagged protein in the supernatants of six independent cultures was determined by quantification of the dot blot luminescence intensity. Western blot analysis was used to resolve the GmGlv distribution between the culture supernatant and cell interior after lysing the cell pellets (1 mL cell suspension) using 1 mL lysis buffer (50 mM Tris, 150 mM NaCl, 1% Nonidet P40).
Optimization of induction conditions
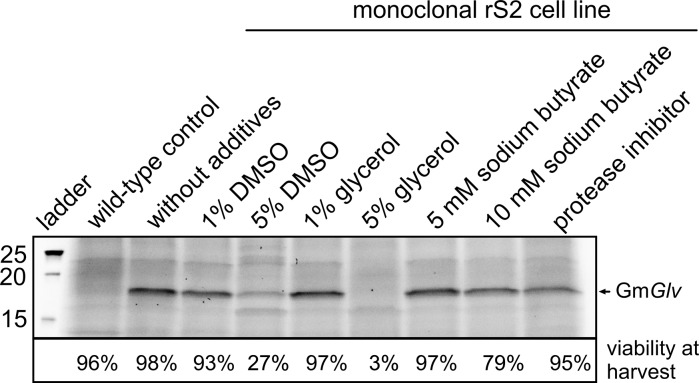
The induction conditions for the production line were optimized by testing the concentration of inducer (CuSO4) and the time of induction (cell concentration). The experiments were carried out in 24-well plates (Infors Celltron) with a 500-µL working volume, shaking at 85 rpm. Based on preliminary experiments, the harvest time was set to 4 days after induction. The response surface method (RSM) was used based on a face-centered, central composite design. The design space covered ranges of 400–1000 µM CuSO4 for induction and 5–10 × 106 cells/mL. The experimental design and statistical evaluation is described in more detail in the “Appendix 2”. In addition to the experimental design, several additives that may enhance protein production in rS2 cells were also evaluated, namely DMSO (1 or 5%), glycerol (1 or 5%), sodium butyrate (5 or 10 mM), and the presence or absence of 2 µL/mL Carl Roth His-tag inhibitor cocktail (Jeon et al. 2012; Lemos et al. 2009; Swiech et al. 2008; Chang et al. 2002; Park et al. 2002).
Protein expression at the 1-L bioreactor scale
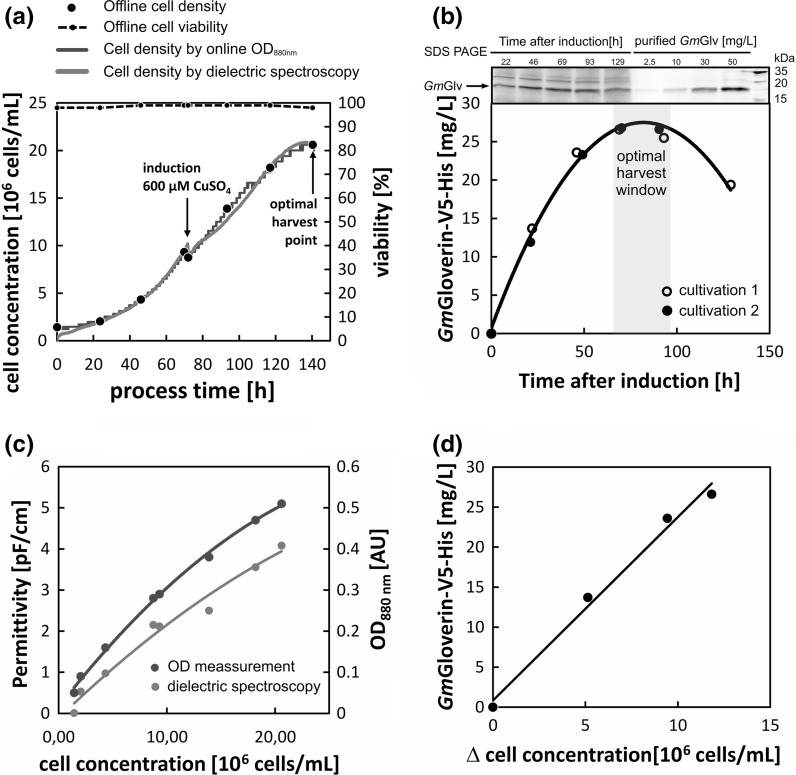
The laboratory-scale expression of recombinant GmGlv was achieved in a 2-L Labfors bioreactor (1-L working volume, Infors HT, Einsbach, Germany) equipped with one pitched-blade impeller (3 × 45°, d = 65 mm) and standard sensors for temperature, pH and oxygen saturation. A bubble-free aeration system was used, comprising a gassed silicon tube (4 m, inner diameter = 0.76 mm, outer diameter = 1.65 mm) and additional head space aeration. The aeration system was provided with a controlled gas mix of compressed air, nitrogen and pure oxygen. The process was kept at 27 °C, pH 6.4 and 40% air saturation. The stirrer speed was increased from 100 to 150 rpm during the process. The cultivation was designed as a two-step procedure: the cells were inoculated at 1.5 × 106 cells/mL and grown to the previously determined optimal density before induction with 600 µM CuSO4 using a bolus feed of 150 mL fresh medium. During cultivation, the offline viable cell count was determined using a Guava flow cytometer (Merck Millipore, Darmstadt, Germany). Living and dead cells were distinguished by propidium iodide staining (5 mg/L, Carl Roth, Karlsruhe, Germany). Cell growth was also monitored online by dielectric spectroscopy (Inycte system, Hamilton, Bonaduz, Switzerland) and optical density probing (ExCell 230, Exner Process Equipment, Ettlingen, Germany). The permittivity of the medium was measured at 1 and 10 MHz, and the difference between the signals was the output of the dielectric spectrometer. The increase in optical density was measured at 880 nm by light transmission through a 5-mm slit. As an additional offline parameter, the glucose concentration was determined using an enzymatic-amperometric analyzer (BiosenC, EKF Diagnostics, Barleben, Germany).
Protein purification from the cell culture supernatant
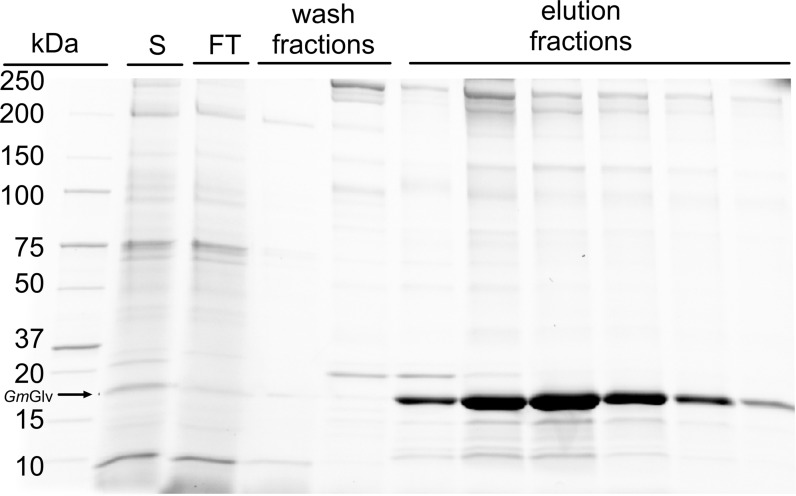
The harvested cell suspension was centrifuged and the supernatant was passed through a sterilizing filter before storing at 4 °C. The protein carried a His6 tag and was therefore purified by immobilized metal ion affinity chromatography (IMAC) using an FPLC system (NGC Discover, Biorad, Munich, Germany) equipped with an affinity column suitable for insect cell culture medium (1-mL HisTrap excel column, flow velocity 1 mL/min, GE Healthcare Life Science, Freiburg, Germany). The column was equilibrated with 5 column volumes (CV) of binding buffer (20 mM sodium phosphate, 0.5 M NaCl, pH 7.4) before the clarified supernatant was applied to the column at a flow rate of 0.3 mL/min. Contaminating proteins were removed by flushing with 50 CV of binding buffer supplemented with 15 mM imidazole, and pure GmGlv was recovered by eluting with 8 CV of elution buffer (20 mM sodium phosphate, 0.5 M NaCl, pH 7.4, 500 mM imidazole). Fractions containing GmGlv were identified by SDS PAGE and dialyzed against PBS to remove the imidazole (Slide-a-Lyzer, Thermo Fisher Scientific). This three-step procedure comprised sequential dialysis steps of 2, 2 and 12 h. The concentrations of pure GmGlv were determined by spectrophotometry at 280 nm (Synergy HTX Multi-Mode Reader, Biotek, Bad Friedrichshall, Germany) using the predicted extinction coefficient for Gloverin (http://web.expasy.org/protparam/) and by using a standard Bradford assay with BSA as the reference (Applichem, Darmstadt, Germany). High-purity fractions were used as standards to enable direct protein quantification by SDS-PAGE in further experiments.
SDS-PAGE, dot blot and western blot analysis
Proteins from the cell culture supernatants and cell lysates were resolved by reducing SDS-PAGE on 4–20% polyacrylamide gradient gels (Criterion XT™, Biorad) and detected using Biorad stain-free technology (Kazmin et al. 2002; Ladner et al. 2004). After electrophoresis for 25 min at 250 V, the gels were transferred to the imaging system (ChemiDoc™, Biorad) and activated for 2.5 min with UV light for documentation. For western blotting, proteins were transferred to polyvinylidine difluoride membranes (7 min at 25 V and 2.5 A, Trans-Blot® Turbo™, Biorad). The membranes were blocked for 1 h with PBS containing 5% BSA, stained for 2 h with a His5-HRP-antibody conjugate (Qiagen, Hilden, Germany) diluted 1:5000 in PBS with 0.05% Tween 20, and washed three times with PBS containing 0.1% Tween 20. Proteins were detected by enhanced chemoluminescence (Clarity Western ECL substrate, Biorad). The same staining procedure was also used for vacuum-assisted dot-blot experiments (VWR dot blot device, Darmstadt, Germany) in which culture supernatants from different cell lines were directly blotted onto a membrane (Amersham Protan 0.2 µm, GE Healthcare).
In vitro antimicrobial assay
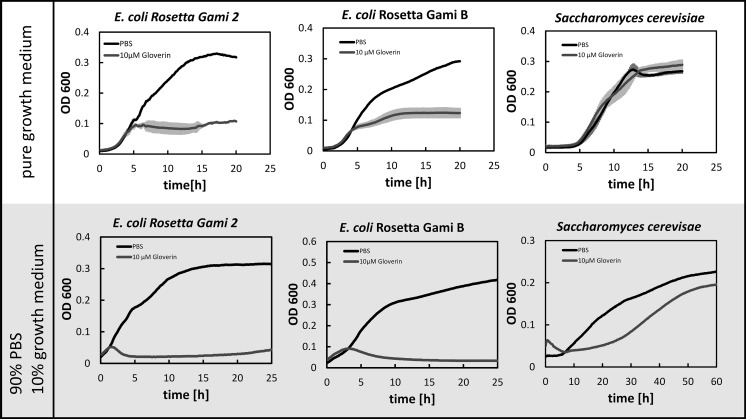
The antimicrobial activity of GmGlv was investigated using two separate growth assays based on previous reports (Xu et al. 2012; Yi et al. 2013). The assays were carried out using shake-flask cultures (37 °C, 25 mL, 250 rpm) of E. coli Rosetta Gami B (B-strain derivative) and E. coli Rosetta Gami 2 (K-12 derivative), both grown in LB medium (Sigma-Aldrich). Cultures of Saccharomyces cerevisiae, grown in YPD medium (Sigma-Aldrich) were used as a non-bacterial reference. For the first assay, cells were collected from mid-exponential phase cultures and diluted to OD600 = 0.01 in fresh medium containing either 10 µM GmGlv (dissolved in PBS) or same amount of pure PBS as a reference. Three replicates of 100 µL each were transferred to a 96-well plate and the OD600 was monitored for 20 h during incubation in a well-humidified environment at 37 °C (Synergy HTX Multi-Mode Reader, Biotek). The dose-dependent activity of GmGlv (2.8, 8.5, 11.0 and 17.8 µM) against E. coli Rosetta Gami 2 was determined in the same way, but with a shorter incubation time of 10 h. For the second assay, actively growing cells from all three cultures were centrifuged and resuspended in 10% medium diluted in PBS to simulate non-ideal growth conditions. Again, the mixtures contained either 10 µM GmGlv or PBS as a reference. The cells were cultivated and monitored as described above.
Results
Single-cell cloning for the generation of a highly productive cell line
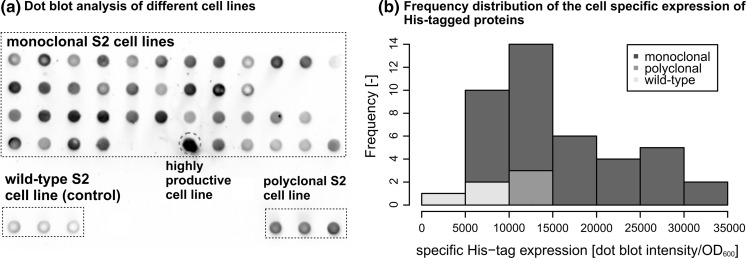
The successful generation of stable polyclonal transformants (Kollewe 2013) was followed by a limiting dilution assay to generate stable clones suitable for scaling up to monocultures. The polyclonal cultures were seeded into 960 wells and we recovered 42 monoclonal cell lines, which were induced with 900 µM CuSO4. Dot blot analysis of the corresponding supernatants revealed that most of the clones were no more productive than the original polyclonal culture (Fig. 2a), as confirmed by the frequency distribution of cell-specific GmGlv expression (Fig. 2b). However, a small number of monoclonal lines showed much greater productivity as well as vigorous growth, and the most promising clone (indicated by a red circle in Fig. 2a) was selected for expansion and was preserved for further experiments.
Fig. 2.
a Dot blot analysis for the screening of single cell clones. The signal intensity is a direct measure of the overall expression of His6-tagged proteins and is determined by staining with a His5-HRP antibody followed by chemiluminescent detection. The red circle indicates a highly productive clone. b Frequency distribution of the cell-specific expression of His6-tagged proteins for wild-type, polyclonal and the different monoclonal S2 cell lines
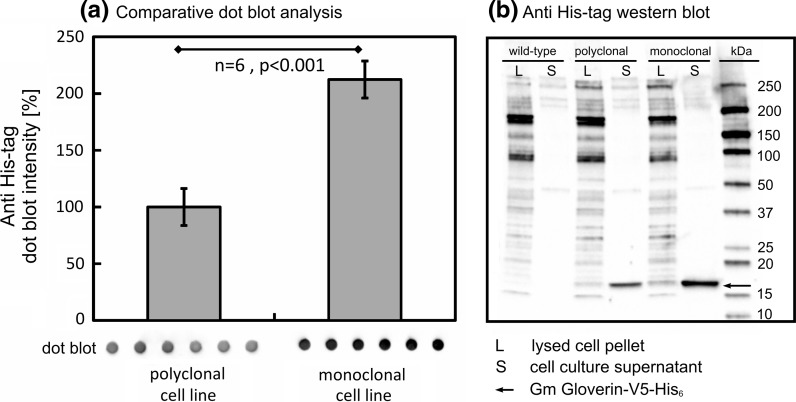
Subsequent statistical verification experiments showed that the monoclonal cell line expressed and secreted significantly more His6-tagged protein than the parental polyclonal cells (Fig. 3a, “Appendix 1”). The difference between the mean expression values of the monoclonal and polyclonal cell lines was ~100% with a 99% confidence interval of 82–142%. Western blotting was carried out using an antibody that recognizes the His6-tag, revealing a band of the anticipated molecular weight (~17.4 kDa) in both recombinant cell lines but no band in the wild-type S2 control. The presence of the correct band in the supernatant indicated the successful synthesis of full-length GmGlv and correct processing, i.e. propeptide cleavage and secretion.
Fig. 3.
Expression screening of the parental polyclonal cells and the derived monoclonal cell line. a Comparison of dot blot intensity representing cell culture supernatants from six independent experiments indicates statistically significant differences using Student’s two-sample t test by assuming normality and homoscedasticity (mean ± SD, p < 0.001). b Western blot of cell lysates (L) and cell culture supernatants (S) from wild-type S2 cells, polyclonal rS2 cells and monoclonal rS2 cells, stained with a His5-HRP antibody
Optimization of expression conditions and harvest time in a small-scale culture
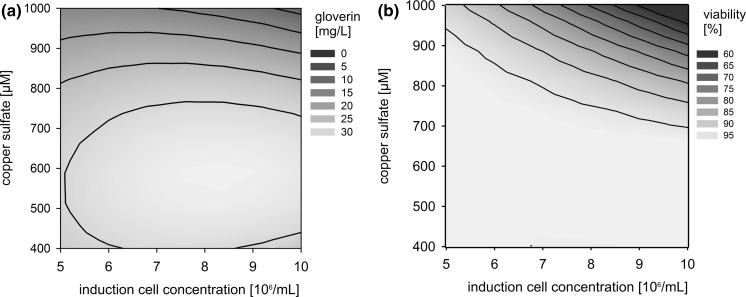
The monoclonal S2 cells were induced with different concentrations of CuSO4 at different cell densities according to a central composite design. The resulting data were used to generate a response surface showing GmGlv production as function of both input variables (Fig. 4a, “Appendix 2”). The resulting model predicted that the maximum protein yield would be achieved by inducing the cells with 600 µM CuSO4 at a density of 8–9.5 × 106 cells/mL. Additional investigations (Fig. 4b) showed that high concentrations of CuSO4 are cytotoxic, and the effect is more severe at higher cell densities. This may reflect the partial exhaustion of the medium when induction is carried out at high cell densities, which restricts cell growth and regeneration capacity.
Fig. 4.
Contour plots of the response surfaces. a GmGlv concentration after 4 days of expression as a function of CuSO4 concentration and induction cell density. b Cell viability after 4 days of expression as a function of CuSO4 concentration and induction cell density
The presence of additives (DMSO, glycerol or sodium butyrate) did not boost the production of GmGlv and even had a negative effect on cell growth and viability at elevated concentrations (Fig. 5). Based on these data, the three additives were excluded from subsequent experiments and the concentration of CuSO4 was kept below cytotoxic levels.
Fig. 5.
The influence of different medium additives on GmGlv expression based on the analysis of supernatants by SDS-PAGE following induction with 600 µM CuSO4
Scale up to a well-monitored 1-L bioreactor culture
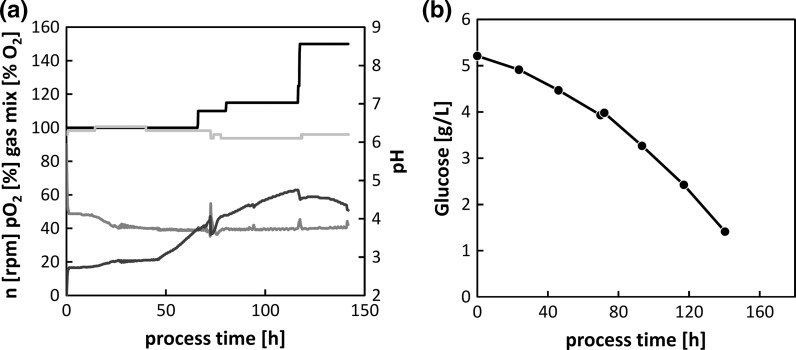
The data from the small-scale experiments allowed us to scale up the process to 1-L bioreactors. Standard parameters such as dissolved oxygen, pH and temperature were maintained at constant levels throughout the cultivation to ensure optimal cell growth (“Appendix 3”). For the first 3 days, the cells proliferated with a specific growth rate of 0.027 h−1, which is in the range of previous reports (Moraes et al. 2012). Based on the RSM data, the cells were induced with 600 µM CuSO4 before the density reached 1 × 107 cells/mL. The post-induction growth rate dropped to 0.016 h−1, but viability remained above 95% until harvest. Signals from both online cell monitoring methods correlated with the offline values and could be fitted using second-order polynomials (Fig. 6a, c).
Fig. 6.
Analysis of cell growth and GmGlv production. a Representative time course of cell growth in a GmGlv-expressing monoclonal rS2 cell line at the 1-L bioreactor scale. The two-step procedure comprised a 3-day growth phase followed by induction with 600 µM CuSO4 and a subsequent 3-day protein expression phase. b GmGlv production as function of time reveals an optimal harvest window 3–4 days after induction. c Online optical density and permittivity signals plotted against the corresponding offline cell densities and corresponding second-order polynomial fits. d GmGlv production shows a linear dependency on cell growth
The GmGlv concentration in the supernatant was monitored to determine the optimal window for harvesting (Fig. 6b). The highest concentration of GmGlv (25 mg/L) accumulated on days 3–4 after induction. The growth curve then flattened as the cell population entered the stationary phase. During the production phase, the amount of GmGlv showed a linear relationship with cell growth, indicating a strong relationship between cell proliferation and product generation (Fig. 6d). Prolonged cultivation during the stationary phase led to product degradation and should be avoided in an optimized process.
Dielectric spectroscopy and the online measurement of optical density were both suitable for the accurate timing of key operational transitions (induction and harvest). However, the measurement of residual glucose concentration was not suitable for this purpose. Cell growth declined before the main carbon source was exhausted and no drop in glucose consumption was detected at the optimal harvest time (“Appendix 3”). The early decline in cell growth may reflect either the prolonged exposure to the cytotoxic CuSO4 or the depletion of another growth-limiting substrate.
Capture of recombinant GmGloverin from the supernatant
Recombinant GmGlv was captured from culture supernatant by IMAC. The protein fraction was then dialyzed against PBS. Analysis of the fractions by SDS-PAGE indicated the successful binding of GmGlv to the column and its concentrated elution with 500 mM imidazole. However, several host cell proteins that are naturally rich in histidine residues were secreted during cultivation, and these were also captured on the column and co-eluted with GmGlv, as shown by the minor contaminating bands (Fig. 7).
Fig. 7.
SDS-PAGE analysis of IMAC fractions confirming the successful capture and concentration of GmGlv from the culture supernatant. Lanes culture supernatant (S), flow through (FT), wash fractions 1–2, and elution fractions 1–6
Characterization of antimicrobial activity of GmGlv
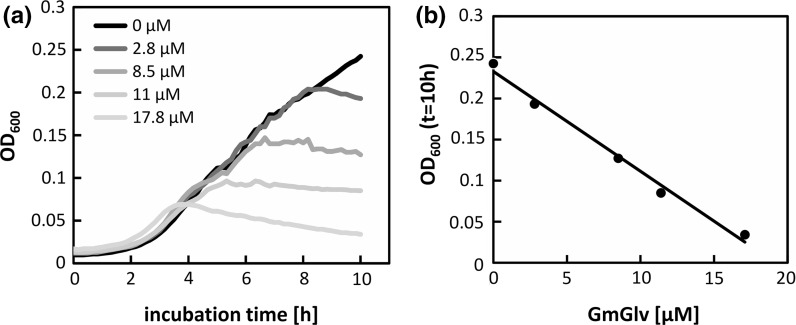
Two different microbial growth assays were used to confirm the activity of the recombinant GmGlv under optimal growth conditions and nutrient-delimited conditions, respectively. Both of the E. coli strains showed significantly inhibited growth in the presence of 10 µM GmGlv (Fig. 8 upper panel) but the effect was more severe under nutrient-delimited conditions, where growth was nearly completely inhibited (Fig. 8 lower panel). Incubation with different concentrations of GmGlv showed that its antimicrobial activity against the K12 derivative E. coli Rosetta Gami 2 was concentration dependent, and could be observed to concentrations as low as 2.8 µM (Fig. 9).
Fig. 8.
Growth profiles of E. coli Rosetta Gami 2, E. coli Rosetta Gami B and S. cerevisiae following treatment with 10 µM GmGlv (blue) or PBS as a reference (black). The test was performed either in fresh medium (upper panel, n = 3, mean ± SD) or in 10% medium diluted in PBS (lower panel, single measurement). Cultivation and time-resolved OD measurements were carried out in a tightly-sealed 96-well plate with a working volume of 100 µL
Fig. 9.
Concentration-dependent activity of GmGlv. a Growth profiles of E. coli Rosetta Gami 2 in fresh LB medium following treatment with 0, 2.8, 8.5, 11 or 17.8 µM GmGlv. Cultivation and time-resolved OD measurements were carried out in a tightly sealed 96-well plate with a working volume of 100 µL. b Optical density after 10 h growth in GmGlv-containing LB medium as function of the GmGlv concentration
As already described in other studies, the C-terminal tag has a negligible effect on the activity of Gloverin (Xu et al. 2012, 2015). In contrast to Gram-negative bacteria, S. cerevisiae with its mannan-based outer barrier was not susceptible to GmGlv under ideal growth conditions and only partially affected under nutrient-delimited conditions. Our results suggest that GmGlv binds specifically to Gram-negative bacteria, most likely through an interaction with bacterial lipopolysaccharide (Axén et al. 1997; Yi et al. 2013; Xu et al. 2015).
Discussion
Early process optimization achieved by single-cell cloning and screening induction conditions
Stable polyclonal cell lines can be generated easily and used as a straightforward basis for protein production, but the expression level in a polyclonal cell population is often heterogeneous and the most productive clones become outnumbered by the less-productive but faster-growing majority (Cherbas and Cherbas 2007). The isolation of single-cell clones is therefore an important step in early process optimization, and is often achieved by limiting dilution (Wang et al. 2012; Uribe et al. 2013). However, for insect cells no data are available thus far that show a comprehensive comparison between different clones and the polyclonal parental culture. Furthermore, single-cell cloning does not always achieve enhanced protein production in rS2 cells, and the considerable effort may therefore be wasted (Schetz and Shankar 2004). We therefore isolated more than 40 monoclonal cell lines and compared their expression profiles. The resulting frequency distribution (Fig. 2b) confirms that most clones do not outperform the parental polyclonal culture. However, this is unsurprising because the frequency of highly-productive cells in the parental cell pool is usually low and the likelihood of selecting such cells reflects this initial ratio. Nevertheless, some clones showed the expected increase in productivity and were used to seed monocultures that produced significantly larger amounts of target protein. It is therefore advisable to isolate and screen an appropriate number of clones. Because limiting dilution usually takes several weeks to months, less cumbersome alternatives have been evaluated by other groups. One is the re-selection of rS2 subpopulations by repeated treatment with high concentrations of the selection antibiotic (Lemos et al. 2009). This is a straightforward approach but it does not prevent further rearrangements in the still polyclonal culture, so the most suitable application may be as an additional step before single-cell cloning to enhance the final yield of highly productive clones. Another recently introduced method is immune-magnetic selection, which achieves the rapid selection of highly productive cells but is limited to rS2 cells expressing membrane-bound proteins (Santos et al. 2016).
The second step in early process development is the optimization of induction conditions. For the Mt promoter, induction is carried out with CuSO4 or CdCl2, both of which act in a dose-dependent manner but are cytotoxic at high concentrations (Bunch et al. 1988; Lim and Cha 2006). Although Cd2+ is a potent inducer (Li et al. 2012b; Yang et al. 2012; Wang et al. 2012; Uribe et al. 2013) it also activates endogenous heat-shock promoters (Bunch et al. 1988) and is highly toxic to humans, a reason why this substance was not used routinely in the past. The evaluation of 37 publications dealing with the expression of proteins in rS2 cells under the control of the Mt promoter revealed that the majority of authors (n = 24, 65%) used CuSO4 at concentrations of 400–600 µM, with smaller numbers using higher (n = 6, 16%) or lower concentrations (n = 3, 8%) of CuSO4 and 11% (n = 4) used CdCl2 (“Appendix 4”). Our RSM results confirmed that the optimal CuSO4 dose is within the common range of 400–600 µM. We also showed that there is an optimal induction cell density, but this parameter has less impact on the final yield. The main reason for the decline in protein production at higher inducer concentrations is the loss of cell viability. Efficient protein production therefore requires a balance between induction strength and toxicity. The relationship between cell growth and recombinant protein production exists because both processes require efficient protein synthesis (Gnoth et al. 2007). This was clearly demonstrated in our bioreactor-scale experiment, where there was a linear correlation between cell growth and GmGlv production.
Certain medium additives are often used to enhance protein expression in rS2 cells. The histone deacetylase inhibitor sodium butyrate inhibits chromatin condensation and thus promotes transcription in less active regions of the genome (Dorner et al. 1989; Chen et al. 2002; Li and Li 2006; Zhao et al. 2006). Protein expression in rS2 cells is thus enhanced if the transgene is integrated into silent regions of the genome (Chang et al. 2002; Lemos et al. 2009). However, our results show that this effect is negligible if the insert is located in an active genomic region, as it is the case for highly productive single-cell clones. Other common additives include DMSO (Chang et al. 2002; Jeon et al. 2012) and glycerol (Swiech et al. 2008). Both substances have been shown to stabilize proteins and act as chemical chaperones during folding (Yoshida et al. 2002). DMSO is also an effective permeabilizing agent that promotes the release of intracellular products into the medium. However, neither of these compounds affected GmGlv production at concentrations of 1%, indicating that GmGlv requires no further stabilization or assisted release. The protease inhibitor cocktail also showed no effect, supporting reports that Gloverins are generally intrinsically stable (Axén et al. 1997). Accordingly we found that GmGlv was secreted efficiently from rS2 cells as demonstrated by western blot analysis. Higher concentrations of the additives (5% glycerol or DMSO, and 10 mM sodium butyrate) reduced cell viability and thus the protein yield. Our data emphasize the importance of maintaining physiological conditions for optimal cell growth in order to maximize protein expression even in the presence of cytotoxic inducers. Special additives are not required in the case of stable AMPs such as GmGlv but may be necessary for membrane-bound proteins that are otherwise expressed at low levels (Smith 2011; Santos et al. 2016).
Optimal timing guided by process analytical technology is necessary during scale up
Appropriate process analytical technology is required during scale-up to monitor the physiological state of the cells, which is a critical process parameter for protein production (Junker et al. 1994). Dielectric spectroscopy (Druzinec et al. 2013, 2014; Justice et al. 2011) and online OD measurement (Akhnoukh et al. 1996; Bédard et al. 1994) are valuable tools for the real-time monitoring of processes based on insect cells, such as the baculovirus mediated protein expression in Spodoptera frugiperda cells. Exemplarily, in a corresponding process for recombinant adeno-associated virus vectors, the permittivity signal was used to determine the optimal time of infection and harvest (Negrete et al. 2007). The same techniques were successfully adapted herein to report the physiological status of a stable rS2 cell line. Both techniques were able to determine optimal windows for induction and harvest and their outputs could be used interchangeably. However, using both techniques simultaneously provides added value, because the signal ratio is an indicator of cell viability.
The optimal induction point in our bioreactor system was during the mid-exponential growth phase 70 h after inoculation. This is comparable to simulations of human transferrin production using rS2 cells in a spinner flask system, where the optimal induction time point was similarly in the exponential phase 70–80 h after inoculation (Lim and Cha 2006). The optimal time for harvest was determined to be between 75 and 100 h post-induction in our system. Other studies have shown that yields reach a peak at 100–160 h post-induction (Park et al. 1999, 2008; Lim and Cha 2006; Lee et al. 2007). However, these cultivations were carried out in non-controlled cultivation systems, with prolonged growth phases. Furthermore, when GmGlv was expressed in shake-flask cultures the optimal harvest time shifted to 100–120 h post-induction (data not shown). The production peak may therefore correspond to the end of the exponential growth phase as previously reported (Lee et al. 2007), which is consistent with our GmGlv expression profile. Other studies suggest that the production peak corresponds to the beginning stationary phase (Lim and Cha 2006; Park et al. 2008). Nevertheless, most of the protein is synthesized during exponential cell proliferation in all systems and further process intensification could therefore be achieved by extending the growth phase after induction, e.g. using an extended fed batch process (Søndergaard 1996) or a perfusion culture (Wang et al. 2012).
Assessment of the achievable AMP yield
In order to compete with other platforms, a sufficient protein yield is necessary. Common yields for the recombinant production of different mature AMPs (including those of non-insect origin) using the hosts E. coli and P. pastoris varied between 10 µg/L and 700 mg/L (Parachin et al. 2012). If only AMPs of insect origin were considered, the same hosts showed expression levels between 0.5 and 68 mg/L (Parachin et al. 2012; Müller et al. 2015). Despite potential high yields, the susceptibility of microorganisms to AMPs can hamper their suitability as expression hosts and higher eukaryotic expression systems are necessary, as proposed in this study. Therefore, mammalian cell lines, such as CHO-K1 (Horwitz et al. 2000; Chiou et al. 2009), and insect cell lines, such as S2 or Sf9 (Lundström et al. 2002; Kawaoka et al. 2008; Carballar-Lejarazú et al. 2008; Xu et al. 2012, 2015), were frequently used for AMP expression. However, there are less comprehensive data on achieved yields because the main focus of these studies was basic functional analysis of the proteins. Generally, the expression level for stably transformed insect cell lines and the baculovirus expression vector system varies between 0.2 and 20 mg/L (Wickham et al. 1995; Kollewe 2013), optimized processes can also exceed 100 mg/L.
As a concrete example, a spider peptide toxin (4.7 kDa) was expressed in recombinant S2-cells at the 12-L scale and yielded 0.48 mg/L after purification (Escoubas et al. 2003). The recombinant expression of a bacteria binding C-type lectin (~16 kDa) in rS2-cells resulted in 1.5 mg/L (Uribe et al. 2013), while another C-type lectin-like protein (14–17 kDa) was expressed at around 95 mg/L (Scotter et al. 2006). The latter is an example of rS2 cells surpassing all previous attempts to produce this protein in E. coli, baculovirus-infected fall armyworm cells or P. pastoris (Scotter et al. 2006). Another approach is the expression in CHO cells, but despite their widespread and very successful use in antibody production they are not always superior to rS2 cells. Exemplarily a recent comparison showed that rS2 cells were more suitable for the production of human coagulation factor IX (Vatandoost and Bos 2016). With respect to the production of an antimicrobial protein, named rBPI, CHO-K1 cells yielded 6 mg/L (Horwitz et al. 2000).
In context with the information discussed above, the achieved Gloverin yield of 25 mg/L is not superior but still competitive to other expression hosts, especially when considering the easy recovery of the active product. As discussed before, the yield reported in this study is also not a dead end, but will increase with further process intensification and the use of advanced process modes (Wang et al. 2012).
Specific antimicrobial activity of GmGlv
GmGlv is a member of the Gloverin family. These are heat-stable proteins of approximately 14 kDa. They are rich in glycine but lack cysteine residues, hence there are no disulfide bonds. The Gloverins usually have a basic isoelectric point (pI). GmGlv showed the typical characteristics of this family: 17.4 kDa (14.7 kDa without tags), a predicted pI of 9.74 (9.79 without tags) and ~50% sequence identity with other Gloverins in the UniProt database (http://www.uniprot.org/blast/). Gloverins have been identified in several lepidopteran species, including Hyalophora gloveri (Axén et al. 1997), Helicoverpa armigera (Mackintosh et al. 1998), Trichoplusia ni (Lundström et al. 2002), M. sexta (Xu et al. 2012), B. mori (Yi et al. 2013), Diatraea saccharalis (Silva et al. 2010), P. xylostella (Xia et al. 2015) and Spodoptera exigua (Hwang and Kim 2011). The general mechanism of their antimicrobial activity is not fully understood, but it is based on interactions with bacterial LPS that increase membrane permeability or inhibit the formation of the bacterial outer membrane. The activity of Gloverins differs according to the origin of each protein. Gloverins are often identified in the hemolymph of larvae challenged with E. coli or LPS, suggesting that most of them are more or less active against Gram-negative bacteria (e.g. HgGlv, HaGlv, TnGlv, BmGlv, DsGlv and PxGlv). However, some Gloverins are essentially inactive against E. coli, but are active against Gram-positive bacteria or fungi (e.g. MsGlv and SeGlv). In addition to its antibacterial activity, TnGlv was also shown to inactivate a common insect virus: Autographa californica multiple nucleopolyhedrovirus (AcMNPV) (Moreno-Habel et al. 2012). Little is known about the activity of GmGlv, although the hemolymph of LPS-challenged G. mellonella larvae contains GmGlv and significantly inhibits the growth of E. coli on agar plates (Altincicek et al. 2007). This effect may reflect the presence of a mixture of GmGlv and other AMPs (Altincicek et al. 2007). We found that pure GmGlv also specifically inhibits the growth of two E. coli strains that are frequently used for protein expression, suggesting that E. coli would be an unsuitable host for the expression of this AMP. Antimicrobial activity was observed down to a concentration of 2.4 µM GmGlv, which is consistent with the minimum inhibitory concentration of 0.5–3 µM reported for other Gloverins (Axén et al. 1997; Mackintosh et al. 1998; Lundström et al. 2002). According to the antimicrobial peptide database (http://aps.unmc.edu/AP/main.php), AMPs are classified as active, if they have a minimum inhibitory concentration below 100 µM (Wang et al. 2016). In terms of this definition GmGlv is regarded as active and suitable for further research. Our data strongly support the likelihood that GmGlv acts specifically against Gram-negative bacteria such as E. coli because it had a negligible effect on the growth of S. cerevisae, as previously shown for BmGlv (Yi et al. 2013). This specificity reflects the binding of Gloverin to LPS (“Appendix 5”) which probably induces conformational changes in the AMP (Axén et al. 1997; Yi et al. 2013). The complete mechanism of action is not yet understood, and crystallization studies will be required to determine the specific nature of these interactions.
Conclusion
The establishment of a robust and efficient production process for functional AMPs is a major challenge, but this is required to produce enough material for crystallization studies and for the development of AMPs as anti-infective drugs. We found that rS2 cells were ideal for the production of GmGlv and that the production system benefited significantly from single-cell cloning. Optimized induction conditions and process monitoring facilitated the reliable production of pure GmGlv that can be used for further research or for the development of complete downstream processes. The incorporation of online monitoring techniques at this early stage not only helps us to understand the process characteristics, but can also be used for the validation and qualification that becomes necessary when the product enters clinical development.
Acknowledgements
We would like to thank the Hessen State Ministry of Higher Education, Research and the Arts (Grant No. LOEWE AZ: III L5-518/19.004) for financial support within the Hessen initiative for scientific and economic excellence (LOEWE-Program). The authors acknowledge Dr. Richard M. Twyman for editing the paper.
Author contributions
JZ conceived, designed and conducted the experiments, and wrote the manuscript; TW helped to draft and to revise the manuscript; PC helped to draft and to revise the manuscript, and supervised the research; All authors have given their approval for this final version of the manuscript.
Appendix 1: Statistical assessment of normality and homoscedasticity for the comparison of the expression of the parental polyclonal cells and the monoclonal cell line D7
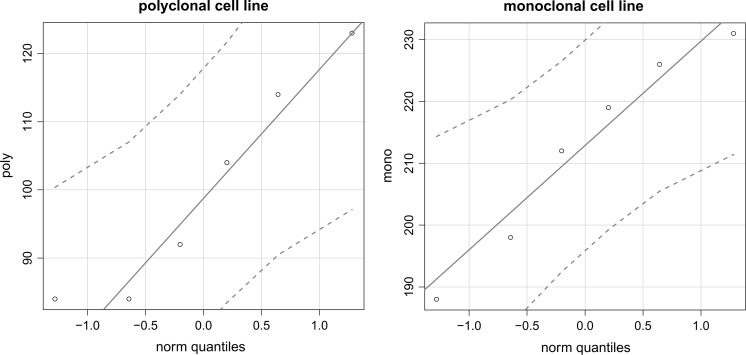
Statistical assessment of expression data was conducted using R v3.2.3 (R Core Team 2014). Because the normal q–q plots in Fig. 10 do not show any points outside their confidence intervals, there is no reason to doubt the validity of the assumption of normality for the data in both groups. The high p value of 0.9619 in a subsequent F-test indicates that there is insufficient evidence to reject the assumption of homoscedasticity. Consequently, Student’s two-sample t test was used to compare the means of both datasets.
Fig. 10.
Normal q–q plots for the expression datasets used to compare the monoclonal cell line and polyclonal culture
Appendix 2: Response surface method for the determination of optimal induction conditions
Table 1.
ANOVA for the quadratic model that describes GmGlv production after 4 days as a function of cell concentration (A) and CuSO4 concentration (B)
| Source | Sum of squares | df | Mean square | F value |
p value Prob > F |
|
|---|---|---|---|---|---|---|
| Model | 2387.31975 | 6 | 397.886626 | 125.119645 | <0.0001 | Significant |
| A | 16.6083246 | 1 | 16.6083246 | 5.22266277 | 0.0301 | |
| B | 1434.54256 | 1 | 1434.54256 | 451.107035 | <0.0001 | |
| AB | 49.5245589 | 1 | 49.5245589 | 15.5735197 | 0.0005 | |
| A2 | 107.989939 | 1 | 107.989939 | 33.958575 | <0.0001 | |
| B2 | 724.809437 | 1 | 724.809437 | 227.92397 | <0.0001 | |
| AB2 | 56.4182078 | 1 | 56.4182078 | 17.7413003 | 0.0002 | |
| Residual | 89.0413776 | 28 | 3.1800492 | |||
| Lack of fit | 28.45301 | 7 | 4.06471571 | 1.40883528 | 0.2537 | Not significant |
| Pure error | 60.5883676 | 21 | 2.88516036 | |||
| Cor total | 2558.3453 | 35 |
Table 2.
ANOVA for the cubic model that describes cell viability after 4 days as a function of cell concentration (A) and CuSO4 concentration (B)
| Source | Sum of squares | df | Mean square | F value | p value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 20,039.7564 | 8 | 2504.96955 | 282.350427 | <0.0001 | Significant |
| A | 49.0105715 | 1 | 49.0105715 | 5.52428104 | 0.0251 | |
| B | 116.81813 | 1 | 116.81813 | 13.1672853 | 0.0010 | |
| AB | 1851.79606 | 1 | 1851.79606 | 208.727251 | <0.0001 | |
| A2 | 23.3543994 | 1 | 23.3543994 | 2.63241709 | 0.1145 | |
| B2 | 4474.0554 | 1 | 4474.0554 | 504.298127 | <0.0001 | |
| A2B | 35.7292093 | 1 | 35.7292093 | 4.02725753 | 0.0533 | |
| AB2 | 828.976863 | 1 | 828.976863 | 93.4390485 | <0.0001 | |
| B3 | 580.280857 | 1 | 580.280857 | 65.4070018 | <0.0001 | |
| Residual | 283.899077 | 32 | 8.87184616 | |||
| LACK OF FIT | 151.95109 | 11 | 13.8137355 | 2.19850604 | 0.0581 | Not significant |
| Pure error | 131.947987 | 21 | 6.28323746 | |||
| Cor total | 20,469.4054 | 41 |
Appendix 3: Time course of additional process variables during the cultivation of rS2 cells for the production of GmGlv
See Fig. 11.
Fig. 11.
Time course of standard bioreactor parameters: a stirrer speed (n, black), dissolved oxygen (pO2, red), gas mix composition (blue) and pH (gray). b Glucose consumption during cultivation
Appendix 4: Comparison of the induction conditions in 37 studies
See Table 3.
Table 3.
Comparison of the induction conditions in 37 studies using the Mt promoter in rS2 cells
| Inducer and concentration | Number | Proportion (%) | References |
|---|---|---|---|
| CuSO4 < 400 µM | 3 | 8.1 | Deml et al. (1999), Valle et al. (2001), Lieberman et al. (2007) |
| CuSO4 400–600 µM | 24 | 64.8 | Bernard et al. (1994), Nilsen and Castellino (1999), Park et al. (1999, 2008), Shin and Cha (2002), Friry et al. (2002), Chang et al. (2002), Shin et al. (2003), Schetz et al. (2003), Shin and Cha (2003), Cho et al. (2004), Lim and Cha (2006), Johansson et al. (2007), Lee et al. (2007, 2009, 2011), Kim et al. (2008), Seok et al. (2010), Gutsche et al. (2011), Chung et al. (2011), Jeon et al. (2012), Uribe et al. (2013), Xu et al. (2015), Vatandoost and Bos (2016) |
| CuSO4 601–800 µM | 4 | 10.8 | Dubreuil et al. (1996), Dubreuil and Grushko (1999), Perret et al. (2003), Santos et al. (2007) |
| CuSO4 > 800 µM | 2 | 5.4 | Bunch et al. (1988), Graziano et al. (1998) |
| CdCl2 1–100 µM | 4 | 10.8 | Uribe et al. (2013), Li et al. (2012b), Yang et al. (2012), Wang et al. (2012) |
Appendix 5: Site-specific binding of GmGlv to LPS
There is no broad agreement on the exact position at which Gloverin binds to LPS. The latter comprises three major parts: lipid A, a carbohydrate core region, and the O-specific antigen. If all parts are present, the LPS is regarded as smooth. If the outer parts are absent, LPS is regarded as rough or deep rough depending on the degree of truncation (Ra, Rc, Rd, and Re LPS). BmGv is inactive against E. coli DH5alpha but active against the rough mutants E. coli D21 (Ra-LPS), E. coli D21e7 (Rc-LPS), E. coli D21f1 (Rd-LPS) and E. coli D21f2 (Re-LPS) (Yi et al. 2013). Furthermore, TnGlv (Lundström et al. 2002) and HgGlv (Axén et al. 1997) also show activity primarily against E. coli D21f2. These results indicate that most (positively charged) Gloverins probably bind to the (negatively charged) inner core region or lipid A. The two E. coli strains used in this study have not been comprehensively characterized in terms of their LPS phenotype, but their parental stains carry short LPS (Chart et al. 2000). Furthermore E. coli Rosetta Gami 2 carries the galE–galK mutation, which blocks the synthesis of UDP-galactose and its incorporation in the LPS, thus producing a truncated LPS (van Die et al. 1984). These data suggest that GmGlv also binds to the inner part of the LPS. However, MsGlv binds to the outer core region and the O-specific antigen of LPS and also to other fungal and bacterial membrane compounds, but it does not interact with Rc, Rd or Re LPS or with lipid A (Xu et al. 2012).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Akhnoukh R, Kretzmer G, Schügerl K. On-line monitoring and control of the cultivation of Spodoptera frugiperda Sf9 insect cells and β-galactosidase production by Autographa californica virus vector. Enzyme Microb Technol. 1996;18:220–228. doi: 10.1016/0141-0229(95)00093-3. [DOI] [Google Scholar]
- Altincicek B, Linder M, Linder D, et al. Microbial metalloproteinases mediate sensing of invading pathogens and activate innate immune responses in the lepidopteran model host Galleria mellonella. Infect Immun. 2007;75:175–183. doi: 10.1128/IAI.01385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki W, Kuroda K, Ueda M. Next generation of antimicrobial peptides as molecular targeted medicines. J Biosci Bioeng. 2012;114:365–370. doi: 10.1016/j.jbiosc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Axén A, Carlsson A, Engström Å, Bennich H. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur J Biochem. 1997;247:614–619. doi: 10.1111/j.1432-1033.1997.00614.x. [DOI] [PubMed] [Google Scholar]
- Bédard C, Jolicoeur M, Jardin B, et al. Insect cell density in bioreactor cultures can be estimated from on-line measurements of optical density. Biotechnol Tech. 1994;8:605–610. doi: 10.1007/BF00241682. [DOI] [Google Scholar]
- Bernard AR, Kost TA, Overton L, Cavegn C, Young J, Bertrand M, et al. Recombinant protein expression in a Drosophila cell line: comparison with the baculovirus system. Cytotechnology. 1994;15:139–144. doi: 10.1007/BF00762388. [DOI] [PubMed] [Google Scholar]
- Brown SE, Howard A, Kasprzak AB, et al. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem Mol Biol. 2009;39:792–800. doi: 10.1016/j.ibmb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Grinblat Y, Goldstein LS. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 1988;16:1043–1061. doi: 10.1093/nar/16.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballar-Lejarazú R, Rodríguez MH, de la Cruz Hernández-Hernández F, et al. Recombinant scorpine: a multifunctional antimicrobial peptide with activity against different pathogens. Cell Mol Life Sci. 2008;65:3081–3092. doi: 10.1007/s00018-008-8250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KH, Park JH, Lee YH, Kim JH, Chun HO, Kim JH, et al. Dimethylsulfoxide and sodium butyrate enhance the production of recombinant cyclooxygenase 2 in stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett. 2002;24:1353–1359. doi: 10.1023/A:1019841829667. [DOI] [Google Scholar]
- Chart H, Smith HR, La Ragione RM, Woodward MJ. An investigation into the pathogenic properties of Escherichia coli strains BLR, BL21, DH5α and EQ1. J Appl Microbiol. 2000;89:1048–1058. doi: 10.1046/j.1365-2672.2000.01211.x. [DOI] [PubMed] [Google Scholar]
- Chen T, Sun H, Lu J, et al. Histone acetylation is involved in hsp70 gene transcription regulation in Drosophila melanogaster. Arch Biochem Biophys. 2002;408:171–176. doi: 10.1016/S0003-9861(02)00564-7. [DOI] [PubMed] [Google Scholar]
- Cherbas L, Cherbas P. Transformation of Drosophila cell lines. In: Murhammer D, editor. Baculovirus and insect cell expression protocols. New York: Humana Press; 2007. pp. 317–340. [Google Scholar]
- Chiou M-J, Chen L-K, Peng K-C, et al. Stable expression in a Chinese hamster ovary (CHO) cell line of bioactive recombinant chelonianin, which plays an important role in protecting fish against pathogenic infection. Dev Comp Immunol. 2009;33:117–126. doi: 10.1016/j.dci.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Cho HS, Kim YK, Cha HJ. Expression of double foreign protein types following recombinant baculovirus infection of stably transfected Drosophila S2 cells. Enzyme Microb Technol. 2004;35:525–531. doi: 10.1016/j.enzmictec.2004.08.004. [DOI] [Google Scholar]
- Chung HY, Hwang-Bo J, Kim S-K, Baek NI, Lee YH, Chung IS, et al. Functional expression of Arabidopsis thaliana sterol glycosyltransferase from stably transformed Drosophila melanogaster S2 cells. Biotechnol Bioprocess Eng. 2011;16:801–807. doi: 10.1007/s12257-010-0445-9. [DOI] [Google Scholar]
- de Jongh WA, Salgueiro S, Dyring C. The use of Drosophila S2 cells in R&D and bioprocessing. Pharm Bioprocess. 2013;1:197–213. doi: 10.4155/pbp.13.18. [DOI] [Google Scholar]
- Deml L, Wolf H, Wagner R. High level expression of hepatitis B virus surface antigen in stably transfected Drosophila schneider-2 cells. J Virol Methods. 1999;79:191–203. doi: 10.1016/S0166-0934(99)00021-X. [DOI] [PubMed] [Google Scholar]
- Dorner AJ, Wasley LC, Kaufman RJ. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989;264:20602–20607. [PubMed] [Google Scholar]
- Druzinec D, Salzig D, Brix A, et al. Optimization of insect cell based protein production processes—online monitoring, expression systems, scale up. Adv Biochem Eng Biotechnol. 2013;136:65–100. doi: 10.1007/10_2013_205. [DOI] [PubMed] [Google Scholar]
- Druzinec D, Weiss K, Elseberg C, et al. Process analytical technology (PAT) in insect and mammalian cell culture processes: dielectric spectroscopy and focused beam reflectance measurement (FBRM) In: Pörtner R, et al., editors. Animal cell biotechnology. New York: Humana Press; 2014. pp. 313–341. [DOI] [PubMed] [Google Scholar]
- Dubreuil RR, Grushko T. Neuroglian and DE-cadherin activate independent cytoskeleton assembly pathways in Drosophila S2 cells. Biochem Biophys Res Commun. 1999;265:372–375. doi: 10.1006/bbrc.1999.1689. [DOI] [PubMed] [Google Scholar]
- Dubreuil RR, MacVicar G, Dissanayake S, Liu C, Homer D, Hortsch M. Neuroglian-mediated cell adhesion induces assembly of the membrane skeleton at cell contact sites. J Cell Biol. 1996;133:647–655. doi: 10.1083/jcb.133.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P, Bernard C, Lambeau G, et al. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci Publ Protein Soc. 2003;12:1332–1343. doi: 10.1110/ps.0307003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friry C, Feliciangeli S, Richard F, Kitabgi P, Rovere C. Production of recombinant large proneurotensin/neuromedin N-derived peptides and characterization of their binding and biological activity. Biochem Biophys Res Commun. 2002;290:1161–1168. doi: 10.1006/bbrc.2001.6308. [DOI] [PubMed] [Google Scholar]
- Gnoth S, Jenzsch M, Simutis R, Lübbert A. Process Analytical Technology (PAT): batch-to-batch reproducibility of fermentation processes by robust process operational design and control. J Biotechnol. 2007;132:180–186. doi: 10.1016/j.jbiotec.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Graziano MP, Broderick DJ, Tota MR. Expression of G protein-coupled receptors in Drosophila Schneider 2 cells. In: Lynch KR, editor. Receptor biochemistry and methodology series, identification and expression of G protein-coupled receptors. New York: Wiley; 1998. pp. 181–195. [Google Scholar]
- Gutsche I, Coulibaly F, Voss JE, Salmon J, d’Alayer J, Ermonval M, et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci USA. 2011;108:8003–8008. doi: 10.1073/pnas.1017338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AH, Carroll SF, Williams RE, Liu P. Inclusion of S-sepharose beads in the culture medium significantly improves recovery of secreted rBPI21 from transfected CHO-K1 cells. Protein Expr Purif. 2000;18:77–85. doi: 10.1006/prep.1999.1163. [DOI] [PubMed] [Google Scholar]
- Hwang J, Kim Y. RNA interference of an antimicrobial peptide, Gloverin, of the beet armyworm, Spodoptera exigua, enhances susceptibility to Bacillus thuringiensis. J Invertebr Pathol. 2011;108:194–200. doi: 10.1016/j.jip.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Jeon H-B, Park J-H, Lee H-H, Kim D-H, Lee H-Y, Shon D-H, et al. Enhancement of protein productivity of recombinant hepatitis A virus VP1 in stably transfected Drosophila melanogaster S2 cells. J Bacteriol Virol. 2012;42:69–75. doi: 10.4167/jbv.2012.42.1.69. [DOI] [Google Scholar]
- Johansson DX, Drakenberg K, Hopmann KH, Schmidt A, Yari F, Hinkula J, et al. Efficient expression of recombinant human monoclonal antibodies in Drosophila S2 cells. J Immunol Methods. 2007;318:37–46. doi: 10.1016/j.jim.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Junker BH, Reddy J, Gbewonyo K, Greasham R. On-line and in situ monitoring technology for cell density measurement in microbial and animal cell cultures. Bioprocess Eng. 1994;10:195–207. doi: 10.1007/BF00369530. [DOI] [Google Scholar]
- Justice C, Brix A, Friemark D, et al. Process control in cell culture technology using dielectric spectroscopy. Biotechnol Adv. 2011;29:391–401. doi: 10.1016/j.biotechadv.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kawaoka S, Katsuma S, Daimon T, et al. Functional analysis of four Gloverin-like genes in the silkworm, Bombyx mori. Arch Insect Biochem Physiol. 2008;67:87–96. doi: 10.1002/arch.20223. [DOI] [PubMed] [Google Scholar]
- Kazmin D, Edwards RA, Turner RJ, et al. Visualization of proteins in acrylamide gels using ultraviolet illumination. Anal Biochem. 2002;301:91–96. doi: 10.1006/abio.2001.5488. [DOI] [PubMed] [Google Scholar]
- Kim KR, Kim YK, Cha HJ. Recombinant baculovirus-based multiple protein expression platform for Drosophila S2 cell culture. J Biotechnol. 2008;133:116–122. doi: 10.1016/j.jbiotec.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Kollewe C. Production of recombinant proteins in insect cells. Am J Biochem Biotechnol. 2013;9:255–271. doi: 10.3844/ajbbsp.2013.255.271. [DOI] [Google Scholar]
- Ladner CL, Yang J, Turner RJ, Edwards RA. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal Biochem. 2004;326:13–20. doi: 10.1016/j.ab.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Lee JM, Jeon H-B, Sohn BH, Chung IS. Functional expression of recombinant canstatin in stably transformed Drosophila melanogaster S2 cells. Protein Expr Purif. 2007;52:258–264. doi: 10.1016/j.pep.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Lee JM, Lee HH, Hwang-Bo J, Shon DH, Kim W, Chung IS. Expression and immunogenicity of recombinant polypeptide VP1 of human hepatitis A virus in stably transformed fruitfly (Drosophila melanogaster) Schneider 2 cells. Biotechnol Appl Biochem. 2009;53:101–109. doi: 10.1042/BA20080019. [DOI] [PubMed] [Google Scholar]
- Lee JM, Chung HY, Kim KI, Yoo KH, Hwang-Bo J, Chung IS, et al. Synthesis of double-layered rotavirus-like particles using internal ribosome entry site vector system in stably-transformed Drosophila melanogaster. Biotechnol Lett. 2011;33:41–46. doi: 10.1007/s10529-010-0390-x. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/S0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- Lemos MAN, dos Santos AS, Astray RM, et al. Rabies virus glycoprotein expression in Drosophila S2 cells. I: design of expression/selection vectors, subpopulations selection and influence of sodium butyrate and culture medium on protein expression. J Biotechnol. 2009;143:103–110. doi: 10.1016/j.jbiotec.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Li RW, Li C. Butyrate induces profound changes in gene expression related to multiple signal pathways in bovine kidney epithelial cells. BMC Genom. 2006;7:234. doi: 10.1186/1471-2164-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiang Q, Zhang Q, et al. Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides. 2012;37:207–215. doi: 10.1016/j.peptides.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Li YZ, Counor D, Lu P, et al. A specific and sensitive antigen capture assay for NS1 protein quantitation in Japanese encephalitis virus infection. J Virol Methods. 2012;179:8–16. doi: 10.1016/j.jviromet.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Lieberman MM, Clements DE, Ogata S, Wang G, Corpuz G, Wong T, et al. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine. 2007;25:414–423. doi: 10.1016/j.vaccine.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HJ, Cha HJ. Observation and modeling of induction effect on human transferrin production from stably transfected Drosophila S2 cell culture. Enzyme Microb Technol. 2006;39:208–214. doi: 10.1016/j.enzmictec.2005.10.021. [DOI] [Google Scholar]
- Lundström A, Liu G, Kang D, et al. Trichoplusia ni Gloverin, an inducible immune gene encoding an antibacterial insect protein. Insect Biochem Mol Biol. 2002;32:795–801. doi: 10.1016/S0965-1748(01)00162-X. [DOI] [PubMed] [Google Scholar]
- Mackintosh JA, Gooley AA, Karuso PH, et al. A Gloverin-like antibacterial protein is synthesized in Helicoverpa armigera following bacterial challenge. Dev Comp Immunol. 1998;22:387–399. doi: 10.1016/S0145-305X(98)00025-1. [DOI] [PubMed] [Google Scholar]
- Moraes ÂM, Jorge SAC, Astray RM, et al. Drosophila melanogaster S2 cells for expression of heterologous genes: from gene cloning to bioprocess development. Biotechnol Adv. 2012;30:613–628. doi: 10.1016/j.biotechadv.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Moreno-Habel DA, Biglang-awa IM, Dulce A, et al. Inactivation of the budded virus of Autographa californica M nucleopolyhedrovirus by Gloverin. J Invertebr Pathol. 2012;110:92–101. doi: 10.1016/j.jip.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Salzig D, Czermak P. Considerations for the process development of insect-derived antimicrobial peptide production. Biotechnol Prog. 2015;31:1–11. doi: 10.1002/btpr.2002. [DOI] [PubMed] [Google Scholar]
- Negrete A, Esteban G, Kotin RM. Process optimization of large-scale production of recombinant adeno-associated vectors using dielectric spectroscopy. Appl Microbiol Biotechnol. 2007;76:761–772. doi: 10.1007/s00253-007-1030-9. [DOI] [PubMed] [Google Scholar]
- Nilsen SL, Castellino FJ. Expression of human plasminogen in Drosophila schneider S2 cells. Protein Expr Purif. 1999;16:136–143. doi: 10.1006/prep.1999.1045. [DOI] [PubMed] [Google Scholar]
- Parachin NS, Mulder KC, Viana AAB, et al. Expression systems for heterologous production of antimicrobial peptides. Peptides. 2012;38:446–456. doi: 10.1016/j.peptides.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee JM, Chung IS. Production of recombinant endostatin from stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett. 1999;21:729–733. doi: 10.1023/A:1005510821928. [DOI] [Google Scholar]
- Park JH, Kyung-Hwa C, Lee YH, Hae-Yeong K, Jai-Myung Y, In-Sik C. Production of recombinant rotavirus capsid protein VP7 from stably transformed Drosophila melanogaster S2 cells. J Microbiol Biotechnol. 2002;12:563–568. [Google Scholar]
- Park J-H, Hwang I-S, Kim K-I, Lee J-M, Park Y-M, Park C-H, et al. Functional expression of recombinant human ribonuclease/angiogenin inhibitor in stably transformed Drosophila melanogaster S2 cells. Cytotechnology. 2008;57:93–99. doi: 10.1007/s10616-008-9126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret BG, Wagner R, Lecat S, Brillet K, Rabut G, Bucher B, et al. Expression of EGFP-amino-tagged human mu opioid receptor in Drosophila Schneider 2 cells: a potential expression system for large-scale production of G-protein coupled receptors. Protein Expr Purif. 2003;31:123–132. doi: 10.1016/S1046-5928(03)00140-2. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Santos MG, Jorge SAC, Brillet K, Pereira CA. Improving heterologous protein expression in transfected Drosophila S2 cells as assessed by EGFP expression. Cytotechnology. 2007;54:15–24. doi: 10.1007/s10616-007-9060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos NGL, Rocca MP, Pereira CA, et al. Impact of recombinant Drosophila S2 cell population enrichment on expression of rabies virus glycoprotein. Cytotechnology. 2016;68:2605–2611. doi: 10.1007/s10616-016-9984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetz JA, Shankar EPN. Protein expression in the Drosophila Schneider 2 cell system. In: Gerfen CR, Holmes A, Sibley D, Skolnick P, Wray S, editors. Current protocols in neuroscience. New York: Wiley; 2004. pp. 4.16.1–4.16.15. [DOI] [PubMed] [Google Scholar]
- Schetz JA, Kim O-J, Sibley DR. Pharmacological characterization of mammalian D1 and D2 dopamine receptors expressed in Drosophila schneider-2 cells. J Recept Signal Transduct Res. 2003;23:99–109. doi: 10.1081/RRS-120018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FR. Recombinant expression systems in the pharmaceutical industry. Appl Microbiol Biotechnol. 2004;65:363–372. doi: 10.1007/s00253-004-1656-9. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972;27:353–365. [PubMed] [Google Scholar]
- Scotter AJ, Kuntz DA, Saul M, et al. Expression and purification of sea raven type II antifreeze protein from Drosophila melanogaster S2 cells. Protein Expr Purif. 2006;47:374–383. doi: 10.1016/j.pep.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Seitz V, Clermont A, Wedde M, et al. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol. 2003;27:207–215. doi: 10.1016/S0145-305X(02)00097-6. [DOI] [PubMed] [Google Scholar]
- Seok YJ, Kim KI, Yoo KH, Hwang-Bo J, Lee HH, Shon DH, et al. Expression and immunogenicity of a recombinant chimeric protein of human colorectal cancer antigen GA733-2 and an Fc antibody fragment in stably transformed Drosophila melanogaster S2 cells. Appl Biochem Biotechnol. 2010;162:1435–1445. doi: 10.1007/s12010-010-8909-0. [DOI] [PubMed] [Google Scholar]
- Shin HS, Cha HJ. Facile and statistical optimization of transfection conditions for secretion of foreign proteins from insect Drosophila S2 Cells using green fluorescent protein reporter. Biotechnol Prog. 2002;18:1187–1194. doi: 10.1021/bp025533l. [DOI] [PubMed] [Google Scholar]
- Shin HS, Cha HJ. Statistical optimization for immobilized metal affinity purification of secreted human erythropoietin from Drosophila S2 cells. Protein Expr Purif. 2003;28:331–339. doi: 10.1016/S1046-5928(02)00685-X. [DOI] [PubMed] [Google Scholar]
- Shin HS, Lim HJ, Cha HJ. Quantitative monitoring for secreted production of human Interleukin-2 in stable insect Drosophila S2 cells using a green fluorescent protein fusion partner. Biotechnol Prog. 2003;19:152–157. doi: 10.1021/bp0255614. [DOI] [PubMed] [Google Scholar]
- Silva JLC, Barbosa JF, Bravo JP, et al. Induction of a Gloverin-like antimicrobial polypeptide in the sugarcane borer Diatraea saccharalis challenged by septic injury. Braz J Med Biol Res. 2010;43:431–436. doi: 10.1590/S0100-879X2010005000010. [DOI] [PubMed] [Google Scholar]
- Smith SM. Strategies for the purification of membrane proteins. Methods Mol Biol. 2011;681:485–496. doi: 10.1007/978-1-60761-913-0_29. [DOI] [PubMed] [Google Scholar]
- Søndergaard L. Drosophila cells can be grown to high cell densities in a bioreactor. Biotechnol Tech. 1996;10:161–166. doi: 10.1007/BF00158939. [DOI] [Google Scholar]
- Swiech K, Rossi N, Astray RM, Suazo CAT. Enhanced production of recombinant rabies virus glycoprotein (rRVGP) by Drosophila melanogaster S2 cells through control of culture conditions. Cytotechnology. 2008;57:67–72. doi: 10.1007/s10616-008-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe E, Venkatesan M, Rose DR, Ewart KV. Expression of recombinant Atlantic salmon serum C-type lectin in Drosophila melanogaster Schneider 2 cells. Cytotechnology. 2013;65:513–521. doi: 10.1007/s10616-012-9505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle MA, Kester MB, Burns AL, Marx SJ, Spiegel AM, Shiloach J. Production and purification of human menin from Drosophila melanogaster S2 cells using stirred tank reactor. Cytotechnology. 2001;35:127–135. doi: 10.1023/A:1017586523710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die IM, Zuidweg EM, Bergmans JE, Hoekstra WP. Transformability of galE variants derived from uropathogenic Escherichia coli strains. J Bacteriol. 1984;158:760–761. doi: 10.1128/jb.158.2.760-761.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatandoost J, Bos MHA. Efficient expression of functional human coagulation factor IX in stably-transfected Drosophila melanogaster S2 cells; comparison with the mammalian CHO system. Biotechnol Lett. 2016;38:1691–1698. doi: 10.1007/s10529-016-2156-6. [DOI] [PubMed] [Google Scholar]
- Vogel H, Altincicek B, Glöckner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genom. 2011;12:308. doi: 10.1186/1471-2164-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hu H, Yang J, Wang F, Kaisermayer C, Zhou P. High yield of human monoclonal antibody produced by stably transfected Drosophila Schneider 2 cells in perfusion culture using wave bioreactor. Mol Biotechnol. 2012;52:170–179. doi: 10.1007/s12033-011-9484-5. [DOI] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T, Nemerow G, Wood HAZ, Shuler ML. Comparison of different cell lines for the production of recombinant baculovirus proteins. In: Richardson C, editor. Baculovirus expression protocols. New York: Humana Press; 1995. pp. 385–395. [DOI] [PubMed] [Google Scholar]
- Xia X, Yu L, Xue M, et al. Genome-wide characterization and expression profiling of immune genes in the diamondback moth, Plutella xylostella (L.) Sci Rep. 2015;5:9877. doi: 10.1038/srep09877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-X, Zhong X, Yi H-Y, Yu X-Q. Manduca sexta Gloverin binds microbial components and is active against bacteria and fungi. Dev Comp Immunol. 2012;38:275–284. doi: 10.1016/j.dci.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XX, Jin FL, Wang YS, Freed S, Hu QB, Ren SX. Molecular cloning and characterization of Gloverin from the diamondback moth, Plutella xylostella L. and its interaction with bacterial membrane. World J Microbiol Biotechnol. 2015;31:1529–1541. doi: 10.1007/s11274-015-1901-7. [DOI] [PubMed] [Google Scholar]
- Yang L, Song Y, Li X, et al. HIV-1 virus-like particles produced by stably transfected Drosophila S2 cells: a desirable vaccine component. J Virol. 2012;86:7662–7676. doi: 10.1128/JVI.07164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H-Y, Deng X-J, Yang W-Y, et al. Gloverins of the silkworm Bombyx mori: structural and binding properties and activities. Insect Biochem Mol Biol. 2013;43:612–625. doi: 10.1016/j.ibmb.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Yoshizawa T, Shibasaki F, et al. Chemical chaperones reduce aggregate formation and cell death caused by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch. Neurobiol Dis. 2002;10:88–99. doi: 10.1006/nbdi.2002.0502. [DOI] [PubMed] [Google Scholar]
- Zhao YM, Chen X, Sun H, et al. Effects of histone deacetylase inhibitors on transcriptional regulation of the hsp70 gene in Drosophila. Cell Res. 2006;16:566–576. doi: 10.1038/sj.cr.7310074. [DOI] [PubMed] [Google Scholar]