Abstract
Background
Heavy alcohol consumption frequently causes liver inflammation/injury, and certain fatty acids (FAs) may be involved in this liver pathology. In this study, we evaluated the association of heavy drinking and the changes in the FA levels involved in the ω-6 (pro-inflammatory) and ω-3 (anti-inflammatory) state in alcohol-dependent (AD) patients who had no clinical manifestations of liver injury. We aimed to identify sex-based differences in patients with mild or no biochemical evidence of liver injury induced by heavy drinking.
Methods
A total of 114 heavy drinking AD female and male patients aged 21 to 65 years without clinical manifestations of liver injury, who were admitted to an alcohol dependence treatment program, were grouped by the alanine aminotransferase (ALT) levels: ≤40 IU/l, as no liver injury (GR.1), and >40 IU/l, as mild liver injury (GR.2). Patients were actively drinking until the day of admission. Comprehensive metabolic panel, comprehensive FA panel, and drinking history data were evaluated.
Results
Elevated ALT and aspartate aminotransferase (AST) showed close association with markers of heavy alcohol intake. In the patients with mild biochemical liver injury (GR.2), females showed significantly higher AST level than males. Significant association of AST and total drinks in past 90 days (TD90) in females, and AST and heavy drinking days in past 90 days (HDD90) in males was observed. The ω-6:ω-3 ratio showed a significant pro-inflammatory response only in females with mild liver injury (GR.2) when adjusted by drinking history marker, TD90. Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) were increased in males with liver injury, while females did not show any comparable rise in EPA; and DHA levels were lower.
Conclusions
Measures of heavy drinking, TD90 and HDD90, predicted changes in liver injury. Changes in the ω-3 and ω-6 FA levels and the ω-6:ω-3 ratio showed a pro-inflammatory shift in patients with biochemical liver injury with a significant effect in females. Changes in FAs involved in the inflammatory state may represent one mechanism for liver inflammation/injury in response to heavy alcohol drinking.
Keywords: Alcohol, Sex, Heavy Drinking Markers, Fatty Acids, Liver Injury
Alcoholic Liver Disease (ALD) is a leading cause of chronic liver injury (Walsh and Alexander, 2000; World Health Organization, 2014). A majority of heavy drinkers develop hepatic steatosis, and up to 30% of these individuals progress to more severe forms of ALD, such as steatohepatitis, advanced fibrosis, and cirrhosis. Several risk factors that could modify the course of the disease have been identified; of relevance to this study are high unsaturated fat diet and drinking profile/patterns (Gao and Bataller, 2011; Kirpich et al., 2016; Tsukamoto et al., 2009). Among the factors related to ALD progression, fatty acids (FAs) involved in the pro-inflammatory and anti-inflammatory responses could play a critical role in the onset of liver inflammation/injury (Zhu et al., 2014). Indeed, FA supplements (ω-3) have been shown to suppress pro-inflammatory cytokine production and lymphocyte recruitment (Meydani, 1996).
Consumption of long-chain ω-3 polyunsaturated fatty acids (PUFAs; such as eicosapentaenoic acid [EPA]) can replace arachidonic acid (AA), an ω-6 PUFA, in immune cell membrane phospholipids, can inhibit hydrolysis of AA from membrane phospholipids and can compete with AA for cyclooxygenase and 5-lipoxygenase metabolic pathways (thus stimulating anti-inflammatory effects) (Calder, 2001). It is important to determine whether FA levels are altered in heavy drinking and lead to inflammation, and whether or not FAs could potentially function as liver injury biomarkers. Alterations in FA levels could be attributed to the dysregulation of lipid metabolism due to heavy drinking (Browning and Horton, 2004). As alcohol drinking causes liver injury, understanding the potential interactions of the levels of drinking and the pattern of drinking with the changes in the FA levels in the context of liver injury could potentially identify the role of alcohol consumption in FA metabolism.
To this end, we assessed the FAs that are involved in ω-3 and ω-6 pathways as potential biomarkers of inflammation in mild liver injury in heavy drinkers. Our primary aim was to evaluate the changes in FAs with mild biochemical liver injury in alcohol-dependent (AD) patients vis-à-vis their association with drinking profile. Some studies have shown changes in the FA levels and have used FA supplementation to treat alcohol-related liver injury in animal models (Chen et al., 2015). However, no such corresponding changes have been studied in patients at the onset of liver inflammation/injury. Additionally, there are few studies which describe any sex differences in FA metabolism in humans. Thus, we further aimed to identify and characterize the sex-based susceptibility to inflammation and liver injury in this cohort. Understanding these early changes in FA levels that participate in inflammation and associating these changes with liver injury adds value to our present understanding of the nature of ALD.
MATERIALS AND METHODS
Patient Population and Enrollment
This study was approved by the Institutional Review Board of National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda MD, under the screening protocol 98-AA-0009. The study was indexed at the National Clinical Trial Web site (www.dinicaltrials.gov:NCT00001673). This study included 114 male and female patients, aged 21 to 65 years (Table 1). All the assessments included in this study were collected at admission. Patients were diagnosed with alcohol dependence according to DSM-IV, based on the alcohol dependence module of the SCID-I interview, and alcohol withdrawal for either: (i) clinically manifest significant alcohol withdrawal symptoms, as observed by CIWA-Ar scores of ≥9, with or without detectable blood alcohol concentrations (BACs); or (ii) in the absence of the above, current intoxication above 0.1 g/dl BAC, self-reported history of continuous alcohol use >1 month, and self-reported previous episodes of significantly distressful alcohol withdrawal symptoms. DSM-IV is a manual published by the American Psychiatric Association that includes all currently recognized mental health disorders. The SCID-I is the Structured Clinical Interview for DSM-IV axis 1 disorders. It is a clinical examination for diagnosis of mental conditions. More information on its reliability and background can be found at: http://www.scid4.org/psychometric/scidI_reliability.html. CIWA-Ar is a revised version of the Clinical Institute Withdrawal Assessment of alcohol scale that is used to evaluate severity in withdrawal. We used ≥9 as our eligibility criteria for a diagnosis based on the needs of the several treatment studies. Further literature on the CIWA can be found at: http://www.aafp.org/afp/2004/0315/p1443.html.
Table 1.
Demographic, Drinking History Assessment, and Liver Injury Markers in Alcohol-Dependent Patients.
| Measures | Group 1 (Normal ALT, GR.1)
|
Group 2 (Elevated ALT, GR.2)
|
p-Value (GR.1 and GR. 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Males (34; 58.6%) | Females (24; 41.4%) | Within-gr.sex diff. p-value | Total (58; 50.4%) | Males (40; 70.2%) | Females (16; 29.8%) | Within-gr. sex diff. p-value | Total (56; 49.6%) | ||
| Age (years) | 37.6 ± 10.5 | 42.3 ± 10.6 | NS | 39.6 ± 10.7 | 43.0 ± 10.3 | 43.1 ± 10.3 | NS | 43.0 ± 10.2 | 0.080 |
| BMI (kg/m2) | 27.7 ± 4.2 | 25.7 ± 7.3 | NS | 26.9 ± 5.7 | 25.7 ± 3.8 | 26.3 ± 5.0 | NS | 25.9 ± 4.1 | NS |
| Drinking history | |||||||||
| TD90 | 957.2 ± 622.5 | 925.7 ± 735.1 | NS | 946.1 ± 656.9 | 1186.7 ± 528.5 | 950.3 ± 411.3 | NS | 1119.8 ± 505.9 | NS |
| HDD90 | 62.1 ± 26.9 | 62.7 ± 20.8 | NS | 62.3 ± 24.7 | 73.5 ± 20.7 | 76.8 ± 14.6 | NS | 74.5 ± 19.1 | 0.007 |
| AvgDD90 | 13.9 ± 7.9 | 13.0 ± 7.7 | NS | 13.6 ± 7.7 | 15.6 ± 5.9 | 12.1 ± 5.1 | NS | 14.6 ± 5.8 | NS |
| NDD90 | 67.4 ± 24.9 | 67.4 ± 21.0 | NS | 67.4 ± 23.4 | 76.7 ± 17.9 | 78.2 ± 13.6 | NS | 77.1 ± 16.7 | 0.017 |
| NNDD90 | 22.4 ± 24.9 | 22.5 ± 21.1 | NS | 22.4 ± 23.4 | 13.2 ± 17.9 | 11.8 ± 13.6 | NS | 12.8 ± 16.7 | 0.019 |
| Liver injury markers | |||||||||
| ALT (IU/l) | 28.9 ± 6.9 | 22.0 ± 8.3 | 0.001 | 26.0 ± 8.2 | 86.4 ± 35.3 | 111.9 ± 77.7 | NS | 93.6 ± 51.6 | NA |
| AST (IU/l) | 27.4 ± 16.2 | 28.8 ± 15.1 | NS | 28.0 ± 15.7 | 113.8 ± 79.5 | 181.4 ± 116.8 | 0.016 | 133.1 ± 95.7 | ≤0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; TD90, total drinks past 90 days; HDD90, heavy drinking days past 90 days; AvgDD90, average drinks per drinking day past 90 days; NDD90, number of drinking days in past 90 days; NNDD90, number of nondrinking days in past 90 days; NS, not significant; NA, not applicable.
Age was significantly different only in males between GR.1 and GR.2 groups (p = 0.030). Males also showed significant differences in the BMI values between the GR.1 and GR.2 (p = 0.044). Markers of drinking history: HDD90, NNDD90, and NDD90 showed significant difference between the study arms. Data are presented as mean ± SD. Statistical significance was set at p ≤ 0.05; trend level of significance (noted in italics) at 0.05 ≤ p ≤ 0.1 (for presentation only).
Patients were excluded from the study if they had clinical manifestations of liver injury/disease. Patients were also excluded from the study if they were diagnosed with a severe psychiatric illness such as dementia or active psychotic disorder including delirium, psychotic phase of bipolar disorder; if they were currently suicidal or violent; if they had agitation requiring immediate clinical treatment and/or a Mini Mental Status Examination) of <22 (cognitive grading with at least mild deficit); or if they had other clinically significant psychiatric illness. Diagnosis of clinically relevant systemic illness or HIV was another exclusionary criterion. Day of assessment exclusionary criteria also included: confirmed pregnancy or ongoing breastfeeding and/or positive urine screen for any illicit drug.
Demographics, Drinking, and Laboratory Assessments
On the day of evaluation, blood samples were drawn for a serum chemistry panel and comprehensive FA assessment (Table 2). Serum chemistry was used to determine potential liver injury, and FA panel was used to assess the ω-3 and ω-6 FAs.
Table 2.
Fatty Acid Levels in Alcohol-Dependent Patients with Normal and Elevated Levels of Alanine Aminotransferase.
| Measures | GR.1
|
GR.2
|
p-Value (GR.1 and GR. 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Within-gr. sex diff. p-value | Total | Male | Female | Within-gr. sex diff. p-value | Total | ||
| Omega—3 | |||||||||
| α-Linolenic acid | 79.9 ± 29.6 | 75.5 ± 34.8 | NS | 78.2 ± 31.5 | 92.2 ± 38.2 | 83.9 ± 36.3 | NS | 89.8 ± 37.5 | 0.083 |
| EPA | 97.3 ± 63.2 | 94.1 ± 49.7 | NS | 96.1 ± 58.0 | 146.2 ± 87.0 | 117.4 ± 89.9 | NS | 137.8 ± 88.0 | 0.004 |
| DPA5 3ω | 94.7 ± 48.9 | 100.1 ± 54.1 | NS | 96.8 ± 50.5 | 123.6 ± 47.3 | 103.4 ± 51.6 | NS | 117.8 ± 49.0 | 0.029 |
| DHA | 176.2 ± 79.2 | 243.5 ± 111.6 | 0.012 | 201.9 ± 97.6 | 235.3 ± 113.5 | 232.2 ± 118.5 | NS | 234.4 ± 113.9 | NS |
| Total ω-3 | 0.450 ± 0.16 | 0.514 ± 0.21 | NS | 0.475 ± 0.19 | 0.597 ± 0.25 | 0.544 ± 0.27 | NS | 0.582 ± 0.26 | 0.013 |
| Omega—6 | |||||||||
| Linoleic acid | 3311.4 ± 752.6 | 3054.8 ± 770.7 | NS | 3213.4 ± 762.9 | 3340.7 ± 757.8 | 3406.9 ± 761.3 | NS | 3360.0 ± 752.4 | NS |
| γ-Linolenic acid | 74.5 ± 40.1 | 73.6 ± 34.1 | NS | 74.1 ± 37.6 | 91.2 ± 45.4 | 78.8 ± 48.3 | NS | 87.6 ± 46.1 | 0.096 |
| DGLA | 109.7 ± 39.1 | 127.2 ± 41.4 | NS | 116.4 ± 40.5 | 129.8 ± 37.3 | 136.3 ± 57.9 | NS | 131.7 ± 43.8 | 0.060 |
| Arachidonic acid | 1156.7 ± 341.8 | 1194.6 ± 540.1 | NS | 1171.2 ± 424.0 | 1265.3 ± 429.9 | 1205.9 ± 292.7 | NS | 1248.0 ± 393.2 | NS |
| DTA | 25.9 ± 8.4 | 28.4 ± 18.6 | NS | 26.8 ± 13.2 | 34.1 ± 16.7 | 39.3 ± 28.1 | NS | 33.9 ± 15.9 | 0.013 |
| DPA5 6ω | 32.4 ± 18.7 | 42.1 ± 26.5 | NS | 36.1 ± 22.3 | 35.6 ± 19.1 | 34.9 ± 16.9 | NS | 35.4 ± 18.3 | NS |
| Total ω-6 | 4.7 ± 0.98 | 4.5 ± 1.27 | NS | 4.635 ± 1.09 | 4.89 ± 1.1 | 4.9 ± 0.99 | NS | 4.895 ± 1.05 | NS |
| ω6:ω3 ratio | 11.8 ± 4.8 | 8.97 ± 4.5 | NS | 10.5 ± 5.0 | 8.9 ± 3.5 | 10.8 ± 5.0 | NS | 9.4 ± 4.0 | NS |
Statistically significant differences between GR.1 and GR.2 group patients are shown in the last column. Data are presented as mean ± SD. Statistical significance was set at p ≤ 0.05; trend level at 0.05 ≤ p ≤ 0.1. Variability in the decimal points is based on the sensitivity of the markers.
DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; DTA, docosatetraenoic acid; NS, not significant. Units for fatty acids: ALA: 50 to 130 nmol/ml; EPA: 14 to 100 nmol/ml; DPA5 3ω: 20 to 210 nmol/ml; DHA: 30 to 250 nmol/ml; LA: 2,270 to 3,850 nmol/ml; GLA: 16 to 150 nmol/ml; DGLA: 50 to 250 nmol/ml; AA: 520 to 1,490 nmol/ml; DTA: 10 to 80 nmol/ml; DPA5 6ω: 10 to 70 nmol/ml; Total ω-3: mmol/l; Total ω-6: mmol/l.
Demographics (age, sex, body mass index [BMI]) and recent drinking history were collected and used in the analysis as factors and covariates. Recent drinking measures were collected from Time-line Follow-back questionnaire (Sobell et al., 2003) that included total drinks past 90 days (TD90), number of drinking days past 90 days (NDD90), number of nondrinking days past 90 days (NNDD90), average drinks per drinking day past 90 days (AvgDD90), and heavy drinking days past 90 days (HDD90).
Alanine aminotransferase (ALT) levels were used as the reference measure to assess biochemical liver injury in this study (Medline Plus-National Institutes of Health; https://medlineplus.gov/ency/article/003473.htm). Thus, ALT was used as a primary discriminating factor for liver injury in this study, with values of 40 IU/l as the upper limit of normal (patients with ALT ≤40 IU/l were grouped as GR.1) and values >40 indicated mild liver injury (GR.2).
Statistical Analysis
One-way analysis of variance (ANOVA) was used to evaluate demographic characteristics and drinking history. Univariate analysis of covariance was used to evaluate differences in the FAs between liver injury groups (overall) and by sex within the liver injury groups as factors. Modifiers of liver injury, drinking history, and demographic factors were tested in this study to estimate their roles as confounders. Post hoc analysis was conducted with ANOVA to identify the underlying factors. Regression analysis was used to characterize the association between liver injury markers and drinking history measures by sex differences. Linear regression analysis with either single independent variable or multiple independent variables was used to associate liver injury and representative ω-3 and ω-6 FAs that were significant. SPSS 22.0 (IBM, Chicago, IL) and Microsoft Excel 2013 (Microsoft Corp, Redmond, WA) were used for statistical analysis and data computation. Statistical significance was established at p ≤ 0.05. Data are expressed as mean ± SD (standard deviation) in the tables and mean ± SE (standard error) in the figures.
RESULTS
Patient Description and Characterization of Drinking Profile
A total of 114 patients were assigned to 2 groups: GR.1 (males, n = 34; and females, n = 24) who had normal ALT; and GR.2 (males, n = 40; and females, n = 16) with elevated ALT levels (Table 1). There was a trend toward significance in age differences between the 2 groups. BMI did not show any significant difference between the study arms (Table 1). Some markers of drinking history showed significant differences between the study arms, but TD90 and AvgDD90 did not (Table 1). There was no sex difference in the heavy drinking HDD90 marker in GR.2 patients; however, AvgDD90 measure showed a near significant sex difference (with 20% higher elevation in males than in females, p = 0.053). GR.2 males showed a near significant elevation in HDD90 values compared to GR.1 males (p = 0.051) while females showed a significant difference in this marker between-groups (p = 0.036). Males with liver injury showed a numerical elevation in the NDD90 marker compared to GR.1 male patients (p = 0.076).
Characterization of Liver Injury by Sex and Drinking History
We examined liver injury in the context of drinking history markers, and further with sex differences using both between-group, and within-group analyses. In GR.2, females showed significantly higher aspartate aminotransferase (AST) levels than males (Fig. 1). There was no statistical difference in the ALT levels between males and females in GR.2. In the GR.2 patients (elevated ALT group), the increase in ALT levels in females compared to the females without the liver injury (5-fold) was much higher than the increase observed in the males of the 2 groups (2.8-fold) (Table 1).
Fig. 1.
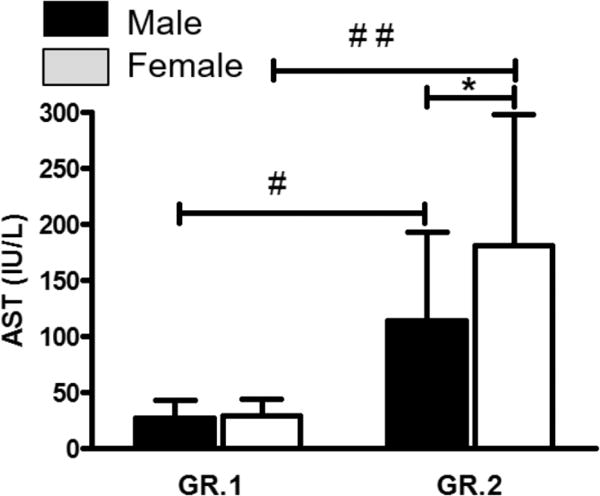
Distribution of patients by the levels of liver injury markers (mean ± SE), p ≤ 0.05. Significant sex and drinking history-based liver injury differences were observed across the GR.1 and GR.2 patients by aspartate aminotransferase (AST) levels. AST levels by liver injury status and sex. *GR.2 study arm female patients showed significantly elevated AST at p = 0.016 compared to GR.2 male patients; this sex difference was absent in GR.1 patients. #GR.2 male patients showed significant AST elevation, p ≤ 0.001 compared to GR.1 male patients. ##Significant AST increase, p ≤ 0.001 in GR.2 female patients compared to GR.1.
In GR.2, ALT correlated with HDD90 (adjusted R2 = 0.153, p = 0.004); and with NDD90 (R2 = 0.174, p = 0.002). In GR.2, AST showed significant association with NDD90 (R2 = 0.165, p = 0.001); and with HDD90 (adjusted R2 = 0.193, p = 0.001). Data not shown.
When AST was tested between the 2 sexes in the AD patients with liver injury (GR.2) using ANOVA and adjusted by HDD90, we found a significant elevation in females (p = 0.025) (Fig. 1). Post hoc testing showed that the difference is due to the underlying role of female sex only. Using linear regression, females showed a high association between AST and TD90 (Fig. 2), which males did not exhibit. On the other hand, males showed a high association with HDD90 (Fig. 2), which females did not. The association between ALT and drinking by sex was not as strong as that observed with AST.
Fig. 2.
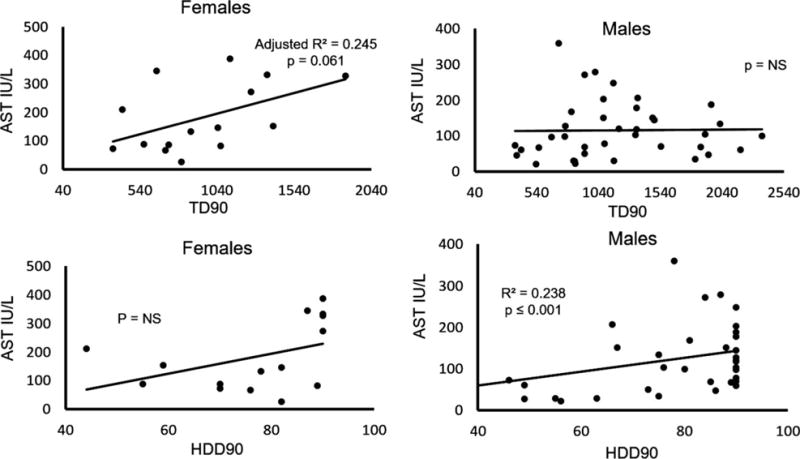
Sex and liver injury progression (aspartate aminotransferase [AST] levels) characterized by drinking markers in GR.2 (mildly elevated alanine aminotransferase group). Females showed a statistically significant moderate effect with the total drinks past 90 days (TD90) marker; however, males showed a statistically significant moderate effect with the heavy drinking days past 90 days (HDD90) marker. Patient data are presented by the levels of the liver injury marker, AST, comparing its association with drinking history markers using multiple regression analysis, p ≤ 0.05.
In the GR.1 patients (without liver injury), none of the drinking history markers showed any significant association with the ALT levels, and this is not unexpected. GR.1 patients did show a significant association between AST and NDD90 (p = 0.020) and HDD90 (p = 0.019); however, the effect size of the association was very mild and no sex differences could be identified. Total bilirubin and alkaline phosphatase were in the normal range and were not evaluated further.
FA Evaluation
Individual ω-3 and ω-6 FAs showed significant differences between the 2 study arms (those with and without mild liver injury) (Table 2). First, we analyzed whether an inflammatory shift was present with liver injury (section ω-6:ω-3 Ratio) and assessed variability by sex and drinking history markers. Thereafter, we examined the total ω-3 and total ω-6 FAs and changes in their levels that could have contributed to the inflammatory shifts (section Total ω-3 and Total ω-6 Changes in Liver Injury Group by Sex). Finally, we evaluated individual FAs involved in the ω-3 and ω-6 pathways (section Changes in the Individual FAs Involved in the Inflammatory Pathways) to identify the differences in the FAs that could impact the inflammatory response and to identify sex-based differences, if any.
ω-6:ω-3 Ratio
In GR.2 patients compared to GR.1, there was approximately a 10% decrease in the ω-6:ω-3 ratio. However, this decrease was not statistically significant without taking into account the drinking markers, suggesting that the relation was better explained by including the heavy alcohol drinking patterns. When correlated with HDD90, NDD90, TD90, and AvgDD90 (one at a time), each comparison showed significance at p < 0.05. In the GR.2 mild liver injury arm, the ω-6:ω-3 ratio was approximately 21% higher in females than in males (Fig. 3). This elevation also showed a sex difference effect with drinking markers as covariates: AvgDD90 (R2 = 0.124, p = 0.011) and TD90 (R2 = 0.113, p = 0.016). Further, when we looked at males and females separately for between-group (GR.2 and GR.1) differences, males showed a significant decrease in the ratio (R2 = 0.110, p = 0.004), whereas GR.2 females showed approximately a 20% increase in this ratio compared to GR.1 females (Fig. 3). However, these differences did not reach statistical significance.
Fig. 3.
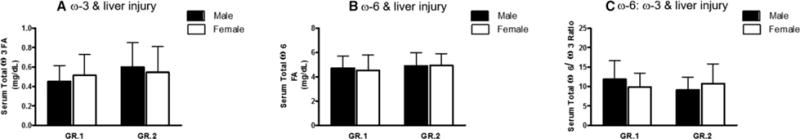
(A–C) ω-6, ω-3, and ω-6:ω-3 ratio in alcohol-dependent patients by liver injury status and sex. In Gr.2, ω-6:ω-3 ratio analysis showed sex difference at near significance p = 0.070 with average drinks per drinking day past 90 days as a factor. In Gr.2, ω-6:ω-3 ratio analysis showed higher in GR.2 females compared to males; this increase was statistically significant with the total drinks past 90 days as a covariate, p = 0.016. Data are plotted as mean ± SE, p ≤ 0.05.
Total ω-3 and Total ω-6 Changes in Liver Injury Group by Sex
On the one hand, the increase in total ω-3 levels in males of GR.2 compared to GR. 1 males was greater than the increase detected in females of GR.2 compared to GR.1 (Fig. 3A). On the other hand, the increase in ω-6 levels in females in GR.2 compared GR. 1 was higher than among the males between the 2 groups. A corresponding shift toward inflammation in GR.2 females was associated with greater increases in the levels of ω-6 FAs (Fig. 3B) and comparatively diminished increases in total ω-3 FA levels. There was more variability in ω-3 values in females with liver injury.
Lowering of the ω-6:ω-3 ratio in GR.2 males was strong (~2.9 units) (Fig. 3C). It was anti-inflammatory compared to females who exhibited a pro-inflammatory response of approximately +2.2 units. This result led us to evaluate individual FAs that are known to participate in inflammation.
Changes in the Individual FAs Involved in the Inflammatory Pathways
We evaluated specific FAs of ω-3 and ω-6 pathways (Table 2) by sex and by liver injury group. We also looked at differences in EPA and docosahexaenoic acid (DHA) by liver injury and sex.
Eicosapentaenoic Acid
EPA was significantly higher in the GR.2 patients compared to the GR.1 (p = 0.004) (Table 2 and Fig. 4A,B). We further reviewed the sex differences in the liver injury group. They were no statistically significant differences in the EPA levels between males and females (p = 0.275); however, when TD90 and AvgDD90 were factored in individually, the expression of this difference became statistically significant (p = 0.048 and p = 0.017, respectively). EPA was significantly elevated in GR.2 males compared to GR.1 males (p = 0.008, Table 2, Fig. 4). This difference was not significant in females.
Fig. 4.

Differences in the ω-3 and ω-6 pathways in GR.1 and GR.2 study arm alcohol-dependent patients by sex. Overall values of all fatty acids (FAs) showed differences by liver injury status.
 shows clinically significant levels of the FAs.
shows clinically significant levels of the FAs.
 shows clinically borderline elevated FAs. (A, B) α-Linoleic acid (ALA) did not show a sex-based difference in the GR.1 patients. ALA elevation was significant in GR.2 male patients only when co-factored with the heavy drinking history measures, HDD90, TD90, and NDD90. Sex differences in EPA indicated a trend-like impact with the heavy drinking markers, TD90 and AvgDD90 as factors in patients with liver injury. Changes in EPA levels were both statistically and clinically significant in male patients with and without liver injury; in GR.2 patients, the level was increased by 50%. In females, this difference was clinically significant with a minor increase in patients with liver injury. DHA levels were reduced in females in GR.2 compared to those in GR.1. Sex differences in DHA levels were significant in patients with no liver injury (GR.1); there was an increase in DHA levels in males without a similar increase in females. AvgDD90, average drinks per drinking day past 90 days; HDD90, heavy drinking days past 90 days; NDD90, number of drinking days past 90 days; TD90, total drinks past 90 days.
shows clinically borderline elevated FAs. (A, B) α-Linoleic acid (ALA) did not show a sex-based difference in the GR.1 patients. ALA elevation was significant in GR.2 male patients only when co-factored with the heavy drinking history measures, HDD90, TD90, and NDD90. Sex differences in EPA indicated a trend-like impact with the heavy drinking markers, TD90 and AvgDD90 as factors in patients with liver injury. Changes in EPA levels were both statistically and clinically significant in male patients with and without liver injury; in GR.2 patients, the level was increased by 50%. In females, this difference was clinically significant with a minor increase in patients with liver injury. DHA levels were reduced in females in GR.2 compared to those in GR.1. Sex differences in DHA levels were significant in patients with no liver injury (GR.1); there was an increase in DHA levels in males without a similar increase in females. AvgDD90, average drinks per drinking day past 90 days; HDD90, heavy drinking days past 90 days; NDD90, number of drinking days past 90 days; TD90, total drinks past 90 days.
Docosahexaenoic Acid
DHA did not show any overall significant differences between GR.2 and GR.1 as a main effect. However, GR.2 males showed a large elevation in DHA levels compared to GR.1 males (p = 0.013) (Table 2, Fig. 4A,B). This elevation was not significant in females (p = 0.768), indicating that females with liver injury have a lower response in DHA (which is protective and anti-inflammatory in nature). There was no significant difference between the males and females in the GR.2 group with regard to the DHA levels.
We evaluated ω-6 FAs, and could not determine any significant differences, either clinically (of the normal range of values) or statistically as related to the early onset of liver injury. There was a 400-unit elevation in the linoleic acid levels in females with liver injury, but no significant change in the males. Linoleic acid levels likely contributed to the elevation in total ω-6 levels in females.
Association of DHA and EPA with Liver Injury by Sex
There was a nonsignificant association in ALT levels and DHA levels in all the GR.2 arm patients (R2 = 0.025, p = 0.127). There was no significant difference in the DHA levels between the males and females in GR.2. This result supports the idea that the difference could be an outcome of lowered DHA levels in females with mild liver injury when compared to AD females without any liver injury (Table 2). In contrast, males with mild liver injury showed a significant (p = 0.013) 25% elevation in DHA levels compared to the males of GR.1.
Hence, it is important to examine the association of liver injury and specific ω-3 FAs separately in males and females. There was no significant association between DHA and ALT in females in GR.2, while in the same group, there was a significant association of DHA and ALT levels in males, albeit at a mild effect (Fig. 5A,B). EPA levels had a nonsignificant negative correlation with ALT in GR.2 females; however, GR.2 male patients showed a significant association with a mild effect between ALT and EPA levels (Fig. 5C,D).
Fig. 5.
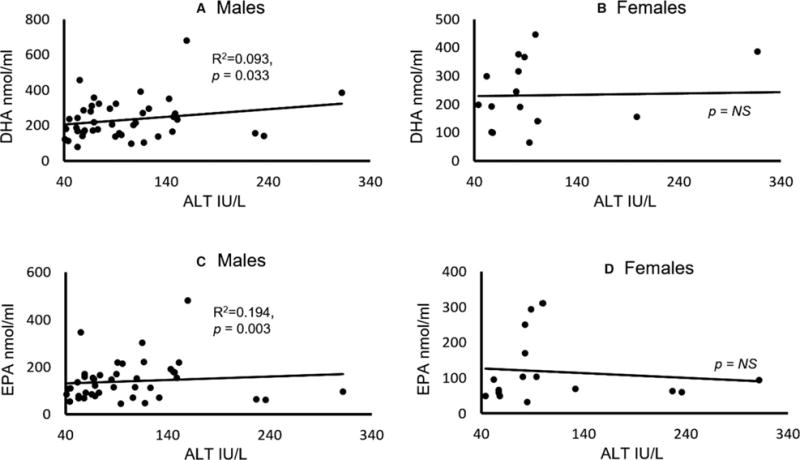
Association of candidate ω-3 fatty acids and the liver injury marker ALT in males and females. (A) DHA and ALT association in males. (B) DHA and ALT association in females. (C) EPA and ALT association in males. (D) EPA and ALT association in females. Statistical significance was set at p ≤ 0.05. ALT, alanine aminotransferase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
DISCUSSION
In our study of 114 heavy drinkers, we found that 40 of 74 males and 16 of 40 females had developed mild liver injury (ALT > 40 IU/l) in response to very heavy drinking. Importantly, these patients had no overt clinical signs of liver injury. As might be expected, several drinking history markers showed variable degrees of significant association with liver injury markers, ALT and AST, suggesting that the rate, frequency, and amount of alcohol intake were likely to be important determinants in liver injury (Table 1).
Females showed greater liver injury compared to the males, as assessed by the AST and ALT levels (Table 1). Sex-specific differences in the development of ALD have long been noted, but incompletely characterized. Results from human and animal studies suggest that females are more susceptible to liver injury and show a more progressive nature of ALD than males. Thus, females may be at greater risk of developing ALD than men, even when consuming a lesser amount of alcohol (Banerjee et al., 2006; Becker et al., 1996; Lieber, 1993; Wagnerberger et al., 2013). In addition, females are often at an advanced stage of liver disease at the time of diagnosis, even though they have a shorter history of alcohol consumption and a lower intake of alcohol than men (Cheong et al., 2016; Wilkinson, 1980).
A dose-dependent relation between alcohol intake and the risk of developing ALD has been observed in both males and females; and the relative risk increases earlier and more sharply for women than men with increased alcohol intake, suggesting that the drinking pattern may differentially affect the progression of ALD (Becker et al., 1996). On the one hand, our data show that females have an elevation in AST related to the amount of drinking over the recent past period of time. On the other hand, for males, both the number of drinks and the pattern of drinking are important. Thus, assessing the pattern of drinking at a similar level of intake over the same period of time in males might be more meaningful than only looking at the total amount. The frequency of heavy alcohol intake likely predicts negative consequences on the liver in men. To the best of our knowledge, the effects of sex differences on liver injury in response to very heavy drinking in AD patients have not been reported before. The mechanism(s) underlying sex-specific differences in susceptibility to ALD remain poorly understood. In fact, data from the National Alcohol Survey of a large cohort of drinkers conducted during the decade of 2000 to 2010 showed an upward trend in alcohol consumption in the United States, with a 25% increase by volume, which was likely due to a shift from a moderate drinking pattern in the first 5 years to a subsequent heavy drinking pattern (Kerr et al., 2014).
The dysregulated ω-6:ω-3 ratio could be attributed to either the role of alcohol intake or dietary intake of FAs (Simopoulos, 2002b), or perhaps a combination of both alcohol and FA intake. In our study, we did not see an overall statistically significant difference in this ratio between the 2 major study groups; however, the ratio was high (or toward inflammatory state) in the liver injury group (GR.2) with a distinct sex difference. In the GR.2 males, a pro-inflammatory response could not be detected in terms of the ω-6:ω-3 ratio. However, female AD patients in GR.2 showed an elevation in the ω-6:ω-3 ratio.
It could be that sex-specific FA metabolism leads to the differences in ω-3 and ω-6 FA levels seen. This has been supported in an in vitro study that showed sex hormones may affect FA synthesis by regulating FA desaturase and elongase expression epigenetically (Sibbons et al., 2014). Omega-3 FAs were lower in females than in males among the AD patients with liver injury (GR.2). Female patients did not show significant differences in ω-3 levels between the 2 groups. And importantly, we found that sex-specific differences in the severity of liver injury were closely related to the relative serum levels of ω-6 and ω-3 FAs.
Omega-6 and ω-3 FAs are essential FAs, and their metabolites have potentially pro-inflammatory and anti-inflammatory roles, respectively. AA, the major source of ω-6-derived eicosanoids, was elevated in both males and females in GR.2 compared to GR.1, and there was no significant difference between the 2 sexes within either GR.1 or GR.2. EPA and DHA were significantly higher in males in GR.2, and corresponding changes were not observed in females. EPA and DHA are precursors for specialized proresolving lipid mediators (SPMs) which stimulate resolution of inflammation and wound repair (Serhan et al., 2015). Females also did not show a corresponding increase in the ω-3 FAs, which may be a mechanistic difference contributing to the relatively greater liver injury seen in females as compared to males.
Our study has several limitations. Demographic data showed that race was shifted toward patients who reported as Caucasian (n = 61), with smaller numbers reportingas Asian (n = 2), Latino (n = 12), and African American (n = 34). Among the African American in our cohort, 27 were males and only 7 were females. This created a statistical problem for analysis, so they were not analyzed as a separate group. Another limitation of this study is that we do not have the dietary history on intake of FAs. However, a previous study suggested that daily macronutrient and micronutrient intake does not seem to be responsible for the sex-specific susceptibility in the development of ALD (Wagnerberger et al., 2008). Further, none of these patients was overtly malnourished to indicate a variability in nutrition (we tested CONUT score, there was no clinical, statistical, or numerical difference), and both groups (and their subgroups divided by sex) showed equivalent borderline overweight average BMIs. Individual variability among females was observed (Fig. 5B,D) and likely there are female-specific factors that might come into play that could only be identified with mechanistic studies. ALD is a complex disease, and there are several pathways that also come into play in the initiation and progression of ALD (McClain et al., 2012; Szabo, 2015). In this study, we found that the between-group and sex differences, and the within-group and within sex-based associations were significant; however, they were mild to moderate in size, which could indicate that FAs are only 1 of many factors involved in liver injury/repair. However, given the cohort size (more than 100), this study support the potential importance of FAs involved in liver inflammation/injury/repair. Importantly, specific ω-3 FAs (SPMs) play a critical role in the anti-inflammatory/wound repair process (Serhan et al., 2015). These specific lipids were not assayed in this study but may be reduced, especially in females in GR.2, due to relative decreases in EPA and DHA which are precursors for specific SPMs, such as resolvins.
In conclusion, we previously reported that alcohol intake increased serum levels of specific PUFAs (Johnson et al., 1985). The current study characterized specific markers of drinking that might be responsible for altered PUFA levels and delineated how these changes are manifested in males and females. Patients with inflammatory conditions usually respond to EPA and DHA supplementation by showing attenuated levels of pro-inflammatory cytokines (Simopoulos, 2002a). Our study is the first to report key differences in the extent of liver injury in heavy drinkers and the potential sex-specific roles of pro- and anti-inflammatory FAs (Fig. 4). An increase in ω-3 FAs could be an early compensatory anti-inflammatory response to the hazardous effects of heavy drinking. Markers of heavy drinking that were closely associated with liver injury were also found to contribute to the changes in the levels of FAs that are involved in ω-3 and ω-6 pathways. Last, females (regardless of the state of liver injury) did not show a significant increase in DHA with heavy drinking compared to males, and females showed increased susceptibility to liver injury.
Acknowledgments
This investigation was supported by the following grants: Z99-AA999999 (VV), U01-AA021901, U01AA021901-01, P50AA024337-01 (CJM), ZIA-AA000213 (DTG), and ZIA-AA000466 (VAR). Authors thank NIAAA inpatient clinical staff for their patient care, and the study participants. Ms. Marion McClain provided editorial support for the manuscript.
Footnotes
AUTHOR CONTRIBUTION
VV and CJM are responsible for the study concept and design. MLS and VV contributed to the acquisition of clinical data. MLS and VV conducted validation and quality assurance of clinical data and information. VV performed data analysis. VV and MS interpreted the data analysis. VV and MS drafted the manuscript. CJM, VAR, DTG, SSB, MLS, and MCC provided scientific contribution. All authors critically reviewed content and approved final version for publication.
CONFLICT OF INTERESTS
The authors have declared that no competing interests exist.
References
- Banerjee A, Apte UM, Smith R, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. J Pathol. 2006;208:473–485. doi: 10.1002/path.1917. [DOI] [PubMed] [Google Scholar]
- Becker U, Deis A, Sorensen T, Gronbaek M, Borch-Johnsen K, Muller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Investig. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- Chen P, Torralba M, Tan J, Embree M, Zengler K, Stärkel P, van Pijkeren J-P, DePew J, Loomba R, Ho SB. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148:e216. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J, Stein E, Bataller R. Diagnostic approaches and clinical end points of treatment in alcoholic liver disease. In: Chalasani N, Szabo G, editors. Alcoholic and Non-Alcoholic Fatty Liver Disease. Springer International Publishing; Switzerland: 2016. pp. 195–209. [Google Scholar]
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Gordon E, McClain C, Low G, Holman RT. Abnormal polyunsaturated fatty acid patterns of serum lipids in alcoholism and cirrhosis: arachidonic acid deficiency in cirrhosis. Proc Natl Acad Sci USA. 1985;82:1815–1818. doi: 10.1073/pnas.82.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr WC, Mulia N, Zemore SE. US trends in light, moderate, and heavy drinking episodes from 2000 to 2010. Alcohol Clin Exp Res. 2014;38:2496–2501. doi: 10.1111/acer.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Petrosino J, Ajami N, Feng W, Wang Y, Liu Y, Beier JI, Barve SS, Yin X, Wei X, Zhang X. Saturated and unsaturated dietary fats differentially modulate ethanol-induced changes in gut microbiome and metabolome in a mouse model of alcoholic liver disease. Am J Pathol. 2016;186:765–776. doi: 10.1016/j.ajpath.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Susceptibility to alcohol-related liver injury. Alcohol Alcohol Suppl. 1993;2:315–326. [PubMed] [Google Scholar]
- McClain CJ, Hill DB, Marsano L. Liver Disease Present Knowledge in Nutrition. 10th. Wiley-Blackwell; Oxford UK: 2012. [Google Scholar]
- Meydani SN. Effect of (n-3) polyunsaturated fatty acids on cytokine production and their biologic function. Nutrition. 1996;12:S8–S14. [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution, in. In: Mantovani A, editor. Seminars in Immunology. 3. Vol. 27. Elsevier; Amsterdam Netherlands: 2015. pp. 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbons CM, Brenna JT, Lawrence P, Hoile SP, Clarke-Harris R, Lillycrop KA, Burdge GC. Effect of sex hormones on n-3 polyunsaturated fatty acid biosynthesis in HepG2 cells and in human primary hepatocytes. Prostagland Leukot Essent Fatty Acids. 2014;90:47–54. doi: 10.1016/j.plefa.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002a;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002b;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Connors GJ, Agrawal S. Assessing drinking outcomes in alcohol treatment efficacy studies: selecting a yardstick of success. Alcohol Clin Exp Res. 2003;27:1661–1666. doi: 10.1097/01.ALC.0000091227.26627.75. [DOI] [PubMed] [Google Scholar]
- Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. In: Berk PD, editor. Seminars in Liver Disease. 02. Vol. 29. © Thieme Medical Publishers; New York, NY: 2009. pp. 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnerberger S, Fiederlein L, Kanuri G, Stahl C, Millonig G, Mueller S, Bischoff SC, Bergheim I. Sex-specific differences in the development of acute alcohol-induced liver steatosis in mice. Alcohol Alcohol. 2013;48:648–656. doi: 10.1093/alcalc/agt138. [DOI] [PubMed] [Google Scholar]
- Wagnerberger S, Schäfer C, Schwarz E, Bode C, Parlesak A. Is nutrient intake a gender-specific cause for enhanced susceptibility to alcohol-induced liver disease in women? Alcohol Alcohol. 2008;43:9–14. doi: 10.1093/alcalc/agm161. [DOI] [PubMed] [Google Scholar]
- Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J. 2000;76:280–286. doi: 10.1136/pmj.76.895.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. Sex differences in morbidity of alcoholics. In: Kalant OJ, editor. Alcohol and Drug Problems in Women. Plenum Press; New York: 1980. pp. 331–364. [Google Scholar]
- World Health Organization. Global Status Report on Alcohol 2004. 2004 Available at: http://apps.who.int/iris/handle/10665/42971. Accessed August 16, 2016.
- Zhu X, Xiao Z, Chen X, Li Y, Zhang X, Xu Y, Feng X, Wang J. Parenteral nutrition-associated liver injury and increased GRP94 expression prevented by ω-3 fish oil-based lipid emulsion supplementation. J Pediatr Gastroenterol Nutr. 2014;59:708–713. doi: 10.1097/MPG.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]


