Abstract
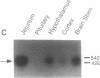
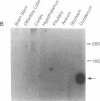
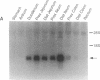
Secretin is a 27-amino acid gastrointestinal hormone that stimulates the secretion of bicarbonate-rich pancreatic fluid. The unusually high number of serine, leucine, and arginine residues in secretin has precluded the use of oligonucleotides to screen cDNA libraries to isolate a secretin cDNA. In the present study, a short cDNA encoding porcine secretin was amplified from duodenal mucosal first-strand cDNA template by using 16,384- and 4096-fold degenerate primers in the DNA polymerase chain reaction. From the sequence of the amplified cDNA, an unambiguous oligonucleotide probe was designed to screen a cDNA library. Here we report the sequences of cDNAs encoding the porcine and rat secretin precursors. The predicted amino acid sequences reveal that each precursor consists of a signal peptide, an N-terminal peptide, secretin, and a 72-amino acid C-terminal peptide. Secretin has been highly conserved through evolution. Rat secretin differs from its porcine counterpart by a single glutamine-for-arginine substitution at position 14. In contrast, the amino acid sequences of the C-terminal peptides are only 39% conserved between the two species, suggesting that the C-terminal peptide does not have an essential physiologic function. RNA blot hybridizations reveal that the rat secretin gene is expressed throughout the small intestine. Although secretin immunoreactivity has been localized in the central nervous system by some laboratories, we are unable to detect secretin mRNA in tissues of the central nervous system by Northern blot hybridization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss W. M., Starling E. H. The mechanism of pancreatic secretion. J Physiol. 1902 Sep 12;28(5):325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bloom S. R. Hormones of the gastrointestinal tract. Br Med Bull. 1974 Jan;30(1):62–67. doi: 10.1093/oxfordjournals.bmb.a071169. [DOI] [PubMed] [Google Scholar]
- Bond C. T., Nilaver G., Godfrey B., Zimmerman E. A., Adelman J. P. Characterization of complementary deoxyribonucleic acid for precursor of porcine motilin. Mol Endocrinol. 1988 Feb;2(2):175–180. doi: 10.1210/mend-2-2-175. [DOI] [PubMed] [Google Scholar]
- Carlquist M., Jörnvall H., Mutt V. Isolation and amino acid sequence of bovine secretin. FEBS Lett. 1981 May 5;127(1):71–74. doi: 10.1016/0014-5793(81)80343-2. [DOI] [PubMed] [Google Scholar]
- Chang T. M., Berger-Ornstein L., Chey W. Y. Presence of biologically and immunologically active secretin-like substance in the mammalian brain. Peptides. 1985 Mar-Apr;6(2):193–198. doi: 10.1016/0196-9781(85)90039-7. [DOI] [PubMed] [Google Scholar]
- Chelly J., Concordet J. P., Kaplan J. C., Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W. Y., Chang T. M., Park H. J., Lee K. Y., Escoffery R. Secretin-like immunoreactivity and biological activity in the antral mucosa. Endocrinology. 1983 Aug;113(2):651–656. doi: 10.1210/endo-113-2-651. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gafvelin G., Carlquist M., Mutt V. A proform of secretin with high secretin-like bioactivity. FEBS Lett. 1985 May 20;184(2):347–352. doi: 10.1016/0014-5793(85)80636-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Gossen D., Vandermeers A., Vandermeers-Piret M. C., Rathé J., Cauvin A., Robberecht P., Christophe J. Isolation and primary structure of rat secretin. Biochem Biophys Res Commun. 1989 Apr 28;160(2):862–867. doi: 10.1016/0006-291x(89)92514-x. [DOI] [PubMed] [Google Scholar]
- Gould S. J., Subramani S., Scheffler I. E. Use of the DNA polymerase chain reaction for homology probing: isolation of partial cDNA or genomic clones encoding the iron-sulfur protein of succinate dehydrogenase from several species. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1934–1938. doi: 10.1073/pnas.86.6.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Huang C. G., Eng J., Yalow R. S. Ontogeny of immunoreactive cholecystokinin, vasoactive intestinal peptide, and secretin in guinea pig brain and gut. Endocrinology. 1986 Mar;118(3):1096–1101. doi: 10.1210/endo-118-3-1096. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Eng J., Yalow R. S. Ontogeny of immunoreactive CCK, VIP and secretin in rat brain and gut. Biochem Biophys Res Commun. 1983 May 16;112(3):891–898. doi: 10.1016/0006-291x(83)91701-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter A. B., Toder A., Wolfe H. J., Taylor I. L., Cooperman S., Mandel G., Goodman R. H. Peptide YY. Structure of the precursor and expression in exocrine pancreas. J Biol Chem. 1987 Sep 25;262(27):12984–12988. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. A., Llanos O. L., Swierczek J. S., Rayford P. L., Thompson J. C. Concentrations of gastrin and secretin in the alimentary tract of the cat. Surgery. 1978 Jan;83(1):90–93. [PubMed] [Google Scholar]
- Mutt V., Carlquist M., Tatemoto K. Secretin-like bioactivity in extracts of porcine brain. Life Sci. 1979 Nov 12;25(20):1703–1707. doi: 10.1016/0024-3205(79)90472-7. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E., Magnusson S. Structure of porcine secretin. The amino acid sequence. Eur J Biochem. 1970 Sep;15(3):513–519. doi: 10.1111/j.1432-1033.1970.tb01034.x. [DOI] [PubMed] [Google Scholar]
- O'Donohue T. L., Charlton C. G., Miller R. L., Boden G., Jacobowitz D. M. Identification, characterization, and distribution of secretin immunoreactivity in rat and pig brain. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5221–5224. doi: 10.1073/pnas.78.8.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette T. L., Shulman D. F., Alpers D. H., Jaffe B. M. Postnatal development of intestinal secretin in rats and guinea pigs. Am J Physiol. 1982 Dec;243(6):G511–G517. doi: 10.1152/ajpgi.1982.243.6.G511. [DOI] [PubMed] [Google Scholar]
- Shinomura Y., Eng J., Yalow R. S. Dog secretin: sequence and biologic activity. Life Sci. 1987 Sep 7;41(10):1243–1248. doi: 10.1016/0024-3205(87)90202-5. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Immunoreactive secretin in gastrointestinal mucosa of several mammalian species. Gastroenterology. 1978 Sep;75(3):401–404. [PubMed] [Google Scholar]
- Yanaihara N., Sakagami M., Sato H., Yammamoto K., Hashimoto T. Immunological aspects of secretin, substance P, and VIP. Gastroenterology. 1977 Apr;72(4 PT2):803–810. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]