Abstract
Adolescence is associated with the continued maturation of dopamine neurotransmission and is implicated in the etiology of many psychiatric illnesses. Adolescent exposure to neurotoxicants that distort dopamine neurotransmission, such as methylmercury (MeHg), may modify the effects of chronic d-amphetamine (d-AMP) administration on reversal learning and attentional-set shifting. Male C57Bl/6n mice were randomly assigned to two MeHg-exposure groups (0 ppm and 3 ppm) and two d-AMP-exposure groups (saline and 1 mg/kg/day), producing four treatment groups (n = 10–12/group): Control, MeHg, d-AMP, and MeHg + d-AMP. MeHg exposure (via drinking water) spanned postnatal day 21–59 (the murine adolescent period), and once daily i.p. injections of d-AMP or saline spanned postnatal day 28–42. As adults, mice were trained on a spatial-discrimination-reversal (SDR) task in which the spatial location of a lever press predicted reinforcement. Following two SDRs, a visual-discrimination task (extradimensional shift) was instated in which the presence of a stimulus light above a lever predicted reinforcement. Responding was modeled using a logistic function, which estimated the rate (slope) of a behavioral transition and trials required to complete half a transition (half-max). MeHg, d-AMP, and MeHg + d-AMP exposure increased estimates of half-max on the second reversal. MeHg exposure increased half-max and decreased the slope term following the extradimensional shift, but these effects did not occur following MeHg + d-AMP exposure. MeHg + d-AMP exposure produced more perseverative errors and omissions following a reversal. Adolescent exposure to MeHg can modify the behavioral effects of chronic d-AMP administration.
Keywords: adolescence, methylmercury, d-amphetamine, reversal learning, extradimensional shift
The adolescent period is a time of dynamic psychobiological maturation (Spear, 2000) and is implicated in the development of a variety of psychiatric illnesses (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003), such as schizophrenia, attention deficit hyperactivity disorder (ADHD), and substance-abuse disorders. In rodent models, the adolescent period has been defined as broadly ranging from postnatal day (PND) 21 to 60 (Spear, 2000), though more nuanced definitions exist (Laviola, Macrì, Morley-Fletcher, & Adriani, 2003).
Neurochemical signaling, particularly dopamine (DA) neurotransmission, undergoes dramatic region-specific changes during adolescence in both humans and nonhumans (Andersen, 2003). DA-receptor expression in the rat prefrontal cortex (Andersen, Thompson, Rutstein, Hostetter, & Teicher, 2000; Tarazi & Baldessarini, 2000) and striatum (Andersen, Rutstein, Benzo, Hostetter, & Teicher, 1997; Teicher, Andersen, & Hostetter, 1995) peaks around PND 40 and decreases into adulthood. By comparison, caudate volume in humans peaks around 10 to 14 years of age for females and males, respectively (Lenroot et al., 2007). Adolescent-onset changes in DA signaling co-occur with distortions in behavior that is critically dependent on prefrontal-cortex function, such as choice and decision-making (Dalley, Cardinal, & Robbins, 2004). For example, adolescent humans and nonhumans display increased impulsive choices (Green, Fry, & Myerson, 1994; Pinkston & Lamb, 2011), perseverative errors on reversal-learning and attentional-set shifting tasks (Newman & McGaughy, 2011; Overman, 2004), and a higher likelihood of using and abusing stimulant drugs (Johnston, O’Malley, Miech, Bachman, & Schlenberg, 2014) compared to adults. Disturbing DA signaling during adolescence could give rise to enduring psychological dysfunction later in life. One way DA signaling can be altered is through exposure to environmental neurotoxicants that directly interfere with DA signaling and enhance sensitivity to DAergic drugs (Jones & Miller, 2008), but the long-term impact of contaminant exposure during adolescence and its potential to cause behavior disorders is woefully understudied.
Methylmercury (MeHg) is a ubiquitous environmental neurotoxicant that can have long-lasting neurobehavioral effects when exposure occurs in utero (Newland, Reed, & Rasmussen, 2015). However, the long-term behavioral consequences of adolescent MeHg exposure have not been explored until recently (Boomhower & Newland, 2016). Adolescent MeHg exposure is especially concerning for a number of reasons. First, relative to other age groups, human adolescents consume more fish (Nielsen, Aoki, Kit, & Ogden, 2015), such as tuna, that can have high mercury concentrations (Tran, Barraj, Smith, Javier, & Burke, 2004; Wang et al., 2013). Second, adolescents are encouraged to consume even more fish for health reasons (Gidding et al., 2005). Third, the consumption of high-mercury seafood is related to blood mercury levels in adolescents (Nielsen et al., 2015). Finally, adolescents use DAergic drugs, such as d-amphetamine (d-AMP), more frequently relative to other age groups (Johnston et al., 2014). Determining whether exposure to MeHg during adolescence disturbs later neurobehavioral functioning, and whether MeHg’s effects are modified by concurrent exposure to d-AMP would have significant implications for public health.
In nonhuman models, gestational MeHg exposure slows the acquisition of choice (Newland, Reile, & Langston, 2004) and increases perseverative errors following spatial- and visual-discrimination reversals (Paletz, Day, Craig-Schmidt, & Newland, 2007; Reed, Paletz, & Newland, 2006) while leaving errors on a spatial-to-visual discrimination task (i.e., extradimensional shift) unaffected in adulthood (Paletz et al., 2007). MeHg-induced disruptions in choice co-occur with increased sensitivity to dopamine transporter (DAT) blockers, such as d-amphetamine (Rasmussen & Newland, 2001) and cocaine (Reed & Newland, 2009). Further, MeHg inhibits DAT (Dreiem, Shan, Okoniewski, Sanchez-Morrissey, & Seegal, 2009) and stimulates DA efflux both in vitro (Tiernan, Edwin, Goudreau, Atchison, & Lookingland, 2013) and in vivo (Faro, Do Nascimento, San José, Alfonso, & Durán, 2000).
As developmental MeHg exposure distorts DA neurotransmission and choice behavior, reversal learning and attentional-set shifting, both critically dependent on proper DA signaling (Kehagia, Murray, & Robbins, 2010), could be especially sensitive to adolescent MeHg and d-AMP exposure. Impairments in reversal learning and attentional-set shifting are associated with a variety of psychiatric illnesses (Arnsten & Rubia, 2012; Wegbreit et al., 2016). The effects of adolescent MeHg exposure on reversal learning and attentional-set shifting remain unexplored, whereas the literature on the effects of adolescent d-AMP exposure on reversal learning and attentional set shifting is mixed. Some investigations report no impairments on either task following exposure to a dl-AMP mixture in adolescent monkeys (Soto et al., 2012) and humans (Ersche, Roiser, Robbins, & Sahakian, 2008), and others report both impaired and improved reversal learning in rats (Hankosky, Kofsky, & Gulley, 2013). Adolescent exposure to cocaine, a stimulant that also blocks DAT, results in enhanced perseverative errors following a reversal (Kantak, Barlow, Tassin, Brisotti, & Jordan, 2014; Pope, Boomhower, Hutsell, Teixeira, & Newland, 2016). Using nonhuman models to determine whether environmental neurotoxicants produce deficits in reversal learning and attentional-set shifting, and whether chronic psychostimulant administration exacerbates these deficits enhances understanding of the variables that may contribute to human psychiatric illness.
The present study was designed to determine the extent to which d-AMP administration and MeHg exposure during adolescence affected reversal learning and attentional-set shifting in a mouse model. To assess reversal learning, we employed a spatial-discrimination-reversal (SDR) procedure (Pope et al., 2016) in which the spatial location of a lever (e.g., left or right) predicted reinforcement. Attentional-set shifting (i.e., an extradimensional shift) was assessed using a spatial-to-visual discrimination procedure (Paletz et al., 2007) in which the spatial location of a lever no longer predicted reinforcement and instead an illuminated stimulus light above a lever did.
Method
Subjects and exposures
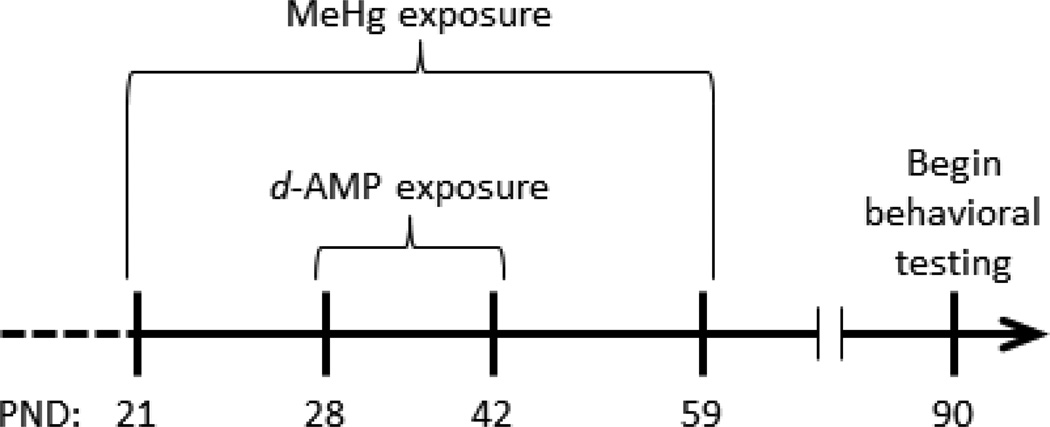
Forty-eight male C57BL6 mice (12 litters, each producing 4 littermates) were ordered from a commercial vendor (Envigo, Indianapolis, IN) and arrived on postnatal day (PND) 21. The mice were housed in a humidity- and temperature-regulated, AAALAC-accredited animal facility under a 12-h light/dark cycle (lights on at 6:00 AM). Mice were provided ad libitum access to food and water and were randomly assigned to two methylmercury (MeHg) exposure groups (n = 24 in each): 0 ppm (control) and 3 ppm MeHg (calculated as Hg). MeHg was delivered as methylmercuric chloride (Sigma) dissolved in drinking water. Exposure lasted from PND 21 through PND 59 (see Figure 1). A dose of 3 ppm MeHg was used because the daily dosing that it produces in mice results in permanent behavioral impairment without overt signs of toxicity in rats exposed during gestation (Paletz et al., 2007; Reed et al., 2006) or mice exposed during adolescence (Boomhower & Newland, 2016).
Figure 1.
Mice were given tap water (control) or water containing 3 ppm methylmercury (MeHg) from postnatal day (PND) 21 through 59. Within these groups, mice received acute i.p. injections of saline or d-amphetamine (d-AMP; 1.0 mg/kg/day) from PND 28 through 42.
Within each MeHg exposure group, half the mice were given injections of saline or 1.0 mg/kg d-AMP sulfate (calculated as the salt), which resulted in a 2 (MeHg) x 2 (d-AMP) full-factorial design and produced four treatment groups (n = 12 per cell): Control, MeHg, d-AMP, and MeHg + d-AMP. Littermates were divided equally among treatment groups so each group had only one representative from a litter, making litter the statistical unit as recommended for developmental studies (Maurissen, 2010). D-amphetamine sulfate (Sigma) was dissolved in 0.9% saline. Acute i.p. injections of d-AMP or saline were given once daily at 5:00 PM from PND 28 through PND 42 (Figure 1), which is similar to past studies employing chronic stimulant exposures in adolescent rodents (Hankosky et al., 2013; Pope et al., 2016). Further, this d-AMP dosing regimen encompasses the time in which DA-receptor expression in the rodent prefrontal cortex (Andersen et al., 2000; Brenhouse, Sonntag, & Andersen, 2008; Tarazi & Baldessarini, 2000) and striatum (Andersen et al., 1997; Gelbard, Teicher, Faedda, & Baldessarini, 2000; Teicher et al., 1995) peaks in adolescence. Two weeks prior to and throughout behavioral testing, mice were maintained at a body mass of 26 (±1) g by restricting food intake to approximately 2.4 (±1) g chow daily. During behavioral testing, mice were fed after sessions. Three mice died before behavioral testing (two from the d-AMP only group and one from the Control group) because of reasons unrelated to the experiment. All procedures were approved by the Auburn University Institutional Animal Care and Use Committee.
Apparatus
Experiments were conducted in ten operant chambers (Med Associates Inc., St. Albans, VT) enclosed in sound-attenuating cabinets. A non-retractable lever was mounted on the rear wall. Two retractable levers were mounted on the front wall with stimulus lights (LEDs) above each. An alcove was centered between the two front levers where a dipper delivered 5 sec of .01-cc droplets of a 3:1 solution of water and sweetened-condensed milk. Two Sonalert® tone generators were mounted at the top of the front wall. In an adjacent room, a computer with Med Associates IV programming recorded and controlled all experimental events with a .01-s resolution.
Procedure
Autoshaping and chain training
On PND 90, lever pressing was trained using an autoshaping procedure similar to Pope et al. (2016). Autoshaping began on either the front-left or front-right lever, counterbalanced across mice. Responses were reinforced on a fixed-ratio (FR) 1 schedule of reinforcement. Following 40 reinforced responses on each lever (i.e., left, right, and back), response-chain training began. Each trial began with a pulsating tone, and a back-lever response within 5 min caused one of the front levers to extend into the chamber. A response on the front lever within 5 min was reinforced followed by a 10-sec intertrial interval (ITI). Whether the left or right lever was extended following a back-lever response was pseudorandomly determined such that an equal number of back-left and back-right response chains were reinforced during a session. Chain training was considered complete following 50 reinforced response chains within 60 min.
Spatial discrimination reversal (SDR)
The SDR procedure (Pope et al., 2016) proceeded in three phases: original discrimination (OD), first reversal (R1), and second reversal (R2). In the OD, sessions comprised 60 trials separated by a 10-sec ITI. A trial began with a pulsating tone, and a back-lever (trial-initiation) response within 15 sec extended both front levers and illuminated both stimulus lights. Here, the spatial location (left or right) of the front lever served as the discriminative stimulus. A response within 15 sec on the “correct” lever (e.g., right) resulted in 5-sec access to milk, and an “error” (e.g., left-lever press) resulted in termination of the trial and the 10-sec ITI. Whether a left- or right-lever press was reinforced throughout the OD was counterbalanced across mice. A trial was considered an omission if either a trial-initiation response or a front-lever press did not occur within the 15 sec limited holds. After three consecutive OD sessions with ≥51 correct responses, the first reversal (R1) was imposed by switching the location of the correct lever (e.g., left to right or right to left). Following three consecutive R1 sessions with ≥51 correct responses, the R2 phase was imposed by switching again the location of the correct lever. In some cases the ≥51 correct response criterion was not reached within 20 sessions due to trial omissions (i.e., ≥10 trials were repeatedly omitted in a session). In these scenarios, the next phase was imposed following the completion of three consecutive sessions at ≥85% accuracy (i.e., ≥85% correct responses of all responses produced), provided at least 30 responses occurred.
Visual discrimination (extradimensional shift, EDS)
Following completion of the OD, R1, and R2 phases of the SDR procedure, a visual discrimination (Paletz et al., 2007) was implemented to assess an extradimensional shift (EDS). Here, the presence of an illuminated stimulus light over a lever served as the discriminative stimulus, and the spatial location of the lever was irrelevant. The visual discrimination proceeded in an identical manner as the SDR procedure. Following a trial-initiation response, both levers were extended and one stimulus light was illuminated. A lever press under the illuminated lever was correct, and a response on the unilluminated lever was an error. The location (left or right) of the illuminated stimulus light was randomly determined each trial. If a trial did not result in reinforcement, a correction trial commenced in which the position of the stimulus light was unchanged until a response was reinforced. Correction trials were necessary because, without them, a mouse that exclusively responded on one lever (e.g., right) could earn half a session’s reinforcers (this was not a concern in the SDR phase). The visual discrimination proceeded until three consecutive sessions with ≥51 correct responses were made.
Data analysis
The primary dependent variables were the outcomes of a trial in each phase: correct, error, and omission. The change in responding following a reversal and extradimensional shift can be conceptualized as a behavioral transition (Newland & Reile, 1999; Pope et al., 2016), in which the probability of a correct response plummets after a change in reinforcement contingencies and gradually increases throughout the new phase (e.g., from OD to R1). To quantify the behavioral transition, we used Equation 1, a three-term logistic (S-shaped) function that has been successful in past work describing the effects of neurotoxicants on behavior in transition because the free parameters are easily interpretable (Newland et al., 2004).
| (1) |
Here, the probability of a correct response [p(correct)] is a function of trial (X). This probability corresponds to the proportion correct as determined experimentally. X0 is the trial at which half the transition is complete (termed “half-maximum”), k is the maximum slope of the transition after it begins, and Ym is the maximum asymptote after a transition. The value 1/k is the number of trials required to get from 1/e to 2/e of the transition, where e is the natural logarithm (approximately 2.72). Thus, Eq. 1 provides important information concerning the trial at which a transition is centered (X0), how quickly a transition proceeds (k), and the final degree of accuracy once a transition is complete (Ym). These free parameters described differences in responding following a spatial-discrimination reversal in mice exposed to cocaine in adolescence (Pope et al., 2016).
Eq. 1 was fit to response data from R1, R2, and EDS using least-squares regression (Microsoft Excel® Solver, v14.6.9, Microsoft Corporation, USA). A correct (or reinforced) response was coded as 1, an error was coded as 0, and omitted trials were not coded. Performance in the last three sessions of the prior phase was included in the analyses to establish a baseline of responding. Free-parameter estimates were compared across groups using 20% Winsorized means and variance, which decreases the influence of outliers and stabilizes variability without reducing sample size (for details, see Wilcox & Keselman, 2003; Wilcox, 1998, 2012). Eq. 1 was not fit to responding across the OD, which was different from the R1, R2, and EDS in that responding was acquired rapidly by all mice and did not undergo a transition from a low-to-high probability of being correct in the OD. The restricted range of the probability of a correct response (0.5 to 1.0) in the OD coupled with the rapid acquisition made it difficult to estimate parameters of the logistic function using nonlinear least-squares regression.
Both errors and omissions to criterion were also compared across groups for OD, R1, R2, and EDS, as both measures revealed neurotoxicant- and drug-induced impairment following the first reversal in past work (Paletz et al., 2007; Pope et al., 2016; Reed et al., 2006). To determine the obtained dose of MeHg across adolescence, water consumption and body mass were measured. Because mice were pair-housed, water consumption was estimated for an individual mouse after controlling for spillage, identical to Boomhower and Newland (2016).
All dependent measures were analyzed using a linear mixed effects (LME) model with group (Control, MeHg, d-AMP, and MeHg + d-AMP) and phase (OD, R1, R2, and EDS) or postnatal day (for water-consumption and body-mass measures) as within-subjects fixed effects and litter as a random effect (Systat Software Inc., Richmond, CA, USA). LME was chosen because it is able to model incomplete repeated-measures data more effectively than traditional repeated-measures ANOVA.
Results
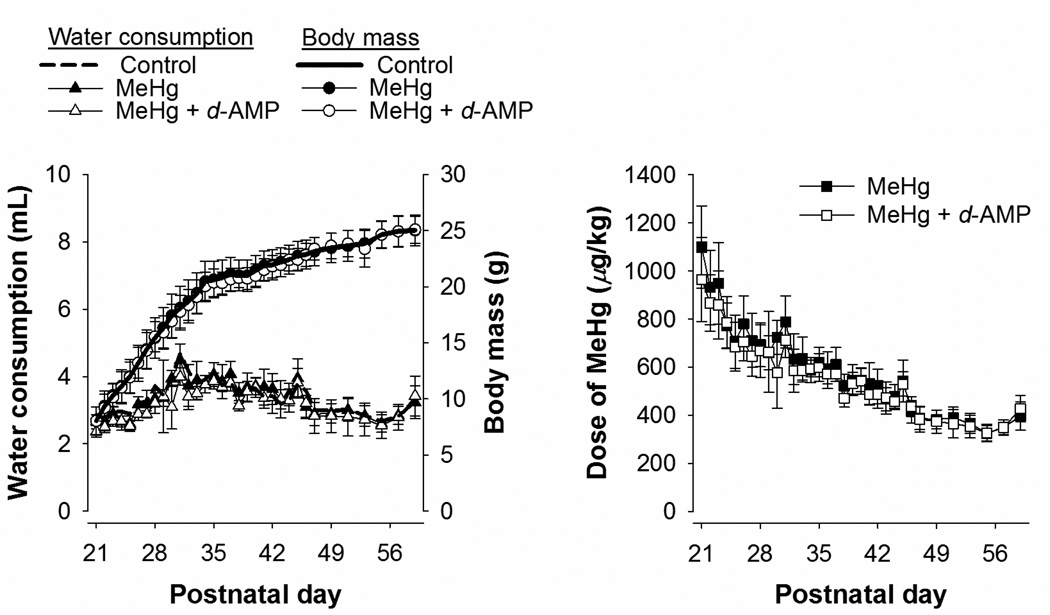
Figure 2 shows mean estimated water consumption, body mass, and MeHg dose as a function of postnatal day for mice exposed to MeHg and MeHg + d-AMP during adolescence. Water consumption and body mass for the Control group are shown also for reference. All treatment groups consumed a similar amount of water and displayed similar body growth across adolescence. For both groups, MeHg dose was highest at the beginning of exposure, and then stabilized at about 400 µg/kg/day at the end of exposure. The dose of MeHg was similar for both the MeHg and MeHg + d-AMP groups.
Figure 2.
Left panel: Mean (±SD) estimated water consumption (triangles, left axis) and body mass (circles, right axis) for mice exposed to MeHg alone and MeHg + d-AMP during adolescence. Mean water consumption and body mass for Control are shown also for comparison. Right panel: Mean estimated dose of MeHg for mice exposed to MeHg alone and MeHg + d-AMP during adolescence. Note all error bars represent one standard deviation.
There were no group differences in completing autoshaping, chain training, and the original discrimination (OD). In the original discrimination, approximately 3–4 mice in each exposure group did not achieve the ≥51 correct response criterion before the twentieth session; thus, the more lenient ≥85% accuracy criterion for three consecutive sessions was imposed. Cases in which the more lenient criterion was used were entirely confined to the OD, as no mice required more than 20 sessions to complete the first reversal, second reversal, and extradimensional shift.
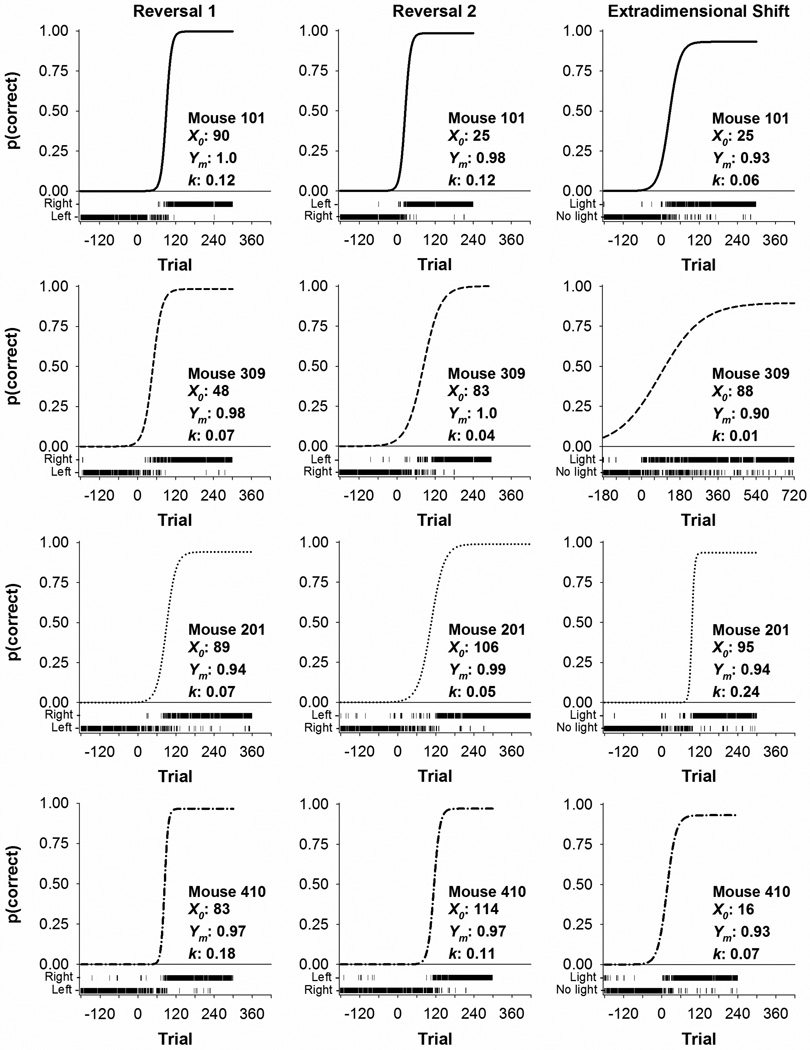
Figure 3 shows representative responding across the first and second reversals and extradimensional shift for four mice. Each row of panels shows data from a single mouse. Each panel is divided into the best-fit predictions of Eq. 1, shown as a solid line representing the probability of a correct response as a function of trial for an entire phase. Trial-by-trial outcomes are displayed just below the abscissa of each panel. A correct response was coded as 1 and an error was coded as 0. Thus, data in Figure 3 show a response on the right lever as correct in the first reversal, a response on the left lever as correct in the second reversal, and a response below a cue light (“Light”) as correct in the extradimensional shift. For example, for the top left panel, a long run of Left presses changes to a mix of Left and Right responses at about trial 90 and then is followed by a long run of Right presses. The center panel shows responding transition from the right lever to the left lever, and the right panel shows the extradimensional shift to a light/no-light discrimination. The first correct lever (left or right) experienced was counterbalanced, but data are presented as if all subjects experienced the same lever first. In both reversals, responding that was maintained on one lever (e.g., left) transitioned to responding on the correct lever (e.g., right). This behavioral transition is reflected in near exclusive responding on one lever in the last three sessions of the previous phase and, following a reversal, perseverative responding on that lever until switching to the opposite lever. For the EDS, responses were classified as occurring on a lever with either a light illuminated above it (“Light”) or no light illuminated (“No light”).
Figure 3.
Representative data from four mice for the first reversal (left column), second reversal (middle column) and extradimensional shift (right column). Each row of panels presents data from an individual mouse in Control (Mouse 101), MeHg (Mouse 309), d-AMP (Mouse 201), and MeHg + d-AMP (Mouse 410). The best fit of Eq. 1, showing the probability of a correct response [p(correct)] as a function of trial, is shown as the solid line with its corresponding parameter estimates. Trial-by-trial responses are shown as vertical dashes at the bottom of each panel, classified as left and right responses (for the reversals) or light and no-light responses (for the extradimensional shift). Trial 0 indicates when a reversal or extradimensional shift was imposed, and data from trials before Trial 0 show performance in the last three sessions of the previous experimental phase. The first correct lever (left or right) experienced was counterbalanced, but data are presented as if all subjects experienced the same lever first. Note the X-axis for Mouse 309 in the extradimensional shift had to be expanded to incorporate his entire data set.
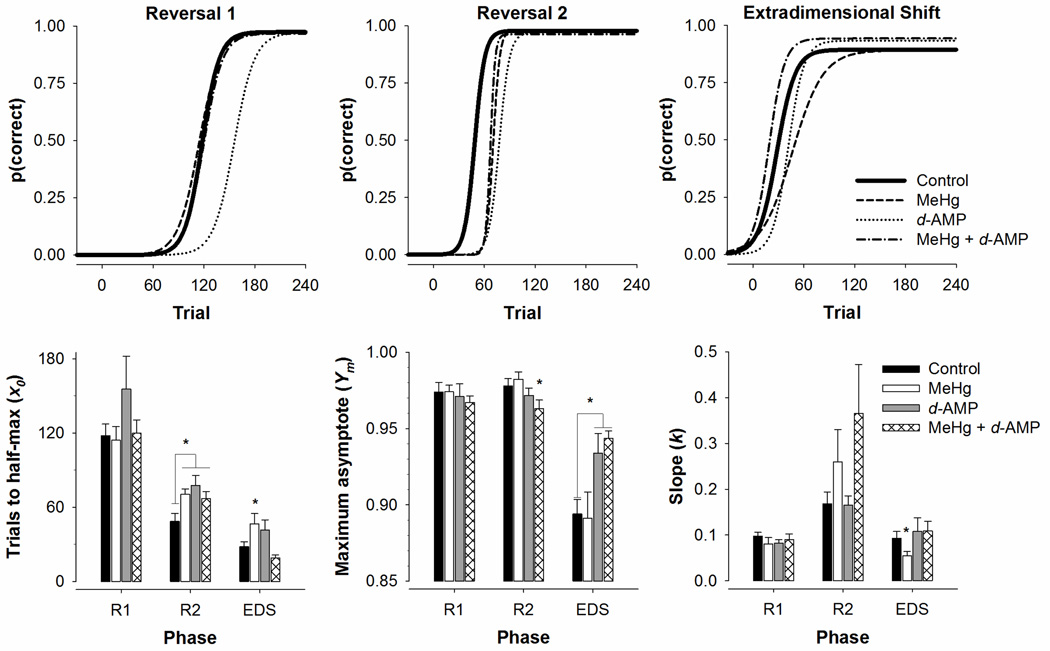
Figure 4 shows the probability of a correct response as a function of trial using mean parameter estimates for each treatment group (top row) and Winsorized mean parameter estimates from Eq. 1 (bottom row) across each phase. For trials to half-max (X0), a main effect of phase, F(2, 120) = 65.12, p < .001, indicated that trials to half-max decreased from R1 to the EDS, which can be visualized as a leftward shift of each function. A main effect of exposure, F(3, 120) = 3.52, p = .01, revealed that mice exposed to MeHg and d-AMP (both alone and in combination) on average required more trials for the probability of a correct response to reach half its maximum. Post-hoc contrasts revealed that MeHg, d-AMP, and MeHg + d-AMP mice had higher X0 estimates than controls following the second reversal, p < .05, which can be visualized as a rightward shift in the curves relative to the Control curve. MeHg-exposed mice in the EDS also had higher X0 estimates than Control, p < .05. For the maximum asymptote (Ym), there was a main effect of phase, F(2, 120) = 56.12, p < .001, and a significant exposure*phase interaction, F(6, 120) = 5.20, p < .001. Post-hoc contrasts revealed the MeHg + d-AMP group had lower Ym estimates relative to control in R2, and both d-AMP and MeHg + d-AMP mice had higher Ym estimates relative to control in the EDS, all p’s < .05. Differences in Ym estimates can be visualized as differing heights of the curves. For the slope of the transition (k), there was a main effect of phase, F(2, 120) = 16.42, p < .001, in that transitions proceeded more quickly in the second reversal. Post-hoc contrasts revealed that MeHg-exposed mice had lower k estimates relative to controls in the EDS, which can be visualized as a shallower ascending limb of the function for the MeHg group relative to Control.
Figure 4.
Top row: Probability of a correct response [p (correct)] as a function of trial across each experimental phase for the four treatment groups. Lines were generated using mean parameter estimates from Eq. 1.
Bottom row: Parameter estimates (+SEM) derived from Eq. 1 of trials to half-max (left), asymptotic accuracy (middle), and slope of the transition (right) for the first reversal (R1), second reversal (R2), and extradimensional shift (EDS). *p < .05 relative to Control
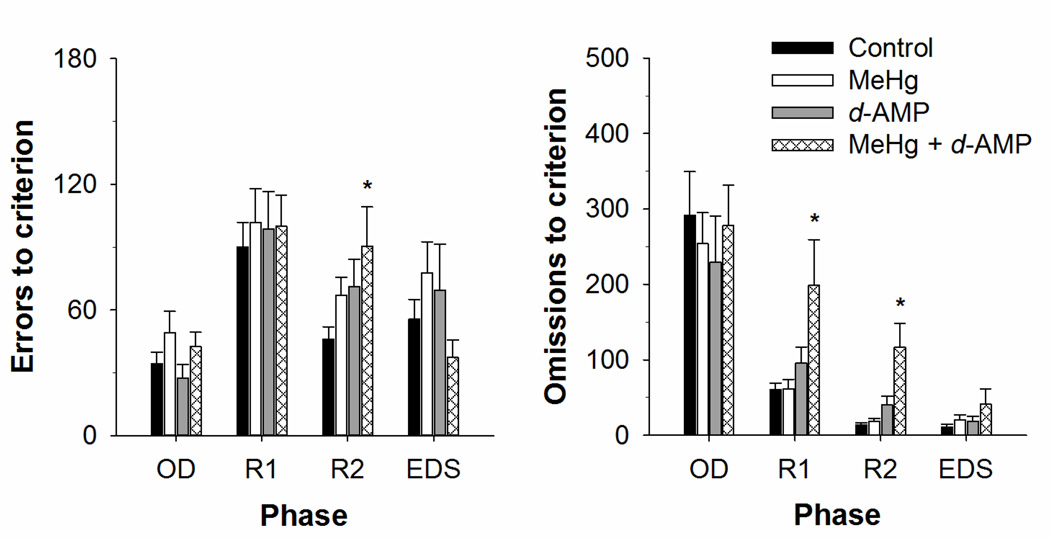
Figure 5 shows mean errors (left) and omissions (right) to criterion at the end of each experimental phase. For errors to criterion, a main effect of phase indicated that more errors on average occurred following a reversal and extradimensional shift, F(3, 153) = 15.33, p < .001. Post-hoc contrasts revealed that mice exposed to MeHg + d-AMP made more errors following the second reversal relative to controls, p < .05. For omissions to criterion, a main effect of phase, F(3, 153) = 45.75, p < .001, indicated that omissions decreased substantially from original discrimination to the extra dimensional shift. The MeHg + d-AMP mice omitted more trials but especially after a reversal, as evidenced in a main effect of exposure, F(3, 153) = 4.00, p < .01.
Figure 5.
Mean (+SEM) errors (left) and omissions (right) to criterion for mice exposed to MeHg, d-AMP, MeHg + d-AMP, and controls during adolescence. The original discrimination (OD), first reversal (R1), and second reversal (R2) of the spatial discrimination as well as the extradimensional shift (EDS) to a visual discrimination are shown. *p < .05 relative to Control
Discussion
The present study compared performance on a spatial-discrimination reversal (SDR) and then an extradimensional shift to a visual discrimination among mice exposed to MeHg and d-AMP, both alone and concurrently, during adolescence. In both MeHg-exposure groups, the dose of MeHg was higher at the beginning of adolescence, and then tapered off to about 400 µg/kg/day. The pattern of dosing observed here is most likely due to a rapid rise in body mass coupled with a relatively stable level of water consumption throughout the adolescent period (Adriani, Macrì, Pacifici, & Laviola, 2002; Boomhower & Newland, 2016). Administration of d-AMP for two weeks (PND 28–42) during adolescence did not alter the dose of MeHg consumed relative to the MeHg-only group.
Responding in reversal-learning and attentional-set shifting procedures is disrupted by damage to specific regions of the prefrontal cortex and can be conceptualized as behavior in transition, which is particularly sensitive to developmental neurotoxicant or psychostimulant exposure (Newland & Reile, 1999; Newland, Yezhou, Logdberg, & Berlin, 1994; Pope et al., 2016). Reversal learning is impaired by orbitofrontal lesions (Bissonette et al., 2008; McAlonan & Brown, 2003) whereas infralimbic cortical lesions disrupt extradimensional shifts (Birrell & Brown, 2000; Bissonette et al., 2008; Chudasama & Robbins, 2003; Dalley et al., 2004). Determining the extent to which exposure to neurotoxicants and psychostimulants, either alone or in combination, impair reversal learning and attentional-set shifting enhances understanding of the factors that may influence behavior implicated in psychiatric illness. To describe performance throughout two spatial discrimination reversals and an extradimensional shift, we used a logistic function (Newland et al., 2004, 1994). Asymptotic accuracy (Ym) was maintained above 0.85 in all phases reflecting the ≥85% accuracy criterion. Overall, performance improved from the first to the second reversal as evidenced by a reduction in the number of trials required to complete half a transition (X0) and a decrease in perseverative errors and omissions in some groups from R1 to R2. The overall improvement in performance from the first to second reversal noted in the present study is consistent with previous work employing similar procedures and analyses (Paletz et al., 2007; Pope et al., 2016; Reed et al., 2006). The slope (k) was generally steep for R2, reflecting a rapid transition for that reversal, but shallow for both R1 and the transition to EDS. Enhanced perseverative errors following an EDS is traditionally indicative of an attentional-set shift, but we did not observe a significant increase in errors to criterion or trials to complete half a transition from R2 to the EDS. Though the spatial-to-visual procedure we employ to assess an EDS is identical to past work with rodents (Paletz et al., 2007), it could be that more experience with the SDR procedure was necessary to produce greater errors in the EDS. For example, Paletz et al. (2007) imposed seven spatial-discrimination reversals before introducing the EDS, whereas we imposed only two reversals. Regardless, we did observe a decrease in the asymptotic accuracy as well as the slope from R2 to the EDS, which indicates a decrement in performance.
Mice exposed to MeHg, d-AMP, and MeHg + d-AMP all required more trials to achieve half a transition (i.e., higher X0 estimates) relative to controls on the second reversal. Interestingly, the combination of MeHg and d-AMP did not have a greater effect than either treatment alone, suggesting that they do not interact or combine additively on this measure in mice. Control animals required about 50 trials to complete half a transition in the second reversal, while exposed animals required about 70–80 trials. Though neither MeHg nor d-AMP alone reduced asymptotic accuracy (Ym), the combination of MeHg + d-AMP exposure did reduce Ym estimates in the second reversal. The combined treatment also increased the number of perseverative errors and omissions relative to control in the second reversal. MeHg and d-AMP alone produced intermediate changes on errors to criterion, placing these groups between the controls and the mice exposed to the joint treatment. While the MeHg and d-AMP alone groups were not statistically distinguishable from controls, they were also not distinct from the combined treatment, so it is possible that MeHg and d-AMP alone produced subtle changes on errors to criterion that were difficult to distinguish from background variability. The logistic analysis, which revealed that both the MeHg and d-AMP groups had higher X0 estimates than controls, was more sensitive than errors and omissions to criterion in capturing impairment following a reversal.
Previous reports have shown that gestational MeHg exposure impairs performance on both spatial- (Paletz et al., 2007; Reed et al., 2006) and visual-discrimination reversals (Paletz et al., 2007) in rats by increasing perseverative errors and omissions primarily in the first reversal. Chronic d-AMP administration in adolescence has been associated with increased perseverative errors following a reversal in rats, but these effects were dependent on the methodological arrangement of the reversals (Hankosky et al., 2013). That is, adolescent d-AMP exposure increased perseverative digging errors in an odor-discrimination-reversal procedure but did not affect perseverative lever-press errors in a spatial-discrimination-reversal task (Hankosky et al., 2013). It is unclear why the second reversal in the present study was more sensitive to MeHg-and d-AMP-induced impairment relative to the first reversal, though this is not the only case in which subsequent reversals resulted in greater impairment than the first reversal (Clarke, Dalley, Crofts, Robbins, & Roberts, 2004).
A visual-discrimination procedure in which a light above a lever predicted reinforcement was imposed to assess an extradimensional shift, or a change in the modality of a stimulus (e.g., space to visual cue) to predict reinforcement. Asymptotic accuracy (Ym) in the extradimensional shift was lower than Ym estimates in the first two reversals in all groups, but administration of d-AMP buffered this effect. That is, the d-AMP only and MeHg + d-AMP groups had significantly greater Ym estimates relative to controls. Adolescent MeHg exposure impaired the extradimensional shift by increasing the trials required to complete half the transition (X0) and decreasing the speed of the transition (k). Both of these effects were due to an increase in errors to criterion following adolescent MeHg exposure relative to controls. Though the increase in errors to criterion did not reach statistical significance, the MeHg group displayed on average the highest number of errors during the extradimensional shift than any other group. Thus, the logistic analysis of the transition was more sensitive in revealing MeHg-induced impairment following an extradimensional shift than other conventional measures of task performance, such as omissions or errors to criterion. The improved sensitivity of the logistic analysis could reflect its ability to integrate the contributions of behavior that slowed the transition, such as errors and omissions, and of behavior that facilitated the acquisition, such as the appearance of correct responses. Administration of d-AMP eliminated the effects of MeHg exposure alone on the extradimensional shift, as the MeHg + d-AMP group had similar X0 and k estimates relative to controls. This protective effect of d-AMP was confined to the extradimensional shift, whereas d-AMP and MeHg exposure acted additively to impair the second reversal.
Past work has demonstrated that gestational MeHg exposure selectively increases perseverative errors following a reversal and not an extradimensional shift in rats (Paletz et al., 2007). In the present study, the logistic analysis revealed subtle MeHg-induced deficits in the absence of enhanced perseverative errors following an extradimensional shift. Paletz et al. (2007) did not employ a logistic analysis, which could explain why they did not report impaired performance on an extradimensional shift following MeHg exposure in rats. This seems unlikely, however, since the performance of the different groups was nearly identical in Paletz et al. (2007). Alternatively, adolescent MeHg exposure may produce a different pattern of behavioral effects compared to gestational MeHg exposure, perhaps due to different vulnerable neurobiological substrates—especially the orbitofrontal and infralimbic cortices (Birrell & Brown, 2000; Bissonette et al., 2008; Chudasama & Robbins, 2003; Dalley et al., 2004). Adolescent rats require more trials to acquire a reversal and an extradimensional shift relative to adults (Newman & McGaughy, 2011), suggesting the neurocircuitry that supports these behaviors is still immature. Sex differences in the development of DA neurotransmission (Andersen et al., 1997) also may play a role in behavioral vulnerability to adolescent MeHg and d-AMP exposure. Comparing reversal and extradimensional-shift performance of male and female mice will be necessary to determine whether females display a similar sensitivity to the adolescent exposures we employ in the present study. Regardless, the finding that adolescent d-AMP administration did not affect trials to half-max or perseverative errors following an extradimensional shift is consistent with past work in monkeys (Soto et al., 2012) and rats to an extent (Hankosky et al., 2013).
The present study demonstrated that the behavioral effects of adolescent MeHg exposure can be enhanced or eliminated by d-AMP administration depending on the behavioral task. MeHg and d-AMP concomitantly impaired reversal learning to a greater extent than exposure to either chemical alone. Conversely, d-AMP administration prevented MeHg-induced deficits on the extradimensional shift. It is well established that MeHg exerts its neurotoxicity in part by distorting dopamine (DA) neurotransmission (Newland et al., 2015; Tiernan et al., 2015). MeHg inhibits DA uptake via DAT (Dreiem et al., 2009; Faro, do Nascimento, Alfonso, & Durán, 2002) and stimulates presynaptic DA efflux in vitro (Kalisch & Racz, 1996; Tiernan et al., 2013). Similarly, gestational MeHg exposure increases sensitivity of response rates to d-AMP (Rasmussen & Newland, 2001) and cocaine (Reed & Newland, 2009) in adult rats. Gestational MeHg exposure seems to alter the behavioral effects of DAT-inhibiting drugs selectively, as MeHg-exposed rats had similar dose-response curves to unexposed animals for other non-dopaminergic drugs and for selective D1 and D2 agonists (Rasmussen & Newland, 2001; Reed & Newland, 2009). In the present study, the effects of d-AMP administration on MeHg-induced behavioral impairment depended on the procedure—that is, whether a reversal or an extradimensional shift was in effect—and may be the result of brain-region-specific neurotoxicity. Performance during a reversal and extradimensional shift relies on different regions of the prefrontal cortex (Birrell & Brown, 2000; Bissonette et al., 2008; Chudasama & Robbins, 2003; Dalley et al., 2004), which may explain the opposing behavioral effects of combined exposure to MeHg and d-AMP following a reversal and extradimensional shift.
The present study demonstrated that adolescent exposure to MeHg and d-AMP, both alone and in combination, affected reversal learning and attentional-set shifting in adulthood. The combination of adolescent MeHg + d-AMP exposure impaired the second reversal to a greater extent than exposure to either substance alone. Further, d-AMP administration reversed MeHg-induced deficits on the extradimensional shift. The present study provides support for the notion that adolescence is a developmental period that is susceptible to the behavioral effects of psychostimulant and neurotoxicant exposure, whose effects can be modified when exposure occurs concomitantly. Determining the extent to which psychopharmaceuticals interact with environmental contaminants to exacerbate behavior related to psychiatric illness is crucial to future public health.
Public Significance Statement.
This study suggests that the adolescent period is vulnerable to neurobehavioral effects of neurotoxic and psychoactive chemicals, specifically, methylmercury and d-amphetamine. When administered together, the extent to which methylmercury and d-amphetamine impaired behavior in male mice depended on the behavioral task. This study demonstrates that adolescent exposure to methylmercury and d-amphetamine can interact to change behavior later in life.
Acknowledgments
This research was financially supported by the National Science Foundation Graduate Research Fellowship Program (DGE-1414475) and ES 024845 from NIEHS.
All authors contributed in a significant way to the preparation of the manuscript, and all authors have read and approved the final manuscript.
The authors would like to thank Kate Johnson, Joe McIlwain, and Alex Sauer for help with data collection.
Footnotes
The authors declare no conflicts of interest.
The research contained herein has been presented at the International Society for Developmental Psychobiology and Society for Neuroscience.
References
- Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27(2):212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. http://doi.org/10.1016/S0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–1498. doi: 10.1097/00001756-199704140-00034. http://doi.org/10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson A, Rutstein M, Hostetter J, Teicher M. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. http://doi.org/10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. http://doi.org/10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. The Journal of Neuroscience. 2000;20(11):4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of Neuroscience. 2008;28(44):11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. http://doi.org/10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomhower SR, Newland MC. Adolescent methylmercury exposure affects choice and delay discounting in mice. Neurotoxicology. 2016;57:136–144. doi: 10.1016/j.neuro.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. The Journal of Neuroscience. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23(25):8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. http://doi.org/23/25/8771[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304(5672):878–880. doi: 10.1126/science.1094987. http://doi.org/10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. http://doi.org/10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience & Biobehavioral Reviews. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. http://doi.org/10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Shan M, Okoniewski RJ, Sanchez-Morrissey S, Seegal RF. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicology and Teratology. 2009;31(5):312–317. doi: 10.1016/j.ntt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. http://doi.org/10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faro L, do Nascimento J, Alfonso M, Durán R. Mechanism of action of methylmercury on in vivo striatal dopamine release: Possible involvement of dopamine transporter. Neurochemistry International. 2002;40(5):455–465. doi: 10.1016/s0197-0186(01)00098-5. [DOI] [PubMed] [Google Scholar]
- Faro L, Do Nascimento JLM, San José JM, Alfonso M, Durán R. Intrastriatal administration of methylmercury increases in vivo dopamine release. Neurochemical Research. 2000;25(2):225–229. doi: 10.1023/a:1007571403413. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. International Journal of Developmental Neuroscience. 2000;18(1):29–37. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Gidding SS, Dennison BA, Birch LL, Daniels SR, Gilman MW, Lichtenstein AH, Rattay KT, Steinberger J, Stettler N, Van Horn L. Dietary recommendations for children and adolescents: A guide for practitioners consensus statement from the American Heart Association. Circulation. 2005;112(13):2061–2075. doi: 10.1161/CIRCULATIONAHA.105.169251. http://doi.org/10.1161/CIRCULATIONAHA.105.169251. [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Hankosky E, Kofsky N, Gulley J. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behavioural Brain Research. 2013;252:117–125. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schlenberg JG. Monitoring the Future national results on adolescent drug use: 1975–2013: Overview key findings on adolescent drug use. Ann Arbor; 2014. [Google Scholar]
- Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochemical Pharmacology. 2008;76(5):569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Kalisch BE, Racz WJ. The effects of methylmercury on endogenous dopamine efflux from mouse striatal slices. Toxicology Letters. 1996;89(1):43–49. doi: 10.1016/s0378-4274(96)03787-3. http://doi.org/10.1016/S0378-4274(96)03787-3. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Barlow N, Tassin DH, Brisotti MF, Jordan CJ. Performance on a strategy set shifting task in rats following adult or adolescent cocaine exposure. Psychopharmacology. 2014;231(23):4489–4501. doi: 10.1007/s00213-014-3598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Current Opinion in Neurobiology. 2010;20(2):169–192. doi: 10.1016/j.conb.2010.01.007. http://doi.org/10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macrì S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience and Biobehavioral Reviews. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbox AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. http://doi.org/10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurissen J. Practical considerations on the design, execution and analysis of developmental neurotoxicity studies to be published in Neurotoxicology and Teratology. Neurotoxicology and Teratology. 2010;32(2):121–123. doi: 10.1016/j.ntt.2009.09.002. http://doi.org/10.1016/j.ntt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reed MN, Rasmussen E. A hypothesis about how early developmental methylmercury exposure disrupts behavior in adulthood. Behavioural Processes. 2015;114:41–51. doi: 10.1016/j.beproc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Reile PA. Learning and behavior change as neurotoxic endpoints. Target Organ Series: Neurotoxicology. 1999:311–337. [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicology and Teratology. 2004;26(2):179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methylmercury: Reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicology and Applied Pharmacology. 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- Newman L, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011;53(4):391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SJ, Aoki Y, Kit BK, Ogden CL. More than half of US youth consume seafood and most have blood mercury concentrations below the EPA reference level, 2009–2012. The Journal of Nutrition. 2015;145(2):322–327. doi: 10.3945/jn.114.203786. http://doi.org/10.3945/jn.114.203786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55(1):134–147. doi: 10.1016/S0278-2626(03)00279-3. http://doi.org/10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Day JJ, Craig-Schmidt MC, Newland MC. Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n-3 polyunsaturated fatty acids. Neurotoxicology. 2007;28(4):707–719. doi: 10.1016/j.neuro.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: Adolescent-limited and life-persistent patterns of impulsivity. Behavioral Neuroscience. 2011;125(2):194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope DA, Boomhower SR, Hutsell BA, Teixeira K, Newland MC. Chronic cocaine exposure in adolescence: Effects on spatial discrimination reversal, delay discounting, and performance on fixed-ratio schedules in mice. Neurobiology of Learning and Memory. 2016;130:93–104. doi: 10.1016/j.nlm.2016.01.017. http://doi.org/10.1016/j.nlm.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC. Developmental exposure to methylmercury alters behavioral sensitivity to D-amphetamine and pentobarbital in adult rats. Neurotoxicology and Teratology. 2001;23(1):45–55. doi: 10.1016/s0892-0362(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Gestational methylmercury exposure selectively increases the sensitivity of operant behavior to cocaine. Behavioral Neuroscience. 2009;123(2):408–417. doi: 10.1037/a0014595. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: Effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27(5):721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: Effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012;37(12):2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2, and D4 receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18(1):29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tiernan CT, Edwin Ea, Goudreau JL, Atchison WD, Lookingland KJ. The role of de novo catecholamine synthesis in mediating methylmercury-induced vesicular dopamine release from rat pheochromocytoma (PC12) cells. Toxicological Sciences. 2013;133:125–132. doi: 10.1093/toxsci/kft025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiernan CT, Edwin EA, Hawong HY, Rios-Cabanillas M, Goudreau JL, Atchison WD, Lookingland KJ. Methylmercury impairs canonical dopamine metabolism in rat undifferentiated pheochromocytoma (pc12) cells by indirect inhibition of aldehyde dehydrogenase. Toxicological Sciences. 2015;144(2):347–356. doi: 10.1093/toxsci/kfv001. http://doi.org/10.1093/toxsci/kfv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NL, Barraj L, Smith K, Javier A, Burke TA. Combining food frequency and survey data to quantify long- term dietary exposure: a methyl mercury case study. Risk Analysis. 2004;24(1):19–30. doi: 10.1111/j.0272-4332.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- Wang HS, Xu WF, Chen ZJ, Cheng Z, Ge LC, Man YB, Giesy JP, Du J, Wong CKC, Wong MH. In vitro estimation of exposure of Hong Kong residents to mercury and methylmercury via consumption of market fishes. Journal of Hazardous Materials. 2013;248–249(1):387–393. doi: 10.1016/j.jhazmat.2012.12.060. http://doi.org/10.1016/j.jhazmat.2012.12.060. [DOI] [PubMed] [Google Scholar]
- Wegbreit E, Cushman GK, Weissman AB, Bojanek E, Kim KL, Leibenluft E, Dickstein DP. Reversal-learning deficits in childhood-onset bipolar disorder across the transition from childhood to young adulthood. Journal of Affective Disorders. 2016;203:46–54. doi: 10.1016/j.jad.2016.05.046. http://doi.org/10.1016/j.jad.2016.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RR. How many discoveries have been lost by ignoring modern statistical methods? American Psychologist. 1998;53(3):300–314. http://doi.org/10.1037/0003-066X.53.3.300. [Google Scholar]
- Wilcox RR. Introduction to robust estimation and hypothesis testing. Academic Press; 2012. [Google Scholar]
- Wilcox RR, Keselman HJ. Modern robust data analysis methods: measures of central tendency. Psychological Methods. 2003;8(3):254–274. doi: 10.1037/1082-989X.8.3.254. http://doi.org/10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]