Abstract
Background
The Tokyo guidelines recommend initial cholecystostomy tube drainage, antibiotics, and delayed cholecystectomy in patients with grade III cholecystitis.
Study Design
We used Medicare data (1996–2010) to identify patients ≥66 years admitted with grade III acute cholecystitis. We evaluated adherence to the Tokyo guidelines and compared mortality, readmission, and complication rates with and without cholecystostomy tube placement in a propensity-matched (1:3) cohort of patients with grade III cholecystitis.
Results
8,818 patients were admitted with grade III cholecystitis; 565 patients (6.4%) had a cholecystostomy tube placed. Cholecystostomy tube placement increased from 3.9% to 9.7% over the study period. Compared to 1,689 propensity-matched controls, patients with cholecystostomy tube placement had higher 30-day (HR 1.26, 95% CI 1.05–1.50), 90-day (HR 1.26, 95% CI 1.08–1.46) and 2-year mortality (HR 1.19, 95% CI 1.04–1.36) and were less likely to undergo cholecystectomy in the 2-years after initial hospitalization (33.4% vs. 64.4%, HR 0.26, 95% CI 0.21–0.31). Readmissions were also higher at 30 days (HR 2.93, 95% CI 2.12–4.05), 90 days (HR 3.48, 95% CI 2.60–4.64), and 2 years (HR 3.08, 95% CI 2.87–4.90).
Conclusions
Since introduction of the Tokyo Guidelines (2007), use of cholecystostomy tubes in patients with grade III cholecystitis has increased, but the majority of patients do not get cholecystostomy tube drainage as first line therapy. Cholecystostomy tube placement was associated with lower rates of definitive treatment with cholecystectomy, higher mortality and higher readmission rates. These data suggest a need for further evaluation and refinement of the Tokyo guidelines.
Keywords: Cholecystostomy, Cholecystitis, Cholecystectomy, Tokyo Guidelines, Organ dysfunction
Precis
The Tokyo guidelines recommend initial cholecystostomy tube placement and delayed cholecystectomy in patients with grade III cholecystitis. This was associated with lower rates of definitive treatment with cholecystectomy, and higher mortality and readmission rates. The data suggests a need for further evaluation and refinement of the Tokyo guidelines.
INTRODUCTION
For patients presenting with acute calculous cholecystitis, cholecystectomy is the preferred treatment, but there remains a subset of patients in whom cholecystectomy may be deemed too high risk, due to either the severity of their cholecystitis and/or their underlying acute and chronic medical comorbidities. To guide the management of patients with acute cholecystitis, the Tokyo Guidelines were developed in 2007 (TG07) and refined in 2013 (TG13).1,2 These guidelines developed a consensus methodology for assessing and describing the severity of acute cholecystitis. Patients with grade I cholecystitis have inflammatory changes in the gallbladder and no associated organ dysfunction. Patients with grade II acute cholecystitis have leukocytosis, a palpable tender mass, and/or marked local inflammation, with no associated organ dysfunction. Patients with grade III cholecystitis have associated organ dysfunction including cardiovascular hypotension, neurologic disturbances, respiratory failure, oliguria, hepatic dysfunction, and/or thrombocytopenia.
In the critically ill subset of patients with grade III cholecystitis, the Tokyo guidelines recommend urgent gallbladder drainage with a cholecystostomy tube as initial treatment, followed by antibiotics, and delayed cholecystectomy. However, the Tokyo guidelines base management decisions on the severity of cholecystitis and do not factor in baseline patient comorbidities beyond the acute physiologic changes associated with the disease process. Conversely, U.S. studies evaluating outcomes after cholecystostomy tube placement place more emphasis on patient comorbidities or contraindications to surgery as the indication for cholecystostomy tube placement rather than severity of disease;3–11 these studies do not incorporate the TG13 grading of cholecystitis or address management based on the Tokyo guidelines for cholecystostomy tube placement. In the U.S., significant controversy remains regarding management of these critically ill patients, including the decision to place a cholecystotomy tube as well as the decision for subsequent cholecystectomy.
The goal of our study was to evaluate adherence to the Tokyo guidelines in Medicare patients presenting with grade III cholecystitis and compare outcomes in patients with grade III (severe) acute cholecystitis who did or did not have a cholecystostomy tube placed. Propensity score matching based on comorbidities, organ failure, and other factors were used to create a comparable cohort based on severity of disease and patient comorbidities in order to control for potential selection or indication bias.
METHODS
Data Source
This retrospective cohort study used enrollment and claims data from a 5% national sample of Medicare beneficiaries from 1995 to 2011. Demographic and enrollment data were obtained from the Denominator file. The Medicare Provider Analysis and Review file (MEDPAR) was used to obtain inpatient hospital admission claims. Outpatient claims and claims submitted by non-institutional providers were obtained from the Outpatient Standard Analytic File (OUTSAF) and Carrier Standard Analytic File (SAF).
Cohort Identification
The study cohort included all patients admitted to the hospital for acute gallstone disease between 1996 and 2009. We used International Classification of Disease, 9th Edition, Clinical Modification (ICD-9-CM) codes to identify acute gallstone disease (Table 1). We applied the following inclusion criteria to derive the final cohort: (1) age 66 and older (2) continuously enrolled in Medicare Part A and Part B for at least 12 months before and 24 months after the index hospitalization and (3) patients with a diagnosis of acute cholecystitis and organ dysfunction (grade III cholecystitis). Based on the Tokyo guidelines, patients were classified as having “organ dysfunction” if they had an ICD-9-CM code for one or more of the following diagnoses during the index admission: hypotension, renal failure, respiratory failure, neurological dysfunction, hepatic dysfunction, or hematological dysfunction (Table 1).
Table 1.
The International Classification of Diseases, 9th Edition, Clinical Modification and CPT Codes to Identify Patients, Procedures, and Complications
| Procedure | ICD-9-CM | CPT |
|---|---|---|
| Cholecystostomy tube insertion | 51.0, 51.00, 51.01, 51.03, 51.04 | |
| Initial diagnosis | ||
| Acute calculous cholecystitis | 574.0 (574.00, 574.01), 574.1 (574.10, 574.11), 575.0, 575.1, 575.2, 575.3, 575.4 |
|
| Open cholecystectomy | ||
| Open cholecystectomy | 51.2, 51.22 | 47600 |
| Cholecystectomy with cholangiography | 47605 | |
| Cholecystectomy with exploration of common duct | 47610 | |
| Cholecystectomy with choledochoenterotomy | 47612 | |
| Laparoscopic cholecystectomy | ||
| Laparoscopic cholecystectomy | 51.23 | 47562 |
| Cholecystectomy with cholangiography | 51.21 | 47563 |
| Cholecystectomy with exploration of common duct | 51.24 | 47564 |
| Cholecystoenterostomy | 47570 | |
| Unlisted laparoscopy procedure, biliary tract | 47579 | |
| Complications | ||
| SSI | 998.5, 998.51, 998.59 | |
| UTI | 1122, 590.1, 590.11, 5903, 590.8, 590.81, 595.0, 595.3, 599.0, 99664 |
|
| Pneumonia | 0391, 1124, 1179, 1363, 46619, 480.0, 480.1, 480.2, 480.3, 480.8, 480.9, 481, 481.0, 482.0, 482.1, 482.2, 482.30, 482.31, 482.32, 482.39. 482.40, 482.41, 482.42, 482.49, 482.81, 482.82, 482.83, 482.84, 482.89, 482.9, 483.0, 483.1, 483.8, 484.1, 484.3, 484.5, 484.6, 484.7, 484.8, 487.0, 486, 485, 4841, 4846,4847, 485, 486, 4870, 507*, 5130, 5168, 99731, 99739 |
|
| Sepsis | 038, 038.1, 038.2, 038.3, 038.4, 038.8, 038.9, 78552, 99591, 99592, 9980, 99859, 99931 |
|
| Deep vein thrombosis | 451.1, 451.2, 451.8, 451.9, 453.4, 453.41. 453.42, 453.8, 453.9 |
|
| Pulmonary embolism | 415.1, 415.11, 415.12, 415.19 | |
| Myocardial infarction | 410, 410.0, 410.00, 410.01, 410.02, 410.10, 410.11, 410.12, 410.20, 410.21, 410.22, 410.30, 410.40, 410.41, 410.42, 410.50, 410.51, 410.52, 410.60, 410.61, 410.62, 410.70, 410.71, 410.72, 410.80, 410.82, 410.90. 410.91, 410.92 |
|
| Cardiovascular dysfunction | ||
| Hypotension | 458 | |
| Respiratory dysfunction | ||
| Acute Respiratory failure | 518.8 | |
| Acute on Chronic Respiratory Failure | 518. 83 | |
| Other pulmonary insufficiency (ARDS is coded under here) |
518.82 | |
| Neurological dysfunction | ||
| Altered mental status | 780.0 | |
| Other alternation of consciousness | 780.09 | |
| Renal dysfunction | ||
| Acute kidney failure | 584 | |
| Renal failure, unspecified | 586 | |
| Disorders resulting from impaired renal function | 588 | |
| Chronic kidney disease | 585 | |
| Hepatic dysfunction | ||
| Acute hepatic failure | 570.0 | |
| Chronic liver disease, unspecified | 571.9 | |
| Hematological dysfunction | ||
| Secondary thrombocytopenia | 287.4 | |
| Other secondary thrombocytopenia | 287.49 | |
| Thrombocytopenia, unspecified | 287.5 |
ICD-9-CM, International Classification of Diseases, 9th Edition, Clinical Modification.
Exposure
The primary exposure was cholecystostomy tube placement during the index hospitalization. The ICD-9-CM and Current Procedural Terminology (CPT) codes used to identify tube placement are reported in Table 1.
Outcomes
The following outcomes were compared in this study: (1) mortality (in-hospital, 30-day, 90-day), (2) 2-year survival, (3) gallstone-related readmissions (30-day, 90-day and 2-year), (4) length of stay during the index hospitalization and global hospital stay within 90 days including the index hospitalization, and (5) cholecystectomy during the index hospital hospitalization or anytime in the 2-years following discharge. Cholecystectomy rates and complication rates were identified using ICD-9-CM and CPT codes (Table 1).
Covariates
The following covariates were included in the study: patient’s age at the index hospitalization, race/ethnicity, education, income, comorbidity, diagnosis of sepsis during the index hospitalization, history of prior gallstone-related hospitalization, source of admission (emergency department, referral from another physician, skilled nursing facility, or other), and number of Intensive Care Unit (ICU) days during the index hospitalization. Patient comorbidities were identified in the year prior to the index hospitalization using the Elixhauser comorbidity score and a summary score was constructed.12
Statistical Analysis: Propensity Score Matching
Cohort characteristics were described using the appropriate descriptive statistics (mean + standard deviation, or proportions). Baseline characteristics were compared between patients who had a cholecystostomy tube placed versus patients who did not using the standardized difference. A standardized difference of less than 10% was indicative of a good balance between the study groups.7 The balance of measured covariates in the matched cohort mimics pseudorandomization.
Propensity score analysis was done to account for selection bias in those who underwent cholecystostomy tube placement, as bivariate comparisons between the cholecystostomy tube versus no cholecystostomy tube groups showed significant differences across the covariates listed above (Table 2). In the first step, a patient’s propensity for receiving a cholecystostomy tube was calculated for each patient using a logistic regression model. In this model, the probability of cholecystostomy tube placement was estimated by using the aforementioned set of covariates. We then performed Greedy 1:3 matching to match those who underwent cholecystostomy tube placement with those who did not. In the propensity score matched cohort, we checked the balance of covariates using standardized differences.13
Table 2.
Balance of Covariates in the Original Cohort and Propensity Score Matched Cohort
| Original cohort | Propensity score matched cohort (1:3 matching) | |||||
|---|---|---|---|---|---|---|
| Cholecystostomy tube placement, n (%) |
No cholecystostomy tube placement, n (%) |
Standardized difference |
Cholecystostomy tube placement, n (%) |
No cholecystostomy tube placement, n (%) |
Standardized difference |
|
| n | 565 | 8,253 | 563 | 1,689 | ||
| Age, y, mean (SD) | 80.1 (7.8) | 78.3 (7.3) | 0.24 | 80.1(7.8) | 80.3(7.4) | −0.02 |
| Sex | 0.24 | −0.02 | ||||
| Male | 333 (58.9) | 4,195 (50.8) | 331 (58.8) | 982 (58.2) | ||
| Female | 232 (41.1) | 4,058 (49.2) | 232 (41.2) | 707 (41.9) | ||
| Race | 0.03 | 0.03 | ||||
| White | 480 (85.0) | 7,078 (85.8) | 480 (85.3) | 1,443 (85.4) | ||
| Black | 56 (9.9) | 788 (10.0) | 56 (10.0) | 175 (10.3) | ||
| Hispanic | 15 (2.7) | 181 (2.2) | 14 (2.5) | 43 (2.6) | ||
| Other | 14 (2.5) | 206 (2.5) | 13 (2.3) | 28 (1.7) | ||
| Education, quartile* | 0.19 | 0.04 | ||||
| 1-Lowest | 179 (31.7) | 2,056 (24.9) | 179 (31.8) | 514 (30.4) | ||
| 2 | 149 (26.4) | 2,117 (25.7) | 147 (26.1) | 452 (26.8) | ||
| 3 | 133 (23.5) | 2,054 (24.9) | 133 (23.6) | 420 (24.9) | ||
| 4-Highest | 104 (24.0) | 2,010 (24.3) | 104 (18.5) | 303 (17.9) | ||
| Income, quartile* | 0.22 | 0.03 | ||||
| 1-Lowest | 99 (17.5) | 1,964 (23.8) | 99 (17.6) | 295 (17.5) | ||
| 2 | 118 (20.9) | 1,949 (23.6) | 118 (21.0) | 365 (21.6) | ||
| 3 | 137 (24.3) | 2,033(24.6) | 137 (24.3) | 390 (23.1) | ||
| 4-Highest | 195 (34.5) | 2,077 (25.2) | 193 (34.3) | 588 (34.8) | ||
| Summary Elixhauser comorbidity score |
8.3(8.5) | 6.6(7.6) | 0.21 | 8.3(8.4) | 8.1(8.3) | 0.02 |
| Elixhauser comorbidity | 0.16 | 0.07 | ||||
| 0 | 43 (7.6) | 905 (11.0) | 43 (7.6) | 155 (9.2) | ||
| 1 | 64 (11.3) | 1,121 (13.6) | 64 (11.4) | 171 (10.1) | ||
| 2 | 81 (14.3) | 1,317 (16.0) | 81 (14.5) | 260 (15.4) | ||
| ≥3 | 377 (66.7) | 4,910 (59.4) | 375 (66.6) | 1,103 (65.3) | ||
| Sepsis at diagnosis | −0.12 | −0.05 | ||||
| Yes | 227 (40.2) | 1,532 (18.6) | 225 (40.0) | 672 (39.8) | ||
| No | 338 (59.8) | 6,721 (81.2) | 338 (60.0) | 1,017 (60.2) | ||
| Prior hospitalization | −0.12 | −0.05 | ||||
| Yes | 120 (21.2) | 1,307 (15.8) | 119 (21.1) | 361 (21.4) | ||
| No | 445 (78.8) | 6,946 (84.2) | 444 (78.9) | 1,328 (78.6) | ||
| Source of admission | 0.37 | 0.02 | ||||
| Emergency | 292 (51.7) | 4,197 (50.8) | 292 (51.9) | 878 (52.0) | ||
| Referral | 103 (18.2) | 2,578 (31.2) | 103 (18.3) | 319 (18.9) | ||
| SNF | 132 (23.4) | 1,181 (14.3) | 130 (23.1) | 387 (22.9) | ||
| Other | 38 (6.7) | 297 (3.6) | 38 (6.7) | 105 (6.2) | ||
| ICU Stay, d, mean (SD) | 5.3 (7.6) | 4.8 (8.7) | 0.12 | 5. 3(7.6) | 5. 4(9.6) | 0.06 |
Quartile has missing values (not presented in the table).
In the matched sample, we did bivariate analysis comparing the outcomes in the two groups. We used Kaplan-Meier curves for 2-year survival and 2-year cumulative incidence curves for receipt of cholecystectomy to compare these outcomes between the cholecystostomy and no cholecystostomy groups.
Conditional logistic regression models were used to determine the association between cholecystostomy tube placement and 30-day mortality. Cox regression models were used to determine the association between cholecystostomy tube placement and receipt of cholecystectomy at 2 years, 90-day and 2-year mortality, and readmissions at all time points. When evaluating the cumulative incidence of cholecystectomy, we censored patients who died, as they were no longer at risk for undergoing a cholecystectomy. For readmissions, patients were censored if they died and were no longer at risk for readmission. Linear regression was used for length of stay and logistic regression for dichotmous, non-time dependent outcomes including cholecystectomy during index admission, and in-hospital mortality.
All statistical analyses were conducted using SAS 9.4. Statistical significance was accepted at the p <0.05 level.
RESULTS
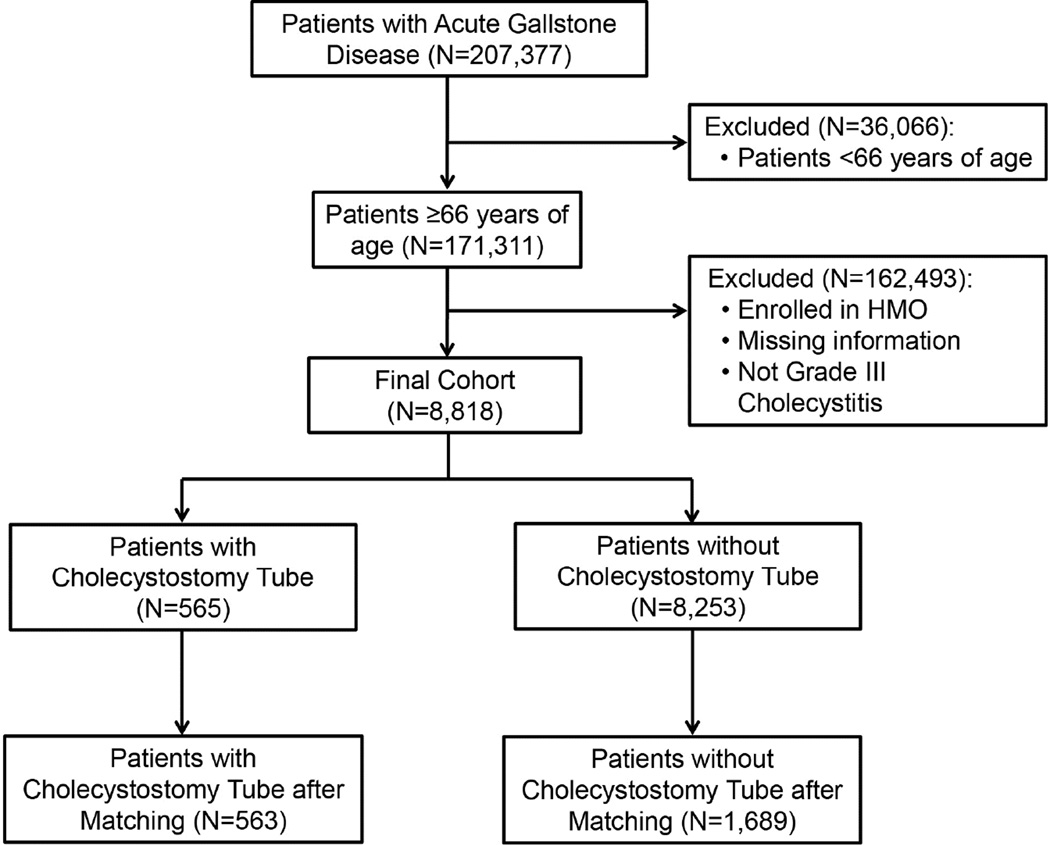
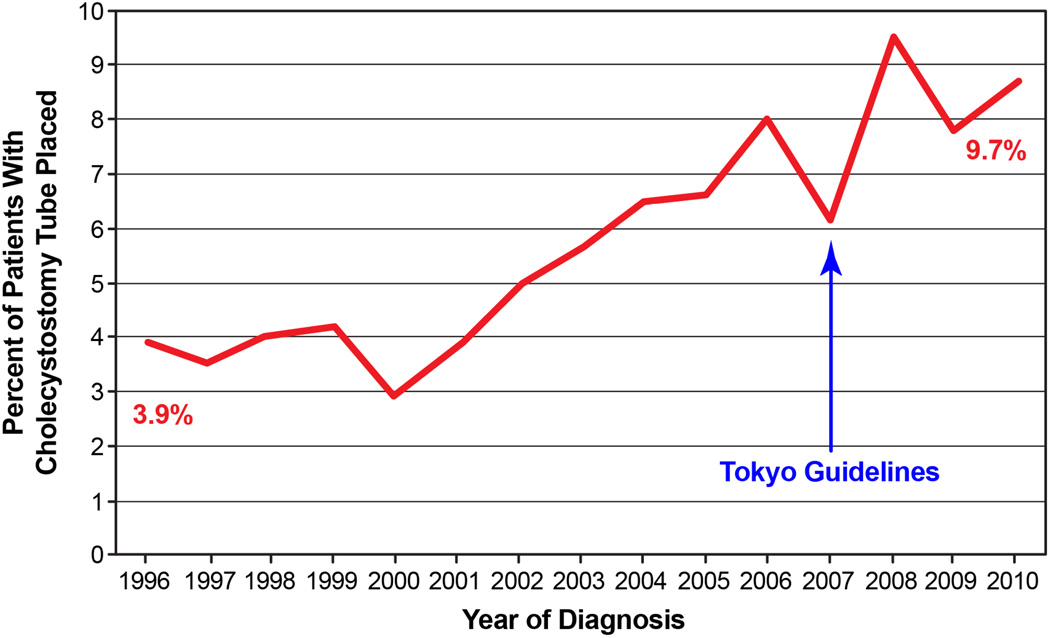
The cohort derivation is summarized in Figure 1. We identified 8,818 elderly patients hospitalized for grade III cholecystitis. 565 (6.4%) patients had placement of a cholecystostomy tube on their index hospitalization. The incidence of tube placement increased from 3.9% to 9.7% in 2010 (Figure 2). Several differences were observed in both groups before propensity score matching (Table 2). Patients undergoing cholecystostomy tube placement were more likely to be older, with lower education, lower income, and greater number of comorbidities. They were also more likely to have sepsis and be admitted through the emergency room or from a skilled nursing facility. The mean number of days in the ICU was greater in those with a cholecystostomy tube (5.3 ± 7.6 days) compared to those without (4.8 ± 8.7 days).
Figure 1.
Cohort selection diagram for patients diagnosed with acute gallstone disease between 1996 and 2011.
Figure 2.
Incidence of cholecystostomy tube use in older patients (1996 to 2011) increased from 3.9% to 9.7% in 2010.
In the propensity score model, we were able to successfully match 563 of the 565 patients in the cholecystostomy tube group to similar controls from the 8,253 patients in the no cholecystostomy tube group (N=1,689). Table 2 reports the baseline characteristics of patients before and after propensity score matching. After propensity score matching, all covariates were balanced with the greatest standardized difference being 7% for Elixhauser comorbidity score.
Given both groups were well balanced after propensity score matching, we did not control for covariates in the conditional regression models. Table 3 reports the proportion of patients who experienced outcomes and odds ratios, hazard ratios, or beta coefficients as appropriate for the matched cohort (eTable 1 reports the same outcomes in the unmatched cohort). The in-hospital mortality was similar between the two groups (24.0% in the cholecystostomy group vs. 22.6% in the control group; OR 1.08, 95% CI 0.86–1.35). However, the odds of 30-day and 90-day mortality were significantly higher in patients who underwent cholecystostomy tube placement compared to patients who did not (Table 3). 30-day mortality was 38.9% in those who underwent tube placement versus 32.7% in those who did not undergo tube placement (HR 1.26, 95% CI 1.05–1.50); 90-day mortality was 46.7% versus 39.6% (HR 1.26, 95% CI 1.08–1.46).
Table 3.
Comparison of Outcomes in Propensity Score Matched Cohort
| Outcomes | Cholecystostomy tube placement |
No cholecystostomy tube placement |
Odds ratio, hazard ratio, or beta coefficient |
|---|---|---|---|
| Mortality, n (%) | |||
| In-hospital | 135 (24.0) | 382 (22.6) | OR 1.08 (0.86–1.35) |
| 30-d | 219 (38.9) | 553 (32.7) | HR 1.26(1.05–1.50)* |
| 90-d | 263 (46.7) | 668 (39.6) | HR 1.26(1.08–1.46)* |
| 2-y | 365 (64.8) | 998 (59.1) | HR 1.19(1.04–1.36)* |
| Readmission, n (%) | |||
| 30 d | 82 (14.6) | 89 (5.3) | HR 2.93(2.12–4.05)* |
| 90 d | 121 (21.5) | 115 (6.8) | HR 3.48(2.60–4.64)* |
| Within 2 y | 158 (28.1) | 154 (9.1) | HR 3.08(2.87–4.90)* |
| Length of stay, median (q1,q3) | |||
| During index hospital admission | 13 (6, 16) | 10 (4, 12) | Beta 0.91 (0.63)† |
| During 90-d period | 15 (7, 20) | 11 (4, 13) | Beta 2.56 (1.29)† |
| Cholecystectomy, n (%) | |||
| On index admission | 63 (11.2) | 995 (58.9) | OR 0.08 (0.06–0.11) |
| Any time during 2 y | 188 (33.4) | 1,091 (64.6) | HR 0.26(0.21–0.31)* |
| In-hospital occurrences, n (%) | |||
| Surgical site infection | 34 (6.4) | 66 (3.9) | OR 1.57 (1.03–2.39) |
| Urinary tract infection | 192 (34.1) | 608 (36.0) | OR 0.92 (0.75–1.12) |
| Pneumonia | 193 (34.3) | 590 (34.9) | OR 0.97 (0.89–1.19) |
| Deep vein thrombosis | 47 (8.4) | 135 (8.0) | OR 1.05 (0.74–1.50) |
| Embolism | 26 (4.6) | 66 (3.9) | OR 1.20 (0.75–1.92) |
| MI | 76 (13.5) | 175 (10.4) | OR 1.35 (1.02–1.81) |
Hazard ratio derived from Cox Proportional Hazard models.
Point estimation or the difference of the pooled mean.
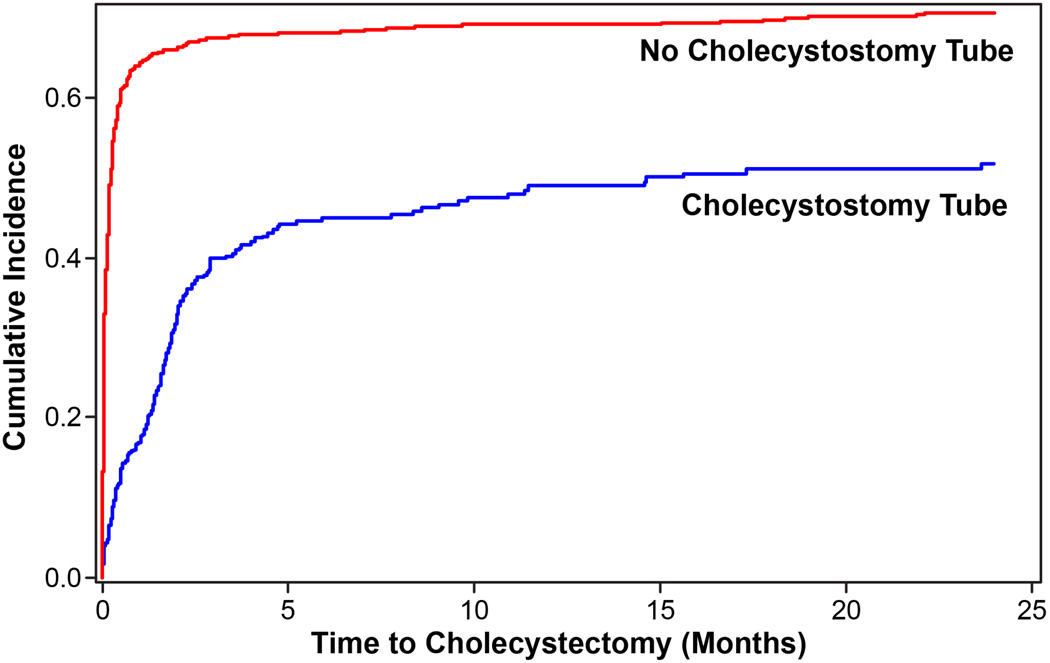
Patients with cholecystostomy tube placement were less likely to undergo cholecystectomy during the index admission (11.2% vs. 58.9%; OR 0.08, 95% CI 0.06–0.11), or anytime during the 2-year study period, including the index admission (33.4% vs. 64.6%; HR 0.26, 95% CI 0.21–0.31). The median time to cholecystectomy was also significantly longer in those who had a cholecystostomy tube compared to those without (4.6 months vs. 0.23 months; p<0.0001; Figure 3).
Figure 3.
Rates of cholecystectomy in patients who underwent cholecystostomy tube placement vs those who did not. Median time to cholecystectomy was significantly longer in those who had a tube placed vs those who did not (4.6 months vs 0.23 months, p<0.0001).
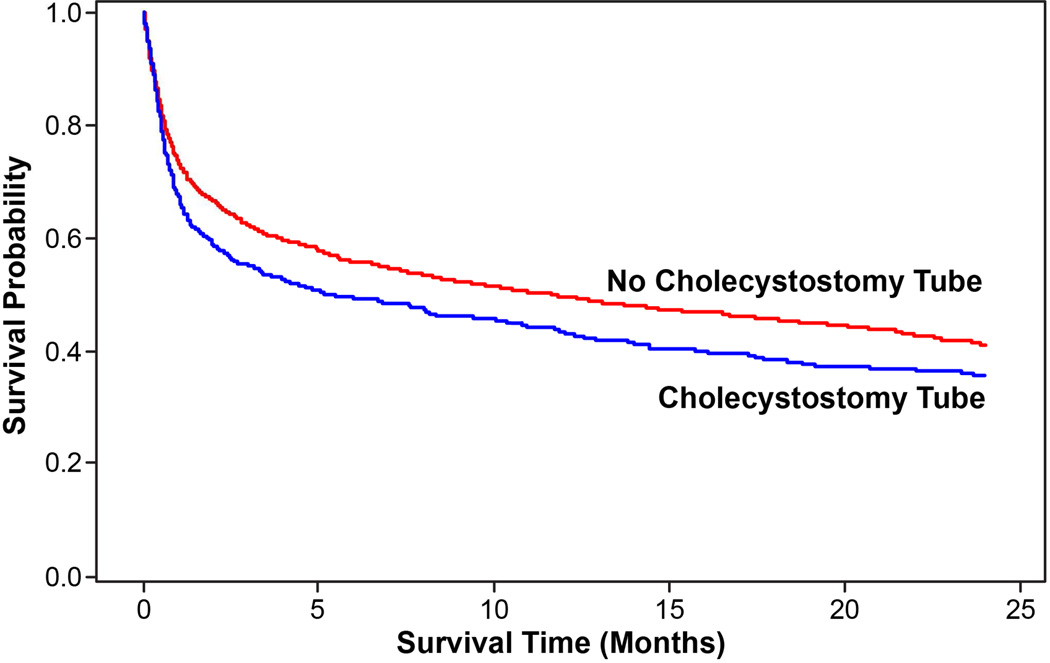
The median length of stay during the index hospitalization (Beta 0.91; 13 vs. 10 days, p<0.001) and global 90-day length of stay (including index hospitalization, Beta 2.56; 15 vs. 11 days, p<0.001) were significantly higher in patients with tube placement compared to patients without. Readmission at 30 days (14.6% vs. 5.3%; HR 2.93, 95% CI 2.12–4.05), 90 days (21.5% vs. 6.8%; HR 3.48, 95% CI 2.60–4.64), and 2 years (28.1% vs. 9.1%; HR 3.08, 95% CI 2.87–4.90) were significantly higher in patients with a cholecystostomy tube compared to those without a cholecystostomy tube. The 2-year survival was significantly lower in patients who underwent tube placement (35.7% with a cholecystostomy tube vs. 41.0% without; HR 1.19, 95% CI 1.04–1.36; Figure 4).
Figure 4.
Two-year survival rates in patients who underwent cholecystostomy tube placement were significantly shorter compared to those who did not (35% vs 41%, p<0.0059).
DISCUSSION
The management of severely ill patients with acute gallbladder disease represents a difficult clinical challenge for physicians. Per the Tokyo guidelines (TG13),2,14 patients with grade III cholecystitis should undergo urgent management of organ dysfunction and management of the severe local inflammation by means of percutaneous cholecystostomy tube drainage, with optimal medical treatment; the TG13 guidelines recommend delayed elective cholecystectomy when cholecystectomy is indicated.
Despite these guidelines, our data demonstrate that greater than 90% of Medicare patients with grade III cholecystitis do not get cholecystostomy tube drainage as a first line therapy. We were able to propensity score match each patient with a cholecystostomy tube to three controls, further reinforcing the fact that use of a cholecystostomy tube is not an automatic first line therapy in the U.S. for grade III cholecystitis. Additionally, only one-third undergo definitive treatment with cholecystectomy in the two years after the initial episode.
In this propensity-matched cohort of patients with grade III cholecystitis, our data suggest that cholecystostomy tube placement is associated with worse short and long-term (2-year) outcomes, with higher 30- and 90-day mortality, lengths of hospital stay, complications and readmission rates. Many prior studies demonstrate that cholecystostomy tube placement is a safe alternative to cholecystectomy for patients with acute gallbladder disease, but primarily report this intervention as “safe” and “feasible.”4–6,8,10,11,15–19 Yet, simply reporting feasibility of an intervention does not translate to overall outcomes in these patients and still may have severe consequences including mortality.
Mortality rates following cholecystostomy tube placement widely vary and are primarily from small, retrospective studies. In-hospital mortality has been reported anywhere from 4% to 17%,20–25 whereas 30-day mortality rates are just as broad from 7% to 26%.19,21,26–28 However, these studies do not address mortality from grade III cholecystitis or compare outcomes to those without a tube. One randomized controlled trial by Hatzidakis and colleagues29 did compare cholecystostomy tube placement versus conservative management in 123 patients with calculous or acalculous cholecystitis deemed too high risk for surgery. There was no significant difference in 30-day mortality between those who underwent tube placement versus those who did not (18% vs. 17%). Our in-hospital and 30-day mortality rates were higher, exceeding 24%, but we only included patients with organ failure and true grade III cholecystitis according to TG13.
Only one retrospective study reported long-term mortality of 71 patients with a median follow-up of 37 months and an overall mortality rate of 32% during that time period,24 which is consistent with our overall 2-year survival rate of 35% in the cholecystostomy group and 41% in the no cholecystostomy group. The poor long-term survival data reflect the underlying severity of comorbid illness. To our knowledge, our study is the first to compare longer-term outcomes between patients with grade III cholecystitis who did or did not undergo cholecystostomy tube placement.
The only definitive treatment for calculous cholecystitis is cholecystectomy. The TG13 guidelines recommend delayed elective cholecystectomy when cholecystectomy is indicated, but do not clearly define indications or optimal timing.14 This uncertainty is reflected in our study results; only one-third of patients undergo cholecystectomy in the two years after the initial episode. The lack of definitive treatment in these patients is likely related to patient factors that influenced the original decision to forego cholecystectomy. It may also represent hesitancy towards performing a cholecystectomy in patients once they have undergone tube placement, which can be difficult and often requires conversion to an open procedure.
Our study and others also definitively show that, without cholecystectomy, disease recurrence and readmission rates associated with cholecystostomy tube placement are far greater.10,15,20,21,30 The >20% gallstone-related readmission demonstrates that recurrence of disease and/or problems with the cholecystostomy tubes are high without definitive treatment (cholecystectomy). Our data are consistent with previous studies report recurrence of cholecystitis in 11% to 41% of patients who undergo tube placement10,15,20,21,30 and readmission rates ranging from 23% to 41%.21,31 Readmission rates for patients without a cholecystostomy tube were only 9%, likely due to the higher rate of cholecystectomy in this cohort. In addition to cost to the healthcare system, the cost to the patient in the long-term when considering hospitalstay, repeated procedures, and discomfort should be considered.
Underlying patient comorbidities can directly affect a patient’s physiologic ability to withstand cholecystectomy and the insult of severe acute cholecystitis may significantly increase surgical morbidity and mortality. However, many patients with chronic disease will experience significant physiologic improvement after source control with a cholecystostomy tube; our data suggest that attempts to optimize a patient’s physiologic status and perform cholecystectomy, either during initial admission or in the weeks after tube placement, are indicated.
There are several limitations to our study. We cannot determine if an operation on initial admission was contraindicated because of the severity of their gallbladder disease, overall medical condition, failure of treatment with antibiotics alone, or a combination of these factors. While we were able to determine the number of ICU days, we cannot determine when patients were admitted to the ICU (before or after surgery, etc.). Coding only reports the total number of ICU days and does not account for the escalation of care in patients with worsening medical conditions, cholecystitis, or post-procedural complications. However, the total number of ICU days provides information regarding the critical nature of this patient population and the acuity of care required.
While we can measure gallstone-related hospitalizations, we cannot definitively deduce whether this is recurrence of disease or issues with the tube itself. Nonetheless, patients who had a cholecystostomy tube had significantly higher readmission rates compared to those without a tube. Also, we did not attempt to evaluate overall healthcare utilization including outpatient radiology studies, interventional radiology procedures, gastroenterologic interventions, and outpatient visits.
Lastly, claims data cannot accurately capture information regarding antibiotic administration, duration of symptoms, and tube management/removal. Identification of antibiotics is understandably an important point given patients without tube placement had improved outcomes and if only receiving antibiotics, it would aid in better defining utilization of cholecystostomy tube placement in this subset of patients. Yet, given the severity of disease in these patients, it is reasonable to assume that the majority of these patients received antibiotics at some point during their treatment.
CONCLUSIONS
Current practice patterns in the U.S. do not reflect the Tokyo guidelines; cholecystostomy tube placement in older patients is not routinely performed even in critically ill patients with severe grade III cholecystitis. Our data demonstrate worse outcomes with cholecystostomy tube placement in a matched cohort of patients with grade III disease, with increased mortality, survival, length of stay, complications, and readmissions after cholecystostomy tube placement. As current practice patterns in the U.S. do not reflect the Tokyo guidelines and demonstrate worse outcomes with cholecystostomy tube placement, re-evaluation and revision of the Tokyo guidelines is indicated. Clarification of the role of cholecystostomy tube placement in patients with grade III cholecystitis and identification of the subset of patients who would most benefit from tube placement is essential.
Supplementary Material
Acknowledgments
Support: This work was supported by the University of Texas Medical Branch Clinical and Translational Science Award #UL1TR000071; the NIH T-32 Grant # T32DK007639; and the AHRQ Grant # 1R24HS022134
ABBREVIATIONS
- CPT
Current Procedural Terminology
- ICD-9-CM
International Classification of Disease, 9th Edition, Clinical Modification
- ICU
Intensive Care Unit
- MEDPAR
Medicare Provider Analysis and Review
- OUTSAF
Outpatient Standard Analytic File
- SAF
Carrier Standard Analytic File
- TG07
Tokyo Guidelines 2007
- TG13
Tokyo Guidelines 2013
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 128th Annual Meeting, Palm Beach, FL, December 2016.
REFERENCES
- 1.Mayumi T, Takada T, Kawarada Y, et al. Results of the Tokyo Consensus Meeting Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:114–121. doi: 10.1007/s00534-006-1163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokoe M, Takada T, Strasberg SM, et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:35–46. doi: 10.1007/s00534-012-0568-9. [DOI] [PubMed] [Google Scholar]
- 3.Bala M, Mizrahi I, Mazeh H, et al. Percutaneous cholecystostomy is safe and effective option for acute calculous cholecystitis in select group of high-risk patients. Eur J Trauma Emerg Surg. 2016;42:761–766. doi: 10.1007/s00068-015-0601-1. [DOI] [PubMed] [Google Scholar]
- 4.Byrne MF, Suhocki P, Mitchell RM, et al. Percutaneous cholecystostomy in patients with acute cholecystitis: experience of 45 patients at a US referral center. J Am Coll Surg. 2003;197:206–211. doi: 10.1016/S1072-7515(03)00143-1. [DOI] [PubMed] [Google Scholar]
- 5.Başaran O, Yavuzer N, Selçuk H, et al. Ultrasound-guided percutaneous cholecystostomy for acute cholecystitis in critically ill patients: one center’s experience. Turk J Gastroenterol. 2005;16:134–137. [PubMed] [Google Scholar]
- 6.Atar E, Bachar GN, Berlin S, et al. Percutaneous cholecystostomy in critically ill patients with acute cholecystitis: complications and late outcome. Clin Radiol. 2014;69:e247–e252. doi: 10.1016/j.crad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Cha BH, Song HH, Kim YN, et al. Percutaneous cholecystostomy is appropriate as definitive treatment for acute cholecystitis in critically ill patients: a single center, cross-sectional study. Korean J Gastroenterol. 2014;63:32–38. doi: 10.4166/kjg.2014.63.1.32. [DOI] [PubMed] [Google Scholar]
- 8.Davis CA, Landercasper J, Gundersen LH, Lambert PJ. Effective use of percutaneous cholecystostomy in high-risk surgical patients: techniques, tube management, and results. Arch Surg. 1999;134:727–731. doi: 10.1001/archsurg.134.7.727. discussion 731–732. [DOI] [PubMed] [Google Scholar]
- 9.Spira RM, Nissan A, Zamir O, et al. Percutaneous transhepatic cholecystostomy and delayed laparoscopic cholecystectomy in critically ill patients with acute calculus cholecystitis. Am J Surg. 2002;183:62–66. doi: 10.1016/s0002-9610(01)00849-2. [DOI] [PubMed] [Google Scholar]
- 10.Chang YR, Ahn YJ, Jang JY, et al. Percutaneous cholecystostomy for acute cholecystitis in patients with high comorbidity and re-evaluation of treatment efficacy. Surgery. 2014;155:615–622. doi: 10.1016/j.surg.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Al-Jundi W, Cannon T, Antakia R, et al. Percutaneous cholecystostomy as an alternative to cholecystectomy in high risk patients with biliary sepsis: a district general hospital experience. Ann R Coll Surg Engl. 2012;94:99–101. doi: 10.1308/003588412X13171221501302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta HB, Sura SD, Sharma M, et al. Comparative performance of diagnosis-based and prescription-based comorbidity scores to predict health-related quality of life. Med Care. 2016;54:519–527. doi: 10.1097/MLR.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 13.Parsons L. Performing a 1:N Case-Control Match on Propensity Score. Canada: Montreal; 2004. [Google Scholar]
- 14.Yamashita Y, Takada T, Strasberg SM, et al. TG13 surgical management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:89–96. doi: 10.1007/s00534-012-0567-x. [DOI] [PubMed] [Google Scholar]
- 15.Jang WS, Lim JU, Joo KR, et al. Outcome of conservative percutaneous cholecystostomy in high-risk patients with acute cholecystitis and risk factors leading to surgery. Surg Endosc. 2015;29:2359–2364. doi: 10.1007/s00464-014-3961-4. [DOI] [PubMed] [Google Scholar]
- 16.Berber E, Engle KL, String A, et al. Selective use of tube cholecystostomy with interval laparoscopic cholecystectomy in acute cholecystitis. Arch Surg. 2000;135:341–346. doi: 10.1001/archsurg.135.3.341. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Li N, Ji W, et al. Percutaneous cholecystostomy is a definitive treatment for acute cholecystitis in elderly high-risk patients. Am Surg. 2013;79:524–527. doi: 10.1177/000313481307900529. [DOI] [PubMed] [Google Scholar]
- 18.Ha JP, Tsui KK, Tang CN, et al. Cholecystectomy or not after percutaneous cholecystostomy for acute calculous cholecystitis in high-risk patients. Hepatogastroenterology. 2008;55:1497–1502. [PubMed] [Google Scholar]
- 19.Joseph T, Unver K, Hwang GL, et al. Percutaneous cholecystostomy for acute cholecystitis: ten-year experience. J Vasc Interv Radiol. 2012;23:83–88. doi: 10.1016/j.jvir.2011.09.030. e.1. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh Y-C, Chen C-K, Su C-W, et al. Outcome after percutaneous cholecystostomy for acute cholecystitis: a single-center experience. J Gastrointest Surg. 2012;16:1860–1868. doi: 10.1007/s11605-012-1965-8. [DOI] [PubMed] [Google Scholar]
- 21.McKay A, Abulfaraj M, Lipschitz J. Short- and long-term outcomes following percutaneous cholecystostomy for acute cholecystitis in high-risk patients. Surg Endosc. 2012;26:1343–1351. doi: 10.1007/s00464-011-2035-0. [DOI] [PubMed] [Google Scholar]
- 22.Paran H, Zissin R, Rosenberg E, et al. Prospective evaluation of patients with acute cholecystitis treated with percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Int J Surg. 2006;4:101–105. doi: 10.1016/j.ijsu.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Patel M, Miedema BW, James MA, Marshall JB. Percutaneous cholecystostomy is an effective treatment for high-risk patients with acute cholecystitis. Am Surg. 2000;66:33–37. [PubMed] [Google Scholar]
- 24.Pang KW, Tan CH, Loh S, et al. Outcomes of percutaneous cholecystostomy for acute cholecystitis. World J Surg. 2016;40:2735–2744. doi: 10.1007/s00268-016-3585-z. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Bower M, Cassaro S, et al. Tube cholecystostomy before cholecystectomy for the treatment of acute cholecystitis. JSLS. 2015;19 doi: 10.4293/JSLS.2014.00200. e2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang WS, Lim JU, Joo KR, et al. Outcome of conservative percutaneous cholecystostomy in high-risk patients with acute cholecystitis and risk factors leading to surgery. Surg Endosc. 2015;29:2359–2364. doi: 10.1007/s00464-014-3961-4. [DOI] [PubMed] [Google Scholar]
- 27.Nikfarjam M, Shen L, Fink MA, et al. Percutaneous cholecystostomy for treatment of acute cholecystitis in the era of early laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2013;23:474–480. doi: 10.1097/SLE.0b013e318290142d. [DOI] [PubMed] [Google Scholar]
- 28.Saeed SA, Masroor I. Percutaneous cholecystostomy (PC) in the management of acute cholecystitis in high risk patients. J Coll Physicians Surg Pak. 2010;20:612–615. [PubMed] [Google Scholar]
- 29.Hatzidakis AA, Prassopoulos P, Petinarakis I, et al. Acute cholecystitis in high-risk patients: percutaneous cholecystostomy vs conservative treatment. Eur Radiol. 2002;12:1778–1784. doi: 10.1007/s00330-001-1247-4. [DOI] [PubMed] [Google Scholar]
- 30.Abi-Haidar Y, Sanchez V, Williams SA, Itani KM. Revisiting percutaneous cholecystostomy for acute cholecystitis based on a 10-year experience. Arch Surg. 2012;147:416–422. doi: 10.1001/archsurg.2012.135. [DOI] [PubMed] [Google Scholar]
- 31.Horn T, Christensen SD, Kirkegard J, et al. Percutaneous cholecystostomy is an effective treatment option for acute calculous cholecystitis: a 10-year experience. HPB (Oxford) 2015;17:326–331. doi: 10.1111/hpb.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.