Abstract
Objective
To identify a genetic basis for markedly reduced bone density and multiple fractures in an adult patient with hypophosphatemia and hypercalciuria.
Subjects
A 54-year-old Vietnamese man, his unaffected two daughters and wife.
Methods
We performed biochemical studies and sequenced the SLC34A3 gene using genomic DNA from peripheral blood mononuclear cells.
Results
Biochemical evaluation of the proband revealed hypophosphatemia with increased renal phosphate wasting, hypercalciuria, low serum parathyroid hormone (PTH) and an elevated serum 1,25(OH)2D level. Mutation analysis of SLC34A3 gene revealed that the patient was a compound heterozygote for two nonsynonymous nucleotide substitutions: a novel c.571G>A (p.G191S) damaging mutation and the previously reported c.200G>A (p.R67H) polymorphism, consistent with the clinical diagnosis of late-onset hereditary hypophosphatemic rickets with hypercalciuria (HHRH). His wife and older daughter both carried the p.R67H polymorphism, while his younger daughter was compound heterozygous for p.R67H and p.G191S.
Conclusions
HHRH is an uncommon autosomal recessive disease that generally manifests in childhood as rickets or nephrolithiasis, but an adult onset phenotype may occur in heterozygous carriers of SLC34A3 mutations. The severe presentation of this proband in adulthood with marked nephrolithiasis, multiple fractures and low bone density emphasizes the importance of measuring the serum phosphorus level in patients with suspected but unexplained osteoporosis and/or recurrent renal stones. The recognition of late-onset HHRH facilitates timely institution of appropriate therapy.
Keywords: HHRH, SLC34A3, hypophosphatemia, nephrocalcinosis, osteoporosis, osteomalacia
INTRODUCTION
The evaluation of an adult male patient with osteoporosis focuses on specific risk factors that include hypogonadism, glucocorticoid steroid excess, and alcoholism, as well as primary hyperparathyroidism, malignancy and vitamin D insufficiency. Hypercalciuria is another risk factor for osteoporosis [1] that is often overlooked despite its known association with low bone density as well as nephrolithiasis and renal calcifications. Although the basis for excessive urinary excretion of calcium remains unknown in most patients, recent genetic studies have identified an expanding list of monogenic causes of so-called “idiopathic hypercalciuria”: (i.e., CLCN5, CASR, CLDN16, CLDN19, ADCY10, SLC9A3R1, GLUT2, HSPG2, FN1, CYP24A1, SLC34A1 and SLC34A3) [2–4]. Mutations in some of these genes (e.g. SLC34A1 and SLC34A3) lead to hypercalciuria indirectly through pathophysiological consequences of defective renal tubular reabsorption of phosphate, and result in a phenotype characterized by hypophosphatemia, elevated levels of 1,25(OH)2D, impaired growth with rickets and/or osteomalacia, and rarely, adult osteoporosis [4–6]. In particular, biallelic mutations in SLC34A3 encoding the renal sodium-dependent phosphate cotransporter 2c (NPT2c) represent an important cause of hypercalciuria and metabolic bone disease, and are associated with an uncommon autosomal recessive disorder termed hereditary hypophosphatemic rickets with hypercalciuria (HHRH) [7–9]. In HHRH, renal phosphate wasting results in hypophosphatemia with suppressed levels of FGF23, and the consequent elevation in serum levels of 1,25(OH)2D levels leads to absorptive hypercalciuria [10]. Patients who are heterozygous carriers of SLC34A3 mutations can show less severe biochemical features and may be indistinguishable from patients with idiopathic hypercalciuria (IH), including osteopenia [8, 9, 11]. Although patients with HHRH typically present in childhood with rickets and/or nephrolithiasis, occasional patients can present as adults with low bone density [4]. Here we describe an adult patient referred to us for evaluation of non-healing rib and sacral fractures, severe osteoporosis and recurrent bilateral renal calculi. Genetic analysis indicated he was compound heterozygous for two nonsynonymous nucleotide substitutions, a novel c.571G>A (p.G191S) damaging mutation and the previously reported c.200G>A (p.R67H) polymorphism, consistent with SLC34A3 haploinsufficiency and the diagnosis of late-onset HHRH manifesting as markedly reduced bone density and multiple fractures.
Methods and Case Report
The protocol was approved by the appropriate institutional review boards, and informed consent was obtained from the proband and relatives. The patient diagnosis and disease course together with sequencing and biochemical analyses are described below.
Case Report
A 54-year-old Vietnamese man with past medical history of hepatitis B was referred for evaluation of osteoporosis and multiple rib and sacral fractures (Figure 1). A prior bone densitometry (May 2012) by dual-energy X-ray absorptiometry (DXA) (Table 1) had shown T-scores at the lumbar spine of −2.8 and at the left total hip −1.1, and he had been begun on treatment with alendronate 10 mg/d. The patient continued to experience significant bone pain and he was referred for evaluation in September 2013. There was no history of bone deformities or bone pain during childhood, and no family history of a metabolic bone disorder. He had a history of recurrent, bilateral renal stones that were discovered incidentally in adulthood. The patient denied any history of glucocorticoid use or excess alcohol usage, but he was chronic smoker, and had smoked 1 pack of cigarettes per day for 30 years. He had a normal diet but took no vitamin D or calcium supplements. He had 8 brothers and sisters, 6 deceased and 2 alive, and two daughters, each of whom lacked a clinical history of bone deformity or renal stones and had normal studies of mineral metabolism (Table 2). The patient was of normal stature (165 cm) and weight (60 kg). Physical examination revealed tenderness on palpation over the sixth and seventh ribs bilaterally and lumbosacral tenderness. No skeletal deformities were noted on exam. He had normal dentition.
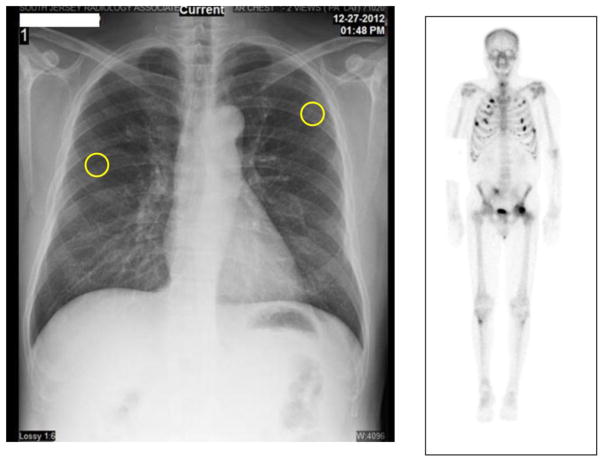
Figure 1. Imaging Studies.
Chest radiograph (left) shows fractures of sixth and seventh ribs bilaterally. Technetium bone scan (right) shows multiple foci of increased uptake consistent with rib fractures and pelvic stress fractures.
Table 1.
Bone Densitometry
| Scan Date | Age | BMD L1–2* (g/cm2) | T-score L1–2 Spine Total | BMD L1–2 Change | BMD Total hip (g/cm2) | T-score Total Hip | BMD Total hip Change |
|---|---|---|---|---|---|---|---|
| 05/30/2012 Pretreatment | 53 | 0.747 | −2.8 | 0.870 | −1.1 | ||
| 06/07/2014 Posttreatment | 55 | 0.911 | −1.3 | 22.0% | 1.106 | 0.5 | 27.2% |
Bone sclerosis in L3 and L4 prevented use of L3–4 in the analysis.
Table 2.
Biochemical Analyses
| Proband I-1 | Proband I-1 | I-2 | II-1 | II-2 | |
|---|---|---|---|---|---|
| Feature | At presentation | After phosphate treatment | Baseline | Baseline | Baseline |
| Age, years | 54 | 55 | 48 | 21 | 19 |
| Calcium (8.5 to 10.5 mg/dL) | 8.9 | 9.3 | 9.3 | 9.1 | 9.9 |
| Phosphorus (2.5 – 4.5 mg/dL) | 1.7 | 4.4 | 4.1 | 3.8 | 4.5 |
| 1,25(OH)2 D (21 ng/mL to 65 ng/mL) | 188.2 | 63.2 | 74 | 57 | 62 |
| 25(OH) D (20–100 ng/mL) | 35 | 43 | 28 | 14 | 11 |
| iPTH (10–65 pg/mL) | 18 | 54 | 27 | 34 | 25 |
| Creatinine (0.5 to 1.0 mg/dL) | 1.1 | 0.6 | 0.5 | 0.6 | |
| Alkaline Phosphatase (39 – 117 IU/L) | 111 | 54 | 65 | 61 | |
| 24 hour urine calcium | 864 | 402 | 89 | 151 | 80 |
| Urine ca/cr ratio (<0.21 mg2/mg2) | 0.64 | 0.25 | 0.15 | 0.15 | 0.18 |
| TRP (> 90%) | 60% | 94% | 91% | 93% | |
| TmP/GFR (Male 45–55 yrs, 0.9–1.35 mmol/L; Female 45–55 yrs, 0.88–1.44; young females 0.96– 1.5) | 0.33 | 1.5 | 1.2 | 1.5 | |
| Genotype | R67H/G191S | R67H/WT | R67H/WT | R67H/G191S |
A chest radiograph (Figure 1) revealed right and left sixth and seventh rib fractures. MRI of the sacrum and coccyx showed possible healing fracture of the sacrum. A technetium bone scan (Figure 1) showed numerous foci of increased uptake in the axial and appendicular skeleton. Computed tomography of chest, abdomen, and pelvis showed non-displaced rib fractures in various stages of healing described as non-malignant. A renal ultrasound showed 5-mm non-obstructing stones bilaterally.
Renal, thyroid, and hepatic functions were normal, and the serum testosterone was 720 ng/dL (350–890 ng/dL). Studies of mineral metabolism (Table 2) showed low serum levels of phosphorus with reduced tubular resorption of phosphate (60%, normal > 80%) and TmP/GFR (0.33 mmol/L; normal for age 0.9–1.35 mmol/L). The serum PTH concentration was low normal with normal serum levels of calcium and alkaline phosphatase. The serum level of 1,25(OH)2D was elevated and urine studies showed marked hypercalciuria (864 mg/24 hr). The serum concentration of FGF-23 was low normal at 60 RU/mL (normal 44 –215 RU/mL) and PTHrP was low (< 0.74 pmol/L, normal <2.0 pmol/L). Based on these studies a diagnosis of hypophosphatemic osteomalacia was made, and alendronate was discontinued and therapy with neutral phosphate 250 mg four times per day was begun.
Genetic Analysis
All 13 exons (1 non-coding and 12 coding), as well as the adjacent exon/intron boundary sequences, of SLC34A3 were amplified using genomic DNA from peripheral blood mononuclear cells and subjected to Sanger sequencing as previously described [12]. We also utilized an array-based assay to detect potential copy number variants. To confirm that the two variants identified in SLC34A3 (see below) fit a compound heterozygous recessive model, a fragment (957 bp) that contained the two variants was amplified by PCR from genomic DNA from the proband (I-1, Figure 2) and his younger daughter (II-2; Figure 2) and subcloned into pGEM-T Vector (Promega Corp, Madison, WI). We transformed DH5α E. coli with the plasmids and sequenced DNA from ten unique clones from both subjects using standard techniques [13].
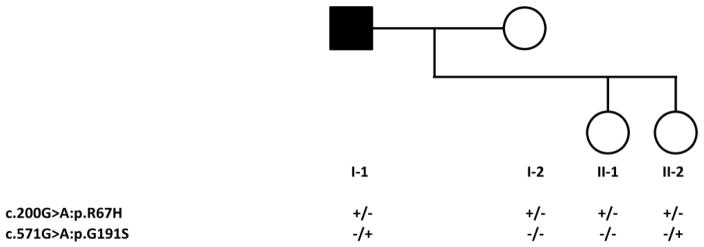
Figure 2. Family Pedigree and Genotypes.
Circles denote females; square denotes male. Black symbol indicate affected subject; open symbols indicate healthy individuals. Genotypes are denoted below pedigree symbols.
Studies of Bone and Mineral Metabolism
Serum levels of 25(OH)D were measured by LS-MS/MS and 1,25(OH)2D levels were measured by immunoassay. Serum intact PTH levels were determined by electrochemiluminescence immunoassay, and FGF23 levels were measured by an ELISA that detects the intact hormone and C-terminal fragments of FGF23 (Immutopics). Tubular maximum phosphate reabsorption per GFR (TmP/GFR) was estimated using the Walton and Bijvoet nomogram [14]. Lumbar spine (LS), and hip bone mineral content (BMC) and BMD were measured using the same Hologic QDR 4500A DXA scanner (Hologic, Inc., Bedford MA).
Results and Discussion
The presence of hypophosphatemia with a reduced TmP/GFR, multiple fractures and low bone density in this adult patient with no prior history of metabolic bone disease initially suggested a diagnosis of oncogenous osteomalacia, but serum levels of FGF23 were not elevated. Further evaluation disclosed markedly elevated levels of urinary calcium and serum 1,25(OH)2D, and the diagnosis of HHRH was subsequently confirmed through genetic analyses that identified two nonsynonymous nucleotide changes in the SLC34A3 gene, a novel c.571G>A (p.G191S) mutation in exon 7 and the previously reported c.200G>A (p.R67H) variant in exon 4 [15]. Using an array-based copy-number assay that can detect deletions of less than 100 bp, we confirmed that there were no intragenic deletions in the proband’s SLC34A3 gene. The proband’s wife (I-2) and the older daughter (II-1) aged 21 years were heterozygous for c.200G>A while the younger daughter (II-2) aged 19 years had a similar biallelic genotype as her father, having inherited c.200G>A from her mother and c.571G>A from her father.
Several lines of evidence indicate that the nonsynonymous c.571G>A transition is a disease-causing mutation rather than a polymorphism. First, this nucleotide substitution was not present in the 1000 Genomes Project [16], dbSNP [17], the National Heart, Lung, and Blood Institute Gene Ontology Exome Sequencing Project (http://evs.gs.washington.edu/EVS/), and the ExAC databases. Second, amino acid p.G191 is conserved in the SLC34A1, SLC34A2 and SLC34A3 genes encoding Npt2a, Npt2b and Npt2c sodium-dependent phosphate co-transporters from multiple vertebrate species (not shown). Third, p.G191S is predicted to be damaging by in silico analyses using both Polyphen2 (0.926) [18] and SIFT (0.02) [19]. On the other hand, it is more difficult to ascribe a pathogenic role for the previously reported c.200G>A (p.R67H) variant in exon 4 [15]. This variant has a minor allele frequency (MAF) of 0.1 (rs34372115), and is predicted by SIFT (0.19) and Polyphen2 (0) to be tolerated or benign, hence suggesting that p.R67H is a polymorphism rather than a mutation. The p.R67H variant has been previously reported in a patient with hypercalciuria and biochemical findings suggestive of a PTH- and FGF23-independent renal phosphate leak, but without a history of rickets [7]. This subject carried the p.R67H variant on the paternal SLC34A3 allele, which also contained a pathological 310-bp deletion in exon 13, c1571_1880del, while there was no evidence for a second mutation on the maternal allele. Hence, it was not possible to determine whether this amino acid substitution had a functional consequence. Nevertheless, similar to the proband in our report, the clinical features of this subject suggest that haploinsuffiency for SLC34A3 may be sufficient in some cases to produce a clinical phenotype.
Although it is possible that a second mutation on the p.R67H allele escaped our analyses, perhaps due to location within an intron [11, 20] or in the promoter, we propose that the clinical features in the proband we describe here are most likely to be the result of a heterozygous mutation (i.e., p.G191S) in SCL34A3. It is interesting to note that the proband (I-1) and his younger daughter (II-2) (Table 2) had such different phenotypes despite sharing the same genotypes, and we surmise that the lack of disease in II-2 may reflect her relatively young age, incomplete penetrance, or a more complicated compensatory mechanism.
Although classical HHRH is typically a metabolic disorder of childhood, recent studies indicate that patients with SLC34A3 mutations can experience a late onset of biochemical and/or clinical abnormalities [4, 12] that is reminiscent of what has been reported in some patients with mutations in FGF23 and Autosomal Dominant Hypophosphatemic Rickets (ADHR) [21]. We suspect that reduced bone mass or symptomatic osteoporosis/osteomalacia, as demonstrated by our patient in a very severe presentation, may be an important and often unrecognized initial symptom in patients with SLC34A3 mutations. Previous studies have shown reduced bone density by DXA and by bone histology in other patients with classical HHRH as well as in their heterozygous relatives [8], who have an intermediate biochemical phenotype [4]. Hence, it is conceivable that a considerable number of adults with the combination of hypercalciuria/nephrolithiasis and reduced bone density have late-onset HHRH due to heterozygous SLC34A3 mutations and not idiopathic osteoporosis. In the case we report, the previous misdiagnosis of osteoporosis led to the prescription of bisphosphonates, with subsequent worsening of bone pain. Once the diagnosis of HHRH was confirmed the bisphosphonate was discontinued and treatment with oral phosphate supplements was begun. Within 3 months of treatment his rib and back pain resolved completely and he no longer required narcotics for pain control. Subsequent biochemical evaluation revealed an increased serum phosphate level and markedly reduced levels of serum 1,25(OH)2D and 24-hour urinary calcium excretion (Figure 2). Repeat bone densitometry showed improved bone density (Table 1). These features suggest that osteomalacia and not osteoporosis was the proper diagnosis.
Our study has several limitations that should be acknowledged. First, we did not perform functional studies of the p.G191S missense mutation. Nevertheless, the in silico analyses predict that this amino acid substitution is highly damaging. And second, we did not sequence genes encoding other phosphate transporters, such as SLC34A1 [22], to ensure that the proband’s phenotype is not the result of a digenic disorder.
In summary, the case we describe highlights the variability of the biochemical profile of HHRH in adults and the important role that genetic testing can play in the evaluation of patients with bone disorders that are not responsive to usual care, in this case bisphosphonates for presumed osteoporosis. Here the critical key to proper diagnosis was the previously overlooked hypophosphatemia, and once a genetic diagnosis of HHRH was established in this patient, bisphosphonate therapy was discontinued and treatment with neutral phosphate commenced, which led to rapid resolution of bone pain and fractures that likely represented osteomalacia and not osteoporosis. This case emphasizes the importance of considering uncommon conditions such as SLC34A3 mutations in adult patients with the combination of hypercalciuria and low bone density.
Highlights.
Mutations in the SLC34A3 gene encoding the sodium-phosphate cotransporter 2c (NPT2c) cause hypercalciuria and metabolic bone disease
Early onset disease is typically autosomal recessive and manifests as hereditary hypophosphatemic rickets with hypercalciuria (HHRH)
Late onset disease can be heterozygous and present as low bone mass and renal stones.
Acknowledgments
This work was supported in part by a grant from the NIDDK (R01DK079970) (M.A.L.).
Abbreviations
- Npt2c
type 2c sodium phosphate co-transporter
- BMD
bone mineral density
- 25(OH)D
25 hydroxyvitamin D
- 1,25(OH)2D
1,25-dihydroxyvitamin D or calcitriol
- PTH
parathyroid hormone
- bone ALP
bone alkaline phosphatase
- HHRH
hereditary hypophosphatemic rickets with hypercalciuria
- TRP
tubular reabsorption of phosphate
- TmP/GFR
tubular maximum phosphate reabsorption per glomerular filtration rate
Footnotes
Author’s role:
Study design: MAL and DG; Study conduct: MAL and DG. Data collection: MAL, DG and DL. Data analysis and interpretation: MAL, DG, DL and HH; LG. Drafting manuscript: DG, MAL and DL. Revising manuscript content: DG, MAL, DL and HH. Approving final version of manuscript: all authors. DG and MAL take responsibility for the integrity of the data analysis.
Disclosure statement
All the authors state that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sella S, Cattelan C, Realdi G, Giannini S. Bone disease in primary hypercalciuria. Clin Cases Miner Bone Metab. 2008;5:118–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28:120–32. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stechman MJ, Loh NY, Thakker RV. Genetic causes of hypercalciuric nephrolithiasis. Pediatr Nephrol. 2009;24:2321–32. doi: 10.1007/s00467-008-0807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta D, Wee MJ, Reyes M, Li Y, Simm PJ, Sharma A, Schlingmann KP, Janner M, Biggin A, Lazier J, Gessner M, Chrysis D, Tuchman S, Baluarte HJ, Levine MA, Tiosano D, Insogna K, Hanley DA, Carpenter TO, Ichikawa S, Hoppe B, Konrad M, Savendahl L, Munns CF, Lee H, Juppner H, Bergwitz C. Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J Am Soc Nephrol. 2014;25:2366–75. doi: 10.1681/ASN.2013101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page K, Bergwitz C, Jaureguiberry G, Harinarayan CV, Insogna K. A patient with hypophosphatemia, a femoral fracture, and recurrent kidney stones: report of a novel mutation in SLC34A3. Endocr Pract. 2008;14:869–74. doi: 10.4158/EP.14.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, Hulin P, Benque-Blanchet F, Silve C, Grandchamp B, Friedlander G. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–91. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Sanderson SR, Reyes M, Sharma A, Dunbar N, Srivastava T, Juppner H, Bergwitz C. Novel NaPi-IIc mutations causing HHRH and idiopathic hypercalciuria in several unrelated families: long-term follow-up in one kindred. Bone. 2012;50:1100–6. doi: 10.1016/j.bone.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–92. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergwitz C, Juppner H. FGF23 and syndromes of abnormal renal phosphate handling. Adv Exp Med Biol. 2012;728:41–64. doi: 10.1007/978-1-4614-0887-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ. Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab. 2006;91:4022–4027. doi: 10.1210/jc.2005-2840. [DOI] [PubMed] [Google Scholar]
- 12.Tencza AL, Ichikawa S, Dang A, Kenagy D, McCarthy E, Econs MJ, Levine MA. Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/type IIc sodium-phosphate cotransporter: presentation as hypercalciuria and nephrolithiasis. J Clin Endocrinol Metab. 2009;94:4433–4438. doi: 10.1210/jc.2009-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 14.Walton RJ, Bijvoet OLM. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;309:310. doi: 10.1016/s0140-6736(75)92736-1. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM. Hereditary Hypophosphatemic Rickets with Hypercalciuria Is Caused by Mutations in the Sodium-Phosphate Cotransporter Gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genomes Project C. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayers EW, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S, Feolo M, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Madden TL, Maglott DR, Miller V, Mizrachi I, Ostell J, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Yaschenko E, Ye J. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2009;37:D5–15. doi: 10.1093/nar/gkn741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa S, Tuchman S, Padgett LR, Gray AK, Baluarte HJ, Econs MJ. Intronic deletions in the SLC34A3 gene: a cautionary tale for mutation analysis of hereditary hypophosphatemic rickets with hypercalciuria. Bone. 2014;59:53–6. doi: 10.1016/j.bone.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82:674–81. doi: 10.1210/jcem.82.2.3765. [DOI] [PubMed] [Google Scholar]
- 22.Schlingmann KP, Ruminska J, Kaufmann M, Dursun I, Patti M, Kranz B, Pronicka E, Ciara E, Akcay T, Bulus D, Cornelissen EA, Gawlik A, Sikora P, Patzer L, Galiano M, Boyadzhiev V, Dumic M, Vivante A, Kleta R, Dekel B, Levtchenko E, Bindels RJ, Rust S, Forster IC, Hernando N, Jones G, Wagner CA, Konrad M. Autosomal-Recessive Mutations in SLC34A1 Encoding Sodium-Phosphate Cotransporter 2A Cause Idiopathic Infantile Hypercalcemia. J Am Soc Nephrol. 2016;27:604–14. doi: 10.1681/ASN.2014101025. [DOI] [PMC free article] [PubMed] [Google Scholar]