Abstract
Objective
To examine the effects of direct skill training and guided training for promoting independence after stroke.
Design
Single-blind randomized pilot study.
Setting
Inpatient rehabilitation facility.
Participants
Forty-three participants in inpatient rehabilitation with acute stroke and cognitive impairments.
Interventions
Participants were randomized to receive direct skill training (n=22, 10 sessions as adjunct to usual inpatient rehabilitation) or guided training (n=21, same dose).
Main Outcome Measure
The Functional Independence Measure assessed independence at baseline, rehabilitation discharge, and months 3, 6, and 12.
Results
Linear mixed models (random intercept, other effects fixed) revealed a significant intervention by time interaction (F4,150=5.11, p<0.001), a significant main effect of time (F4,150=49.25, p<0.001), and a significant effect of stroke severity (F1,150=34.46, p<.001). There was no main effect of intervention (F1,150=0.07, p=0.79). Change in Functional Independence Measures scores was greater for the DIRECT group at rehabilitation discharge (effect size of between group differences, d=0.28) and greater for the GUIDE group at months 3 (d=0.16), 6 (d=0.39), and 12 (d=0.53). The difference between groups in mean 12 month change scores was 10.57 points.
Conclusions
Guided training, provided in addition to usual care, offered a small advantage in the recovery of independence, relative to direct skill training. Future studies examining guided training in combination with other potentially potent intervention elements may further advise best practices in rehabilitation for individuals with cognitive impairments after acute stroke.
Cognitive impairments occur in as many as one-third to one-half of adults who sustain a first-time stroke,1–2 with some reports estimating even higher proportions.3–4 Regardless of stroke volume or location, stroke-related cognitive impairments are associated with significant functional disability, frequently measured by the inability to regain independence in performing activities of daily living.1,5 This loss of independence is costly because individuals with stroke-related cognitive impairments require more rehabilitation and more resources to support their living, whether in institutional or community settings. Therefore, it is not surprising that a large portion of post-stroke health care expenditures is attributed to individuals with stroke-related cognitive impairments.6–7 Rehabilitation training methods that are effective in promoting independence in these individuals, and do so more expediently than current methods, have the potential to significantly reduce stroke-related health care expenses.
Individuals with stroke-related cognitive impairments benefit from acute rehabilitation (i.e., demonstrate improvements in independence with daily activities) but they do not achieve the same degree of benefit as individuals without cognitive impairments.8–9 There are several potential explanations. Individuals with cognitive impairments often have difficulty learning and recalling new information, and difficulty applying learned information to novel circumstances. As a result, these individuals often require more time to execute tasks, and more practice when learning or applying skills. Identification of optimal training methods to address the needs of individuals with stroke-related cognitive impairments may ameliorate some of these problems.
The best time to initiate training to promote independence and minimize long-term disability may be during acute rehabilitation.10–11 However, the best methods for training individuals with stroke-related cognitive impairments are unclear. When considering training methods focused on the promotion of independence with daily activities, two methods may be considered: direct skill training and guided training. Direct skill training maximizes the expertise of the rehabilitation practitioner, who directs the course of treatment. In other words, rehabilitation practitioners identify and prioritize problematic daily activities (e.g., mobility, self-care), identify barriers to performing these activities, generate strategies to address these barriers, and instruct clients in these strategies. Practitioners then repeat the process with a variety of problematic activities identified during the rehabilitation program. The evidence surrounding direct skill training is mixed. Some studies suggest that direct skill training promotes improved performance with trained activities, but the benefits of direct skill training may be task-specific (i.e., only promote improvement on the trained activity, e.g., tub transfers) and may not be generalizable to other conceptually similar but distinct daily activities (e.g., car transfers).12 Thus, clients who receive direct skill training may acquire specific skills addressed in therapy but may or may not be able to generalize these skills without assistance thereby impeding the restoration of independence over time. Additional studies suggest that the provision of explicit instructions prior to activity performance (a component of direct skill training) may actually impede performance and learning.13–15
Guided training maximizes the expertise of the client, by training clients to actively engage in the direction and focus of their treatment. Thus, clients learn to identify and prioritize problematic daily activities, identify barriers to performing activities, generate their own individualized strategies for addressing these barriers, and apply this process through iterative practice. In doing so, guided training is designed to equip clients with the ability to generalize knowledge and skills in problem identification and problem solving skills to address new but similar problems over time. Importantly, these skills have the potential to generalize beyond activities addressed during the intervention program to conceptually related but distinct problematic activities after the intervention program has ceased,16 thereby promoting continued gains toward long-term independence after the completion of rehabilitation. Although there have been questions as to whether individuals with cognitive impairments can participate in and learn through guided training, we have demonstrated that clients with stroke-related cognitive impairments engaged in acute rehabilitation are able to learn to identify and analyze problems as well as develop strategies to address these problems.17 In fact, guided training, compared to an attention control intervention (each provided in addition to usual acute rehabilitation), was associated with more expedient recovery of independence in daily activities in the first 6 months after stroke.18
While it appears that individuals with cognitive impairments benefit from both directed and guided training, it is unclear whether either method is superior in promoting independence with daily activities over time. Superiority of one method over another may be particularly salient for individuals with cognitive impairments after stroke. Evidence from education and adult learning studies suggests that individuals with cognitive impairments may learn new skills more efficiently with structured direct skill training.19–21 Alternatively, evidence from education studies also suggests that individuals with cognitive impairments better generalize their learning when they have the opportunity to face difficult challenges and overcome them through guided problem solving. If prevented from facing and overcoming challenges, these same individuals may learn how to address a given problem, but may not learn strategies to address the next problem.22–23 Guided training provides opportunities to face problems and develop strategies to address them, and through iterative practice allows individuals to develop skills to work through or around problems. Direct skill training may not provide the same opportunities for the development of problem solving skills, because it provides the “answers,” as identified by the rehabilitation practitioner.
The aim of the proposed pilot study was to compare direct skill training and guided training to determine whether one approach was superior to the other in promoting independence with daily activities after stroke among individuals with cognitive impairments after stroke. In a comparison of this nature, we chose to focus on differences in outcome based on training method, rather than differences in the specific activities (e.g., stair climbing, dressing) used for training or differences in the specific impairments that may be addressed in training (e.g., vision, limb function). We predicted that clients in both groups would demonstrate significant improvement in independence with daily activities in the first year after rehabilitation admission (time main effect), but that clients who received guided training would demonstrate significantly more improvement over time than clients who received direct skill training (group by time interaction).
Methods
We conducted a pilot parallel design randomized trial. Participants were recruited at admission to inpatient rehabilitation at an academic health center. Participants had a diagnosis of first-time stroke within 30 days of rehabilitation admission and who demonstrated cognitive impairments (Executive Interview, 14-item version, score≥3).24 Individuals with severe aphasia (Boston Diagnostic Aphasia Examination Severity Rating 0 or 1),25 prior Mild Cognitive Impairment or dementia, current major depressive disorder, recent substance abuse (previous 3 months), or current psychotic disorder (PRIME-MD, Mini Neuropsychiatric Interview)26–27 were excluded. Individuals with current major depressive disorder, recent substance abuse, or psychosis were referred for treatment for these disorders, and excluded from the study to avoid potentially confounding effects of these psychiatric treatments with the experimental interventions under investigation. To describe the sample and to assess for potential differences between the groups that may influence study outcomes, we assessed stroke severity with the National Institutes of Health Stroke Scale.28 Screening and descriptive measures were administered by independent (i.e., blinded) evaluators trained and closely supervised by licensed neuropsychology and psychiatry research team members.
After baseline testing, participants received usual inpatient rehabilitation care, plus an additional session of either direct skill training (DIRECT) or guided training (GUIDE). Participants were assigned to intervention by the research coordinator using a simple randomization scheme (1:1 allocation ratio) developed with a random number generation program and maintained in an electronic file accessible only to the research coordinator. DIRECT and GUIDE protocols spanned ten 45 minute sessions and were delivered 5 days per week for 2 weeks. [Note. If participants were discharged home before the 10 sessions were completed, the research therapist administered the remaining sessions at home. However, if participants were discharged to acute care due to decline in medical status, or discharged to a skilled nursing facility before the 10 sessions were completed, the intervention protocol was stopped]. Both interventions were administered by licensed occupational and physical therapists (research therapists) who were trained to competency on one or the other standardized intervention protocols. These research therapists were independent contractors who were not members of the usual care rehabilitation team. To avoid cross-contamination, the research therapists were trained in only one protocol, and remained naïve to the opposite protocol.
Both intervention protocols were standardized by the research team prior to recruiting the first participant. To isolate the effects of direct skill training and guided training from the effects of client-centered goal setting, both protocols started with the identification of client-centered goals using the Canadian Occupational Performance Measure.29 Briefly, the research therapist would ask the participant to describe a “typical” routine prior to the stroke, focusing on a typical weekday, typical Saturday and typical Sunday. Based on this discussion, the research therapist then asked the participant to identify 4 – 6 activities that the participant thought were important and likely to be problematic after the stroke. Therapists in both conditions were instructed to focus the subsequent intervention program on these activities.
In the DIRECT protocol, the research therapist selected an activity to practice in the research session that addressed one of the client-identified goals. The DIRECT therapist analyzed the activity using interview and performance-based assessment, and identified barriers to performance of that activity. Based on the identified barriers, the DIRECT therapist then set criteria for performance, developed strategies to improve performance and taught the participant those strategies. Participants practiced the identified strategies until the performance criteria were met (and thus the research therapist moved on to the next activity), or until the end of the 10 sessions. The DIRECT therapist documented each of these steps using pre-established forms in a workbook shared with the participants.
In the GUIDE protocol, the research therapist asked the participant to pick the first activity that he or she wanted to practice that addressed one of the client-identified goals. The GUIDE therapist asked the participant to perform that activity and identify barriers to performance. The GUIDE therapist then taught the participant a global strategy, “Goal-Plan-Do-Check,” and asked the participant to set a goal to address the barriers (i.e., identify a criterion for performance), develop a plan to address the goal, do the plan, and check whether the plan worked or required revising. This process was repeated iteratively until the goal was met (and thus participants moved on to the next activity) or until the end of the 10 sessions. The GUIDE therapist guided participants using prompting questions, as well as workbooks to facilitate learning and aid the participants in implementing the strategy.
Each protocol was standardized to ensure consistent intervention administration within protocol regardless of professional training or the specific activities or targeted function of intervention.17–18 To ensure that actual intervention administration adhered to planned administration, we assessed fidelity to each protocol and differentiation of elements between protocols using standardized checklists applied to a random 20% of video recorded sessions in each condition. We also assessed the degree to which elements of DIRECT and GUIDE protocols were present in selected usual care sessions (one occupational therapy session, one physical therapy session, and one speech therapy session – if being followed by speech therapy, for each study participant).
The primary outcome for the study was the Functional Independence Measure.30 The Functional Independence Measure is the standard tool used to assess recovery of independence in the acute inpatient rehabilitation setting. The measure assesses the amount of assistance necessary for an individual to complete 18 basic self-care, mobility, communication, and cognitive activities deemed necessary for household independence. For the purposes of this study, the Functional Independence Measure was administered by 4 trained independent (i.e., blinded) evaluators at study admission (baseline), inpatient rehabilitation discharge, and months 3, 6, and 12 after study admission. Inter-rater reliability among raters for total Functional Independence Measure scores collected on assessments throughout the duration of the study was excellent (ICC=.948).
Based on a priori sample size estimation, we determined that with a sample of 36 participants (18 per group), we would have 95% power (two-sided hypothesis, confidence level α=.05) to detect a clinically meaningful difference between the two groups in improvement over time (i.e., between-group, within-group, and between-within interaction effects) as measured by the Functional Independence Measure. Our power estimate was based on our plan to use mixed effects models and was computed using pilot study estimates of effect sizes.
Data were managed using REDCap electronic data capture tools hosted at the University of Pittsburgh.31 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
We examined baseline characteristics of both groups using descriptive and inferential statistics. Next, we used SAS PROC MIXED (SAS® version 9.3, SAS Institute, Inc., Cary, North Carolina) to perform a linear mixed model analysis to model change in Functional Independence Measure scores between groups over time, addressing the primary aim.32 Although our dependent variable was slightly skewed, linear mixed models are robust to departures from normality. As an additional precaution, we used an asymptotic approach to estimate empirical standard errors. In the model, the intercept was random, with intervention and time as fixed factors. Stroke severity and cognitive impairment were added to the model to determine their contribution to model fit. We used the Bayesian Information Criterion to assess model fit, and selected the best model to report parameter estimates. Last, we computed effect sizes (d) to examine the differences between groups in the magnitude of change scores over time.33
Results
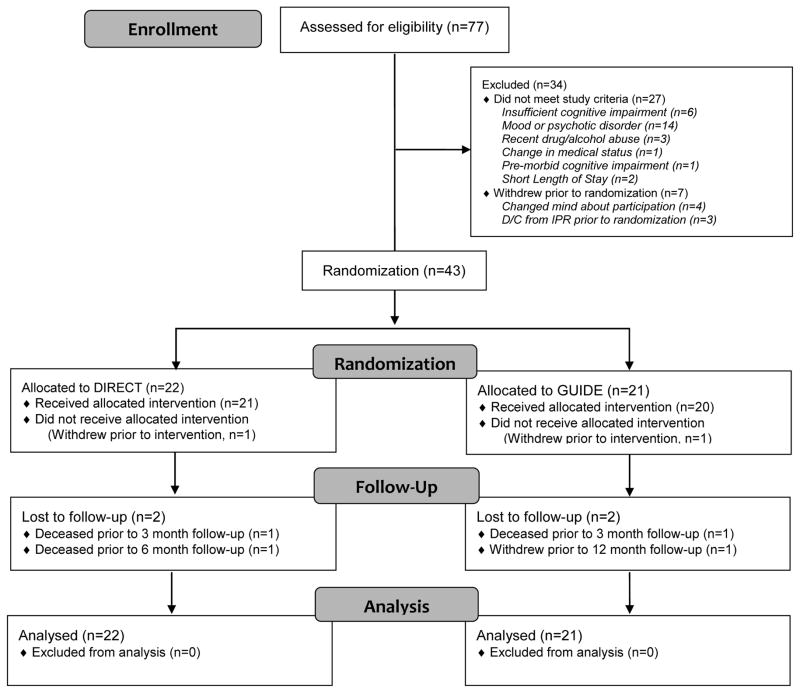
Between August 2012 and December 2014, independent evaluators assessed 77 potential participants for eligibility; 27 potential participants did not meet criteria (Figure 1), and 7 potential participants withdrew prior to randomization. The remaining 43 eligible participants were randomized to the two interventions (DIRECT, n=22; GUIDE, n=21). Figure 1 provides the reasons for exclusion. Table 1 provides an overview of participant characteristics, by group. No differences were detected between groups on any measured baseline or follow-up characteristics (Table 1).
Figure 1.
CONSORT Diagram
Table 1.
Participant Characteristics
| DIRECT (n=22) | GUIDE (n=21) | ||
|---|---|---|---|
| Participant Characteristics at Study Admission | |||
| Sex, Male, n(%) | 13 (62) | 9 (41) | χ21=1.13 |
| Age, Years, M(SD) | 66.73 (14.25) | 65.86 (11.67) | t41=.22 |
| Race, White, n(%) | 21 (95) | 20 (95) | χ21=.00 |
| Stroke onset, Days, M(SD) | 22.36 (30.97) | 16.29 (18.24) | t41=.78 |
| Stroke type, Ischemic, n(%) | 14 (64) | 14 (67) | χ21=.04 |
| Hemisphere, Left, n(%) | 13 (59) | 10 (62) | χ21=.76 |
| Stroke severity, NIHSS (0–42), M(SD)* | 7.86 (5.10) | 7.00 (3.90) | t41=.62 |
| Cognitive impairment, EXIT (0–28), M(SD)* | 10.86 (5.14) | 12.38 (5.11) | t41=.97 |
| Functional Independence Measure (18–126), M(SD) | 67.32 (18.33) | 67.33 (14.72) | t41=.00 |
| Participant Experiences in Inpatient Rehabilitation | |||
| Length of stay, M(SD) | 21.59 (11.53) | 19.62 (7.22) | t41=.68 |
| Research intervention sessions, M(SD) | 7 (3) | 7 (3) | t39=.81 |
| Discharge disposition | χ22=.97 | ||
| Home, no caregiver support, n | 2 | 4 | |
| Home with caregiver support, n | 17 | 15 | |
| Skilled nursing facility, n | 3 | 2 | |
NIHSS= National Institutes of Health Stroke Scale. EXIT=Quick Executive Interview (14-item version).
Lower scores=better health.
p<.05
All participants were retained in the study with the exception of 3 participants in the DIRECT group (one withdrew prior to intervention onset, one died prior to the 3 month follow-up, and one died prior to the 6 month follow-up), and 3 participants in the GUIDE group (one withdrew prior to intervention onset, one died prior to the 3 month follow-up, and one withdrew prior to the 12 month follow-up). Although not statistically significant (perhaps due to power), dropouts were older (mean=73.7 vs 65.1), were earlier in their stroke recovery (days since onset mean=6.5 compared to mean=21.5), had a shorter inpatient rehabilitation length of stay (mean=14.0 days relative to mean=21.7 days), and were more independent at baseline (mean=75.2 relative to mean=66.1). The odds of individuals with right hemisphere stroke of dropping out of the study (n=4; GUIDE=2, DIRECT=2) were greater than the odds of individuals with left hemisphere (n=1) or bilateral hemisphere stroke (n=1; Mantel-Haenszel χ2=3.79, p=0.05). No meaningful differences in sex, race, type of stroke, stroke severity, or cognitive impairment severity were noted. All participants regardless of retention were included in the remaining study analyses.
On average, DIRECT participants and GUIDE participants received a similar number of sessions (t39=0.81, p=.42). For the most part all sessions were administered during inpatient rehabilitation, with only 6% of DIRECT sessions, and 8% of GUIDE sessions completed at home. Data from the sampled sessions (20% of administered sessions) indicated 84% adherence to intervention procedures in the DIRECT group, and 85% in the GUIDE group. The two protocols were sufficiently differentiated, with DIRECT intervention elements present in only one of the sampled GUIDE sessions, and GUIDE intervention elements present in none of the sampled DIRECT sessions. Analysis of the sampled usual care sessions indicated that on average 98% of training in usual care sessions was consistent with direct skill training, and 2% was consistent with guided training.
Participants in both groups selected goals addressing a range of daily activities, including basic self-care (dressing, oral hygiene), mobility (bed transfers, floor transfers), home management (meal preparation, financial management), work/volunteer (accessing and answering electronic mail, scheduling activities), and leisure activities (gardening, adapted exercise). A review of goals revealed no obvious differences between groups in number of goals by activity category (i.e., basic self-care χ2=5.76, p=0.33, mobility χ2=5.89, p=0.21, home management χ2=1.19, p=0.76, work χ2=2.22, p=0.14, leisure χ2=0.00, p=1.00) or total number of goals addressed in research sessions (GUIDE mean=4.10, SD=1.86; DIRECT mean=4.76, SD=2.14).
For the 37 participants who completed the study, exposure to any occupational, physical or speech therapy services in the first 12 months after discharge from inpatient rehabilitation was characterized by setting (skilled nursing, home health, and outpatient rehabilitation) and duration (days). No statistically significant differences were noted in between groups in post-discharge exposure to rehabilitation services by setting (skilled nursing χ21=0.65, p=0.42; outpatient χ21=1.13, p=0.29; home health χ21=1.87, p=0.17) or duration of therapy services (skilled nursing t35=0.17, p=0.89; outpatient t35=0.03, p=0.98; home health t35=0.41, p=0.70).
The final best-fitting model included intervention, time, intervention by time interaction, and stroke severity. Cognitive impairment was not a significant contributor to explaining variability in Functional Independence Measure scores. Analyses revealed a significant intervention by time interaction (F4,150=5.11, p<0.001), a significant main effect of time (F4,150=49.25, p<0.001), and a significant effect of stroke severity (F1,150=34.46, p<.001). There was no main effect of intervention (F1,150=0.07, p=0.791). Examination of residual plots did not reveal any outliers, and residuals were normally distributed. Individual growth trajectories in the overall sample and by group suggested that participants benefitted comparably within each group. Further exploration of least squares means revealed higher Functional Independence Measure scores for the DIRECT group at rehabilitation discharge, and the effect size of between group differences in change from baseline was small (d= −0.28). However, higher scores for the GUIDE group were noted at month 3, month 6, and month 12. The effect sizes of between group differences in change over time were small between baseline and month 3 (d=.0.16), and moderate between baseline and month 6 (d=0.39) and between baseline and month 12 (d=0.53) (Table 2). The difference in mean 12 month Functional Independence Measure change scores was 10.57 points.
Table 2.
Effect Size Estimates for Between Group Differences in Change Scores
| GUIDE | DIRECT | Cohen’s d | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | SE | Mean | SE | ||
| Baseline to Discharge (Mean) | 19.52 | 2.16 | 22.67 | 2.65 | −0.28 |
| Baseline to Month 3 (Mean) | 39.42 | 3.57 | 36.45 | 4.57 | 0.16 |
| Baseline to Month 6 (Mean) | 41.78 | 3.08 | 34.14 | 5.23 | 0.39 |
| Baseline to Month 12 (Mean) | 43.52 | 3.45 | 32.95 | 5.09 | 0.53 |
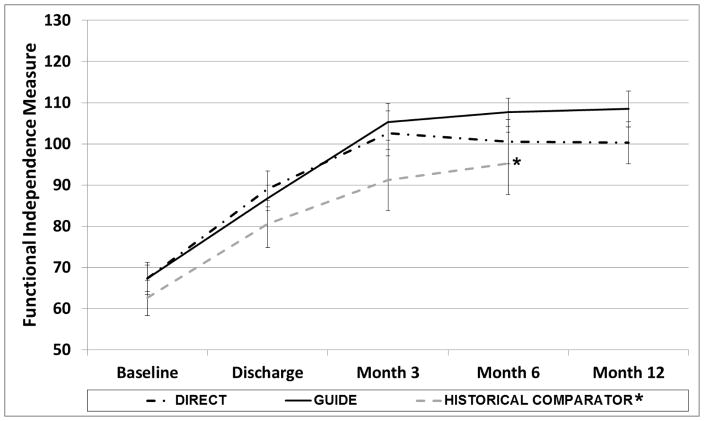
Figure 2 depicts the raw means for both groups over time, and compares these means with a non-randomized historical comparator group from a previous trial.18 Participants were enrolled in the current study and the previous study using the same inclusion and exclusion criteria, and study procedures were identical, with the exception of the intervention. For the non-randomized historical comparator group, participants identified goals using the Canadian Occupational Performance Measure (as described), but then received a non-active control intervention (attention only, described elsewhere),17 in addition to usual care. The plot in Figure 2 suggests that both the DIRECT and the GUIDE interventions resulted in enhanced recovery of independence relative to attention control.
Figure 2. Intervention Group Means by Time.
*Historical comparator group is a non-randomized group from a previous trial, plotted here for comparison. This group received usual inpatient rehabilitation with dose-matched non-active control intervention sessions (attention only).
Discussion
We examined the relative merits of direct skill training and guided training among individuals with cognitive impairments after acute stroke. The findings from this pilot study suggest that there may be an advantage for guided training, relative to direct skill training, for individuals with stroke-related cognitive impairments admitted to acute inpatient rehabilitation, but that this advantage may only emerge over time. The mean change score in the DIRECT group exceeded the minimally clinically important difference of 22 points between baseline and rehabilitation discharge;34 the mean change score in the GUIDE group was less than 22 points during this same time period. Both groups exceeded this change this by month 3 and continued to improve over time, with a slightly greater change in the GUIDE group over 12 months (10.57 points difference in 12 month change scores). The reasons for these differences over time are unclear, but previous studies have suggested that guided discovery, a component of guided training, may be associated with generalization of training over time.16,18 The clinical meaning of these differences is not clear and additional investigation is warranted.
This pilot study was an initial attempt at identifying training methods that might optimize recovery of independence with daily activities over time. In designing this pilot study, we incorporated client-identified goals in both intervention groups. We made this a priori decision specifically to estimate the effect of guided training relative to direct skill training separate from the effects of client-identified goals. Previous studies have demonstrated that client-identified goals positively influence learning and skill development over time.35 Thus, our design permitted isolation of the effects of direct skill training and guided training, two potentially potent elements of training, from client-identified goals, an additional potentially potent element of training. This method highlights the importance of clearly specifying hypothesized active elements of intervention, and examining the effects of these elements alone, and in combination, to identify the best collection and sequence of elements for generating best outcomes. While this pilot study used a fairly simple design to accomplish this goal, more sophisticated methods may be employed when seeking to optimize an intervention method with multiple interacting elements. Multiphase optimization strategy trials (MOST) employing full or fractional factorial designs show merit for identifying which combination of intervention elements may yield the best outcomes.36–37 Sequential, multiple assignment, randomized trials (SMART) show promise for examining the best sequence of intervention elements and/or intervention programs that yield the best outcomes.37 These innovative methods offer new opportunities to clarify and optimize interventions in rehabilitation research.
There are several limitations in this pilot study that can be used as lessons to inform future investigations. First, both experimental interventions were offered in addition to usual care in this study. We designed the study in this manner to examine the effects of direct skill training and guided training in the acute inpatient rehabilitation setting (the intended long-term application of this research), and to do so without altering the implementation of usual care. However, in doing so, it appears that clients in both groups received a high dose of direct skill training in usual care. This in turn may have minimized the overall effect of guided training in our study. That said, the findings suggest that even a small dose of guided training appears to have altered the trajectory of recovery over time. To better estimate the effects of these two methods, future studies should compare higher and more equal doses of guided training and direct skill training.
Second, the study design did not include a prospectively randomized control intervention group, preventing a true estimation of direct skill training and guided training relative to no intervention or a non-active intervention. To address this study limitation, we presented results from a previously published non-active control intervention as a reference point for discussion. The data suggest that participants may have benefited from the additional session of training, whether direct skill training or guided training. This finding is consistent with reports from several large clinical trials in stroke rehabilitation that have concluded that more training is better than less training, but the optimal approach to the training, when examining means within and between groups, is often less clear.38–40 To better assess whether there is a difference among training methods, relative to usual care, future studies should include a no intervention or a non-active control intervention group.
Third, differences among goals among participants and between groups may have influenced intervention outcomes. When using client-identified goals to drive a rehabilitation program, inevitable variances in the type, complexity, and difficulty of activities chosen by the client, in addition to variances in abilities and deficits that might influence goal achievement make it difficult to assess equality of intervention exposure. To address this, we used an objective standardized measure of independence over time, with the assumption that differences in improvements in independence may be due differences in training method, rather than differences in specific goals, per se. That said, it is possible that participants in one group may select more goals related to basic self-care and mobility (the focus of the Functional Independence Measure) and participants in the other group may select more goals related to more complex activities (e.g., home management, work). Our analysis of goal categories did not reveal any differences between groups. However, we are limited in our ability to classify more nuanced features of goals such as complexity or difficulty for a given client, given the client’s abilities and deficits. This limitation may influence study findings.
Finally, this pilot study was not powered to control for all potential confounding factors that may influence intervention response. Stroke severity was a significant predictor of intervention response. Initial cognitive impairment severity was not a significant predictor in this analysis, but in a larger sample, type and severity of cognitive impairment may influence response to each intervention. Guided training may be particularly advantageous, relative to direct skill training, for individuals with impairments in executive functions.41–43 Alternatively, direct skill training, relative to guided training, may be more advantageous for individuals with severe memory impairments.44–46 Other clinical factors (e.g., mood, apathy or motor function) or selected personal and environmental factors (e.g., intelligence, education, socioeconomic status, residential environment, social support), and changes in these factors over time (i.e., progressive cognitive decline) may also be important to examine. Rehabilitation training after discharge from inpatient rehabilitation may also be important. We collected only minimal data on post-discharge residential status, social support and rehabilitation exposure. Although no differences were detected between groups with respect to the data we did collect, the sample was too small to support definitive conclusions. Each of these factors are important areas of investigation for future, larger clinical trials.
Conclusion
In summary, guided training appears to provide an advantage over direct skill training for promoting recovery of independence over the first 12 months after rehabilitation admission. Future studies examining the combination of direct skill training and guided training with other potentially potent intervention elements may further advise best practices in rehabilitation for individuals with cognitive impairments after acute stroke.
Acknowledgments
Supported by the National Institutes of Health (R03 HD073770; R01 HD074693-02S1; UL1 TR000005)
Footnotes
Presented in part at the International Brain Injury Association Meeting March 5, 2106, Hague, NL.
ClinicalTrials.gov Identifier: NCT02766400
References
- 1.Patel M, Coshall C, Rudd AG, Wolfe CD. Natural history of cognitive impairment after stroke and factors associated with its recovery. Clin Rehabil. 2003;17:158–66. doi: 10.1191/0269215503cr596oa. [DOI] [PubMed] [Google Scholar]
- 2.Nys G, van Zandvoort M, de Kort P, Jansen B, de Haan E, Kappelle L. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23:408–16. doi: 10.1159/000101464. [DOI] [PubMed] [Google Scholar]
- 3.Zinn S, Bosworth H, Hoenig H, Swartzwelder H. Executive function deficits in acute stroke. Arch Phys Med Rehabil. 2007;88:173–80. doi: 10.1016/j.apmr.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Jaillard A, Naegele B, Trabucco-Miguel S, LeBas J, Hommel M. Hidden dysfunctioning in subacute stroke. Stroke. 2009;40:2473–79. doi: 10.1161/STROKEAHA.108.541144. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, Wolfe CD. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32:1279–84. doi: 10.1161/01.str.32.6.1279. [DOI] [PubMed] [Google Scholar]
- 6.Flynn RWV, MacWalter RSM, Doney ASF. The cost of cerebral ischaemia. Neuropharmacol. 2008;55:250–56. doi: 10.1016/j.neuropharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Mahler MP, Zuger K, Kaspar K, Haefeli A, Jenni W, Leniger T, Beer JH. A cost analysis of the first year after stroke - early triage and inpatient rehabilitation may reduce long term costs. Swiss Med Wkly. 2008;138:459–68. doi: 10.4414/smw.2008.11845. [DOI] [PubMed] [Google Scholar]
- 8.Heruti RJ, Lusky A, Danker R, Ring H, Dolgopiat M, Barell V, Levenkrohn S, Adunsky A. Rehabilitation outcome of elderly patients after a first stroke: effect of cognitive status at admission on the functional outcome. Arch Phys Med Rehabil. 2002;83:742–749. doi: 10.1053/apmr.2002.32739. [DOI] [PubMed] [Google Scholar]
- 9.Rabadi MH, Rabadi FM, Edelstein L, Peterson M. Cognitively impaired stroke patients do benefit from admission to an acute rehabilitation unit. Arch Phys Med Rehabil. 2008;89:441–448. doi: 10.1016/j.apmr.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, Katz RC, Lamberty K, Reker D. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36:e100–43. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 11.The Ottawa Panel. Ottawa panel evidence-based clinical practice guidelines for post-stroke rehabilitation. Top Stroke Rehabil. 2006;13:1–269. doi: 10.1310/3TKX-7XEC-2DTG-XQKH. [DOI] [PubMed] [Google Scholar]
- 12.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81:409–17. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 13.Boyd LA, Winstein CJ. Impact of explicit information on implicit motor sequence learning following middle cerebral artery stroke. Phys Ther. 2003;83:976–89. [PubMed] [Google Scholar]
- 14.Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem. 2004;11:388–96. doi: 10.1101/lm.80104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidoni ED, Boyd LA. Achieving enlightenment: what do we know about the implicit learning system and its interaction with explicit knowledge? J Neurol Phys Ther. 2007;31:145–52. doi: 10.1097/NPT.0b013e31814b148e. [DOI] [PubMed] [Google Scholar]
- 16.Alfieri L, Nokes-Malach TJ, Schunn CD. Does discovery-based instruction enhance learning? J Educ Psychol. 2013;48:87–113. [Google Scholar]
- 17.Skidmore ER, Dawson DR, Whyte EM, Butters MA, Dew MA, Grattan ES, Becker JT, Holm MB. Developing complex interventions: lessons learned from a pilot study examining strategy training in acute stroke rehabilitation. Clin Rehabil. 2014;28:378–87. doi: 10.1177/0269215513502799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skidmore ER, Dawson DR, Butters MA, Grattan ES, Juengst SB, Whyte EM, Begley A, Holm MB, Becker JT. Strategy training shows promise for addressing disability in the first 6 months after stroke. Neurorehabil Neural Rep. 2015;29:668–76. doi: 10.1177/1545968314562113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brophy J, Good T. Teacher behavior and student achievement. In: Wintrock MC, editor. Handbook of Research on Teaching. 3. New York: Macmillan; 1986. pp. 328–75. [Google Scholar]
- 20.Christenson SL, Ysseldyke JE, Thurlow ML. Critical instructional factors for students with mild handicaps: an integrative review. Remedial Spec Ed. 1989 Sep-Oct;:521–31. [Google Scholar]
- 21.Archer AL, Hughes CA. Explicit instruction: effective and efficient teaching. New York: Guilford Press; 2011. [Google Scholar]
- 22.Swanson HL, Hoskyn M, Lee CM, O’Shaughnessy T. A synthesis of instructional research for children with learning disabilities: a meta-analysis of treatment outcomes. Washington, DC: US Department of Education; 1997. [Google Scholar]
- 23.Swanson HL, Carson C, Sachse-Lee CM. A selective synthesis of intervention research for students with learning disabilities. School Psychol Rev. 1996;25:370–91. [Google Scholar]
- 24.Larson EB, Heinemann AW. Rasch analysis of the Executive Interview (the EXIT-25) and introduction of an abridged version (the Quick EXIT) Arch Phys Med Rehabil. 2010;91:389–94. doi: 10.1016/j.apmr.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Goodglass H, Kaplan E, Barressi B. The assessment of aphasia and related disorders. 3. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 26.Spitzer RL, Woilliams JB, Kroenke K, KLinzer M, deGruy FV, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: the Prime-MD 1000 study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 27.Sheehan DV. The Mini-International Neuropsychiatric Interview (M.I.N. I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatr. 1998;59:22–57. [PubMed] [Google Scholar]
- 28.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moomaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–70. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 29.Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian occupational performance measure (COPM) 5. Toronto: CAOT Publications ACE; 2014. [Google Scholar]
- 30.Stineman MG, Shea JA, Jette A, Tassoni CJ, Ottenbacher KJ, Fiedler R, Granger CV. The Functional Independence Measure: tests of scaling assumptions, structure and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. 1996;77:1101–8. doi: 10.1016/s0003-9993(96)90130-6. [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer; 2009. [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 34.Granger CV, Cotter AC, Hamilton BB, Fiedler RC. Functional assessment scales: a study of persons after stroke. Arch Phys Med Rehabil. 1993;74:133–8. [PubMed] [Google Scholar]
- 35.Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation: a 35-year odyssey. Am Psychol. 2002;57:705. doi: 10.1037//0003-066x.57.9.705. [DOI] [PubMed] [Google Scholar]
- 36.Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med. 2005;30:65–73. doi: 10.1207/s15324796abm3001_8. [DOI] [PubMed] [Google Scholar]
- 37.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32:S112–8. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Larsen DN Excite Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 39.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK LEAPS Investigative team. Protocol for the locomotor experience applied post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007;7:1. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT, Bever CT, Jr, Bravata DM, Duncan PW, Corn BH, Maffucci AD, Nadeau SE, Conroy SS, Powell JM, Huang GD, Peduzzi P. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–83. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, Ellmo W, Kalmar K, Giacino JT, Harley JP, Laatsch L, Morse PA, Catanese J. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86:1681–92. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Dawson DR, Binns MA, Hunt A, Lemsky C, Polatajko HJ. Occupation-based strategy training for adults with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2013;94:1959–63. doi: 10.1016/j.apmr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Dawson D, Richardson J, Troyer A, Binns M, Clark A, Polatajko H, Winocur G, Hunt A, Bar Y. An occupation-based strategy training approach to managing age-related executive changes: a pilot randomized controlled trial. Clin Rehabil. 2014;28:118–27. doi: 10.1177/0269215513492541. [DOI] [PubMed] [Google Scholar]
- 44.Evans J, Wilson BA, Schuri U, Andrade J, Baddeley A, Bruna O, Canavan T, Dl Sala S, Green R, Laaksonen R, Lorenzi L, Taussik I. A comparison of errorless and trial-and-error learning methods for training individuals with acquired memory deficits. Neuropsychol Rehabil. 2000;107:67–101. [Google Scholar]
- 45.Clare L, Jones R. Errorless learning in the rehabilitation of memory impairment: a critical review. Neuropsychol Rehabil. 2008;18:1–23. doi: 10.1007/s11065-008-9051-4. [DOI] [PubMed] [Google Scholar]
- 46.Ehlhardt L, Sohlberg M, Kennedy M, Coelho C, Ylvisaker M. Evidence-based practice guidelines for instructing individuals with neurogenic memory impairments: what have we learned in the past 20 years? Neuropsychol Rehabil. 2008;18:300–42. doi: 10.1080/09602010701733190. [DOI] [PubMed] [Google Scholar]