Abstract
Background: Multiple diet quality scores have been used to evaluate adherence to specific dietary recommendations or to consumption of healthful foods and nutrients. It remains unknown which score can more strongly predict longitudinal changes in cardiometabolic risk factors.
Objective: We aimed to determine associations of 5 diet quality scores [AHA diet score (AHA-DS), Dietary Approaches to Stop Hypertension (DASH), Healthy Eating Index (HEI)-2005, Mediterranean diet score (MeDS), and Alternative Healthy Eating Index (AHEI)] with 2-y changes in cardiometabolic risk factors in adults 45–75 y old.
Methods: Data from the Boston Puerto Rican Health Study were analyzed (n = 1194). Diet quality scores were calculated from a baseline-validated food-frequency questionnaire. Multivariable-adjusted, repeated-subjects, mixed-effects models, adjusted for baseline measures, estimated associations between each z score and 14 individual cardiometabolic factors measured at 2 y.
Results: MeDS was significantly associated with lower 2-y waist circumference (β coefficient ± SE: −0.52 ± 0.26, P = 0.048); body mass index (BMI; −0.23 ± 0.08, P = 0.005); log-insulin (−0.06 ± 0.02, P = 0.005); log–homeostasis model assessment of insulin resistance (HOMA-IR; −0.05 ± 0.02, P = 0.030), and log–C-reactive protein (−0.13 ± 0.03, P = 0.0002). Similar but weaker associations were observed for the AHEI with BMI, insulin, and HOMA-IR. The AHA-DS was inversely associated with BMI (−0.17 ± 0.08, P = 0.033). Neither the HEI-2005 nor DASH was significantly associated with any variable. Traditional Puerto Rican foods consumed by individuals with high MeDSs included vegetables and meats in homemade soups, orange juice, oatmeal, beans and legumes, fish, whole milk, corn oil, and beer.
Conclusions: The MeDS comprises food components and scores associated with a favorable cardiometabolic profile over 2 y in Puerto Rican adults. An overall healthy diet may be particularly beneficial for maintaining a lower BMI. These results can help identify suitable measures of diet quality in epidemiologic studies and craft meaningful nutritional messages and dietary recommendations for the intended population. This study was registered at clinicaltrials.gov as NCT01231958.
Keywords: diet quality, diet quality scores, diet quality indexes, diet quality comparison, cardiometabolic risk factors, Puerto Ricans, Hispanics/Latinos, longitudinal studies, Mediterranean diet, traditional foods
Introduction
Consuming healthful foods is an established strategy to help maintain cardiometabolic risk factors under control and prevent eventual chronic disease (1). Thus, it is important to determine how the overall diet composition of specific populations is associated with their cardiometabolic profile because this may help identify suitable lifestyle factors that prevent and control dysregulation of these biomarkers. Researchers have developed inclusive dietary scores or indexes that comprise multiple foods and nutrients to assess the overall dietary intake (or diet quality) of a group and then assess their association with disease outcomes (2–4). Commonly used diet quality scores are based on scientific evidence of healthfulness of foods or nutrients, such as the Alternative Healthy Eating Index (AHEI)7 (5), the Healthy Eating Index (HEI) (6, 7), and the AHA diet score (AHA-DS) (8). Other scores are based on preventing specific conditions, such as the Dietary Approaches to Stop Hypertension (DASH) score (9), or on adherence to health-promoting dietary patterns, such as the typical Mediterranean lifestyle (10).
These diet quality scores have been independently associated with cardiometabolic risk factors and morbidity and mortality from chronic diseases (2, 4). However, the composition, weight, and cutoffs of intake of the foods and nutrients included in each score vary widely (4, 11), suggesting that their association with disease outcomes may also vary. Studies that have directly contrasted scores have been inconsistent; some show that all scores predict death, risk of diabetes (12–14) and cardiovascular disease (CVD) (13, 15–17), or weight changes (18) with comparable magnitude of association, whereas others show larger differences in the association of various scores and cardiometabolic markers within a population (19–22).
We have previously evaluated the association of 2 diet quality scores and cardiometabolic factors among Puerto Rican adults living in the United States who have excessive prevalence of metabolic syndrome, hypertension, diabetes, obesity, hyperglycemia, and dyslipidemia (23–26). Diet quality as measured by adherence to AHA recommendations was significantly associated with a healthier cardiometabolic profile and lower odds of allostatic load (8, 27). When diet quality was defined with the AHEI in a separate study among Hispanic/Latino ethnic subgroups, we did not detect an association with metabolic syndrome in Puerto Ricans, although it was reported in other subgroups (28). The cross-sectional nature of both studies may affect directionality. The question remains as to which diet score better reflects the dietary components and distribution followed by this at-risk group that would be more predictive of cardiometabolic health.
We aimed to determine the longitudinal associations of 5 commonly used diet quality scores with 2-y changes in 14 cardiometabolic risk factors in Puerto Rican adults living in the United States. Determining the most appropriate score for controlling cardiometabolic markers could identify suitable measures to use in epidemiological studies and to improve the success of nutritional messages and interventions by focusing on meaningful dietary components in at-risk groups.
Methods
Study population.
Data for this analysis were obtained from the Boston Puerto Rican Health Study, a longitudinal cohort that recruited 1500 participants in 2004–2010 with follow-up interviews at 2 y (n = 1258). This study was registered at clinicaltrials.gov as NCT01231958. Details of the study recruitment and protocols have been previously published (23). Recruitment was done using door-to-door enumeration based on 2000 census tracks with additional recruitment through community-based approaches. Participants were self-identified Puerto Rican adults aged 45–75 y, residing in the Boston, Massachusetts, metropolitan area at the time of recruitment, were able to respond to an interviewer-administered home-based interview, and had no severe health conditions or cognitive impairment. All questionnaires and standardized study procedures were performed by trained bilingual personnel. All participants provided written, informed consent. The study was approved by the institutional review boards at Tufts Medical Center, Tufts University, and Northeastern University.
Dietary assessment and dietary scores.
Self-reported usual dietary intake consumed in the past 12 mo was assessed at baseline by using a semiquantitative FFQ previously validated for use in this population (29). Nutrient intakes were calculated from the Nutrition Data System for Research software (Nutrition Coordinating Center). Reported serving equivalents of individual foods were used to create food groups. For mixed dishes, we disaggregated the intake into individual food items that were then added to the appropriate food group. Participants reporting outlying energy intakes (≤600 or ≥4800 kcal/d; ≤2510 or ≥20,083 kJ/d) or with ≥10 questions left blank in the FFQ were excluded (n = 64). Food and nutrient data were used to define the 5 diet quality scores as summarized in Supplemental Table 1.
The AHEI-2010 was defined by following the definition from Chiuve et al. (5). The score included 11 food groups or nutrients with consistent evidence of association with lower risk of chronic diseases. For each food group or nutrient, a participant was given a continuous score between 0 for minimal observance of the recommended intake determined a priori, and 10 points for maximal observance; intermediate values were prorated. The scores of all components were added, and the total AHEI score ranged from 0 (lowest diet quality) to 110 (highest diet quality).
We used a previously defined AHA-DS based on 2006 recommendations for CVD risk reduction (8, 30, 31). We derived 11 subcomponents to reflect adherence to the 7 dietary recommendations. Scores for each component ranged from 0 for minimal adherence to the recommended amount (or sex-specific tertile in the absence of a cutoff) to a maximum of 4, 6, or 10 points for maximal adherence, with intermediate values prorated. The combined scores of all components totaled 90 points, indicative of greater adherence to AHA recommendations.
The DASH score was defined by following the definition from Fung et al. (9). DASH includes 8 foods and nutrients emphasized or minimized in DASH. Quintiles of intake were derived for each of the components, and a point was assigned for each increasing quintile of intake of healthful food groups so that the healthiest quintile had a score of 5. Unfavorable food groups were reverse-coded so that the lowest quintile of intake of an unhealthful food was scored with 5 points. The score of each component was added and the overall DASH score ranged from 8 to 40.
We used the HEI 2005 definition as it corresponded to the dietary guidelines in place at the time of the study. The score has been previously defined in this cohort (32). HEI includes 12 components, each scored by using an energy-density approach according to procedures from the USDA Center for Nutrition Policy and Promotion (6). Six food groups were scored from 0 to 5; 5 were scored from 0 to 10, and 1 was scored from 0 to 20. Summing the 12 components generated a HEI range of 0 to 100 with higher scores indicative of better guidelines adherence.
The Mediterranean diet score (MeDS) was proposed by Trichopoulou et al. (10) and was previously defined in this population (32). We slightly modified the definition by replacing the total-grain group with whole grains because the high intake of refined grains in this population (33) would have confounded the results. The 9 components were scored by using the sex-specific population median adjusted for total energy by using the residual method. A score of 0 was assigned to a participant consuming below the median for healthful components (or above the median for unfavorable components), and 1 point was assigned for the opposite. The added components equaled a range of 0–9; higher values indicate greater observance of a Mediterranean pattern.
Cardiometabolic outcomes.
All outcomes were measured at both timepoints (baseline and 2 y) by using the same procedures. Standing height, weight, and waist and hip circumference were measured in duplicate by using standard protocols; the mean value was used. BMI (in kg/m2) was calculated as weight divided by height squared. Blood pressure (BP) was measured at 3 time points during the interview in duplicate by using an electronic sphygmomanometer (model 8260; Dinamap); the mean of the second and third readings was used.
Blood samples were drawn by trained phlebotomists after a 12-h fast. Laboratory assays were conducted to measure serum C-reactive protein (CRP; Immulite 1000 LKCRP1 kit), serum insulin (Immulite 1000 LKIN1 kit), serum glucose (reagents OSCR6121 on Olympus AU400e), plasma total cholesterol (reagents OSR6116 on Olympus AU400e), plasma HDL cholesterol (reagents OSR6195 on Olympus AU400e), plasma TGs (reagents OSR6133 on Olympus AU400e), and glycosylated hemoglobin (Roche Unimate kit on Cobas FARA). LDL cholesterol was calculated by using the Friedelwald equation. The HOMA-IR was calculated from paired fasting glucose and insulin values by using the HOMA calculator version 2.2.3 (University of Oxford) (34).
Covariates.
All covariates were measured at baseline unless specified. Participants self-reported information on age, sex, educational attainment, marital status, household income, medical history, and use of medication. The ratio of income to poverty was calculated by using the poverty guidelines from the US Department of Health and Human Services based on total household income and household composition. Acculturation was measured with a 10-item psychological acculturation scale that assesses the degree of psychological attachment to either American or Puerto Rican culture (35).
A comprehensive questionnaire gauged frequency and type of smoking behaviors. Smoking status was defined as never, former, or current. Lifetime cigarette use was calculated in packs per year. Physical activity was assessed by a modified Paffenbarger questionnaire from the Harvard Alumni Activity Survey; a score was calculated by multiplying the self-reported hours spent in heavy, moderate, light, or sedentary activities in 24 h by weighing factors that parallel the rate of oxygen consumption of each activity. The FFQ asked participants how often they consumed foods not prepared at home. Medical conditions were defined for diabetes (fasting glucose ≥126 mg/dL or medication use), hypertension (BP ≥140/90 mm Hg or medication use), and CVD (self-reported medical diagnosis of heart attack, heart disease, or stroke).
Statistical analysis.
The sample size ranged between 1137 and 1194, depending on the missing data for food groups available for each score. Descriptive analysis by quintile of each diet quality score was done by using the chi-square test for categorical variables and ANOVA for continuous variables. We converted each diet quality score into z scores to attain comparable ranges. Spearman correlations between the z scores and measures of distribution were calculated. The outcomes for plasma TGs, serum glucose, serum insulin, HOMA-IR, and CRP were log-transformed to achieve normality. To test the associations between each baseline dietary score as predictor and each 2-y cardiometabolic risk factor as outcome, we fitted multivariable-adjusted, repeated-subjects, linear mixed-effects models adjusted for covariates measured at baseline: age, sex, smoking (packs per year), physical activity score, ratio of income to poverty, educational attainment, marital status, frequency of foods away from home, acculturation, CVD, diabetes (except for glucose metabolism parameters, which were adjusted for diabetes medication only), and hypertension (except for BP, which was adjusted for hypertension medication only). Lipid outcomes were additionally adjusted for lipid-lowering medication at baseline. CRP was additionally adjusted for anti-inflammatory medications at baseline and white blood cell count measured at 2 y to account for acute inflammation. Models for the AHA-DS, DASH, and AHEI were additionally adjusted for total energy intake at baseline. Additionally, the baseline measurement of the corresponding outcome and time between measurements were entered in each model to capture the change in each measurement. Sensitivity analysis was conducted by using the difference of the 2-y from the baseline measurement as the outcome. The proportional contribution of food sources to intake of a food group or nutrient component, or category of adherence to a diet quality score, was estimated with SAS PROC RANK. SAS version 9.4 (SAS Institute) was used for all analyses. All tests were 2-tailed; P < 0.05 was considered significant. Data are shown as β coefficients ± SEs or 95% CIs.
Results
The mean, median, and observed range of the 5 diet quality scores varied considerably (Supplemental Table 2). After converting the scores into z scores, the values were comparable. All z scores were significantly correlated with each other (P < 0.0001), with the strongest correlations observed for the AHEI and DASH score (r = 0.68) and the AHEI and AHA-DS (r = 0.63). The weakest correlation was observed for the DASH score and MeDS (r = 0.37).
Participants in the top quintile of the AHA-DS tended to be older and to have a higher income-to-poverty ratio, physical activity score, acculturation, and educational attainment than participants in the lowest quintile (Table 1). Individuals in the top DASH quintile were more likely to be older, nonsmokers, and married and to have a higher income-to-poverty ratio and acculturation. Participants with a higher adherence to the HEI tended to be older, female, nonsmokers, and less likely to consume foods away from home than participants with the lowest adherence. They were also more likely to have diabetes, heart disease, and obesity at baseline and higher BMI but lower diastolic BP at 2 y. Individuals in the top MeDS quintile had higher acculturation and a higher income-to-poverty ratio and were more likely to have attained an eighth-grade or higher education, to have diabetes at baseline, and to have lower waist circumference and glucose but higher BMI at 2 y. Finally, participants in the healthiest AHEI quintile tended to have a higher ratio of income to poverty and obesity; 2-y BP and insulin tended to be lower, but LDL cholesterol tended to be higher for these individuals than for participants with the unhealthiest quintile of AHEI.
TABLE 1.
Characteristics of Puerto Rican adults by lowest and highest quintiles of 5 diet quality scores1
| AHA-DS (n = 1140) |
DASH (n = 1189) |
HEI (n = 1194) |
MeDS (n = 1194) |
AHEI (n = 1137) |
||||||
| Q1 (5.2–20.5) | Q5 (38.3–68.7) | Q1 (11–20) | Q5 (28–36) | Q1 (30.6–64.4) | Q5 (79.7–94.7) | Q1 (0–2) | Q5 (6–9) | Q1 (24.9–46.5) | Q5 (60.9–83.7) | |
| Baseline biomarkers | ||||||||||
| Age, y | 56.0 ± 8.02 | 58.1 ± 7.3* | 55.3 ± 7.1 | 58.8 ± 7.3*** | 55.2 ± 7.0 | 59.4 ± 7.5*** | 56.6 ± 7.9 | 57.2 ± 7.7 | 56.4 ± 7.4 | 57.0 ± 7.1 |
| Female | 67.53 | 77.6 | 75.7 | 73.0 | 64.7 | 85.4*** | 72.8 | 70.9 | 68.3 | 77.5 |
| Current smoker | 28.1 | 18.4 | 31.1 | 13.8** | 42.0 | 11.3*** | 31.0 | 22.3 | 27.8 | 17.7 |
| Ratio of income to poverty4 | 1.13 ± 0.85 | 1.64 ± 1.8** | 1.13 ± 0.89 | 1.64 ± 0.33* | 1.20 ± 0.97 | 1.60 ± 0.34 | 1.09 ± 0.83 | 1.76 ± 0.31*** | 1.21 ± 0.90 | 1.77 ± 0.34*** |
| Physical activity score5 | 31.0 ± 4.5 | 32.2 ± 4.6* | 31.0 ± 3.8 | 32.0 ± 4.6 | 31.2 ± 4.5 | 31.5 ± 4.1 | 30.9 ± 4.4 | 32.0 ± 4.8 | 31.2 ± 4.6 | 32.1 ± 4.3 |
| Psychological acculturation score6 | 17.5 ± 6.4 | 20.1 ± 7.0*** | 17.5 ± 6.5 | 19.5 ± 6.8* | 18.8 ± 7.3 | 18.4 ± 6.8 | 17.1 ± 6.6 | 19.4 ± 7.0** | 17.9 ± 6.6 | 19.2 ± 6.9 |
| Education higher than eighth grade | 54.4 | 64.0** | 52.3 | 59.8 | 56.7 | 49.4 | 50.0 | 61.1** | 52.9 | 59.7 |
| Married/with partner | 30.0 | 31.3 | 24.0 | 36.4* | 30.7 | 29.4 | 29.8 | 37.1 | 26.4 | 26.1 |
| Eat away from home ≥1 time/wk | 25.4 | 22.4 | 28.8 | 19.6 | 33.3 | 15.1*** | 21.7 | 23.0 | 27.8 | 23.4 |
| Diabetes7 | 39.9 | 40.3 | 36.4 | 37.4 | 31.6 | 42.4** | 34.8 | 39.1* | 34.7 | 39.7 |
| CVD7 | 16.3 | 23.0 | 19.4 | 25.9 | 18.9 | 25.4* | 15.2 | 23.5 | 15.9 | 22.5 |
| Hypertension7 | 64.4 | 67.6 | 68.2 | 70.4 | 62.6 | 74.0 | 64.1 | 70.1 | 66.5 | 69.3 |
| Obesity7 | 51.5 | 57.3 | 53.4 | 57.4 | 46.8 | 56.8** | 56.1 | 54.6 | 50.2 | 57.3** |
| 2-y biomarkers | ||||||||||
| Waist circumference, cm | 103 ± 14 | 102 ± 14* | 104 ± 15 | 102 ± 15 | 103 ± 15 | 103 ± 14 | 103 ± 14 | 102 ± 13** | 105 ± 15 | 102 ± 13 |
| Waist-hip ratio | 0.95 ± 0.10 | 0.93 ± 0.08 | 0.95 ± 0.09 | 0.94 ± 0.08 | 0.95 ± 0.09 | 0.94 ± 0.08 | 0.95 ± 0.09 | 0.95 ± 0.08 | 0.95 ± 0.09 | 0.94 ± 0.07 |
| BMI, kg/m2 | 31.1 ± 6.1 | 31.2 ± 5.8 | 31.8 ± 6.9 | 31.7 ± 6.3 | 30.5 ± 6.8 | 32.2 ± 6.1* | 31.4 ± 6.5 | 31.8 ± 5.5*** | 31.8 ± 7.1 | 31.5 ± 5.5 |
| Systolic BP, mm Hg | 135 ± 18 | 136 ± 19 | 135 ± 21 | 136 ± 19 | 136 ± 22 | 137 ± 21 | 135 ± 19 | 137 ± 20 | 136 ± 20 | 136 ± 19* |
| Diastolic BP, mm Hg | 81.2 ± 10.3 | 79.5 ± 10.9 | 82.1 ± 11.9 | 79.4 ± 9.8 | 82.9 ± 11.7 | 78.3 ± 10.1*** | 80.9 ± 11.7 | 80.5 ± 11.4 | 81.4 ± 10.8 | 80.3 ± 10.5* |
| Plasma total cholesterol, mg/dL | 185 ± 43 | 187 ± 42 | 190 ± 46 | 185 ± 43 | 190 ± 40 | 187 ± 44 | 188 ± 42 | 186 ± 45 | 187 ± 45 | 190 ± 43 |
| Plasma HDL cholesterol, mg/dL | 45.7 ± 11.7 | 47.6 ± 11.6 | 46.2 ± 13.3 | 45.5 ± 12.3 | 46.8 ± 12.8 | 47.5 ± 11.6 | 46.3 ± 12.5 | 45.9 ± 12.3 | 44.4 ± 11.7 | 47.5 ± 13.4 |
| Plasma LDL cholesterol, mg/dL | 110 ± 35 | 108 ± 34 | 111 ± 37 | 109 ± 36 | 112 ± 33 | 110 ± 36 | 109 ± 35 | 108 ± 36 | 110 ± 37 | 111 ± 34* |
| Plasma TGs, mg/dL | 151 ± 76 | 160 ± 94 | 168 ± 118 | 157 ± 80 | 163 ± 112 | 150 ± 76 | 163 ± 93 | 165 ± 127 | 168 ± 107 | 162 ± 132 |
| Serum glucose, mg/dL | 122 ± 68 | 113 ± 39 | 120 ± 61 | 110 ± 32 | 111 ± 49 | 115 ± 40 | 115 ± 53 | 112 ± 36** | 119 ± 59 | 114 ± 38 |
| Serum insulin, μIU/mL | 17.1 ± 14.3 | 17.1 ± 22.1 | 17.5 ± 16.1 | 15.8 ± 13.5 | 15.7 ± 143.7 | 17.6 ± 22.3 | 17.1 ± 14.9 | 16.0 ± 20.7 | 18.8 ± 16.0 | 14.7 ± 13.6* |
| HOMA-IR | 2.3 ± 1.9 | 2.4 ± 2.7 | 2.4 ± 2.1 | 2.2 ± 1.8 | 2.1 ± 1.7 | 2.4 ± 2.7 | 2.7 ± 4.3 | 2.2 ± 2.5 | 2.6 ± 2.2 | 2.0 ± 1.7 |
| Glycosylated hemoglobin, % | 6.9 ± 1.8 | 6.7 ± 1.4 | 6.8 ± 1.6 | 6.8 ± 1.4 | 6.6 ± 1.7 | 6.9 ± 1.4 | 6.7 ± 1.3 | 6.8 ± 1.5 | 6.7 ± 1.6 | 6.7 ± 1.5 |
| Serum CRP, mg/L | 7.4 ± 16.8 | 5.4 ± 6.7 | 7.1 ± 14.0 | 5.0 ± 7.4 | 5.8 ± 7.2 | 6.2 ± 8.2 | 6.8 ± 10.2 | 5.1 ± 7.0 | 5.9 ± 7.7 | 5.2 ± 6.7 |
The range of scores are shown in parentheses for Q1 and Q5 for each diet. Differences were calculated by using the chi-square test or ANOVA. *,**,***Significance across quintiles: *P < 0.05, **P < 0.01, ***P < 0.001. To convert total cholesterol, HDL cholesterol, or LDL cholesterol to mmol/L, multiply values by 0.0259. To convert TGs to mmol/L, multiply values by 0.0113. To convert fasting blood glucose to mmol/L, multiply values by 0.0555. AHA-DS, American Heart Association diet score; AHEI, Alternative Healthy Eating Index; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; MeDS, Mediterranean diet score; Q, quintile.
Mean ± SD (all such values).
Percentage (all such values).
Calculated by using the poverty guidelines from the US Department of Health and Human Services based on total household income and household composition.
Assessed by a modified Paffenbarger questionnaire from the Harvard Alumni Activity Survey; the score was defined by multiplying the self-reported hours spent in heavy, moderate, light, or sedentary activities over 24 h by weighing factors that parallel the rate of oxygen consumption of each activity. Higher scores are indicative of greater physical activity.
Measured with a 10-item psychological acculturation scale that assesses the degree of psychological attachment to either American or Puerto Rican culture. Higher scores are indicative of greater acculturation.
Conditions were defined for diabetes (fasting glucose ≥126 mg/dL or medication use), hypertension (BP ≥140/90 mm Hg or medication use), CVD (self-reported medical diagnosis of heart attack, heart disease, or stroke), and obesity [BMI (in kg/m2) ≥25].
We tested the association between each diet quality z score and each of the 14 cardiometabolic risk factors at 2 y, adjusting for baseline measures (Table 2). After adjusting for sociodemographic variables, lifestyle behaviors, use of medications, and several preexisting health conditions at baseline, we observed that the AHA-DS was inversely associated with BMI (−0.17 ± 0.081, P = 0.033) but not with other cardiometabolic risk factors. The AHEI was significantly associated with lower BMI (−0.16 ± 0.08, P = 0.039), log-insulin (−0.05 ± 0.02, P = 0.013), and log–HOMA-IR (−0.05 ± 0.02, P = 0.023). Neither DASH nor HEI was significantly associated with any parameter. The MeDS was significantly associated with lower waist circumference (−0.52 ± 0.26, P = 0.048), BMI (−0.23 ± 0.08, P = 0.005), log-insulin (−0.06 ± 0.02, P = 0.005), log–HOMA-IR (−0.05 ± 0.02, P = 0.030), and log-CRP (−0.13 ± 0.03, P = 0.0002). The associations with log-insulin, log–HOMA-IR, and log-CRP were slightly attenuated but remained significant when further adjusted for BMI (data not shown). The results from sensitivity analysis by using the difference of 2 y from the baseline measurement were similar to the reported results (data not shown).
TABLE 2.
β coefficients for the association of baseline diet quality z scores and 2-y cardiometabolic risk factors among Puerto Rican adults1
| AHA-DS (n = 1140) |
DASH (n = 1189) |
HEI (n = 1194) |
MeDS (n = 1194) |
AHEI (n = 1137) |
||||||
| β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | |
| Anthropometric measures | ||||||||||
| Waist circumference, cm | −0.09 ± 0.28 | 0.75 | 0.05 ± 0.27 | 0.87 | −0.16 ± 0.28 | 0.56 | −0.52 ± 0.26 | 0.048 | −0.26 ± 0.27 | 0.33 |
| Waist-hip ratio | −0.00002 ± 0.002 | 0.99 | −0.001 ± 0.002 | 0.75 | −0.002 ± 0.002 | 0.44 | −0.002 ± 0.002 | 0.22 | −0.001 ± 0.002 | 0.67 |
| BMI, kg/m2 | −0.17 ± 0.08 | 0.033 | −0.07 ± 0.08 | 0.39 | −0.04 ± 0.08 | 0.63 | −0.23 ± 0.08 | 0.005 | −0.16 ± 0.08 | 0.039 |
| BP, mm Hg | ||||||||||
| Systolic | 0.33 ± 0.54 | 0.53 | 0.34 ± 0.53 | 0.50 | 0.23 ± 0.53 | 0.67 | −0.06 ± 0.51 | 0.91 | 0.26 ± 0.52 | 0.61 |
| Diastolic | −0.11 ± 0.30 | 0.72 | −0.18 ± 0.29 | 0.54 | −0.43 ± 0.29 | 0.14 | −0.24 ± 0.28 | 0.40 | −0.05 ± 0.29 | 0.87 |
| Plasma lipids, mg/dL | ||||||||||
| Total cholesterol | −0.27 ± 1.1 | 0.81 | 0.15 ± 1.1 | 0.89 | 0.68 ± 1.1 | 0.55 | 0.01 ± 1.1 | 0.99 | 0.41 ± 1.1 | 0.70 |
| HDL cholesterol | −0.07 ± 0.26 | 0.78 | −0.51 ± 0.26 | 0.05 | 0.13 ± 0.27 | 0.64 | −0.01 ± 0.25 | 0.96 | 0.04 ± 0.26 | 0.87 |
| LDL cholesterol | −0.04 ± 0.94 | 0.96 | 0.80 ± 0.92 | 0.39 | 1.54 ± 0.94 | 0.10 | 0.79 ± 0.90 | 0.38 | 0.71 ± 0.91 | 0.44 |
| Log-TGs | −0.01 ± 0.01 | 0.66 | −0.003 ± 0.01 | 0.81 | −0.02 ± 0.01 | 0.28 | −0.02 ± 0.01 | 0.21 | −0.01 ± 0.01 | 0.34 |
| Glucose metabolism | ||||||||||
| Log-serum glucose, mg/dL | −0.01 ± 0.01 | 0.51 | −0.01 ± 0.01 | 0.59 | −0.01 ± 0.01 | 0.31 | −0.01 ± 0.01 | 0.20 | −0.002 ± 0.01 | 0.80 |
| Log-serum insulin, μIU/mL | −0.02 ± 0.02 | 0.48 | −0.002 ± 0.02 | 0.92 | −0.02 ± 0.02 | 0.36 | −0.06 ± 0.02 | 0.005 | −0.05 ± 0.02 | 0.013 |
| Log–HOMA-IR | −0.01 ± 0.02 | 0.76 | −0.002 ± 0.02 | 0.94 | −0.02 ± 0.02 | 0.31 | −0.05 ± 0.02 | 0.030 | −0.05 ± 0.02 | 0.023 |
| Glycosylated hemoglobin, % | −0.06 ± 0.04 | 0.10 | −0.02 ± 0.04 | 0.67 | −0.06 ± 0.04 | 0.11 | −0.05 ± 0.04 | 0.13 | −0.04 ± 0.04 | 0.32 |
| Inflammation | ||||||||||
| Log-serum CRP, mg/L | −0.04 ± 0.04 | 0.21 | 0.01 ± 0.03 | 0.76 | −0.02 ± 0.04 | 0.67 | −0.13 ± 0.03 | 0.0002 | −0.02 ± 0.03 | 0.63 |
Values were calculated with multivariable-adjusted, repeated-subjects, mixed-effects models adjusted for the following baseline covariates: age, sex, smoking (packs per year), physical activity score, ratio of income to poverty, educational attainment, marital status, frequency of foods away from home, acculturation, cardiovascular disease, diabetes (except for glucose metabolism parameters, which were adjusted for diabetes medication only), hypertension (except for BP, which was adjusted for hypertension medication only), baseline measurements, and time. Lipid outcomes were additionally adjusted for baseline lipid-lowering medication. CRP was additionally adjusted for anti-inflammatory medications at baseline and white blood cell count at 2 y. Models for AHA-DS, DASH, and AHEI were additionally adjusted for energy intake at baseline. To convert total cholesterol, HDL cholesterol, or LDL cholesterol to mmol/L, multiply values by 0.0259. To convert TGs to mmol/L, multiply values by 0.0113. To convert fasting blood glucose to mmol/L, multiply values by 0.0555. AHA-DS, American Heart Association diet score; AHEI, alternative Healthy Eating Index; BP, blood pressure; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; MeDS, Mediterranean diet score.
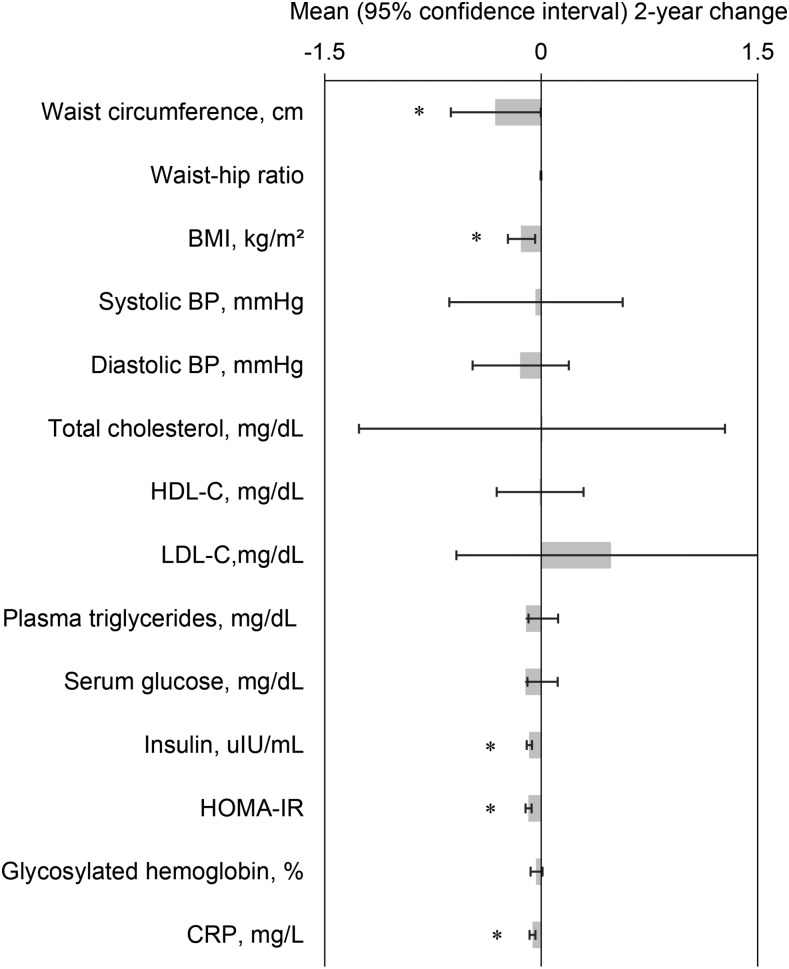
We plotted the mean 2-y change in cardiometabolic risk factors per point of the MeDS, for which we observed the most significant associations with cardiometabolic outcomes (Figure 1). Increasing 1 point, or the equivalent of adhering to 1 additional dietary component of the MeDS, was significantly associated with a lower mean 0.31 cm (0.003, 0.06 cm) of waist circumference, 0.14 units (0.04, 0.23 units) of BMI, 0.08 μIU/mL (0.06, 0.10 μIU/mL) of insulin, 0.08 units (0.07, 0.11 units) of HOMA-IR, and 0.06 mg/dL (0.04, 0.08 mg/dL) of CRP at 2 y, adjusting for baseline measurements.
FIGURE 1.
Changes over 2 y in cardiometabolic risk factor per each healthy dietary component of the Mediterranean diet score followed by Puerto Rican adults. Values are means or back-transformed geometric means for the log-TGs, log-glucose, log-insulin, log–HOMA-IR, log-CRP with corresponding 95% CIs; n = 1194. The following were calculated with multivariable-adjusted, repeated-subjects, mixed-effects models adjusted for baseline covariates: age, sex, smoking (packs per year), physical activity score, ratio of income to poverty, educational attainment, marital status, frequency of foods away from home, acculturation, cardiovascular disease, diabetes (except for glucose metabolism parameters, which were adjusted for diabetes medication only), hypertension (except for BP, which was adjusted for hypertension medication only), baseline measurements, and time. Lipid outcomes were additionally adjusted for baseline lipid-lowering medication. CRP was additionally adjusted for anti-inflammatory medications at baseline and white blood cell count at 2 y. BP, blood pressure; CRP, C-reactive protein; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol. *P < 0.05.
We ranked the contribution of individual food items within a component to the energy intake of the whole component within the MeDS (Table 3). The sex-specific energy-adjusted medians used in this cohort are also shown. In general, a healthy score was estimated at 1.5 servings/d (300 g/d) for vegetable intake, 1.1 servings/d (220 g/d) for fruit, and 2/3 servings/d (21 g/d) for whole grain. Main sources of vegetables included those added to homemade soups, potatoes, iceberg lettuce, root crops, and dark-green leafy vegetables. Fruit sources tended to be juices and green bananas used to prepare pasteles (meat-filled patties). Healthful whole grains were obtained mostly from oatmeal, light bread, and cold cereals. Men had higher median intakes than women for meat (4.7 compared with 4.0 servings/d or 132 compared with 112 g/d) and fish (0.95 compared with 0.81 servings/d or 27 compared with 23 g/d). Main contributors to meat intake included meats added to soups and stews (including poultry), regular beef, and deli meats; contributors to fish intake included regular fish (such as cod), shellfish, and canned tuna. Dairy mostly comprised milk and cheese, both whole- and low-fat, with a median intake of ∼1.7 servings/d (425 mL milk/d or 71 g cheese/d). Foods consumed by those with a high ratio of monounsaturated to saturated fat were corn and olive oils, poultry, tuna, and fried potatoes; sources of alcohol were beer, white wine, and liquors.
TABLE 3.
Population-based energy-adjusted sex-specific median values of each Mediterranean diet score component and top-ranking foods consumed by Puerto Rican adults in the healthy intake category1
| Median cutoff for Mediterranean diet score |
||||
| Dietary components | Men | Women | Top 5 foods contributing to energy intake within the food or nutrient group | Contribution to energy of the top 5 foods to the food or nutrient group, % |
| Vegetables, servings/d | 1.56 | 1.47 | Vegetables added to homemade soups Potatoes, boiled/bakedIceberg lettuceRoot crops (excludes sweet potato)Dark-green leafy vegetables | 49.9 |
| Fruits, servings/d | 1.07 | 1.07 | Orange juiceOther fruit juices (e.g., grape, cranberry)Green bananas for pasteles (meat patties)Apples and pearsGrapefruit juice | 65.6 |
| Whole grains, servings/d | 0.64 | 0.66 | Instant hot oatmealLight whole-wheat breadRegular hot oatmealCold cerealsLight white bread | 80.5 |
| Nuts and legumes, servings/d | 0.77 | 0.65 | Beans and legumesPeanut butterCowpeas, black-eyed peasNuts and seeds (including coconut)Peanuts | 74.4 |
| Meat, servings/d | 4.70 | 4.01 | Meat added to homemade soups (e.g., chicken soup)Meat added to homemade stews (e.g., sancocho)Regular ground beefDeli/luncheon meatRegular beef used for mixed dishes | 41.4 |
| Fish, servings/d | 0.95 | 0.81 | Fish (e.g., cod, haddock)ShellfishShellfish added to homemade stews (e.g., shrimp)Canned tuna, water packedTuna fish in salad or sandwich | 83.7 |
| Dairy, servings/d | 1.76 | 1.56 | Milk, wholeProcessed cheeseMilk, 2%Natural cheeseMilk, skim | 61.7 |
| MUFA:SFA ratio | 1.16 | 1.18 | Corn oilOlive oilChicken/turkey, dark meat, broiled with skinTuna fish in salad or sandwichFried potatoes/French fries | 29.9 |
| Alcohol, drinks/d | ≤2 | ≤1 | Regular beerLight beerWhite wineLiquorsMixed drinks | 98.0 |
Serving sizes are expressed in USDA MyPyramid (https://www.cnpp.usda.gov/mypyramid) equivalents as follows: vegetables = 1 cup (200 g); fruits = 1 cup (200 g) or 0.5 cup dried (100 g); nuts or legumes = 0.5 oz (14 g) nuts, 1 tablespoon (14 g) peanut butter, or 1 cup (200 g) cooked legumes; whole grains = 1 slice of bread or grain product containing 16 g flour or 0.5 cup (31 g) cooked cereal; meat = 1 oz (28 g); fish = 1 oz (28 g); dairy = 1 cup (250 mL) milk, yogurt, or soymilk, 1.5 oz (42 g) of natural cheese or 2 oz (56 g) of processed cheese. One standard drink (wine, beer, or liquor) = 14 g ethanol.
Discussion
In Puerto Rican adults, higher MeDS and AHEI scores were associated with favorable cardiometabolic risk factors at 2 y, adjusted for baseline, including markers of adiposity, insulin, and inflammation. MeDS was associated with more markers, and these associations were stronger than for AHEI. For Puerto Ricans, the components and weights used in the MeDS may be more relevant for cardiometabolic regulation. The DASH score, AHA-DS, and HEI score showed little or no association with the outcomes. It has been posited that the HEI is not adequate for evaluating risk of chronic diseases related to diet (4) because it omits protective FAs or fish intake as a distinct category and combines whole and refined grains. Notably, a lower BMI was consistently observed across 3 of the scores, suggesting that overall healthy eating measured in various ways may reduce adiposity over time. This observation has been consistent in the literature (18).
Differences in the specific food groupings, types of foods within a category, and weights and scoring systems used for each index may explain the observed differences in results. Other studies have reported inconsistent associations across scores. Among Guatemalan young adults and 4 scores that were tested, only the Diet Quality Index-International was associated with BMI and waist circumference, and the Recommended Food Score was associated with TGs and glucose (19). Another study that compared 5 scores showed differential association with lipid markers, central obesity, and BP; the MeDS was more strongly associated with the outcomes (20). Using NHANES, Kant and Graubard (22) showed that the Recommended Food Score and a Dietary Diversity Score were more strongly associated with several biomarkers compared with the HEI.
The aforementioned studies were cross-sectional, which are susceptible to reverse causality and may not be directly compared with our longitudinal results. Alkerwi et al. (20) suggested that reverse causality may be minimal if the population is relatively free of chronic disease, which is not the case for Puerto Ricans. Our results indicate some reverse directionality because more individuals with a preexisting condition at baseline were in the healthiest quintile for the HEI, which is based on widely publicized national dietary recommendations. Higher diet quality among Hispanics with diabetes has been shown in another study (28). Thus, it is important to adjust for preexisting conditions in the association between diet and cardiometabolic markers.
The DASH score and the MeDS were defined by using population-based cutoffs, whereas the other 3 scores were based on dietary recommendations. Puerto Ricans tend to have poor overall diet (8, 28), and it is likely that the limited representation of some foods and nutrients in the guidelines-based indexes influenced the scoring. Although we expected significant associations between the DASH score and BP, a previous study among US Hispanics also failed to detect associations with this DASH definition but observed significant associations by using a simpler DASH score with predefined dichotomous cutoffs and slightly different food groups (36).
Ethnicity and sex also seem to play a role in the association between diet quality scores and health outcomes. In the Multiethnic Cohort, significant associations with type 2 diabetes were observed for DASH in non-Hispanic white men and women, Japanese American women, and Native American men, whereas AHEI and an alternative MeDS were associated with diabetes in non-Hispanic white participants only; the HEI was not significantly associated with diabetes in any ethnic or sex group (21). The authors suggested that differences in associations may have been due to particular consumption patterns and food components, particularly as most scores were originally developed in non-Hispanic white populations. Other studies have also reported sex-specific associations between diet scores and cardiometabolic outcomes (8, 19, 20); however, we did not test for interactions in this analysis.
We slightly modified the MeDS by using whole grains rather than total grains as other researchers have done (12, 18, 37, 38). Other limitations of the MeDS are that it combines full-fat and low-fat dairy, combines all meat in one category (e.g., healthy lean meats as well as unfavorable processed meats), and includes fruit juices in the fruit category despite evidence that some types of juice may have adverse metabolic outcomes (39). Still, MeDSs are fairly easy to define, are suitable to measure diet quality and diet-disease associations (4), may better capture dietary variation in a population as related to cardiometabolic risk (20), and may have potential clinical applications when assessed by using quick screeners (40).
A novel and practical contribution of our work was to identify the most-consumed foods within each component of the MeDS. The typical Mediterranean lifestyle characterized by consumption of olive oil, wine, nuts, whole-grain breads, and fresh fruits and vegetables may not necessarily translate to Puerto Rican food choices. Indeed, we identified foods that are more akin to traditional Puerto Rican cuisine: homemade soups and stews, root crops, fruit juice, green bananas, oatmeal, light bread, beans and legumes, fish and shellfish, canned tuna, milk and cheese, corn and olive oils, and beer. The ranking helped us identify healthful foods that the population already consumes and could be further promoted in public health campaigns and interventions, as well as foods that could be replaced (e.g., processed cheese). One caveat is that the subjective food groupings and disaggregation of mixed dishes may not accurately capture some individual foods.
Notably, the median intakes of each food group of the MeDS reported in this cohort do not align with current dietary guidelines. For example, 2.5 servings/d (500 g/d) of vegetables and 2 servings/d (400 g/d) of fruits are recommended (41), but the median intake was 1.5 servings/d (300 g/d) and 1.1 servings/d (220 g/d), respectively. The recommended and median intakes for whole grains were 3 servings/d (93 g/d) and 0.7 servings/d (22 g/d), respectively. Fish intake (7 oz/wk or 1370 g/wk) was close to the recommended 8 oz/wk (1570 g/wk). Dietary guidelines do not provide amounts for nuts and legumes or meat; however, the median intake of meats here was nearly 6 times higher than that of nuts and legumes. These observations suggest that the diet quality among Puerto Ricans needs to be further improved. Moreover, the observed modest reductions in cardiometabolic markers with a higher MeDS may translate into more meaningful cumulative clinical benefits if people were to follow current dietary recommendations and/or satisfactorily meet more MeDS components.
We should note that measurement error in dietary assessment cannot be ruled out, despite our use of a validated FFQ. Although we included the diet quality scores most commonly used in the literature, it may be possible that other scores include components and scoring appropriate for disease association in this population. The HEI and AHA-DS used here were based on dietary guidelines in place at the time of the study; however, these have been revised recently based on new evidence, and the updated scores may produce different results. We were not able to assess changes in diet; however, the FFQ captures usual long-term intake.
Strengths of our study include using an FFQ validated for this population and linked to a nutrient and food database that allowed identifying traditional Puerto Rican food components within the dietary scores. We were able to directly compare the magnitude of association of the 5 commonly used dietary scores by applying a z score. Our longitudinal analysis enriches the literature on association of diet and cardiometabolic risk factors, which is mostly represented by cross-sectional studies. Finally, our results can further encourage health professionals to counsel individuals to observe a healthy diet with the goal of improving clinical cardiometabolic outcomes. For example, a person at the favorable median intake of just 3 MeDS components may have ∼1-cm lower waist circumference than those with less MeDS adherence in 2 y while maintaining other socioeconomic and behavioral factors constant. One meta-analysis estimated that each centimeter of waist circumference increased the risk of a CVD event by 2% (42). Furthermore, adhering to all MeDS components could translate into a nearly 1.2-kg/m2 lower BMI in 2 y. At the population level, each unit decrease in BMI has been estimated to result in 26 and 28 fewer cases of chronic disease per 1000 men and women, respectively (43).
In conclusion, the MeDS comprises food components and scores that are associated with favorable 2-y changes in cardiometabolic risk factors in Puerto Rican adults. An overall healthy diet may be beneficial for lowering or maintaining a lower BMI. The scoring and grouping of food components used in various diet quality scores seem to influence associations with health outcomes. Population-based cutoffs may be appropriate in capturing distribution of food intake that may be skewed in at-risk populations with low adherence to dietary guidelines, although they may limit comparability across studies. Notably, the MeDS components represented in this Puerto Rican cohort included foods not usually observed in the typical Mediterranean diet, highlighting the need to identify specific foods within scores that were developed in another population. This also helps identify culturally relevant foods for better translational applications. Our results show that choosing the right measure of diet quality affects the results, and assessing them for each population is crucial when crafting nutritional messages and programs with effective, meaningful dietary components for at-risk minority groups.
Acknowledgments
JM designed the analysis, conducted the statistical analysis, interpreted the results, wrote the manuscript, and had primary responsibility for the final content; MS-P, SJB, SEN, and KLT contributed to the study design and interpreted the results; MS-P and SJB analyzed the data. All authors contributed meaningfully to the manuscript and read and approved the final manuscript.
Footnotes
Abbreviations used: AHA-DS, AHA diet score; AHEI, Alternative Healthy Eating Index; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; MeDS, Mediterranean diet score.
References
- 1.Mozaffarian D, Wilson PW, Kannel WB. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation 2008;117:3031–8. [DOI] [PubMed] [Google Scholar]
- 2.Wirt A, Collins CE. Diet quality–what is it and does it matter? Public Health Nutr 2009;12:2473–92. [DOI] [PubMed] [Google Scholar]
- 3.Fransen HP, Ocke MC. Indices of diet quality. Curr Opin Clin Nutr Metab Care 2008;11:559–65. [DOI] [PubMed] [Google Scholar]
- 4.Arvaniti F, Panagiotakos DB. Healthy indexes in public health practice and research: a review. Crit Rev Food Sci Nutr 2008;48:317–27. [DOI] [PubMed] [Google Scholar]
- 5.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guenther PM, Reedy J, Krebs-Smith SM. Development of the healthy eating index-2005. J Am Diet Assoc 2008;108:1896–901. [DOI] [PubMed] [Google Scholar]
- 7.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the healthy eating index-2005. J Am Diet Assoc 2008;108:1854–64. [DOI] [PubMed] [Google Scholar]
- 8.Mattei J, Bhupathiraju S, Tucker KL. Higher adherence to a diet score based on American Heart Association recommendations is associated with lower odds of allostatic load and metabolic syndrome in Puerto Rican adults. J Nutr 2013;143:1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 10.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 11.Alkerwi A. Diet quality concept. Nutrition 2014;30:613–8. [DOI] [PubMed] [Google Scholar]
- 12.de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 2011;34:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwingshackl L, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115:780–800. e5. [DOI] [PubMed] [Google Scholar]
- 14.Cespedes EM, Hu FB, Tinker L, Rosner B, Redline S, Garcia L, Hingle M, Van Horn L, Howard BV, Levitan EB, et al. Multiple healthful dietary patterns and type 2 diabetes in the women’s health initiative. Am J Epidemiol 2016;183:622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation 2015;132:2212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the women’s health initiative observational study: evidence to inform national dietary guidance. Am J Epidemiol 2014;180:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the multiethnic cohort: the dietary patterns methods project. Am J Clin Nutr 2015;101:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung TT, Pan A, Hou T, Chiuve SE, Tobias DK, Mozaffarian D, Willett WC, Hu FB. Long-term change in diet quality is associated with body weight change in men and women. J Nutr 2015;145:1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory CO, McCullough ML, Ramirez-Zea M, Stein AD. Diet scores and cardio-metabolic risk factors among Guatemalan young adults. Br J Nutr 2009;101:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkerwi A, Vernier C, Crichton GE, Sauvageot N, Shivappa N, Hebert JR. Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the observation of cardiovascular risk factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr 2015;113:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs S, Harmon BE, Boushey CJ, Morimoto Y, Wilkens LR, Le Marchand L, Kroger J, Schulze MB, Kolonel LN, Maskarinec G. A priori-defined diet quality indexes and risk of type 2 diabetes: the multiethnic cohort. Diabetologia 2015;58:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kant AK, Graubard BI. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J Am Coll Nutr 2005;24:294–303. [DOI] [PubMed] [Google Scholar]
- 23.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA 2012;308:1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiss G, Snyder ML, Teng Y, Schneiderman N, Llabre MM, Cowie C, Carnethon M, Kaplan R, Giachello A, Gallo L, et al. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: the Hispanic Community Health Study/Study of Latinos. Diabetes Care 2014;37:2391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Pina IL, Ramirez SM, Rodriguez B, et al. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation 2014;130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotos-Prieto M, Bhupathiraju SN, Falcon LM, Gao X, Tucker KL, Mattei J. Association between a healthy lifestyle score and inflammatory markers among Puerto Rican adults. Nutr Metab Cardiovasc Dis 2016;26:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattei J, Sotres-Alvarez D, Daviglus ML, Gallo LC, Gellman M, Hu FB, Tucker KL, Willett WC, Siega-Riz A, Van Horn L, et al. Diet quality and its association with cardiometabolic risk factors vary by Hispanic/Latino ethnic background in the Hispanic Community Health Study/Study of Latinos. J Nutr 2016;146:2035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol 1998;148:507–18. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 31.Bhupathiraju SN, Lichtenstein AH, Dawson-Hughes B, Tucker KL. Adherence index based on the AHA 2006 diet and lifestyle recommendations is associated with select cardiovascular disease risk factors in older Puerto Ricans. J Nutr 2011;141:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, healthy eating index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J Acad Nutr Diet 2013;113:276–81. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Rompay MI, McKeown NM, Castaneda-Sceppa C, Ordovas JM, Tucker KL. Carbohydrate nutrition differs by diabetes status and is associated with dyslipidemia in Boston Puerto Rican adults without diabetes. J Nutr 2013;143:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxford University Diabetes Trials Unit, Centre for Diabetes, Endocrinology, and Metabolism. HOMA Calculator [Internet]. [cited 2016 Nov 30]. Available from: http://www.dtu.ox.ac.uk/homacalculator/.
- 35.Tropp LR, Erkut S, Coll CG, Alarcon O, Vazquez Garcia HA. Psychological acculturation: development of a new measure for Puerto Ricans on the U.S. Mainland. Educ Psychol Meas 1999;59:351–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tangney C, Sarkar D, Staffileno BA. Comparison of three DASH scoring paradigms and prevalent hypertension among older Hispanics. J Hum Hypertens 2016;30:210–5. [DOI] [PubMed] [Google Scholar]
- 37.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steffen LM, Van Horn L, Daviglus ML, Zhou X, Reis JP, Loria CM, Jacobs DR, Duffey KJ. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Br J Nutr 2014;112:1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattei J, Malik V, Hu FB, Campos H. Substituting homemade fruit juice for sugar-sweetened beverages is associated with lower odds of metabolic syndrome among Hispanic adults. J Nutr 2012;142:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Lamuela-Raventós R, Ros E, Salaverría I, Fiol M, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr 2011;141:1140–5. [DOI] [PubMed] [Google Scholar]
- 41.Department of Health and Human Services and USDA. 2015–2020 Dietary Guidelines for Americans [Internet]. 8th ed. [cited 2016 Nov 30]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 42.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007;28:850–6. [DOI] [PubMed] [Google Scholar]
- 43.Kearns K, Dee A, Fitzgerald AP, Doherty E, Perry IJ. Chronic disease burden associated with overweight and obesity in Ireland: the effects of a small BMI reduction at population level. BMC Public Health 2014;14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]