Abstract
During plaque progression, inflammatory cells progressively accumulate in the adventitia, paralleled by an increased presence of leaky vasa vasorum. We here show that next to vasa vasorum, also the adventitial lymphatic capillary bed is expanding during plaque development in humans and mouse models of atherosclerosis. Furthermore, we investigated the role of lymphatics in atherosclerosis progression. Dissection of plaque draining lymph node and lymphatic vessel in atherosclerotic ApoE−/− mice aggravated plaque formation, which was accompanied by increased intimal and adventitial CD3+ T cell numbers. Likewise, inhibition of VEGF-C/D dependent lymphangiogenesis by AAV aided gene transfer of hVEGFR3-Ig fusion protein resulted in CD3+ T cell enrichment in plaque intima and adventitia. hVEGFR3-Ig gene transfer did not compromise adventitial lymphatic density, pointing to VEGF-C/D independent lymphangiogenesis. We were able to identify the CXCL12/CXCR4 axis, which has previously been shown to indirectly activate VEGFR3, as a likely pathway, in that its focal silencing attenuated lymphangiogenesis and augmented T cell presence. Taken together, our study not only shows profound, partly CXCL12/CXCR4 mediated, expansion of lymph capillaries in the adventitia of atherosclerotic plaque in humans and mice, but also is the first to attribute an important role of lymphatics in plaque T cell accumulation and development.
Human and experimental murine atherosclerosis are both hallmarked by chronic inflammation. Several immune cell types, including macrophages, T and B cells, mast cells, and dendritic cells (DCs), are thought to be involved in the development and progression of an atherosclerotic plaque1,2,3,4,5. Although inflammatory responses are largely confined to the intimal plaque itself, there is growing evidence for a crucial role of the adventitia in vascular inflammation and plaque development6.
The adventitia is a highly organized tissue harboring stromal cells, vessels and, in particular in atherosclerosis, (resident) leukocyte subsets7,8,9,10,11,12,13. The overt presence of DC-T cell clusters in adventitia suggests a role as scaffold for antigen presentation12,14. Adventitial vessels, the vasa vasorum, expand with atherosclerosis progression both in humans and in mice, and have important functions in inflammatory cell trafficking, amongst others10,15,16,17.
The adventitia is therefore increasingly viewed as an important gateway for leukocytes, such as T cells10, to the plaque, a notion referred to as the “outside-in” hypothesis17. As we have previously shown, adventitial vessels were more leaky than similarly sized vessels in other organs, potentially causing interstitial fluid buildup, the drainage of which requires functional lymphatics18. Indeed next to vasa vasorum, the adventitia of human atherosclerotic vessels was recently reported to accommodate an extensive lymphatic capillary network19,20, at a density that increased with disease severity19,20. The perivascular lymphatic bed exerts several critical functions, not only in interstitial fluid drainage, but also in reverse cholesterol transport from plaque to liver21, and in cell trafficking in and out of tissues22,23,24. This suggests an important, but so far unresolved, contribution of adventitial lymphatics to plaque inflammation and atherosclerosis.
In this study, we have addressed the role of adventitial lymphatic capillaries in a murine model of atherosclerosis by two independent loss-of-function approaches. Our results show augmented T cell accumulation after inhibiting lymph drainage or VEGFR3 dependent lymphangiogenesis, suggesting that the lymphatic capillaries are responsible for T cell drainage from the atherosclerotic lesion. Furthermore, we identified the CXCL12/CXCR4 axis as an important regulator of adventitial lymphangiogenesis.
Results
Lymphatic capillaries are present in the adventitia of human and mouse atherosclerotic lesions at densities that increase with plaque progression
Previous work by us and others already showed that the adventitial vasculature (aka vasa vasorum) expands with atherosclerosis progression15,16,17,18, and that these vasa vasora are dysfunctional and leaky. Conceivably the enhanced presence of leaky vessels will cause local edema, unless the tissue is properly drained by lymphatics. As lymphangiogenesis and angiogenesis are responsive to partly overlapping cues, this led us to investigate whether adventitial lymphatic vessels expand in response to the atherosclerotic stimulus as well.
Hereto we immunohistochemically screened a cohort of atherosclerotic human aortic artery segments obtained by autopsy, for the presence of adventitial lymphatics. Lymphatic capillaries were abundantly present in the adventitia of human atherosclerotic lesions and their presence was substantially increased in advanced and ruptured lesions compared to non-diseased or early atherosclerotic tissue (Fig. 1A,B). Apparently the pattern of adventitial lymphangiogenesis mirrors that in intimal lesions19,20. Next we investigated whether lymphangiogenesis also occurs in mouse atherosclerosis. The adventitia of atherosclerotic carotid artery segments from ApoE−/− mice featured both vasa vasorum and lymphatic capillaries (Fig. 1C, red and blue, respectively; Suppl. Fig. 1). The lymphatic capillary density in the adventitia was considerably increased at week 4 and remained high at later stages of plaque development (Fig. 1D), suggesting that lymphatic expansion occurs already at an early stage of atherogenesis.
Figure 1. Increased presence of adventitial lymphatic capillaries during human and mouse atherosclerosis development.
(A) Immunohistochemical staining of podoplanin+ (D2-40) lymphatic capillaries (Brown) in human aortic atherosclerotic lesions obtained during kidney transplantation (10x magnification, Insert = 20x magnification). *Shows CD31+ adventitial microvessels (blue). (B) Quantification of adventitial lymph vessel density (LVD) in different stages of human atherosclerotic lesion development. (C) Immunohistochemical staining of Lyve-1+ lymphatic capillaries (Purple) and CD31+ vasa vasorum (Blue) in mouse carotid atherosclerotic lesions. Continued line indicates the outer border of the media; the area between the continuous and dotted line indicates the adventitial area used for quantification (10x magnification, Insert = 20x magnification). (D) Quantification of the percentage Lyve-1+ lymphatic capillaries per adventitial area of different stages of mouse lesion development.
Lymph node and lymph vessel dissection deteriorates atherosclerosis development in ApoE−/− mice by promoting T cell accumulation inside the lesion and adventitia
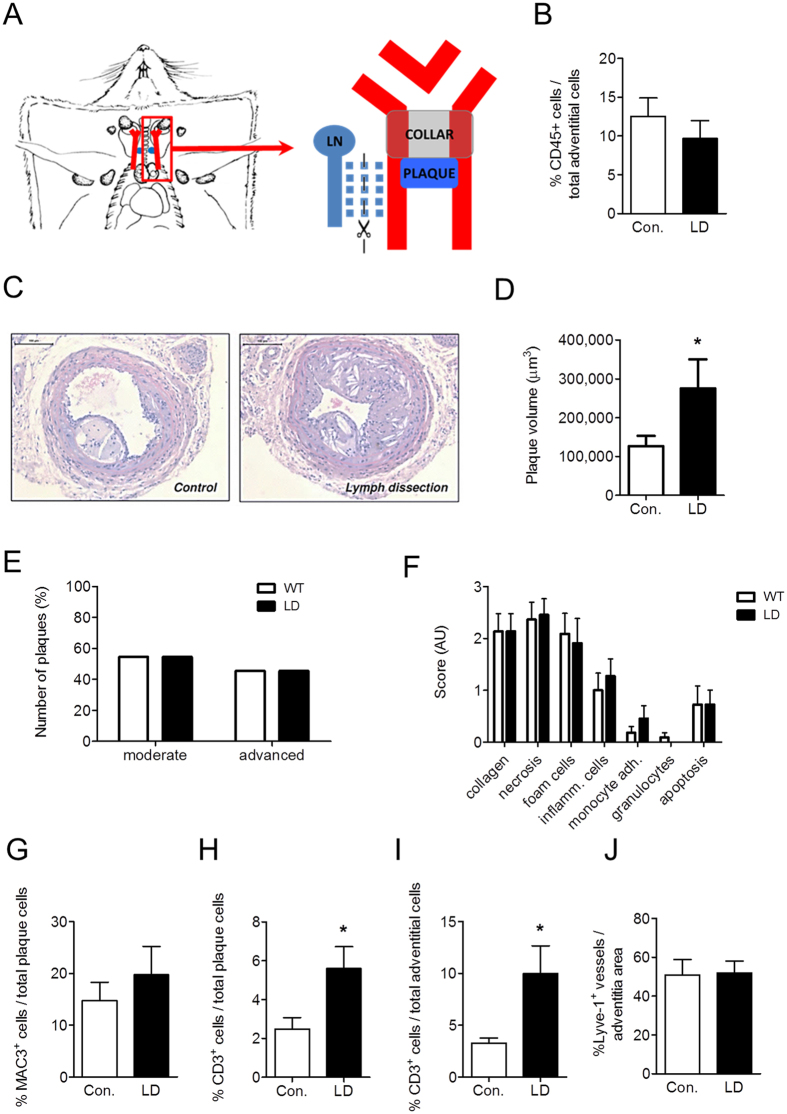
The increased presence of lymph vessels in the adventitia of human and mouse atherosclerotic lesions, together with the fact that lymph vessels have been implicated in the regulation of interstitial fluid drainage and inflammation25,26, argue for an important role for lymph capillaries in atherosclerosis. We therefore studied the impact of dissecting the plaque draining lymph vessels and lymph node on atherosclerosis development in ApoE−/− mice. We opted for the deep cervical lymph node, which is most proximal to the carotid artery bifurcation, and its efferent vessel, running in parallel to the carotid artery (Fig. 2A). Lymph node dissection by itself did neither influence body weight, plasma cholesterol levels (Suppl. Fig. 2A,B) nor induce local inflammation in the adventitia, as judged by the unchanged vascular CD45+ leukocyte contents in lymph node dissected mice (Fig. 2B). Lymph node dissection did not affect body weight (29.23 ± 0.48 g and 27.64 ± 0.52 g in control versus lymph node dissected mice, respectively). Interestingly, dissection aggravated atherosclerotic plaque burden (Fig. 2C,D). No significant changes were found in progression stage or gross composition (Fig. 2E,F). Although plaque macrophage content remained unaltered (Fig. 2G), atherosclerotic plaques displayed increased intimal (Fig. 2H) and adventitial CD3+ T cell content (Fig. 2I). Circulating monocyte numbers (Suppl. Fig. 2C) and peripheral lymph node T cell content (Suppl. Fig. 2D) were unchanged, again illustrating the local reach of the intervention. Lymph node dissection and thus interruption of drainage did not have any effects on lymphatic capillary bed density in the adventitia (Fig. 2J) at endpoint (4 weeks after lymph node dissection), although origin and functionality of these newly formed capillaries could not be assessed. No differences were observed in vasa vasorum development upon lymph node dissection (Suppl. Fig. 2E). Altogether, these data point to a role of adventitial lymphatic vasculature in drainage of plaque derived T cells and/or of T cell targeting chemokines and cytokines from the plaque, translating in reduced T cell but not macrophage accumulation in the plaque, thus dampening plaque growth.
Figure 2. Lymph node dissection results in an aggravation of plaque development in mice, characterized by an accumulation of CD3+ T cells within the vessel wall.
(A) Draining lymph node and efferent vessel were excised in the lymph node dissection group. (B) Percentage of CD45+ leukocytes in the adventitia of control and lymph node dissected (LD) mice. (C) Representative pictures of Hematoxylin-Eosin-stained sections of the carotid arteries from control and lymph node dissected mice. Quantification of (D) plaque volume, (E) plaque classification and (F) routine pathological examination of plaque composition. Percentage of Mac-3+ macrophages in the plaques (G). Percentage of CD3+ T cells in (H) plaque and (I) adventitia. (J) Percentage of Lyve-1+ lymphatic capillary area in the adventitia.
Inhibition of lymphangiogenesis using an AAV-hVEGFR3-Ig construct did not change plaque burden, yet increased lesional T cell content
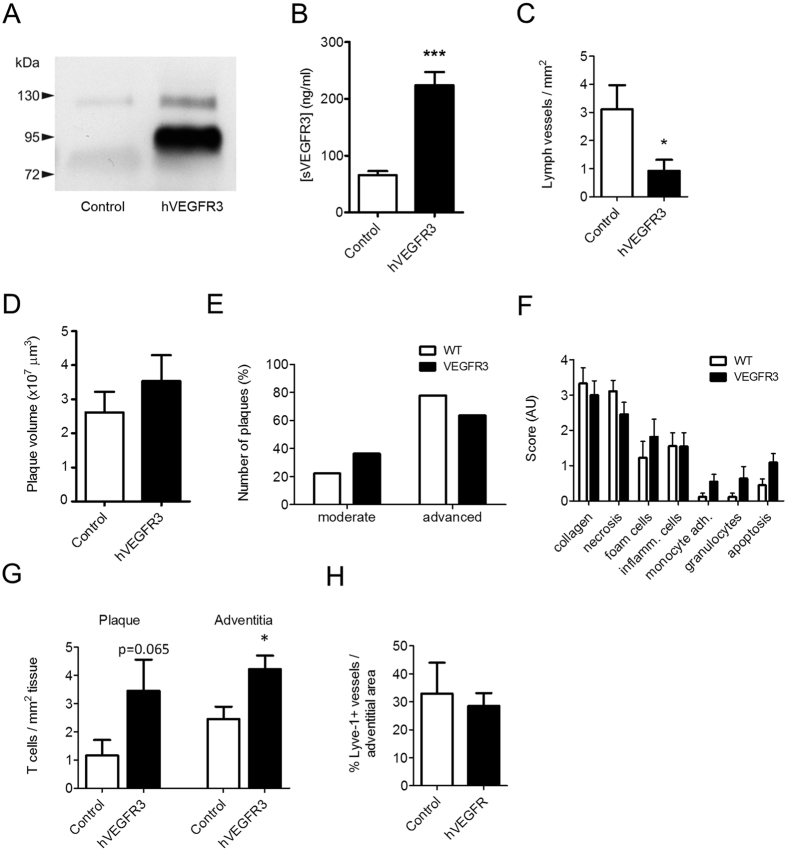
Lymph node dissection did not alter atherosclerosis associated adventitial lymph vessel expansion, suggesting that these newly formed lymphatics are not originating from the main efferent lymph node that runs in parallel to the carotid artery. As this potentially points towards a role of lymphangiogenic factors, we next sought to address the contribution of the major driver of postnatal lymphangiogenesis, VEGF-C/D, to adventitial neolymphangiogenesis by AAV aided transfer of IgG-fused soluble human VEGFR3 (AAV-hVEGFR3-Ig)27, which inactivates VEGF-C/D. Gene therapy led to persistent overexpression of soluble hVEGR3 in serum of treated but not control mice, as assessed by western blotting (Fig. 3A) and ELISA (Fig. 3B). As expected, the achieved sVEGFR3 levels were sufficient to halt the lymphangiogenic response in an in vivo VEGF-C supplemented Matrigel plug assay, as illustrated by the sharp reduction in lymph vessel ingrowth (Fig. 3C). AAV gene therapy did not alter body weight or plasma cholesterol levels (Suppl. Fig. 3A,B), nor did it affect circulating monocyte numbers (Suppl. Fig. 3C) or peripheral lymph node T cell content (Suppl. Fig. 3D). Moreover it did not change plaque volume (Fig. 3D), stage (Fig. 3E) and composition (Fig. 3F). However, plaques of AAV-hVEGFR3-Ig treated mice did show a more inflammatory phenotype, with increased adventitial and intimal CD3+ T cell content (Fig. 3G). Much to our surprise, while halting systemic lymphangiogenesis in the matrigel plug assay, hVEGFR3 overexpression failed to inhibit adventitial lymphatic capillary expansion (Fig. 3H). No effects of hVEGFR3-Ig treatment were observed on intimal or adventitial microvessels (Suppl. Fig. 3E). Collectively, in both the systemic lymphangiogenesis intervention study and the LN dissection study, we observed a striking increment in lesional T cell content, pointing to a role of plaque-associated lymph vessels in regulating local T cell content.
Figure 3. Soluble hVEGFR3 gene therapy did not affect plaque burden and plaque-associated lymphangiogenesis, yet induced an increase in plaque T cell content.
(A) Western blot showing cropped image of blot and (B) ELISA analysis of hVEGFR3 expression in plasma after AAV-hVEGFR3-Ig gene transfer. (C) Lymph vessel formation and ingrowth in an in-vivo matrigel plug assay, four weeks after s.c. administration of AAV-hVEGFR3-Ig gene transfer. Quantification of (D) plaque volume, (E) plaque classification and (F) routine pathological examination of plaque composition of lesion formation in carotid arteries. (G) Quantification of adventitial and plaque T cell content. (H) Percentage of Lyve-1+ lymphatic capillary area in the adventitia.
Plaque-associated lymph vessels are partly derived from non-classical lymphangiogenesis via the CXCL12/CXCR4 axis
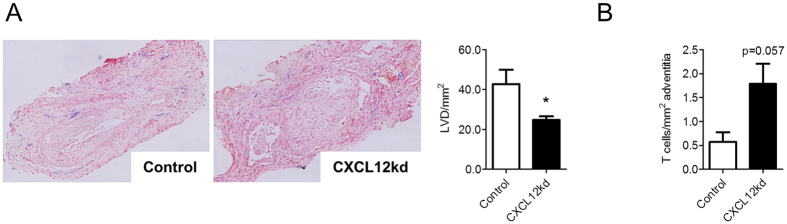
As observed, surgical interruption of lymph drainage as well as gene therapeutic inhibition of lymphangiogenesis by AAV-hVEGFR3-Ig, both affected plaque T cell accumulation, possibly by attenuating their drainage from plaque (or by reducing their recruitment). However, interfering with the classical VEGF-C/D-VEGFR3 pathway of lymphangiogenesis by using the AAV-hVEGFR3-Ig did not abrogate lymph vessel development at plaque adventitia (Fig. 3H), suggesting a role for non-canonical lymphangiogenic backup factors, that come into play once the VEGF-C/D pathway fails. A chemokine pathway that has been implicated in VEGFR3 independent lymphangiogenesis is the CXCL12/CXCR4 pathway28,29. This chemokine axis has already been shown to promote lymphangiogenesis in murine models of corneal neovascularization or cancer28,29. Additionally, CXCL12 has been shown to be a chemoattractant for lymphatic endothelial cells29. To address the involvement of CXCR4 signaling in lymph vessel expansion, we have silenced its ligand CXCL12 in adventitia, at plaque initiation, by local perivascular administration of pluronic gel containing siCXCL12. CXCL12 silencing resulted in a significant 42% reduction in adventitial lymph vessel density, underpinning the causal role that CXCR4/CXCL12 plays in atherosclerosis associated lymph vessel expansion (Fig. 4A). In further support of a role of plaque lymphangiogenesis in T-cell accumulation, CD3+ T cells were increased after siRNA CXCL12 targeting, at borderline significance (Fig. 4B).
Figure 4. Chemokine and lymph vessel dependent accumulation of T cells.
(A) Representative Lyve-1 staining and quantification of lymph vessel density (LVD) after perivascular treatment with siRNA targeting CXCL12. (B) Representative CD3 staining and quantification of adventitial T cell content after perivascular treatment with siRNA targeting CXCL12.
Together, this experiment shows that the lymph angiogenesis is at least partly mediated by CXCL12/CXCR4.
Human plaque data confirms correlations between lymph vessel density and the CXCR4/CXCL12 axis
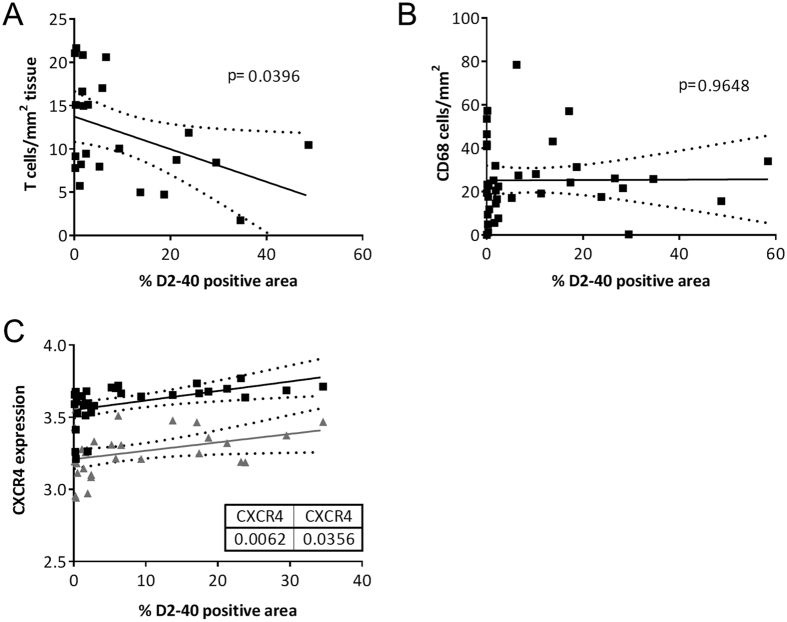
To confirm a potential role of the local lymphatic vessels in T cell trafficking in humans, we performed correlation analysis between the number of T cells and the local lymph vessel area in human carotid endarterectomy plaque tissue. First, we were able to show a tight correlation between D2-40 staining area in human plaque and mRNA expression of lymphatic markers LYVE-1 and podoplanin (the target of D2-40) (Suppl. Fig. 4). Correlation analysis showed a clear inverse correlation between the amount of T cells and lymph vessel presence in human tissue (Fig. 5A). This correlation seemed to be cell type specific, as macrophages did not show any correlation with the amount of lymph vessel presence (Fig. 5B). As described, we found that the CXCL12/CXCR4 axis at least partly mediates the effects on lymph angiogenesis in mice. Indeed also in human tissue we found a substantial correlation between CXCR4 mRNA expression and lymph vessel presence in human carotid endarterectomy plaque tissue (Fig. 5C), arguing for a link of the CXCR4 axis with plaque lymphangiogenesis.
Figure 5. Correlation analysis in human endarterectomy plaque tissues.
Correlation analysis between T cells (A) and macrophages (B) per plaque area and D2-40 positive, lymphatic area in human atherosclerotic lesions. (C) Correlation analysis of CXCR4 expression and D2-40 positive, lymphatic area in human endarterectomy plaque tissue.
In conclusion, our data implicate the CXCR4 rather than VEGF-C/D axis in atherosclerosis associated lymphangiogenesis and identified the regulatory role of adventitial lymphatics in plaque T-cell homeostasis.
Discussion
In this study, we describe a role for adventitial lymphatic capillaries in plaque inflammation and atherosclerosis in mouse and human. As we show, the adventitial lymphatic capillary bed is markedly expanded early on in atherogenesis. Surgical interruption of lymphatic plaque drainage prior to atherogenesis aggravated atherosclerosis development, with increased T cell accumulation in plaque and adventitia as most prominent feature. The latter was also observed after systemic inhibition of VEGFR3-dependent lymphangiogenesis. Interestingly, both interventions did not impact lymph capillary bed density in the adventitia or plaque, suggesting that adventitial lymphatic expansion is not originating from the carotid lymph vessel, and driven by alternative VEGF-C/D independent (backup) mechanisms, possibly through the CXCR4/CXCL12-axis.
As we show, during atherosclerosis, vasa vasorum expansion goes hand in hand with that of the adventitial lymphatic capillary bed. This concurs with previous findings showing progression stage dependent increases in lymphatic capillary content in human carotid artery plaque19,20, and with the recent observation that lymphatic dysfunction precedes the onset of atherosclerosis30. Interestingly, inhibition of VEGFC/D dependent lymphangiogenesis did, in contrast to lymph node/efferent dissection, not affect the atherosclerotic burden, while increasing adventitial and plaque CD3+ T cell content. Conceivably, systemic hVEGFR3‐dependent lymphangiogenesis blockage may initially reduce the draining capacity, eliciting alternative VEGFR3 independent lymphangiogenesis pathways in plaque tissue, such as mechano‐induced β1-integrin signaling31, or the CXCL12-CXCR4 axis28,29. In support of the latter Bot et al. previously observed elevated CXCR4 expression in atherosclerotic lesions, reflecting an increased lymphangiogenic propensity32. Moreover our study shows a positive correlation between CXCR4 expression and lymph vessel presence in human atherosclerotic tissues and reduced adventitial lymph vessel density in mice upon CXCL12 silencing. As such, such VEGF-C/-D independent pathway may also underlie the unchanged adventitial lymph vessel density.
Although CXCR4 appears to be a major cue, in addition to lymphangiogenic growth factors such as VEGF-C and -D33, we cannot exclude the involvement of other inflammatory chemokine pathways or other VEGFR3 independent pathways, such as stretch-dependent β1-integrin signaling in adventitial lymphangiogenesis. The different pathways most likely work in concert to induce lymphangiogenesis, with an initial prominent role for hypoxia and inflammation driven VEGF-C/D and CXCL12 production28,29, followed by an increasing role for stretch-induced β1-integrin signaling upon growth of the plaque and increasing plaque interstitial pressure31.
A second relevant question relates to the consequences of adventitial lymphatic bed expansion on plaque inflammation and atherosclerosis. Adventitial lymphatic capillaries could help to prevent edema, caused amongst others by leaky plaque vasa vasorum. In addition, as pointed out by Randolph and coworkers, lymphatics could exert atheroprotective functions by draining plaque contained lipids during regression21. Our study adds to this notion in that surgical interruption in their draining function or interference with their expansion aggravated disease. The atheroprotection provided by adventitial lymphatics may be partly based on their regulatory role in plaque T cell accumulation. Whether adventitial lymphatics control CD3+ T cell accumulation directly, by mediating egress (as suggested by their close proximity to the adventitial lymph vessels, data not shown), or indirectly by draining T cell chemotactic cues remains to be determined. Dissecting the exact mechanism is complicated by the reciprocal interaction between lymph vessels and T cells with lymph vessels steering T cell trafficking (this study), and T cells in turn inhibiting lymphangiogenesis34.
In conclusion, this study shows that adventitial lymphatic vessels increased at an early stage of plaque development, conferring a protective role by draining and therefore dampening the local inflammatory response. Draining lymph node dissection resulted in aggravation of atherosclerosis, accompanied by increased plaque and adventitial T cell content, probably due to interrupted T cell egress or reduced draining of chemotactic cues. Adventitial lymphangiogenesis could not be halted by VEGF-C/D inhibition, but was at least partly arrested due to non-classical lymphangiogenesis via the CXCL12/CXCR4 axis. Further studies are needed to provide further insight into the precise role and mechanisms of adventitial lymphatic capillary formation in atherosclerosis, and their potential impact on clinical outcome.
Methods
Immunohistochemistry
Human carotid artery plaque tissue was obtained by endarterectomy, for more detail see ref. 35. For studying adventitial lymph vessels, human aortic plaque tissue was obtained during kidney transplantation, as described previously36. Collection, storage and use of tissue and patient data (methods) were performed after informed consent and in agreement with the ‘Code for Proper Secondary Use of Human Tissue in the Netherlands’ and in accordance with the guidelines of, and approved by the medical and ethical committee of Leiden University Medical Center, Leiden, The Netherlands. Lesions were fixed in paraformaldehyde (4%) and paraffin embedded. Histological assessment of human atherosclerotic lesions was performed according to Virmani classification37. Lymph vessel analysis was performed using anti-podoplanin (D2-40) antibody (goat polyclonal, R&D systems), and microvessels were stained using CD31 (clone JC/70a, Dako). Vessels were then counted manually and corrected for plaque or adventitial area.
Immunohistochemical (IHC) staining on mouse paraffin carotid artery sections was performed for lymph vessel marker Lyve-1 (rabbit polyclonal, Abcam), for T cells using CD3 (DAKO), for macrophages using Mac-3 (clone M3/74, BD), and for microvessels using CD31 (clone MEC13.3, BD).
Lyve-1+ lymphatic capillary content (scored on the basis of morphology and positive staining) was expressed as percentage of adventitial area. Lymphatic capillaries were defined as Lyve-1+ cells, circumventing a clearly identifiable lumen. The adventitial area was defined as tissue within a distance of twice the smallest intima-media thickness from the outer elastic lamina. The number of CD3+, CXCR3+ and Mac-3+ cells was expressed as percentage of total plaque or adventitial cells. IHC staining for Lyve-1 was also performed on paraffin embedded matrigel plugs from the AAV-hVEGFR3-Ig study (n = 5/group). Lymph vessel ingrowth into the Matrigel plugs was determined in three slides per plug. All slides were analyzed by a blinded observer (TR) using Leica Qwin software.
Analysis of adventitial lymphatic capillaries and CD3+ T cells
Male ApoE−/− mice (n = 21, 12 weeks of age), backcrossed at least 11 times to C57Bl/6 and obtained from The Jackson Laboratory, were placed on western type diet (WTD) containing 0.25% cholesterol and 15% cacao butter (Special Diets Services, Witham, Essex, UK). Atherosclerotic carotid artery lesions were induced by bilateral placement of semi-constrictive collars38. To study adventitial lymphatic capillary density and CD3+ T cell content in early (n = 9) and advanced lesions (n = 6), mice were sacrificed 4 and 8 weeks after collar placement, respectively. Naïve carotid artery segments from ApoE−/− mice, not equipped with a perivascular collar (20 weeks of age) served as control (n = 6). All animal experiments were approved by the ethics committee of Maastricht University Medical Center (DEC 2010–098 and 2011–093, Maastricht, The Netherlands) and performed according to institutional guidelines.
Lymph node and vessel dissection in mice
Male ApoE−/− mice (n = 27, 12 weeks of age) were placed on WTD and semi-constrictive collars were placed around the carotid arteries to induce atherosclerosis development38. To address the impact of the deep cervical lymph node drainage to leukocyte influx into and efflux from the adjacent plaque, the lymph node and efferent lymph vessel were removed immediately after collar placement (n = 14). Mice which received only collar placement (n = 13) served as control. At sacrifice, mice from the lymph node dissection group did not show any signs of edema in the neck region. In a separate study we investigated whether lymph node/vessel dissection per se induces a local inflammatory response, which could impact lesion formation indirectly. Hereto, we studied effects of the above intervention in normolipidemic wild-type mice (n = 5–6, 12 weeks of age). Both hyper- and normolipidemic mice were sacrificed at 4 weeks after collar placement.
Pharmacological inhibition of lymphangiogenesis in mice
Male ApoE−/− mice (n = 34, 12 weeks of age) were placed on WTD and semi-constrictive collars were placed bilaterally, proximal to the bifurcation of the carotid artery for shear-dependent atherosclerosis induction38. To inhibit VEGFR3-dependent adventitial lymphangiogenesis during plaque development, adeno-associated virus (AAV) encoding a soluble hVEGFR3-Ig (AAV-hVEGFR3-Ig, 1011 virus particles per mouse27) or control virus (AAV-hVEGFR3(D4-7)-Ig) was administered systemically by intravenous injection at the time of collar placement (n = 17 hVEGFR3-Ig vs. n = 17 control). Mice were sacrificed 4 weeks after collar placement.
A subgroup of mice (n = 5 per group) also received a subcutaneous plug of VEGF-C supplemented Matrigel (BD Bioscience) to validate the efficacy of lymphangiogenesis inhibition by the hVEGFR3-Ig construct. In addition, blood was isolated at 4 weeks after collar placement, serum was obtained by centrifugation (10 min, 1,500 g, 4 °C), and plasma transgene expression was assessed by ELISA and western blot.
Focal silencing of adventitial CXCL12 expression
Male ApoE−/− mice (12 weeks of age) were placed on WTD (0.15% cholesterol, Altromin, Lage, Germany) for 6 weeks after which semi-constrictive collars were placed around the carotid arteries to induce atherosclerosis. To address the contribution of adventitial CXCL12 to atherosclerosis development, mice were perivascularly treated with a CXCL12 specific siRNA or non-targeting siRNA control (once weekly, 4 nmol, dissolved in plurogenic gel; Dharmacon) as previously described39.
Histology of mouse atherosclerotic lesions
Mice were euthanized by pentobarbital injection (115 mg/kg) and perfused through the left cardiac ventricle with PBS (NaCl/Na2HPO4/KH2PO4, pH 7.4) containing sodium nitroprusside (0.1 mg/ml, Sigma) and 1% paraformaldehyde (PFA). The right carotid artery was removed, fixed overnight in 1% PFA and paraffin-embedded sections (4 μm) were cut. To determine plaque volume in the carotid artery, sections were stained for haematoxylin and eosin (HE) and plaque area was measured for consecutive cross-sections, at 100 μm intervals, over a carotid artery segment that covered the entire plaque. Slides were analyzed in a blinded manner using a Leica DM3000 light microscope (Leica Microsystems) coupled to computerized morphometry (Leica Qwin 3.5.1)40. Size and progression stage of atherosclerotic lesions was determined as previously described41, with minor modifications. Plaques were classified as early (foam cell rich, but lacking a necrotic core), moderately advanced (containing a fibrotic cap and often a necrotic core, but no medial macrophage infiltration) and advanced lesions, typified by medial macrophage infiltrates, elastic lamina degradation and more pronounced necrosis and fibrosis. HE sections were used for morphometric analysis and routine qualitative examination of collagen content, necrosis, foam cell content and amount of inflammatory cells (using scores from 0 (absent) to 3 (high abundance)).
Flow cytometry and plasma total cholesterol measurement
Blood, spleen and lymph nodes were removed before perfusion (n = 10/group) and used for flow cytometry of monocytes (CD11bhigh Ly6G−, BD), granulocytes (CD11bhigh Ly6Ghigh, BD), T helper cells (CD4+, BD), effector T cells (CD8a+,BD) and activated T cells (CD44high, eBioscience). Briefly, spleen and lymph nodes were homogenized, filtered through a 70 μm mesh. Blood, spleen and lymph nodes were subjected to red blood cell lysis. All cells were stained in flow cytometry buffer (1xPBS, 0.5% BSA, 0.01% NaN3). Staining for blood count analyses was conducted using antibodies to CD45, CD115, Gr1, CD19, and CD3 (all eBiosciences) in Hank’s Balanced Salt Solution (HBSS) with 0.3 mM EDTA and 0.1% bovine serum albumin (BSA). Cell counts were estimated utilizing CountBrightTM absolute counting beads (Invitrogen). All flow cytometry analysis was performed on a FACS CANTO II (BD Bioscience).
Statistical analysis
Data are expressed as mean ± SEM. For correlation analyses of human expression data, Shapiro-Wilk test for normality was performed, after which correlations of Gaussian distributed data were calculated by Pearson and of non-Gaussian data by Spearman correlation test. For group comparisons, data were tested for Gaussian distribution, after which a Student t-test (Gaussian) or Mann-Whitney U test (non-Gaussian) was used to compare individual groups; multiple groups were compared by ANOVA or Kruskall-Wallis tests, with Bonferroni or Dunn’s post-hoc test, respectively. Statistics were performed using Graphpad Prism 5.0. A p-value of < 0.05 was considered statistically significant. *Denotes p < 0.05, **denotes p < 0.01, ***denotes p < 0.001.
Additional Information
How to cite this article: Rademakers, T. et al. Adventitial lymphatic capillary expansion impacts on plaque T cell accumulation in atherosclerosis. Sci. Rep. 7, 45263; doi: 10.1038/srep45263 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
Supported by the CARIM portfolio excellence program “Plaque neoangiogenesis” and the Dutch Heart Foundation (NHS2005B294) to E. Biessen.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.R. designed the study, analysed the data and drafted the manuscript, E.P.C.v.d.V. designed the study, analysed the data and drafted the manuscript, I.T.M.N.D. designed the study and analysed the data, J.J.T.O. offered experimental support, K.T. offered experimental support, T.L.T. offered experimental support, M.G analysed the data, A.A. analysed the data, H.N. analysed the data, J.H.N.L. provided crucial materials, A.S. provided crucial materials, S.H. provided crucial expertise, K.A. provided crucial expertise, E.A.L.B designed the study and revised the manuscript.
References
- Weber C., Zernecke A. & Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nature reviews. Immunology 8, 802–815, doi: 10.1038/nri2415 (2008). [DOI] [PubMed] [Google Scholar]
- Bot I. & Biessen E. A. Mast cells in atherosclerosis. Thrombosis and haemostasis 106, 820–826, doi: 10.1160/TH11-05-0291 (2011). [DOI] [PubMed] [Google Scholar]
- Hansson G. K. & Hermansson A. The immune system in atherosclerosis. Nature immunology 12, 204–212, doi: 10.1038/ni.2001 (2011). [DOI] [PubMed] [Google Scholar]
- Businaro R. et al. Cellular and molecular players in the atherosclerotic plaque progression. Annals of the New York Academy of Sciences 1262, 134–141, doi: 10.1111/j.1749-6632.2012.06600.x (2012). [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 32, 2045–2051, doi: 10.1161/ATVBAHA.108.179705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. A. et al. Lymphocytes and the adventitial immune response in atherosclerosis. Circulation research 110, 889–900, doi: 10.1161/CIRCRESAHA.111.263186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H. et al. B cell depletion reduces the development of atherosclerosis in mice. The Journal of experimental medicine 207, 1579–1587, doi: 10.1084/jem.20100155 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot I. et al. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circulation research 106, 89–92, doi: 10.1161/CIRCRESAHA.109.204875 (2010). [DOI] [PubMed] [Google Scholar]
- de Boer O. J., van der Meer J. J., Teeling P., van der Loos C. M. & van der Wal A. C. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PloS one 2, e779, doi: 10.1371/journal.pone.0000779 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E. et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. The Journal of experimental medicine 203, 1273–1282, doi: 10.1084/jem.20052205 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw T., Tipping P., Toh B. H. & Bobik A. Current understanding of the role of B cell subsets and intimal and adventitial B cells in atherosclerosis. Current opinion in lipidology 22, 373–379, doi: 10.1097/MOL.0b013e32834adaf3 (2011). [DOI] [PubMed] [Google Scholar]
- Moos M. P. et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology 25, 2386–2391, doi: 10.1161/01.ATV.0000187470.31662.fe (2005). [DOI] [PubMed] [Google Scholar]
- Zhao L. et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nature medicine 10, 966–973, doi: 10.1038/nm1099 (2004). [DOI] [PubMed] [Google Scholar]
- Yilmaz A. et al. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Experimental and molecular pathology 83, 93–103, doi: 10.1016/j.yexmp.2007.01.007 (2007). [DOI] [PubMed] [Google Scholar]
- Eriksson E. E. Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation 124, 2129–2138, doi: 10.1161/CIRCULATIONAHA.111.030627 (2011). [DOI] [PubMed] [Google Scholar]
- Langheinrich A. C., Michniewicz A., Bohle R. M. & Ritman E. L. Vasa vasorum neovascularization and lesion distribution among different vascular beds in ApoE−/−/LDL−/− double knockout mice. Atherosclerosis 191, 73–81, doi: 10.1016/j.atherosclerosis.2006.05.021 (2007). [DOI] [PubMed] [Google Scholar]
- Maiellaro K. & Taylor W. R. The role of the adventitia in vascular inflammation. Cardiovascular research 75, 640–648, doi: 10.1016/j.cardiores.2007.06.023 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers T. et al. Plaque-associated vasa vasorum in aged apolipoprotein e-deficient mice exhibit proatherogenic functional features in vivo. Arteriosclerosis, thrombosis, and vascular biology 33, 249–256, doi: 10.1161/ATVBAHA.112.300087 (2013). [DOI] [PubMed] [Google Scholar]
- Drozdz K. et al. Adventitial lymphatics and atherosclerosis. Lymphology 45, 26–33 (2012). [PubMed] [Google Scholar]
- Kholova I. et al. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. European journal of clinical investigation 41, 487–497, doi: 10.1111/j.1365-2362.2010.02431.x (2011). [DOI] [PubMed] [Google Scholar]
- Martel C. et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. The Journal of clinical investigation 123, 1571–1579, doi: 10.1172/JCI63685 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. P., Moussion C. & Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nature reviews. Immunology 12, 762–773, doi: 10.1038/nri3298 (2012). [DOI] [PubMed] [Google Scholar]
- Na I. K. et al. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood 116, e18–25, doi: 10.1182/blood-2009-12-259432 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewalt E. F., Cohen J. N., Rouhani S. J. & Engelhard V. H. Lymphatic endothelial cells - key players in regulation of tolerance and immunity. Frontiers in immunology 3, 305, doi: 10.3389/fimmu.2012.00305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Braun A. & Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends in immunology 33, 271–280, doi: 10.1016/j.it.2012.02.007 (2012). [DOI] [PubMed] [Google Scholar]
- Kim H., Kataru R. P. & Koh G. Y. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol 33, 350–356, doi: 10.1016/j.it.2012.03.006 (2012). [DOI] [PubMed] [Google Scholar]
- Lin J. et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer research 65, 6901–6909, doi: 10.1158/0008-5472.CAN-05-0408 (2005). [DOI] [PubMed] [Google Scholar]
- Du L. L. & Liu P. CXCL12/CXCR4 axis regulates neovascularization and lymphangiogenesis in sutured corneas in mice. Molecular medicine reports 13, 4987–4994, doi: 10.3892/mmr.2016.5179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo W. et al. The CXCL12-CXCR4 chemokine pathway: a novel axis regulates lymphangiogenesis. Clinical cancer research: an official journal of the American Association for Cancer Research 18, 5387–5398, doi: 10.1158/1078-0432.CCR-12-0708 (2012). [DOI] [PubMed] [Google Scholar]
- Milasan A., Dallaire F., Mayer G. & Martel C. Effects of LDL Receptor Modulation on Lymphatic Function. Scientific reports 6, 27862, doi: 10.1038/srep27862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas-Paz L. et al. Mechanoinduction of lymph vessel expansion. The EMBO journal 31, 788–804, doi: 10.1038/emboj.2011.456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot I. et al. CXCR4 blockade induces atherosclerosis by affecting neutrophil function. Journal of molecular and cellular cardiology 74, 44–52, doi: 10.1016/j.yjmcc.2014.04.021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. et al. Lymphangiogenesis promotes inflammation and neointimal hyperplasia after adventitia removal in the rat carotid artery. International journal of cardiology 134, 426–427, doi: 10.1016/j.ijcard.2007.12.098 (2009). [DOI] [PubMed] [Google Scholar]
- Kataru R. P. et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34, 96–107, doi: 10.1016/j.immuni.2010.12.016 (2011). [DOI] [PubMed] [Google Scholar]
- Goossens P. et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell metabolism 12, 142–153, doi: 10.1016/j.cmet.2010.06.008 (2010). [DOI] [PubMed] [Google Scholar]
- van Dijk R. A., Virmani R., von der Thusen J. H., Schaapherder A. F. & Lindeman J. H. The natural history of aortic atherosclerosis: a systematic histopathological evaluation of the peri-renal region. Atherosclerosis 210, 100–106, doi: 10.1016/j.atherosclerosis.2009.11.016 (2010). [DOI] [PubMed] [Google Scholar]
- Virmani R., Kolodgie F. D., Burke A. P., Farb A. & Schwartz S. M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arteriosclerosis, thrombosis, and vascular biology 20, 1262–1275 (2000). [DOI] [PubMed] [Google Scholar]
- von der Thusen J. H., van Berkel T. J. & Biessen E. A. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation 103, 1164–1170 (2001). [DOI] [PubMed] [Google Scholar]
- Akhtar S., Gremse F., Kiessling F., Weber C. & Schober A. CXCL12 promotes the stabilization of atherosclerotic lesions mediated by smooth muscle progenitor cells in Apoe-deficient mice. Arteriosclerosis, thrombosis, and vascular biology 33, 679–686, doi: doi: 10.1161/ATVBAHA.112.301162 (2013). [DOI] [PubMed] [Google Scholar]
- Lutgens E. et al. Requirement for CD154 in the progression of atherosclerosis. Nature medicine 5, 1313–1316, doi: 10.1038/15271 (1999). [DOI] [PubMed] [Google Scholar]
- Gijbels M. J. et al. Progression and regression of atherosclerosis in APOE3-Leiden transgenic mice: an immunohistochemical study. Atherosclerosis 143, 15–25 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.