Abstract
Metabolic syndrome (MetS) is a global epidemic, which involves a spectrum of metabolic disorders comprising diabetes and obesity. The impact of MetS on the brain is becoming to be a concern, however, the poor understanding of mechanisms involved has limited the development of therapeutic strategies. We induced a MetS-like condition by exposing mice to fructose feeding for 7 weeks. There was a dramatic deterioration in the capacity of the hippocampus to sustain synaptic plasticity in the forms of long-term potentiation (LTP) and long-term depression (LTD). Mice exposed to fructose showed a reduction in the number of contact zones and the size of postsynaptic densities (PSDs) in the hippocampus, as well as a decrease in hippocampal neurogenesis. There was an increase in lipid peroxidation likely associated with a deficiency in plasma membrane excitability. Consistent with an overall hippocampal dysfunction, there was a subsequent decrease in hippocampal dependent learning and memory performance, i.e., spatial learning and episodic memory. Most of the pathological sequel of MetS in the brain was reversed three month after discontinue fructose feeding. These results are novel to show that MetS triggers a cascade of molecular events, which disrupt hippocampal functional plasticity, and specific aspects of learning and memory function. The overall information raises concerns about the risk imposed by excessive fructose consumption on the pathology of neurological disorders.
Keywords: Fructose, Diabetes, Metabolic syndrome, Neuronal dysfunction
1. Introduction
In recent decades, there has been a progressive increase in the incidence of metabolic diseases such as metabolic syndrome (MetS), type II diabetes mellitus (T2DM) and obesity. T2DM affects approximately 15% of the global population, comprising over 400 million patients worldwide [1]. The elevated consumption of fructose has been identified as one of the major contributors to the epidemic of MetS [2–6]. The burden of MetS is becoming even more alarming on the light of recent reports suggesting that MetS disrupts brain function and resilience to neurological disorders [7–10]. MetS increases the incidence of cognitive disorders in aging and Alzheimer’s disease (AD) [11, 12], and even in young individuals [13–15]. It has recently been reported that a decrease in gray matter in several brain regions of patients with T2DM, and the extent of this decrease are being proportional to the duration of T2DM [16–19]. These results emphasize the existence of a time period for the development of MetS on the brain, which can be targeted for treatments to prevent the neurological sequel of MetS. Unfortunately, the poor understanding of the cellular and molecular mechanisms underlying the neuropathology of MetS has limited the design of preventive therapies to cope with a potential epidemic of neurological disorders [11].
The ability of neuronal circuits to store and process information is one of the most fundamental pillars of brain function, and the loss of this property conforms the pathology of most cognitive disorders. We induced a condition like-MetS with a diet rich in fructose, a protocol described before and proportional to the intake of fructose by humans [20–22]. Accordingly, we have directed the current studies to understand the impact of MetS on fundamental mechanisms underlying synaptic plasticity and learning and memory in the hippocampus, namely long-term potentiation (LTP) and depression (LTD). Our results provide critical evidence for the impact of MetS on deteriorating neuronal excitability in a brain region crucial for the processing of cognitive information, i.e., hippocampus. We have extended our analysis to determine the impact of MetS on adult neurogenesis as this process is considered a hallmark of the capacity of the brain for plasticity [23]. The subgranular zone (SGZ) of the dentate gyrus displays a continuous generation of new neurons [24] that integrate into the pre-existing hippocampal circuitry [25]. Although some of the peripheral effects of MetS may be treatable [11,26], the potential reversibility of the effects of MetS on brain function is not understood. Accordingly, we sought to determine whether the deleterious consequences of MetS on hippocampal properties are reversible. Reported results are significant to define a mechanism by which fructose-induced MetS compromise the work of neural circuits underlying cognitive function.
2. Methods
2.1. Animals and treatments
We used two-month-old C57BL/6 male mice, the mice were separated into two experimental groups (n = 19 per group) according to the tap water that they received: a control group, which received normal food and water, and a second group that received normal food and water supplemented with 15% fructose by 8 weeks. The animals were housed at the Animal House Facility of the Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, according to the Guide for the Care and Use of Laboratory Animals (NIH-USA Publication 86–23). After treatment the mice were used in the fellow order: 5 animals for electrophysiology experiments; 5 animals for the preparation of synaptosomes, immunoblotting, and immunofluorescence analysis; 9 animals for the cognitive test and the glucose tolerance test, and 3 animals for hippocampal neurogenesis.
2.2. Biochemical analysis
MetS involves a conglomerate of pathological features including obesity, insulin resistance, hypertension, high triglyceride levels, cardiovascular disease, and systemic inflammation [27,28]. Accordingly, we measured several of these features in the animals exposed to fructose. Blood was collected from the tail vein after 6 h of fasting, and then the serum samples were obtained. Glucose levels were measured according to the hexokinase/G-6-PDH method, using Architect Analyzer (Abbott Laboratories, Abbott Park, IL), and insulin levels were measured via chemiluminescence (Beckman Coulter); in both cases, the manufacturers’ instructions were followed. The HOMA, an index of insulin resistance [29,30], was calculated using the following formula: HOMA-R = fasting glucose (mmol l−1) × fasting insulin (μIU ml−1)/22.5. The levels of triglycerides and cholesterol were assessed enzymatically using the Architect c8000 analyzer (Abbott Laboratories, Abbott Park, IL).
2.3. Glucose tolerance test
After 30 days of special or standard diet, animals were fasted for 8 h and then received an injection of glucose (1 g/kg b.w., i.p.). Blood glucose was monitored for 90 min using a glucometer (Accu-Check, Roche) on samples collected from the tip of the tail vein.
2.4. Cognitive task
The Morris Water Maze (MWM) task was performed as we have previously described [31]. Briefly, the mice were trained in a 1.2-m-diameter circular pool (opaque water, 50 cm deep). The maximum trial duration was 60 s, and the animals were positioned on the platform for 10 s at the end of each trial. To test episodic memory, each animal was trained to find the platform per day for 4 days, using a new platform location each day, as described previously [31]. The large open field (LOF) test was used to study locomotor and stress behavior in our mouse model [32]. Each mouse was placed individually in the center of the open field, and its behavior was tracked for a 20 min session. At the end of the session, the mice were returned to their home cages. The parameters measured included: the total time moving and the number of lines that crossed the center area of the platform [33,34].
2.5. Electrophysiological recording
Hippocampal slices were prepared according to previously described standard procedures [31]. Briefly, transverse slices (350 μm) of the dorsal hippocampus were cut under cold artificial cerebrospinal fluid and incubated in ACSF for 1 h at room temperature. In all experiments, 10 μM picrotoxin (PTX) was included. Basal excitatory synaptic transmission was measured using an input/output curve protocol, which consisted of eight stimuli that ranged from 200 to 900 μA (with a 10-s interval between stimuli). LTP was generated using TBS, which consisted of 5 trains of the stimulus using an inter-train interval of 20 s. To generate LTD, we used LFS, consisting of 900 paired pulses at 1 Hz.
2.6. Preparation of synaptosomes
Crude synaptosomal fractions were prepared from the hippocampus of the treated and control mice as described previously [35]. The samples (50 μg of protein per sample) were subjected to SDS–PAGE, followed by immunoblotting using the indicated antibodies. The synaptosomal fraction from the hippocampus was examined using a scanning electron microscope.
2.7. Immunoblotting
The synaptosomal fraction were homogenized in RIPA buffer (50 mM, Tris–Cl, pH 7.5, 150 mM NaCl, 1% NP-40, 0,5% sodium deoxycholate, and 1% SDS), supplemented with a protease inhibitor cocktail (Sigma-Aldrich P8340) and phosphatase inhibitors (50 mM NaF, 1 mM Na3VO4 and 30 μM Na4P207), using a potter homogenizator and then passed sequentially through different caliber syringes. Protein samples were centrifuged at 14,000 rpm at 4 °C twice for 15 min. Protein concentration was determined using the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). 20 μg of synaptosomal fraction were resolved by 10% SDS-PAGE and transferred to a PVDF membrane. The reactions were followed by incubation with a primary antibody; then a secondary anti-goat peroxidase conjugated antibody (Pierce) was used and developed using an ECL kit (Western Lightning Plus ECL, PerkinElmer). Primary antibodies used were mouse anti-GAPDH (Sigma-Aldrich, St. Louis, MO), anti-GluR2 (Sigma-Aldrich, St. Louis, MO), mouse anti-Synaptophysin (SYN) (Life Technologies, Carlsbad, CA), anti-NMDAε1 (Sigma-Aldrich, St. Louis, MO) and mouse anti post-synaptic density protein 95 (PSD-95).
2.8. Immunofluorescence
Immunofluorescence was performed as previously described [36]. The primary antibody used was mouse anti-4-HNE (Life Technologies, Carlsbad, CA). The slices were subsequently mounted on slides using mounting medium and analyzed using a Zeiss LSM 5 Pascal confocal microscope. The images were analyzed using ImageJ software (NIH).
2.9. Analysis of hippocampal neurogenesis
After 4 weeks of control or fructose treatment, the animals were injected i.p. with 100 mg kg − 1 5-Bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich) once per day for 3 consecutive days. After 8 weeks of treatment animals were transcardially perfused with saline, followed by 4% paraformaldehyde (PFA, Sigma-Aldrich) in PBS. Brains were removed and sectioned on a cryostate in 12 sets of serial coronal slices of 40 μm thickness; each set contained 5–7 slices covering the entire length of the hippocampus. Immunodetection of BrdU and NeuN in tissue sections was performed as described previously [37]. The primary antibodies used were rat anti-BrdU (Sigma-Aldrich, St. Louis, MO) and monoclonal anti-NeuN (Millipore). BrdU-positive cells were counted using a fluorescence microscope (Olympus BX51, Tokyo, Japan) as described in [37]. For quantification of net neurogenesis NeuN staining in each BrdU-positive cell was detected using a confocal laser microscope (Olympus FV1000) and z-plane sections of 1–2 sets of coronal sections.
2.10. Studies of MetS remission
To study whether the effect of fructose are revertible we used three groups of 15 animals, from which the first group was treated with normal food plus water for 20 weeks. The second group was treated with normal food plus 15% of fructose in water for 8 weeks, then the fructose was replaced by plain water for another 12 weeks. In this group we monitored the blood levels of glucose, insulin, total cholesterol, glucose tolerance and triglycerides, and the HOMA index was calculated every 30 days. We observed a normalization of the blood parameters after 3 months, and this period was considered a critical time interval for the remission of MetS (Table 2). The third group was treated during the 20 weeks with normal food and 15% of fructose supplemented into the water. All the animals were sacrificed after 20 weeks: 5 animals were used for electrophysiological recording, and 10 animals were used for the cognitive studies and then blood was collected for physiological measurements.
Table 2.
Blood values after remission time.
2.11. Statistical analysis
The results are presented as the means ± standard error. The data were analyzed via one-way or two-way ANOVA followed by Bonferroni post hoc analysis; *p ≤ 0.05 was considered significant. Statistical analysis was performed using Prism software (GraphPad).
3. Results
3.1. Establishment of MetS-like pathogenesis in mice
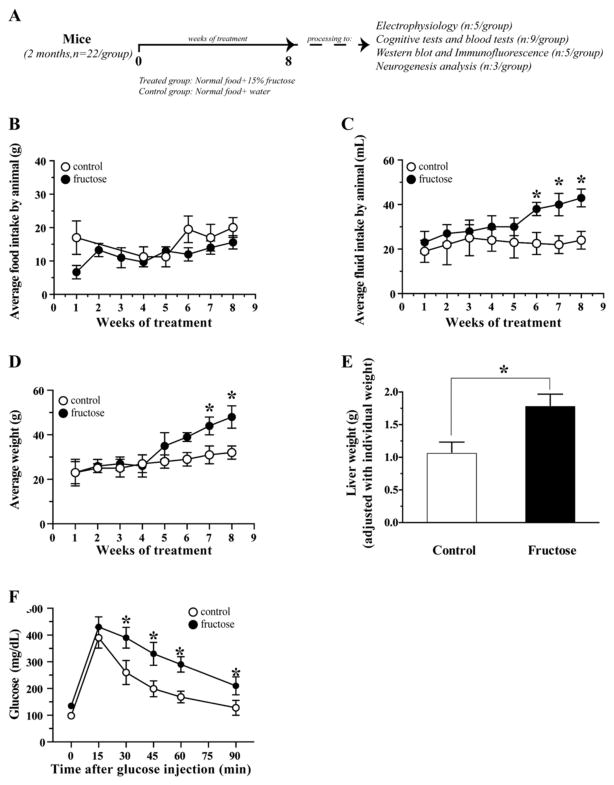
The protocol designed to induce MetS involved the addition of 15% fructose to the drinking water by 8 weeks (Fig. 1A). During the treatment period, food and fructose/water intake were controlled. We do not observed changes in the average of food intake by mice (Fig. 1B). A difference in the fructose/water intake was observed between the two groups in the last three weeks. The group maintained with fructose ingested more liquid compared with the control group (Fig. 1C).
Fig. 1.
Establishment of like-MetS condition. (A) Representative model of the induction of MetS in mice. (B) Average food intake during the 8 weeks of treatment. (C) Water of fructose intake during the treatment time. (D) Average weight of control and treated group. (E) Liver weight in the treated animals (F) Glucose tolerance test by 90 min after glucose injection. The values are expressed as the means ± SEM of n, *p < 0.05 based on ANOVA (one-way) followed by Bonferroni post hoc analysis.
We also controlled the average weight of mice and we observed an increase in the weight of animals supplemented with 15% of fructose in the last two weeks (Fig. 1D). Liver weight provides an indirect assessment of liver function, and fructose treatment induced a 41.5 ± 9.3% increase in liver weight, normalized to the body weight of both groups (Fig. 1D).
To confirm the induction of MetS, we measured several blood parameters including the glucose tolerance test. We observed that the group supplemented with fructose present a decrease in the capacity of regulate the glucose levels after the injection of glucose (Fig. 1F). Also we observed other parameters used in the diagnosis of MetS including the blood levels of glucose, insulin, total cholesterol and triglycerides, and calculated the HOMA ratio. Fructose treatment increased glucose levels (37.7 ± 7%), insulin levels (178 ± 29%), cholesterol levels (54.5 ± 12.2%), and triglyceride levels (69.2 ± 11.3%), and the HOMA index was increased 3-fold in the fructose treated group (Table 1) [38].
Table 1.
Blood values after treatment with fructose 15% by 8 weeks.
| Glucose (mg/dL) | Insulin (μIU/mL) | HOMA index | Cholesterol (mg/dL) | Triglycerides (mg/dL) | |
|---|---|---|---|---|---|
| Control | 98 ± 6 | 0.93 ± 0.20 | 0.23 ± 0.06 | 121 ± 15 | 78 ± 15 |
| Fructose | 135 ± 10* | 2.59 ± 0.15** | 0.86 ± 0.06* | 187 ± 13* | 132 ± 12* |
The values are expressed as the means ± SEM of n: 9 animals per group.
p < 0.05 and
p < 0.01 based on ANOVA (one-way) followed by Bonferroni post hoc analysis.
3.2. MetS impairs spatial memory performance
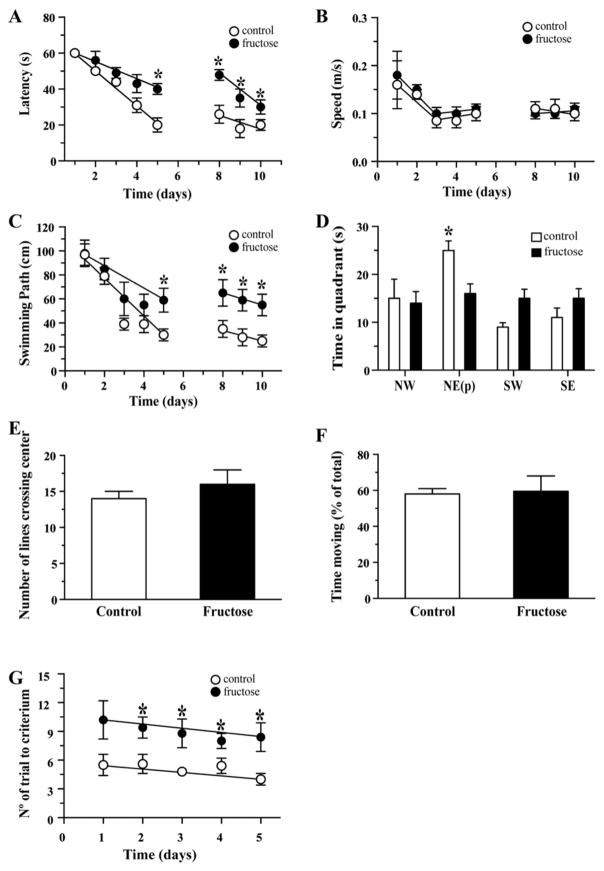
Spatial memory was assessed using the Morris water maze (MWM) paradigm, which was performed in a blinded manner. For the first four days, the mice in both groups took approximately the same length of time to find the platform at the end of the test (latency time); however, by day 5, the MetS group took longer time to locate the platform compared with the control group (40 ± 6 s and 21 ± 3 s, respectively). The trend continued up to day 10, which suggests a decline in the learning and memory abilities of the MetS group (Fig. 2A). The increase in latency time was associated with a longer swimming distance in the MetS group (Fig. 2B). No difference in swimming speed was observed between the two groups; this rules out the possibility that the increased latency time was a result of muscular failure or visual deficiency (Fig. 2C). On the final day of the test, the platform was removed and the time that the animals spent in each of the four quadrants of the pool was recorded: northwest (NW), northeast (NE), southwest (SW) and southeast (SE). In the absence of a platform, the control group spent more time (25 ± 3 s) in the quadrant where the platform had previously been located (NE) than the MetS group (15 ± 2 s), while the MetS group had no preference for any particular quadrant (Fig. 2D).
Fig. 2.
The induction of MetS leads to a decrease in cognitive performance. (A) The time required to find the hidden platform (latency time) was determined for both groups. (B) Speed of swim of both groups during the MWM test. (C) Swimming path during the MWM test by group. (D) The time spent in each quadrant in the absence of the platform. (E and F) In the large open field we measured the number of lines crossing the center and the time of moving. (G) The performance of both groups on the memory-flexibility task was analyzed by assessing the number of trials to meet the criteria. The values are expressed as the means ± SEM of n animals per group and were analyzed via ANOVA (one-way) followed by Bonferroni post hoc analysis.
The LOF test was used to evaluate general locomotor activity and exploration [39]. We evaluated parameters related to spontaneous behavior in mice including the time of moving and the number of lines that crossed the center. No significant difference was observed between the two groups, which suggest MetS does not influence the general status of animals (Fig. 2E and F).
A modified spatial-memory paradigm to assess episodic memory or memory flexibility was used. The assessment of memory flexibility provides a sensitive indication of hippocampal function since the position of the hidden platform is changed every day. The MetS group required significantly more trials to complete the test compared with the control group (10 and 6, respectively) starting the first day and maintained for all 5 days of the test (Fig. 2G).
3.3. MetS impairs hippocampal synaptic plasticity
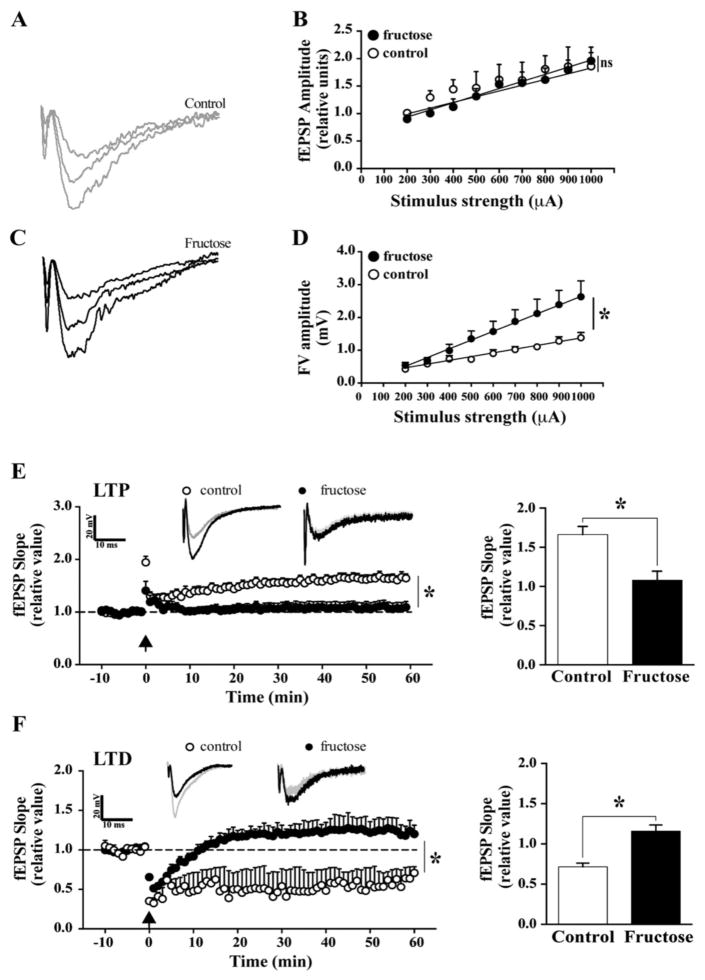
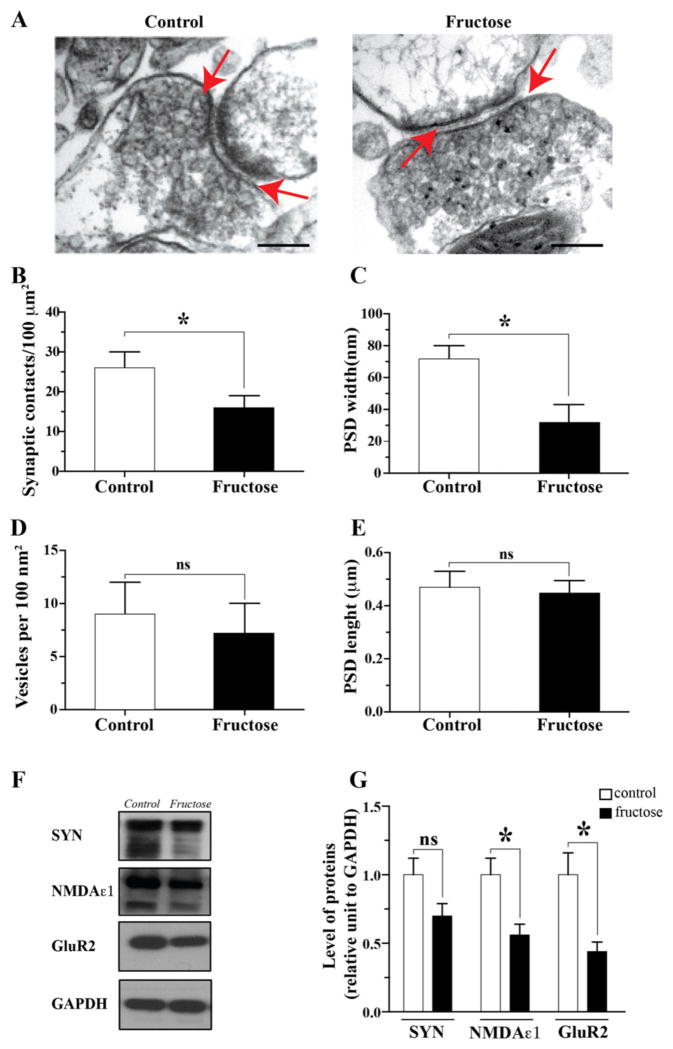
We examined the effects of MetS on hippocampal synaptic plasticity to elucidate a potential mechanism underlying the behavioral results. Synaptic integrity was assessed using electrophysiological recordings for input–output analysis [31]. The field excitatory postsynaptic potential (fEPSP) was not altered in the MetS group (Fig. 3A and B); however, the fiber volley, an indicator of axonal excitability, was decreased in amplitude by 70 ± 19% using a stimulus strength of 1000 μA (Fig. 3C and D), which indicates an alteration in synaptic transmission at the presynaptic level. We measured LTP and LTD, which reflect persistent changes in synaptic connectivity underlying learning and memory functions. A set of protocols for theta burst stimulation (TBS) and low-frequency stimulation (LFS) was used to induce LTP and LTD, respectively. Notably, it was not possible to induce robust LTP in the MetS mice, who displayed a maximum of 1.33 ± 0.12 mV compared with a maximum 1.95 ± 0.12 mV in the control group. Furthermore, in the control group, the induction and maintenance of LTP persisted for at least 60 min of recording (1.67 ± 0.11 mV in the control group and 1.1 ± 0.14 mV in the MetS group; Fig. 3E). In contrast to LTP, LTD indicates a reduction in synaptic strength [40]. It was almost impossible to induce LTD in the fructose-treated animals (0.67 ± 0.054 mV) relative to the control group (1.17 ± 0.13 mV) at 60 min (Fig. 3F). These changes in LTP and LTD suggest that MetS leads to deregulation of hippocampal synaptic transmission and plasticity. We also determined the effects of MetS on synaptic structure by examining the synaptosomal fraction from the hippocampus using electron microscopy (Fig. 4A). We found a decrease in the number of synaptic contacts from 27 ± 3 per 100 μm2 in the control group to 17 ± 4 per 100 μm2 in the treated group (Fig. 4B). We also observed a decrease in the width of postsynaptic density (PSD, n = 5) from 68 ± 9 nm in the control group to 33 ± 13 nm in the fructose treated group (Fig. 4C). We did not observe any changes in the number of vesicles or the length of the PSD regions (Fig. 4D and E). In the synaptosomal fractions, we performed western blot for several pre- and post-synaptic proteins and observed a significant decrease in the expression of the glutamate receptor subunits NMDAε1 and GluR2 (Fig. 4F and G). Together, these results provide clear and novel evidence for the impact of MetS on the structural and molecular composition of hippocampal synapses.
Fig. 3.
MetS reduces the excitability of the hippocampus. (A–D) First, synaptic integrity was evaluated via input–output analysis of the electrophysiological recordings. (E) LTP was measured in the hippocampal slices of the mice in both groups (drinking water with or without supplemental fructose). (F) LTD was determined in both groups. The values are expressed as the means ± SEM of n animals per group. *p < 0.05 based on ANOVA (one-way) followed by Bonferroni post hoc analysis.
Fig. 4.
MetS affects the synaptic structure of the hippocampus. (A–E) Electron microscopy of synaptosomal fractions and quantification of several characteristics of the pre- and post-synaptic regions (red arrows). (F–G) The induction of MetS triggered a decrease in the levels of pre- and post-synaptic proteins. Scale: 200 nm. The values are expressed as the means ± SEM of n animals per group. *p < 0.05 based on ANOVA (one-way) followed by Bonferroni post hoc analysis.
3.4. MetS decreases hippocampal neurogenesis
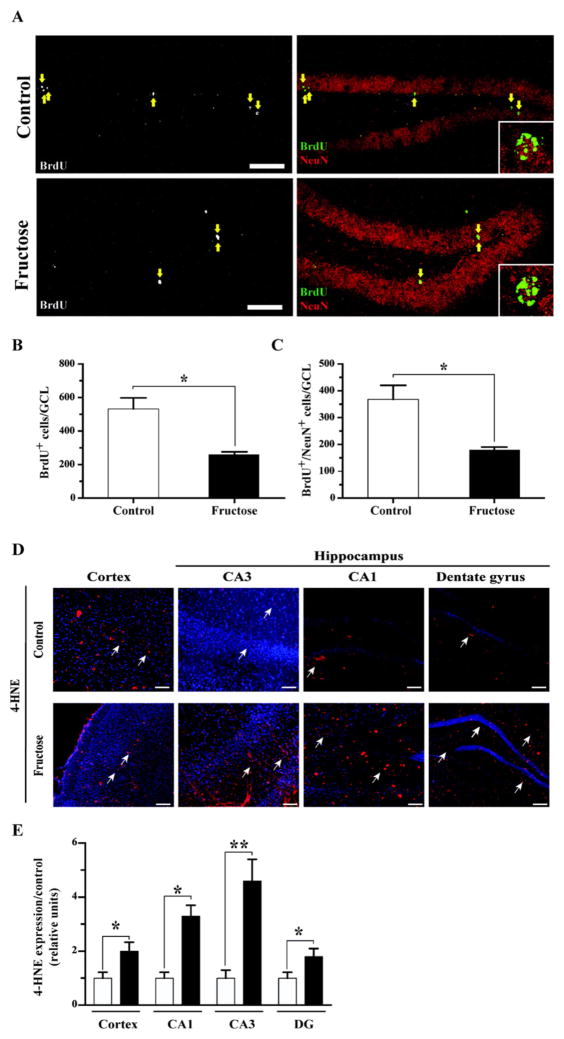
We examined the effects of MetS on hippocampal neurogenesis as the generation of new neurons contributes to neural plasticity and memory [41–43]. First, we evaluated the effect of MetS on cell proliferation in the SGZ by incorporation of the nucleotide analog BrdU. A significant decrease in the total number of BrdU-positive cells was observed in the granule cell layer (GCL) of mice treated with fructose. To evaluate the generation of new neurons, the expression of the mature neuronal marker NeuN in each BrdU-positive cell was evaluated in the hippocampus 4 weeks after BrdU injection (Fig. 5A). There was a significant decrease (50%) in the total number of newborn granule cells (total number of BrdU+/NeuN+ cells in the entire GCL) in the fructose-treated animals (Fig. 5B and C). These data show that MetS decreases adult hippocampal neurogenesis.
Fig. 5.
MetS reduces the generation of new neurons in the adult hippocampus and increase the levels of oxidative stress. (A) Representative immunofluorescence staining for BrdU and NeuN. The inset shows a higher magnification of BrdU-positive cells that are also positive for NeuN. The arrows indicate BrdU+ cells. Scale bar: 50 μm (original magnification 20×). (B–C) The total number of BrdU+ and BrdU+/NeuN+ cells in the GCL was determined. (D–E) Quantification of the immunofluorescence signal normalized to that under the control conditions. 4-HNE, red; nuclear stain Hoechst, blue. The values are expressed as the means ± SEM of n animals per group. *p < 0.05 and **p < 0.01 based on ANOVA (one-way) followed by Bonferroni post hoc analysis. Scale bar: 100 μm.
3.5. MetS increases oxidative stress
Oxidative metabolism is directly associated with the integrity of the plasma membrane and is a factor in almost all neurodegenerative diseases, as well as aging [44]. To determine whether MetS affects oxidative metabolism, we measured the levels of 4-HNE, an aldehyde product of lipid peroxidation that serves as a marker of plasma membrane damage [45]. Plasma membrane integrity is essential for neuronal signaling. Notably, the intensity of 4-HNE immunofluorescence was significantly increased in the cortex (2-fold) and hippocampal CA1 and CA3 (2- and 3-fold, respectively) and displayed an increasing trend in the dentate gyrus (2-fold) of the MetS group compared with the control group (Fig. 5D and E). These results corroborate the effects of MetS on lipid peroxidation with potential repercussion on membrane function.
3.6. Remission of MetS is associated with recovery of hippocampal function
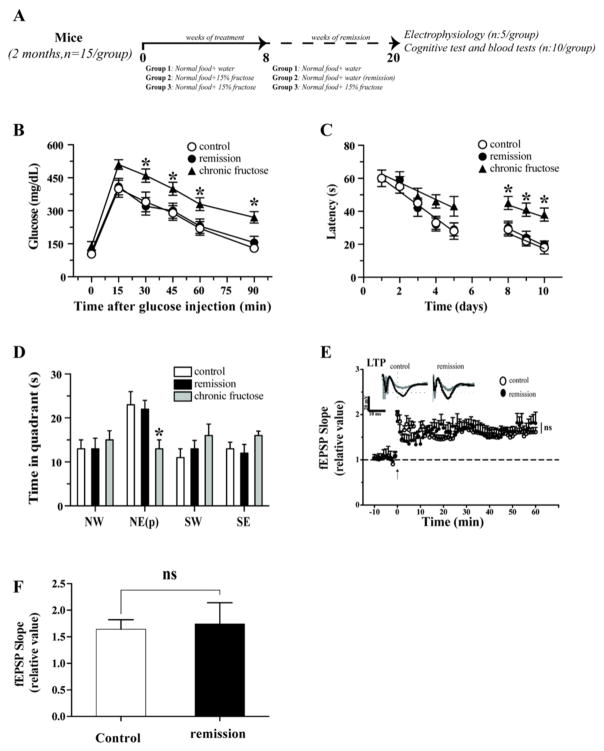
To study if the effects of fructose are reversible, after fructose treatment, we prepare 3 groups of mice (Fig. 6A). In each group we monitored the blood levels of glucose, insulin, total cholesterol, and triglycerides and the HOMA index every 30 days. We observed a normalization of the blood parameters after 3 months, and this period was considered a critical time interval for the remission of MetS (Table 2).
Fig. 6.
MetS remission enabled amelioration of specific aspects of hippocampal deterioration. (A). We used three groups of animals: control with normal diet, fructose-treated for 8 weeks followed by fructose removal for 12 weeks, fructose-treated for 20 weeks. (B) Glucose tolerance test was performed to assess insulin resistance. (C) Behavioral performance on the MWM task was normal after remission and no difference was observed compared to control animals. We observed a significant decrease in the cognitive performance in the group treated with fructose for 20 weeks. (D) In the absence of the platform, we did not observe any differences between the remised and control groups. (E and F) Recovery of LTP depression after MetS remission. The values were expressed as the means ± SEM. *p < 0.05 and **p < 0.01 based on ANOVA (one-way) followed by Bonferroni post hoc analysis.
We observed that the control (group 1) and remission group (group 2) showed a similar capacity of regulate glucose levels after injection of glucose. However the group treated with fructose by 20 weeks showed a several deregulation in the glucose levels control (Fig. 6B).
The remission group exhibited normalized memory performance in the MWM test, as evidenced by spending a time in the platform quadrant nearby to control values. However, the group treated with fructose by 20 weeks showed a increase in the latency time and a decrease in the capacity of remember the localization of the platform (Fig. 6C and D). Likewise the remission of MetS resulted in normalization of synaptic transmission and LTP induction such that no differences were observed between the remission and control groups (Fig. 6D).
4. Discussion
An increasing number of clinical reports depict the impact of altered systemic metabolism on the incidence of a large variety of neurological disorders. We present new mechanistic evidence for the disruptive effects of fructose-induced MetS on crucial aspects of neuronal excitability underlying brain function and plasticity. Animals exposed to fructose lost their ability to sustain hippocampal LTP and LTD, showed a reduction in density and size of active zones at synapses, and reduced the generation of new neurons in the hippocampus. All of these changes are congruent with a reduction in learning and memory performance, and the prospect of elevated vulnerability to neurological disorders. These results are particularly significant on the context of the epidemic of metabolic disorders such as obesity and diabetes [46,47]. The effects of fructose-induced MetS on hippocampal plasticity were partially reversible after ceasing fructose consumption, which suggests the existence of a window of opportunity for the application of preventive programs.
In our model we observed that the treatment with fructose induce changes in several blood markers commonly used for the diagnosis of MetS in humans, including insulin resistance, blood glucose, triglycerides and cholesterol, and obesity. We obtained similar results to those
4.1. Fructose-induced MetS reduces functional and structural synaptic plasticity
Our results show that fructose-induced MetS affects the long-term modulation of basal synaptic transmission and associated axonal excitability of hippocampal synapses [49]. Adaptations in hippocampal synaptic plasticity, such as LTP and LTD, underlie changes in spatial learning and memory processing [50]. The disruption in the balance between LTP and LTD in the MetS group is likely a primary event for the observed failure in learning and memory functions [51]. It is notable that these events were detected in the hippocampus, the locus of associative memory [52], which is heavily compromised during aging and AD. We also observed fewer active zones and smaller PSD regions in the animals exposed to fructose, which harmonize with the dysfunction in LTP and cognition. Taken together, these results suggest that MetS reduces neuronal excitability and hippocampal function, which are necessary to support learning and memory processing.
Hippocampal neurogenesis is important for maintaining cognitive performance in adult mice and is considered a symbol of hippocampal plasticity [41–43]. According to our results, MetS reduced neurogenesis in the SGZ of the dentate gyrus, where newborn neurons integrate into the pre-existing hippocampal circuitry [25] and are involved with LTP and LTD [42,43]. Immature newborn neurons display greater excitability than mature neurons mostly due to reduced inhibition by GABA [53]. These immature neurons appear to contribute to LTP, as suggested by findings that blocking neurogenesis prevents the expression of LTP [41]. In addition, adult-born neurons are involved in certain aspects of hippocampus-dependent learning and memory, such as spatial memory, pattern separation and contextual fear conditioning [54,55]. A decrease in neurogenesis is a feature in the pathology of several neurological disorders [56,57]. Reminiscent of the altered levels of insulin in the MetS condition, it has been reported that insulin plays a central role in the formation of new neurons in the brain [58]. Therefore, it is possible that MetS could impact neural plasticity and cognitive function by altering neurogenesis. MetS could also influence neuronal plasticity and cognition by altering the function of the plasma membrane.
Oxidative metabolism is directly associated with the integrity of the plasma membrane and is a factor in almost all neurodegenerative diseases, as well as in aging [44]. We measured the levels of 4-HNE, an aldehyde product of lipid peroxidation that serves as a marker of plasma membrane damage [45]. The plasma membrane is crucial for supporting neuronal excitability and signaling, which depend largely on the integrity of its phospholipid composition. The oxidative marker 4-HNE is an end-product of lipid peroxidation [59], which increase in the fructose treated mice could be indicative of impaired membrane-related neuronal excitability. Lipid peroxidation affects several processes associated with synaptic activity, including ion uptake, ion-channel activity, glucose metabolism, glutamate uptake and Na+/K+- and Ca2+-ATPase activity [60].
4.2. Fructose-induced MetS reduces learning and memory function
We found that MetS caused a disruption of spatial learning, which is a sequel of Alzheimer’s and Parkinson’s diseases [61,62]. Remarkably, AD might represent a “type 3 diabetes” based on deregulated insulin and glucose metabolism that are components of its pathology [63–65]. On the episodic memory tests, which require the mice to learn and memorize daily alterations in the platform location, the MetS group required more attempts to locate the hidden platform than the control group. These cognitive deficits were likely not a result of visual or muscular deficiencies because the swimming speed of the MetS mice did not differ from that of the control mice. These results are relevant to humans because this type of memory is essential for coping with daily routines, such as remembering specific series of events within contexts. One of the most relevant questions from a public health standpoint is whether the effects of MetS on the brain are reversible. We assessed this possibility by removing fructose from the diet after the establishment of the MetS condition. We monitored the blood glucose levels once a month and found that the levels of glucose tolerance were similar to the control levels 3 months after terminating the fructose-containing diet. Remarkably, spatial-learning performance was nearly completely normalized. In addition, there was complete recovery from LTP. However, other properties did not recover, such as LTD, emphasizing the strong impact of MetS on the brain. Conversely, we also set a group with a chronic exposition to fructose; this groups present a several alteration in the blood parameters as also in the cognitive performance. These data are important for identifying specific aspects of MetS that may serve as targets of therapeutic strategies to reduce its effects on the brain. These findings, upon further validation in humans, are critical for the development of public health policies.
4.3. How fructose affects the brain
The animals exposed to high fructose exhibited several hallmarks of MetS as defined in humans by increases in weight, dyslipidemia, impaired glucose tolerance, and insulin levels. In particular, our results revealed a significant increase in liver weight, which is consistent with the effects of fructose on lipid deposition and the sequel of fatty liver disease [11,38].
MetS in humans has been associated with an increased risk of neurological disorders [66,67], however, the mechanisms by which MetS impairs cognitive processing are poorly understood. Fructose could influence the brain by elevating various peripheral parameters of MetS such as increased adiposity, insulin, and triglycerides [11,21,68], and some of the associated metabolites could reach the brain. An emerging line of evidence indicates that fructose could influence brain function directly, i.e., neuronal cells are able to metabolize fructose uptaken from the extracellular medium [69]. Administration of 20% of fructose induces an increase in the glucose transporter 5 (GLUT5) in glial cells in the hippocampus and cortex in rats. GLUT5 is the main transporter of fructose into the brain and likely important for neuronal function [70–72]. Intraventricular administration of fructose provokes feeding in rodents and suggests a direct action of fructose on hypothalamic function in appetite. The administration of fructose lead to a reduction in cerebral blood flow (CBF) in the thalamus, hippocampus, posterior cingulate cortex, and visual cortex [73]. A reduction in CBF has been associated with alterations in CA1 hippocampal cells including reductions in dendritic arborization and spine density [74].
5. Conclusions
MetS, obesity and diabetes have become worldwide epidemics that affect patients of all ages and result in high health care costs [75]. Our results indicate that fructose induced MetS affects specific aspects of neuronal and synaptic plasticity, and raises concerns about the risk imposed of MetS for the pathogenesis of disorders affecting cognitive abilities such as AD [11] and depression/anxiety [76]. Given the current lack of effective pharmacological approaches to treat these disorders, the most effective therapy is prevention [77], and our results provide some evidence for the reversibility of the neural pathogenesis related to the MetS. The results of this study demonstrate that MetS disrupts the function of neuronal circuits involved in cognitive processing while MetS remission normalizes these alterations. These results are significant to define a targetable mechanism to reduce the risk posed by altered metabolism on neurological disorders.
Acknowledgments
Sources of funding
This work was supported by grants from the Basal Center of Excellence in Aging and Regeneration (CONICYT-PFB 12/2007) to N.C.I. and C.P.V. FONDECYT (no. 1120156) to N.C.I., FONDECYT (no. 1130747) to C.P.V., FONDECYT (no. 1150933) to L.V.N. and the NIH (no. R01 NS50465) to F.G.P. As well a postdoctoral fellowship from FONDECYT (no. 3150475) to P.C. We also thank to Sociedad Química y Minera de Chile (SQM) for special grants “The role of K+ on Hypertension and Cognition” and “The role of lithium in human health and disease”.
Abbreviations
- AD
Alzheimer’s disease
- ACSF
artificial cerebro-spinal fluid
- CBF
cerebral blood flow
- fEPSP
field excitatory postsynaptic potential
- GCL
granule cell layer
- GLUTs
glucose transporters
- 4-HNE
4-hydroxynonenal
- LTP
long-term potentiation
- LTD
long-term depression
- MetS
Metabolic syndrome
- MWM
Morris Water Maze
- PTX
picrotoxin
- PSDs
postsynaptic densities
- SGZ
subgranular zone
- T2DM
type II diabetes mellitus
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr E. International Diabetes Federation Task Force on, Prevention, L. Hational Heart, I. Blood, A. American Heart, F. World Heart, S. International Atherosclerosis, O. International Association for the Study of. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Aydin S, Aksoy A, Aydin S, Kalayci M, Yilmaz M, Kuloglu T, Citil C, Catak Z. Today’s and yesterday’s of pathophysiology: biochemistry of metabolic syndrome and animal models. Nutrition. 2014;30:1–9. doi: 10.1016/j.nut.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Chiavaroli L, Ha V, de Souza RJ, Kendall CW, Sievenpiper JL. Re. “Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis”. Nutrition. 2015;31:419–420. doi: 10.1016/j.nut.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014;30:503–510. doi: 10.1016/j.nut.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Lin F, Lo RY, Cole D, Ducharme S, Chen DG, Mapstone M, Porsteinsson A. I Alzheimer’s Disease Neuroimaging. Longitudinal effects of metabolic syndrome on Alzheimer and vascular related brain pathology. Dement Geriatr Cogn Disord Extra. 2014;4:184–194. doi: 10.1159/000363285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts AS, Loskutova N, Burns JM, Johnson DK. Metabolic syndrome and cognitive decline in early Alzheimer’s disease and healthy older adults. J Alzheimers Dis. 2013;35:253–265. doi: 10.3233/JAD-121168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease—the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89:144–149. doi: 10.1016/j.brainresbull.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Petrov D, Pedros I, Artiach G, Sureda FX, Barroso E, Pallas M, Casadesus G, Beas-Zarate C, Carro E, Ferrer I, Vazquez-Carrera M, Folch J, Camins A. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiencies contribute to Alzheimer disease pathology in rodents. Biochim Biophys Acta. 2015;1852:1687–1699. doi: 10.1016/j.bbadis.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Trevino S, Aguilar-Alonso P, Flores Hernandez JA, Brambila E, Guevara J, Flores G, Lopez-Lopez G, Munoz-Arenas G, Morales-Medina JC, Toxqui V, Venegas B, Diaz A. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse. 2015;69:421–433. doi: 10.1002/syn.21832. [DOI] [PubMed] [Google Scholar]
- 11.Rios JA, Cisternas P, Arrese M, Barja S, Inestrosa NC. Is Alzheimer’s disease related to metabolic syndrome? A Wnt signaling conundrum. Prog Neurobiol. 2014;121:125–146. doi: 10.1016/j.pneurobio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JS, Yan JH, Payne VG. The impact of obesity and exercise on cognitive aging. Front Aging Neurosci. 2013;5:97. doi: 10.3389/fnagi.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, Lambert B, Thompson C, Neubauer S, Clarke K. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr. 2011;93:748–755. doi: 10.3945/ajcn.110.002758. [DOI] [PubMed] [Google Scholar]
- 16.Espeland MA, Brinton RD, Manson JE, Yaffe K, Hugenschmidt C, Vaughan L, Craft S, Edwards BJ, Casanova R, Masaki K, Resnick SM W.-M.S. Group. Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology. 2015 doi: 10.1212/WNL.0000000000001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Annals of the New York Academy of Sciences. 2015 doi: 10.1111/nyas.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang RR, Jia BH, Xie L, Ma SH, Yin JJ, Sun ZB, Le HB, Xu WC, Huang JZ, Luo DX. Spatial working memory impairment in primary onset middle-age type 2 diabetes mellitus: an ethology and BOLD-fMRI study. Journal of magnetic resonance imaging: JMRI. 2015 doi: 10.1002/jmri.24967. [DOI] [PubMed] [Google Scholar]
- 19.Sridhar GR, Lakshmi G, Nagamani G. Emerging links between type 2 diabetes and Alzheimer’s disease. World J Diabetes. 2015;6:744–751. doi: 10.4239/wjd.v6.i5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr. 2013;4:246–256. doi: 10.3945/an.112.003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012;590:2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Pinilla F, Tyagi E. Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care. 2013;16:726–733. doi: 10.1097/MCO.0b013e328365aae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuchlik A. Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Front Behav Neurosci. 2014;8:106. doi: 10.3389/fnbeh.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C L National Heart, I. Blood, A. American Heart, Definition of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 28.Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, Boscoe AN, Ben-Joseph RH, Magid DJ, Okamoto LJ. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord. 2009;7:305–314. doi: 10.1089/met.2008.0070. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Atabek ME. Reliability and validity of homeostasis model assessment for insulin resistance and β-cell dysfunction in critically ill children with hyperglycemia. J Pediatr Endocrinol Metab. 2013;26:1215. doi: 10.1515/jpem-2013-0222. [DOI] [PubMed] [Google Scholar]
- 31.Vargas JY, Fuenzalida M, Inestrosa NC. In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J Neurosci. 2014;34:2191–2202. doi: 10.1523/JNEUROSCI.0862-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker S, Grider G, Cobb M, Li XP, Huff MO, El-Mallakh RS, Levy RS. Open field is more sensitive than automated activity monitor in documenting ouabain-induced hyperlocomotion in the development of an animal model for bipolar illness. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:455–462. doi: 10.1016/s0278-5846(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 33.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Walsh RN, Cummins RA. The open-field test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 35.Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen En Henegouwen PM, Fon EA. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- 36.Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci U S A. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott AC, Calderon Toledo C, Aranguiz FC, Inestrosa NC, Varela-Nallar L. Tetrahydrohyperforin increases adult hippocampal neurogenesis in wild-type and APPswe/PS1DeltaE9 mice. J Alzheimers Dis. 2013;34:873–885. doi: 10.3233/JAD-121714. [DOI] [PubMed] [Google Scholar]
- 38.E. National Cholesterol Education Program Expert Panel on Detection, A. Treatment of High Blood Cholesterol in, Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 39.Stanford SC. The open field test: reinventing the wheel. J Psychopharmacol. 2007;21:134–135. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- 40.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 41.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 42.Mongiat LA, Schinder AF. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci. 2011;33:1055–1061. doi: 10.1111/j.1460-9568.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- 43.Massa F, Koehl M, Wiesner T, Grosjean N, Revest JM, Piazza PV, Abrous DN, Oliet SH. Conditional reduction of adult neurogenesis impairs bidirectional hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2011;108:6644–6649. doi: 10.1073/pnas.1016928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, WO, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab. 2010;299:E879–E886. doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- 46.Efimova I, Efimova N, Lishmanov Y. Cerebral blood flow and cognitive function in patients with metabolic syndrome: effect of antihypertensive therapy. J Clin Hypertens (Greenwich) 2014;16(12):900–906. doi: 10.1111/jch.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin BE, Llabre MM, Dong C, Elkind MS, Stern Y, Rundek T, Sacco RL, Wright CB. Modeling metabolic syndrome and its association with cognition: the northern Manhattan study. J Int Neuropsychol Soc. 2014:1–10. doi: 10.1017/S1355617714000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- 50.Bickler PE, Buck LT. Effects of fructose-1,6-bisphosphate on glutamate release and ATP loss from rat brain slices during hypoxia. J Neurochem. 1996;67:1463–1468. doi: 10.1046/j.1471-4159.1996.67041463.x. [DOI] [PubMed] [Google Scholar]
- 51.Hulme SR, Jones OD, Raymond CR, Sah P, Abraham WC. Mechanisms of heterosynaptic metaplasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130148. doi: 10.1098/rstb.2013.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen P, Morris RD, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book. Oxford Univ. Press; New York: 2007. [Google Scholar]
- 53.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res. 2012;227:391–399. doi: 10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Varela-Nallar L, Aranguiz FC, Abbott AC, Slater PG, Inestrosa NC. Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Birth Defects Res C Embryo Today. 2010;90:284–296. doi: 10.1002/bdrc.20193. [DOI] [PubMed] [Google Scholar]
- 57.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler AN, Levison SW, Wood TL. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol. 2014;11:161–170. doi: 10.1038/nrendo.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar V, Gill KD. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 2014;41C:154–166. doi: 10.1016/j.neuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 61.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blesa J, Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frisardi V. Impact of metabolic syndrome on cognitive decline in older age: protective or harmful. where is the pitfall? J Alzheimers Dis. 2014;41:5. doi: 10.3233/JAD-140389. [DOI] [PubMed] [Google Scholar]
- 64.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol. 2013;88:12. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyd AE, III, Moss LG. When sugar is not so sweet: glucose toxicity. J Clin Invest. 1993;92:2. doi: 10.1172/JCI116550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh GK. Metabolic syndrome in children and adolescents. Curr Treat Options Cardiovasc Med. 2006;8:403–413. doi: 10.1007/s11936-006-0045-3. [DOI] [PubMed] [Google Scholar]
- 68.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 69.Funari VA, Crandall JE, Tolan DR. Fructose metabolism in the cerebellum. Cerebellum. 2007;6:130–140. doi: 10.1080/14734220601064759. [DOI] [PubMed] [Google Scholar]
- 70.Shu HJ, Isenberg K, Cormier RJ, Benz A, Zorumski CF. Expression of fructose sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience. 2006;140:889–895. doi: 10.1016/j.neuroscience.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 71.Messier C, Whately K, Liang J, Du L, Puissant D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav Brain Res. 2007;178:139–145. doi: 10.1016/j.bbr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Payne J, Maher F, Simpson I, Mattice L, Davies P. Glucose transporter Glut 5 expression in microglial cells. Glia. 1997;21:327–331. doi: 10.1002/(sici)1098-1136(199711)21:3<327::aid-glia7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 73.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calvo-Ochoa E, Hernandez-Ortega K, Ferrera P, Morimoto S, Arias C. Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. J Cereb Blood Flow Metab. 2014;34:1001–1008. doi: 10.1038/jcbfm.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor VH, Stonehocker B, Steele M, Sharma AM. An overview of treatments for obesity in a population with mental illness. Can J Psychiatry. 2012;57:13–20. doi: 10.1177/070674371205700104. [DOI] [PubMed] [Google Scholar]
- 76.Farooqui AA, Farooqui T, Panza F, Frisardi V. Metabolic syndrome as a risk factor for neurological disorders. Cell Mol Life Sci. 2012;69:741–762. doi: 10.1007/s00018-011-0840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357–373. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]