Abstract
Patients with advanced cirrhosis experience frequent infections leading to sepsis, which carries high mortality. While innate immune dysfunction underlies this vulnerability, the precise cause remains elusive. We found prostaglandin (PGE2) elevated in acutely decompensated (AD) patients at immunosuppressive levels. Plasma from AD and end-stage liver disease (ESLD) patients suppressed macrophage cytokine secretion and bacteria killing in a PGE2 receptor-dependent manner, effects not seen in stable cirrhosis. Mouse models (bile duct ligation and CCL4-liver injury) also demonstrated elevated PGE2, which when inhibited completely restored immune competence and survival following infection. Importantly, albumin binds/inactivates PGE2 resulting in greater PGE2 bioavailability. This results in enhanced immunosuppressive effects of AD plasma in patients with low albumin levels. Administering albumin to AD patients reversed immunosuppressive properties of their plasma; protective effects recapitulated in rodent survival studies. Thus, elevated PGE2 combined with hypoalbuminemia mediates immunosuppression in AD and ESLD patients, which can be reversed with albumin.
Keywords: Albumin, macrophages, lipid mediators, acute decompensation
Liver cirrhosis is the 6th leading cause of death in high income and the 9th in low income countries1. Cirrhosis patients have an increased predisposition to and mortality from infection2. In 50% of cirrhosis patients infection is the precipitant for hospital admission and a further 15-35% develop nosocomial infections compared to 5-7% of general medical admissions3. Of those who develop sepsis and organ dysfunction, 80-90% will die4. Multimodal defects in the innate immune response have long been recognized5–7, however the underlying cause remains elusive. No effective immune restorative treatment exists. Whilst antibiotic prophylaxis in cirrhosis patients with upper gastrointestinal bleeding reduces bacterial infection and all-cause mortality8, their use in non-bleeding patients has not been substantiated, and the development of antibiotic resistance is a major concern9.
Cirrhosis patients are markedly heterogeneous, with critical variance in both aetiology and stage of disease. Clinical presentation and management is largely dictated by the latter. Key divisions include stable/early cirrhosis (Childs score A), end stage liver disease (ESLD) and acute decompensation (AD). Susceptibility to infection, reflecting underlying immune dysfunction is highest in AD patients and lowest in stable cirrhosis10. Decompensated cirrhosis patients typically present with hepatic encephalopathy, ascites, variceal bleeding, alcoholic hepatitis and hyperbilirubinaemia. AD refers to patients presenting either for the first time or with acute on chronic liver failure (AoCLF, an acute deterioration of pre-existing, chronic liver disease, usually related to a precipitating event and associated with increased mortality at 3 months due to multisystem organ failure11).
Cyclooxygenase (COX)-derived lipid mediators have broad immunosuppressive effects12–15 that could explain the aetiology of infection susceptibility in cirrhosis patients. Thus, we used a number of in vitro and in vivo assays using plasma from patients with AD and ESLD derived from clinical trials to investigate the role of bioactive lipid mediators in immunosuppression as well as animal models of liver injury for survival analyses.
Results
Prostaglandin E2 (PGE2) is elevated in patients with acute decompensation at levels that are immunosuppressive via its effect on the EP2/3 receptor
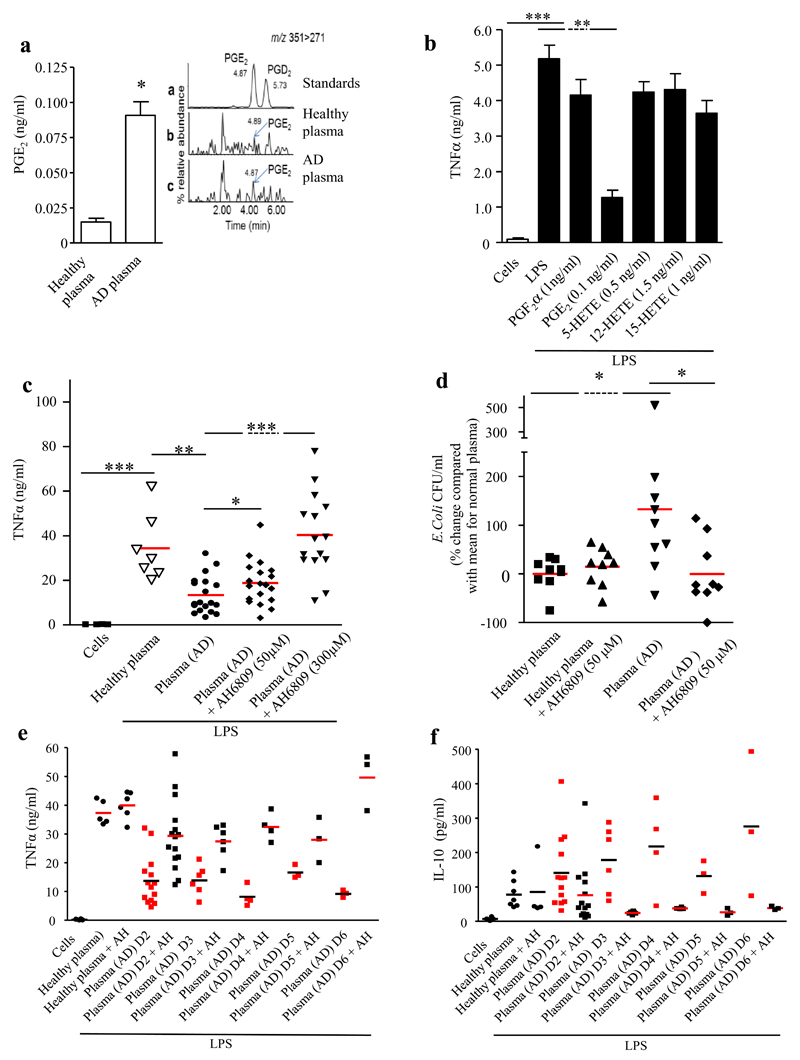
ESI/LC-MS/MS analysis of acutely decompensated patient plasma (day 1-2 of hospital admission) demonstrated significantly elevated PGE2, PGF2α, 5- and 15-HETE compared to HV (Figure 1A and supplementary Figure 1E-G). However, only PGE2 dampened TNFα release from LPS-stimulated human monocyte-derived macrophages when pre-treated with the mean concentrations observed in AD patients (0.1ng/ml) (Figure 1B).
Figure 1. Elevated immunosuppressive PGE2 in the plasma of patients admitted to hospital with Acute Decompensation (AD).
(A) LC/ESI-MS/MS was used to identify, PGE2, among other lipids mediators 1, as being elevated in the plasma of AD patients (see supplementary Figure, n=8 for healthy volunteers and n=14 for AD patients, 3 technical replicates performed) at levels that are immunosuppressive as determined by inhibition of (B) TNFα synthesis from LPS-stimulate human monocyte-derived macrophages, (experiments were carried out on cells from n=7 healthy volunteers in duplicate). (C) Plasma from AD patients inhibited TNFα release from LPS-stimulated (200ng/ml) human monocyte-derived macrophages as well as (D) bacterial killing by these cells in a PGE2 receptor-dependent manner (AH6809 [EP1-3 antagonist], 50μM); plasma from n=35 patients was used with experiments carried out in duplicate). The immune-suppressive effect of AD persisted for at least six days post hospitalization as determined by its EP-receptor dependent suppression of (E) TNF and elevation of (F) anti-inflammatory IL-10 using plasma from (n= 3-15 patients. Data is represented as average ± SEM. * P<0.05 ** P<0.01 ***P<0.001, ANOVA.
Human monocyte-derived macrophages were incubated with culture media supplemented with 25% (vol./vol.) plasma from AD patients (see Table 1 for clinical characteristics). Compared to macrophages treated with media supplemented with HV plasma, AD plasma caused a significant decrease in LPS-stimulated TNFα that was reversed by pre-incubating macrophages with the E-prostanoid (EP) 1-3/D-prostanoid (DP) 1 receptor antagonist, AH6809 (Figure 1C). Additionally, macrophages were incubated with E. coli in the presence/absence of HV or AD plasma. Compared to macrophages treated with HV plasma, those with AD plasma exhibited reduced bacterial killing, an effect reversed by pretreatment with AH6809 (50μM) (Figure 1D). AH6809 had no direct bactericidal effect while cell viability was unaffected by plasma. Baseline TNFα levels within AD plasma were >1000-fold lower than in cell culture.
Table 1.
Patient Clinical Characteristics from DASIMAR study
| ADMIS n=22 (median, IQR) | ADLIS n =13 (median, IQR) | P value | Overall n=35(median, IQR) | |
|---|---|---|---|---|
| % | 63 | 27 | ||
| Age | 49.5 (46.5-64.5) | 54 (48-70) | 0.56 | 52 (47-65) |
| Sex (males) | 16 (73%) | 6 (46%) | 22 (63%) | |
| Albumin | 28 (24-30) | 30 (28-31.5) | 0.0012 | 29 (27-31) |
| INR | 1.7 (1.4-2.04) | 1.6 (1.23-2) | 0.93 | 1.7 (1.4-2) |
| Creatinine | 62 (47-109) | 62 (47.5-122) | 0.54 | 62 (49-108) |
| MELD score | 19.4 (15.6-21.8) | 18.39 (12.1-21.4) | 0.93 | 19 (14.2-23) |
| Positive microbiology | 6 | 3 | 9 | |
| Died during admission | 2 | 1 | 3 | |
| Died within 6 months | 7 | 4 | 11 | |
| Length of stay (days) | 11.5 (8-28) | 10 (5.5-19) | 0.22 | 11 (7-25) |
| CRP | 28 (115-50.5) | 23 (5-43.5) | 0.24 | 26 (13-49) |
| Bilirubin | 78 (34-119) | 53 (19-73) | 0.87 | 67.5 (24-104) |
| Cause of cirrhosis | 15 Alcohol (68%) | 6 Alcohol (46%) | 21 Alcohol (60%) | |
| 3 NASH | 3 NASH | 6 NASH (17%) | ||
| 1 PBC | 1 PBC | 2 PBC | ||
| 1 Sarcoid | 1 Cryptogenic | 2 HCV | ||
| 1 Congenital hepatic fibrosis | 1 HBV | 1 HBV | ||
| 1 HCV | 1 HCV | 1 Congenital hepatic fibrosis | ||
| PGE2 | 0.12 (0.09-0.16); n=7 | 0.065 (0.035-0.085); n=6 | 0.005 | 0.09 (0.065-0.13); n=13 |
| PGE2/albumin | 0.0041 (0.0036-0.0058); n=7 | 0.002 (0.001-0.0028); n=6 | 0.001 | 0.003571 (0.0022-0.0049); n=13 |
| NH3 | 208 (128-260.5); n=5 | 151; n=1 | 204 (133-236); n=6 | |
| Ischemia modified albumin ratio (IMAR) | 0.025 (0.014-0.06); n=10 | 0.011 (0.005-0.02); n=5 | 0.075 | 0.017(0.01-0.04); n=15 |
Plasma from AD patients showed persistent AH6809-mediated immunosuppression up to day six post admission as defined by reduced TNFα and elevated IL-10 (Figure 1E-F). Importantly although ~60% of AD plasma samples from day 1 of admission induced immunosuppression in a PGE2-dependent manner (Figure 1C), all patients’ plasma sampled from days 2-6 did so (Figure 1E-F).
PGE2 mediates immunosuppression in patients with ESLD but not in stable cirrhosis or non-cirrhotic liver disease
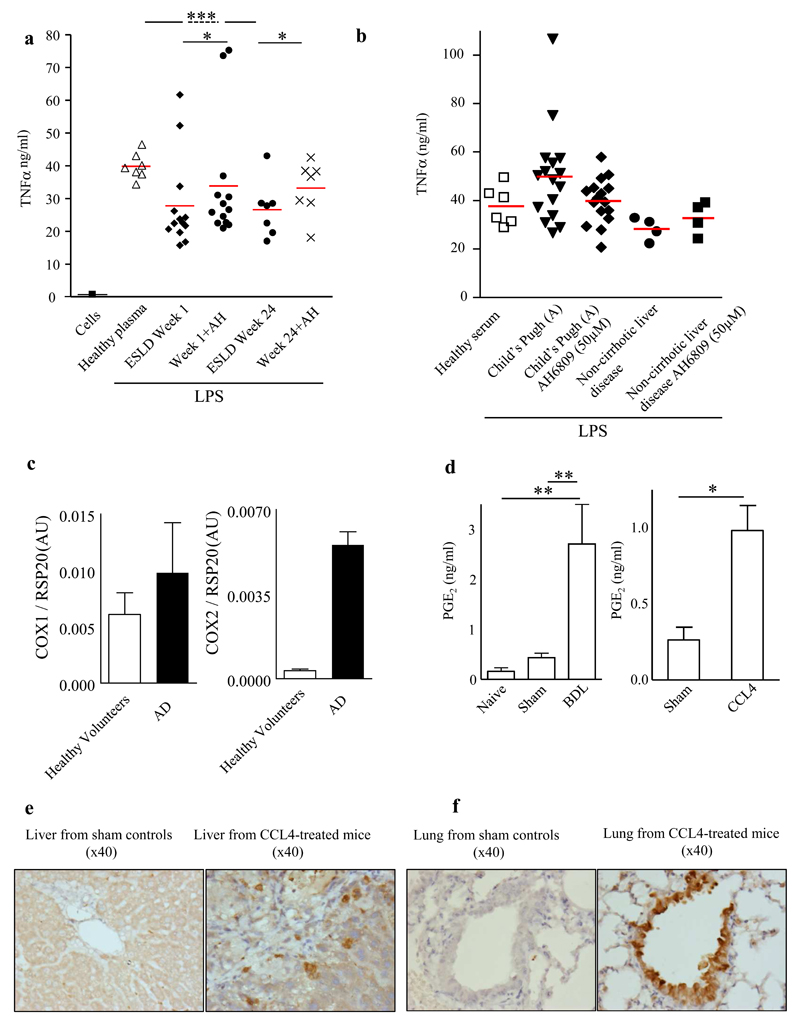
Plasma from patients with ESLD from samples taken at week 1 and week 24 of follow up significantly impaired macrophage TNFα synthesis, which was again reversed by AH6809 (Figure 2A). In contrast, samples from stable cirrhosis (Child score A) or non-cirrhotic liver disease outpatients had no effect on macrophage function (Figure 2B). In stable cirrhosis patients PGE2 levels were found to be twice healthy volunteer levels as opposed to the 7-fold increase in AD patients (Figure 1A and supplementary Table 1).
Figure 2. PGE2 is immunosuppressive in patients with End Stage Liver Disease awaiting liver transplantation, but not in stable cirrhosis or non-cirrhotic liver disease.
(A) Plasma from n=13 patients awaiting liver transplant taken over 24 weeks significantly impaired LPS-stimulated macrophage TNFα synthesis in an EP receptor-dependent manner using AH6809 (EP1-3 antagonist, 50μM), with no such immunosuppression observed in plasma from (B) n=16 stable cirrhosis (Child score A) or non-cirrhotic liver disease outpatients n=5. (C) rtPCR revealed significantly elevated COX-2 (two-tail t-test, p<0.00001), but not COX-1 in peripheral blood mononuclear cells isolated from AD patients compared to healthy volunteers (n=5 per group). Both (D) bile duct ligation (BDL) and carbon tetrachloride (CCL4) liver injury models, both revealed elevated plasma PGE2 (n≥6 mice/group), COX 2 was found in liver Kupffer cells as well as in alveolar macrophages, micrographs from n=3 experiments are presented. * P<0.05 ** P<0.01 ***P<0.001, ANOVA.
COX 2 is elevated in monocytes, liver Kupffer cells and alveolar macrophages implicating these cells as the likely source of PGE2
rtPCR revealed increased expression levels of COX 2 (p<0.00001), but not COX 1 in peripheral blood mononuclear cells of AD patients compared to healthy volunteers (n=5/group, Figure 2C). Immunohistochemical analysis of all organs from CCL4 mice (which have elevated PGE2, Figure 2D) demonstrated up-regulation of COX 2 in Kupffer cells and alveolar macrophages compared to shams but not in gut, aorta, heart or kidneys (Figure 2E and F).
Inhibiting PGE2 increased bacterial killing and restored survival in animal models of liver injury
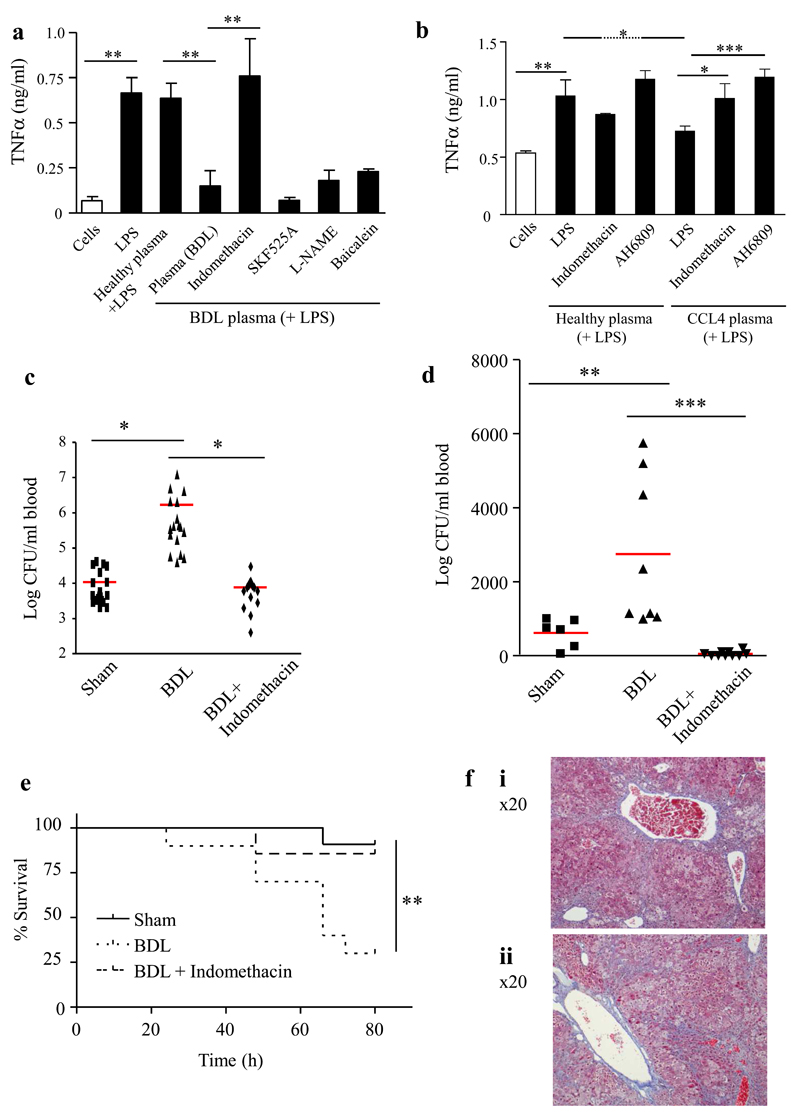
BDL mice exhibit a similar phenotype to AD patients (high bilirubin and low albumin, supplementary Table 3) including >5-fold elevated concentrations of PGE2 (Figure 2D). As in human studies (Figure 1), BDL plasma suppressed macrophage TNFα and IL-6 release, but increased IL-10 (Figure 3A and supplementary Figure 2A and B) without affecting macrophage viability. This was reversed by pretreating mice with the non-selective COX inhibitor indomethacin whereas no reversal was seen in mice given baicalein (lipoxygenase inhibitor), SKF525A (p450 inhibitor) or heat-treated (protein denatured) plasma. Experiments using a second model of CCL4 cirrhosis showed similar findings (Figure 3B).
Figure 3. Inhibiting PGE2 reversed impaired bacterial killing and restored survival following bacterial infection.
Plasma from BDL (A) or CCL4 (B) mice with/without the non-selective cyclooxygenase inhibitor (indomethacin, 3mg/kg p.o. to suppress circulating PGE2 [n≥6 mice/group]) was incubated with peritoneal macrophages from naïve mice. Bile duct ligated mice were administered (C) i.p. or (D) i.v. Group B streptococcus 1h after indomethacin treatment to determine effects of inhibiting PGE2 on bacterial killing in vivo (n≥8 mice/group) while the impact of cyclooxygenase inhibition on animal survival over time is shown in panel (E). The outcome of chronic PGE2 inhibition on cirrhotic mouse liver is shown in (F), micrographs from n=3 experiments are presented. Data is represented as average ± SEM. * P<0.05 ** P<0.01 ***P<0.001, ANOVA.
BDL mice were injected with live Group B streptococcus (GBS, NCTC10/84, serotype V). Consistent with immunosuppression, bacteria were significantly elevated in BDL blood after 3 h compared to shams (Figure 3C and D). However, dosing BDL mice with indomethacin (inhibiting PGE2 concentration by >80%) 1h before GBS administration restored bacterial killing to sham levels when bacteria were injected either i.p. (Figure 3C) or i.v. (Figure 3 D). Indomethacin restored the reduced survival observed following GBS challenge in BDL mice to that of shams (Figure 3E). In the lung PGE2 protects against fibrosis16 and therefore reducing levels may be beneficial for infection but worsen liver fibrosis. Histological analysis with H&E and Masson trichrom staining of the livers however showed no difference between celecoxib (selective COX 2 inhibitor) and non-celecoxib treated CCL4 mice (Figure 3F).
Albumin reverses the immunosuppressive effect of PGE2
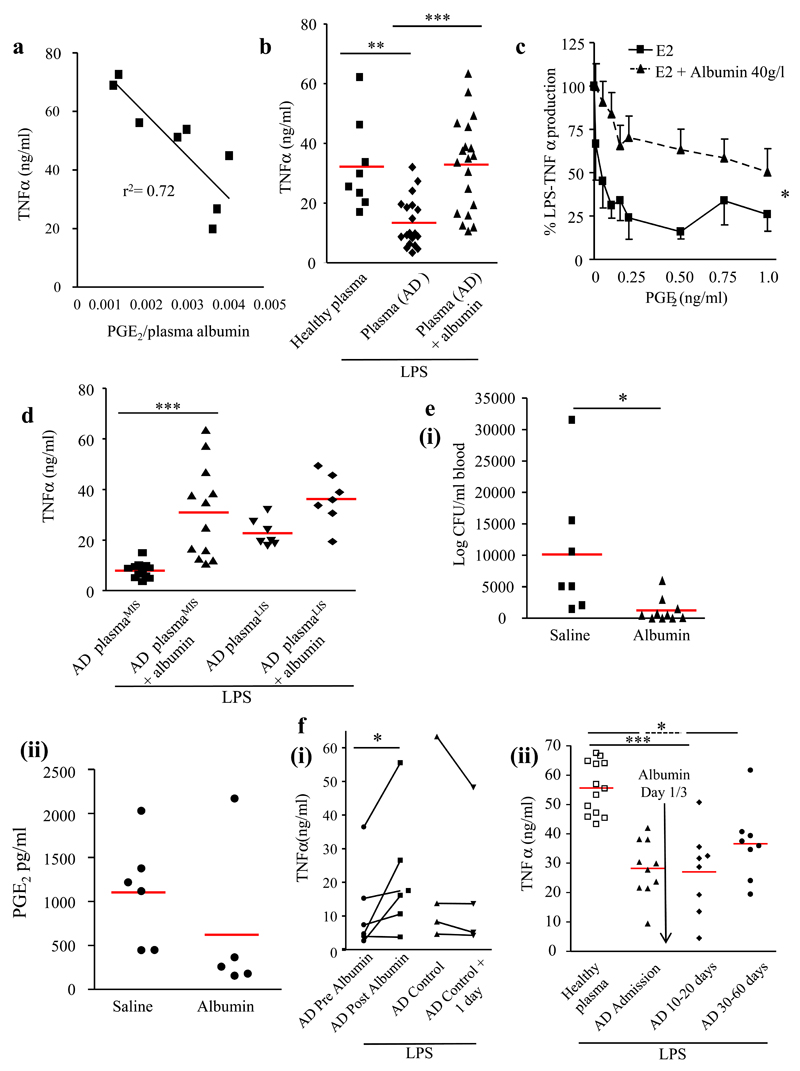
Correlating levels of TNFα from macrophages incubated in the presence of AD plasma with corresponding) plasma PGE2 levels resulted in modest r2 of 0.41 (p=0.085). Albumin binds to A- and E-type PGs by attaching to the ligand-binding site I in subdomain 2A17. Mass spectroscopy measures PGE2 that is both free and albumin-bound and so we postulated that immunosuppression might be a consequence of the bioavailability of PGE2 as determined by the blood albumin concentration. Expressing AD plasma PGE2 levels as a function of corresponding albumin levels and then correlating these values with macrophage TNFα synthesis resulted in a r2 value of 0.72 (p=0.0076) (Figure 4A). Adding albumin to AD plasma in order to restore levels to 40mg/dl, equivalent to HV plasma, reversed AD plasma induced suppression of TNFα generation (Figure 4A). Supplementing culture media with 40mg/dl of albumin impairs PGE2 inhibition of TNFα synthesis (Figure 4C).
Figure 4. PGE2-mediated immunosuppression by AD plasma is reversed by albumin.
(A) AD plasma PGE2 levels were corrected for corresponding albumin levels, which correlated significantly with the effects of these AD samples on LPS-stimulated human monocyte-derived macrophage TNFα synthesis. (B) AD samples were supplemented with human albumin to restore average levels back to 40 g/l (mean albumin levels in AD plasma = 25.4g/l) and then incubated with LPS-stimulated (200ng/ml) human monocyte-derived macrophages, plasma from n=19 patients were used with experiments carried out in duplicate. (C) Suppressed human monocyte-derived macrophage TNFα production caused by increasing concentrations of PGE2 in an albumin-free culture environment is antagonized by 40mg/dl of >99% purified human serum albumin. All (D) AD plasma samples that dampened TNFα below the lowest TNFα exerted by plasma from healthy controls (Figure 1C) were arbitrarily grouped into ADMIS (AD plasma from most immunosuppressed) with the remainder called ADLIS (AD plasma from least immunosuppressed); albumin exerted its greatest reversal of immunosuppression on AD plasmaMIS samples. 0.5ml of 20% Human Albumin Solution (HAS) or normal saline (0.9%) was given to BDL mice (n=10/group) 1h before Group B streptococcus with (E, panel i) bacteria and (E, panel ii) PGE2 plasma levels measured 1h later (n=5). In (F) panel (i) 20% HAS was given to AD patients (n=6) and their pre- and post (1day)-albumin plasma samples incubated with LPS-stimulated human monocyte-derived macrophages. In panel F (ii) AD plasma induced immunosuppression persisted for up to 60 days after discharge and that administration of 1L 20% HAS on days 1 & 3 had no effect on long term immunosuppression (n=10). Data is represented as average ± SEM. * P<0.05 ** P<0.01 ***P<0.001, ANOVA.
A serum albumin levels below 30mg/ml may predict susceptibility to infection
AD plasma samples that dampened TNFα below the lowest TNFα exerted by healthy plasma (Figure 4A) were arbitrarily grouped into ADMIS (AD plasma from most immunosuppressed) and the remainder ADLIS (AD plasma from least immunosuppressed) (Figure 4D). Albumin and AH6809 reversed ADMIS immunosuppression, whereas ADLIS were refractory, (Figure 4D and supplementary Figure 5 A-C). This differential effect of AH6809 on LIS/MIS samples was also observed in ESLD patients (supplementary Figure 5D).
Analysis of AD patient data (Table 1) demonstrated that albumin was the only feature that discriminated between MIS and LIS plasma samples (P=0.0012). Receiver Operating Characteristic (ROC) analysis of this cohort of 35 patients revealed that a cut off level of albumin of <30mg/dl predicted immunosuppression with a sensitivity of 70% (CI 47-87) and a specificity 67% (CI 35-90).
20% HAS enhances bacterial killing in murine bacterial peritonitis
2h prior to GBS challenge BDL mice treated with 20% HAS or saline (0.5ml, i.p.). In albumin treated mice the mean albumin levels rose from 24mg/dl to 32mg/dl. Albumin treatment resulted in significantly lower levels of blood bacteria 3h after GBS challenge compared to saline treated mice, Figure 4E(i), concomitant with lower plasma PGE2 levels, Figure 4E(ii). As albumin has no direct bactericidal effect we propose that it restores immune competence in BDL mice via PGE2 antagonism.
Infusion of 20% HAS reverses immunosuppression in patients admitted to hospital with AD
20% HAS given to patients with AD (median 200 ml) that had serum albumin concentration <30 mg/dl raised albumin levels from 23.7±1.7 to 30.1±3.1 mg/ml and reversed immunosuppression (Figure 4F(i), P<0.05, n=6). Samples taken on consecutive days from patients not given albumin demonstrated persistent immunosuppression.
In patients admitted with acute hepatic encephalopathy we observed that plasma induced immunosuppression persisted for up to 60 days after discharge (P<0.001, Figure 4F(ii)) and that administration of 200ml 20% HAS on days 1 & 3 had no effect on long term immunosuppression (samples taken 8-60 days after albumin). Saline administration also had no effect (supplementary Figure 5E).
Discussion
This study describes for the first time the primary cause of immunosuppression in AD and ESLD patients. Elevated circulating PGE2 concentration secondary to increased production, in combination with hypoalbuminaemia, drives innate immune dysfunction resulting in vulnerability to infection. This phenomenon was seen in all AD patients from day 2 onwards of their admission and persisted for at least 60 days following hospital discharge. We have shown a serum albumin concentration <30mg/dl is predictive of immunosuppression and 20% HAS infusions to achieve a concentration above 30mg/dl reverses immunosuppression in human plasma ex-vivo and in vivo mouse models. We thus propose a new paradigm for the prevention and treatment of infection for patients presenting with AD. All patients should receive stratified HAS therapy to maintain serum concentration >30mg/dl for the duration of their admission. HAS may also represent an effective immune restorative strategy in ESLD patients18.
The significant strengths of our study are that it represents a hypothesis-driven series of in vitro/vivo experiments in mice and humans. We have demonstrated a robust PGE2-mediated immunosuppressive effect in >75 patients with either AD or ESLD on the transplant waiting list, but not in >15 Childs A/non-cirrhotic liver disease patients, with samples provided by 5 different hospitals from two different countries. We selected macrophage production of TNFα as our primary assay of immune-competence as reduced production of pro-inflammatory cytokines after LPS stimulation is associated with adverse outcomes following sepsis19, trauma20 and pediatric cardiopulmonary bypass21. We used several other models of immune dysfunction – macrophage IL10 production and bacterial killing as well as mouse models of bacterial infection/survival to arrive at the conclusion that PGE2 drives immunosuppression in AD and ESLD. Naturally our proposed management paradigm for AD requires validation by a large-scale clinical trial.
PGE2 has been shown to be a key mediator of innate immune dysfunction by inhibition of NADPH oxidase-mediated bacterial killing22,23 via up-regulation of cAMP24 and inhibition of FcγR-mediated phagocytosis25,26. Increased PGE2 production is likely triggered by a combination of the inflammatory cell infiltrate secondary to liver injury and gut-bacterial translocation resulting in elevated COX activity in peripheral blood monocytes and Kupffer cells27. Importantly, the immunosuppressive effect of PGE2 was increased due to reduced circulating levels of albumin in AD patients which is known to bind albumin28, thereby increasing its bioavailability. This may explain why plasma from stable cirrhosis patients had no immunosuppressive effects despite elevated PGE2 (twice control values compared to a 7-fold increase in AD) as these patients had normal albumin concentrations. Our studies revealed the likely source of the PGE2 to be peripheral blood mononuclear cells, liver Kupffer cells and alveolar macrophages. Despite the apparent anti-fibrotic properties of PGE229 chronic COX 2 inhibition (celecoxib at 10mg/kg for five days) did not worsen liver fibrosis.
Given the global increase in liver disease and that sepsis is the major cause of morbidity/mortality in cirrhosis3, treatment and prevention of infection in these patients will become a substantial health care challenge. Although immunosuppression is well documented in cirrhosis, prior to our study no underlying mechanism had been established and therefore no immune restorative treatment exists.
Although effective experimentally, non-steroidal anti-inflammatory drugs (NSAIDS) are contraindicated in patients with decompensated liver cirrhosis due to increased incidence of renal impairment and gastrointestinal bleeding30. Albumin appears to antagonize PGE2’s effects on innate immune cells without compromising its protective role in other tissues. Albumin is already used to treat cirrhosis-associated complications including ascites, type I hepatorenal syndrome and spontaneous bacterial peritonitis (SBP), and its safety is well established31. In SBP, large volume albumin infusions (up to 160g on day 1 and 3 of admission) reduced mortality rates compared to controls32. While fluid resuscitation was undoubtedly beneficial in this setting, an immune restorative effect of albumin cannot be discounted33,34. This regimen is currently recommended in SBP27, however, as PGE2 induced immunosuppression persists throughout admission, albumin on day 1 and 3 may only have a modest benefit. We predict the immune restorative properties of albumin are only likely to last while serum albumin remains >30mg/dl and therefore repeated infusions will be necessary.
In conclusion this is the first study to show that elevated PGE2 represents the underlying cause of immunosuppression in AD and ESLD patients. We propose a new management strategy to reverse immunosuppression in patients presenting with AD. Repurposing albumin as an immune restorative drug in these patients to target a serum albumin >30mg/dl throughout admission will restore immune competency and both augment treatment of infection and prevent nosocomial infection. This would potentially lead to improved mortality, reduction in ICU admission, hospital stay and antibiotic use.
Methods
Human Models
Samples were obtained from several sources representing the spectrum of cirrhosis patients that vary in vulnerability to infection. Non-cirrhotic liver disease patients were used as a control. Informed consent was obtained from all subjects.
-
(i)Acutely decompensated patients:
-
aAoCLF samples - Predictive utility of DASIMAR as a prognostic biomarker in AoCLF (ClinicalTrials.gov: NCT01071746).
-
bAcute encephalopathy - ALFAE (Efficacy of albumin for acute encephalopathy in patients with cirrhosis, Hospital Clinic of Barcelona; ClinicalTrials.gov: NCT00886925).
-
cFirst presentation with decompensation secondary to alcoholic liver disease from University College London Hospital (UCLH).
-
a
-
(ii)
Patients on the liver transplant waiting list, representing ESLD. These were followed up for 24 weeks - MACHT (Effect of midodrine and albumin in the prevention of complications in cirrhotic patients awaiting liver transplantation, Hospital Clinic of Barcelona; ClinicalTrials.gov: NCT00839358).
-
(iii)
Stable cirrhosis (Child’s Pugh A) from The Royal London Hospital outpatient clinic.
-
(iv)
Non-cirrhotic liver disease (Non-Alcoholic Fatty Liver Disease) from The Royal London Hospital outpatient clinic.
Ethical approval was granted for all studies (see supplementary methods).
Lipid mediators were analyzed by liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) based on published protocols35. Human monocyte-derived macrophages36 were stimulated for 24h with 200ng/ml LPS (Salmonella Abortus, Enzo Biochem, Inc) ± plasma/serum (25%) or eicosanoids of interest ± AH6809 (50-300μM, Sigma Aldrich, Dorset, U.K.) or albumin. Multiple mediators or plasma samples were evaluated simultaneously. Supernatants were collected for cytokine analysis. In bacterial killing experiments Escherichia Coli (clinical isolate) was added to macrophages (100:1) ± AD or healthy volunteers (HV) plasma ± AH6809/albumin. Standard real-time polymerase chain reaction (rtPCR) was performed on polymorphonuclear cells from AD patients and healthy volunteers.
Animal
Mice were maintained in a 12h/12h light/dark cycle at 22 ± 1°C and given food and tap water ad libitum in accordance with UK Home Office regulations. Studies were performed in male C57Bl6/J mice (20-25g) from Charles River UK, Margate, UK. Two models of liver injury were used: Bile duct ligation (BDL, 2 weeks) was performed37 or Carbon Tetrachloride (CCL4, Merck, Darmstadt, Germany, 8 weeks) given s.c. (1 ml/kg) twice weekly and 300mg/L phenobarbital in water38. We used these to investigate whether PGE2 inhibitors affected bacterial killing and survival in infection models in vivo.
Statistical analysis
For calculation of group sizes, from experiments with murine peritonitis, cellular profiles, inflammatory protein expression and lipid mediator production is extremely reproducible. We found with random allocation of animals to each group that intra-animal replicate variability is much less than inter-animal biological variability. An effect size of ~40% of parameter mean is considered biologically relevant. Using this and population statistics, to enable statistical determination at a P<0.05 in a primary ANOVA screen followed by post-hoc Bonferroni corrected T-test at 90% power, a group size of 5 animals is necessary with a maximum of 5 groups per experiment. Applying this approach to humans using human cirrhotic plasma on humans cells with bacterial killing and TNFα as a readout, a minimum of n=10/group was required in order to discern significant changes in immune function. Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software). For comparisons between multiple groups, 1-way ANOVA with repeated measures was performed followed by Bonferroni post-test. Comparisons between 2 groups were made by 2-tailed (un)paired t test. Differences between time-response curves were assessed by 2-way ANOVA. Correlations between variables were calculated using linear regression with Pearson statistic. For data not normally distributed (clinical data presented in Table 1) the Mann-Whitney test was used. P < 0.05 was considered statistically significant. No data, either rodent or human, were excluded from analysis.
Randomisation
Cages of animals (n=5/cage) were randomly allocation to surgical or chemical-induced liver injury. Thereafter, groups of mice were allocated to receive drug intervention, infectious stimuli or both. In addition, groups of liver disease mice and sham controls were chosen for plasma extraction for ex vivo bioassays, where appropriate. For human studies, randomisation was not possible as human monocyte-derive macrophages received plasma from either healthy volunteers or from liver disease patients.
Blinding
For all mouse and human cell culture and bacteria experiments and mouse survival experiments, the investigator was blinded to the sample source during both the experiment and analysis of the data.
Supplementary Material
Acknowledgements
We are very grateful to Dr Raj Mookerjee for allowing use of samples from the DASMIAR (NCT01071746), study, Dr Nainah Shah for collecting samples and Dr Nathan Davies for technical assistance. All three are from the Liver Failure Group, Royal Free Hospital, United Kingdom. We also thank Andrew Healey, University of Bradford for technical support. Further, we would like to thank Dr Harry Antoniades (Imperial College, London) for facilitating sample acquisition and Dr Rita Garcia-Marquez (Hospital Clinic Barcelona, Spain) and Juan Cordoba (Instituto de Salud Carlos III, Madrid, Spain) for providing samples from the MACHT (NCT00839358) and ALFAE trials (NCT00886925).
Financial Support
DWG is a Wellcome Trust senior research fellow and support for work presented here was provided by the Wellcome Trust.
Abbreviations
- AD
Acute Decompensation
- ESLD
end-stage liver disease
- AoCLF
acute-on-chronic liver failure
- PG
prostaglandin
- CCL4
carbon tetrachloride
- BDL
bile duct ligation
Footnotes
Author Contributions
DWG/AO’B conceived the idea, AO’B carried out the work. DWG and AO’B co-wrote the paper and JNF edited. JNF/GS/JN/SJ/EK/GA carried out biochemical assays while WA/RG supplied clinical samples. KM/AN carried out electrospray ionization tandem mass spectrometry analysis, AW carried out histological analysis.
References
- 1.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–746. vii. doi: 10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 3.Navasa M, Fernandez J, Rodes J. Bacterial infections in liver cirrhosis. Italian journal of gastroenterology and hepatology. 1999;31:616–625. [PubMed] [Google Scholar]
- 4.O'Brien AJ, Welch CA, Singer M, Harrison DA. Prevalence and outcome of cirrhosis patients admitted to UK intensive care: a comparison against dialysis-dependent chronic renal failure patients. Intensive care medicine. 2012;38:991–1000. doi: 10.1007/s00134-012-2523-2. [DOI] [PubMed] [Google Scholar]
- 5.Fierer J, Finley F. Deficient serum bactericidal activity against Escherichia coli in patients with cirrhosis of the liver. The Journal of clinical investigation. 1979;63:912–921. doi: 10.1172/JCI109391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassner A, et al. Impaired monocyte function in liver cirrhosis. British medical journal. 1981;282:1262–1263. doi: 10.1136/bmj.282.6272.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–262. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 8.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Soares-Weiser K, Uribe M. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. The Cochrane database of systematic reviews. 2010:CD002907. doi: 10.1002/14651858.CD002907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MJ, et al. Antibiotic prophylaxis for spontaneous bacterial peritonitis in cirrhotic patients with ascites, without gastro-intestinal bleeding. The Cochrane database of systematic reviews. 2009:CD004791. doi: 10.1002/14651858.CD004791.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Fagiuoli S, et al. Management of infections in cirrhotic patients: Report of a Consensus Conference. Dig Liver Dis. 2013 doi: 10.1016/j.dld.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Laleman W, et al. Acute-on-chronic liver failure: current concepts on definition pathogenesis, clinical manifestations and potential therapeutic interventions. Expert Rev Gastroenterol Hepatol. 2011;5:523–537. doi: 10.1586/egh.11.47. quiz 537. [DOI] [PubMed] [Google Scholar]
- 12.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009;57:703–708. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 13.Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. 2011;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Kalinski P. Regulation of immune responses by prostaglandin E2. Journal of immunology. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fullerton JN, O'Brien AJ, Gilroy DW. Pathways mediating resolution of inflammation: when enough is too much. J Pathol. 2013;231:8–20. doi: 10.1002/path.4232. [DOI] [PubMed] [Google Scholar]
- 16.Bozyk PD, Moore BB. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:445–452. doi: 10.1165/rcmb.2011-0025RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Petersen CE, Ha CE, Bhagavan NV. Structural insights into human serum albumin-mediated prostaglandin catalysis. Protein science : a publication of the Protein Society. 2002;11:538–545. doi: 10.1110/ps.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romanelli RG, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12:1403–1407. doi: 10.3748/wjg.v12.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall MW, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ploder M, et al. Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock. 2006;25:129–134. doi: 10.1097/01.shk.0000191379.62897.1d. [DOI] [PubMed] [Google Scholar]
- 21.Allen ML, et al. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med. 2006;34:2658–2665. doi: 10.1097/01.CCM.0000240243.28129.36. [DOI] [PubMed] [Google Scholar]
- 22.Serezani CH, et al. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. American journal of respiratory cell and molecular biology. 2007;37:562–570. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourdonnay E, Serezani CH, Aronoff DM, Peters-Golden M. Regulation of alveolar macrophage p40phox: hierarchy of activating kinases and their inhibition by PGE2. J Leukoc Biol. 2012;92:219–231. doi: 10.1189/jlb.1211590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. The Journal of experimental medicine. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goss JA, Mangino MJ, Flye MW. Prostaglandin E2 production during hepatic regeneration downregulates Kupffer cell IL-6 production. Ann Surg. 1992;215:553–559. discussion 559-560. [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Petersen CE, Ha CE, Bhagavan NV. Structural insights into human serum albumin-mediated prostaglandin catalysis. Protein Sci. 2002;11:538–545. doi: 10.1110/ps.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAnulty RJ, Hernandez-Rodriguez NA, Mutsaers SE, Coker RK, Laurent GJ. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-beta isoforms on fibroblast cell cultures. Biochem J. 1997;321(Pt 3):639–643. doi: 10.1042/bj3210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 31.Alves de Mattos A. Current indications for the use of albumin in the treatment of cirrhosis. Annals of hepatology. 2011;10(Suppl 1):S15–20. [PubMed] [Google Scholar]
- 32.Sort P, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 33.Jaisson S, et al. Carbamylated albumin is a potent inhibitor of polymorphonuclear neutrophil respiratory burst. FEBS Lett. 2007;581:1509–1513. doi: 10.1016/j.febslet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Mookerjee RP, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 35.Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:75–83. doi: 10.1002/rcm.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith AM, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. The Journal of experimental medicine. 2009;206:1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgiev P, et al. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008;95:646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 38.Domenicali M, et al. A novel model of CCl4-induced cirrhosis with ascites in the mouse. J Hepatol. 2009;51:991–999. doi: 10.1016/j.jhep.2009.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.