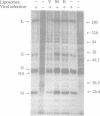
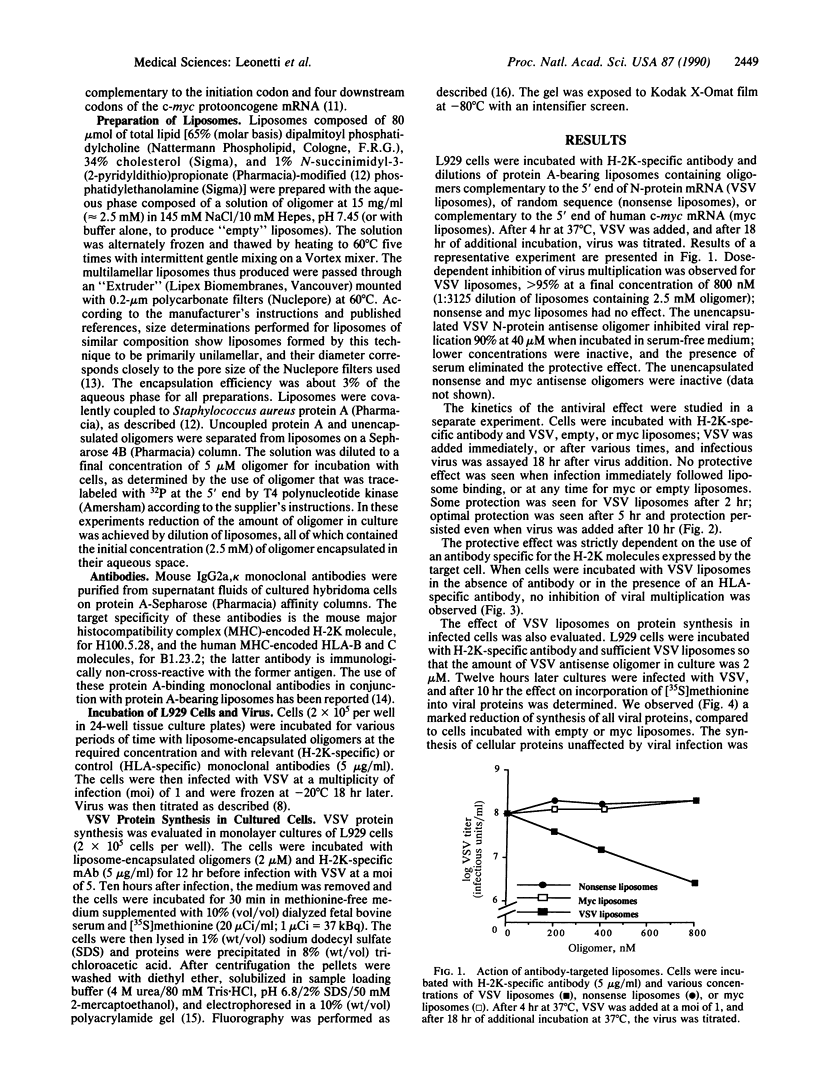
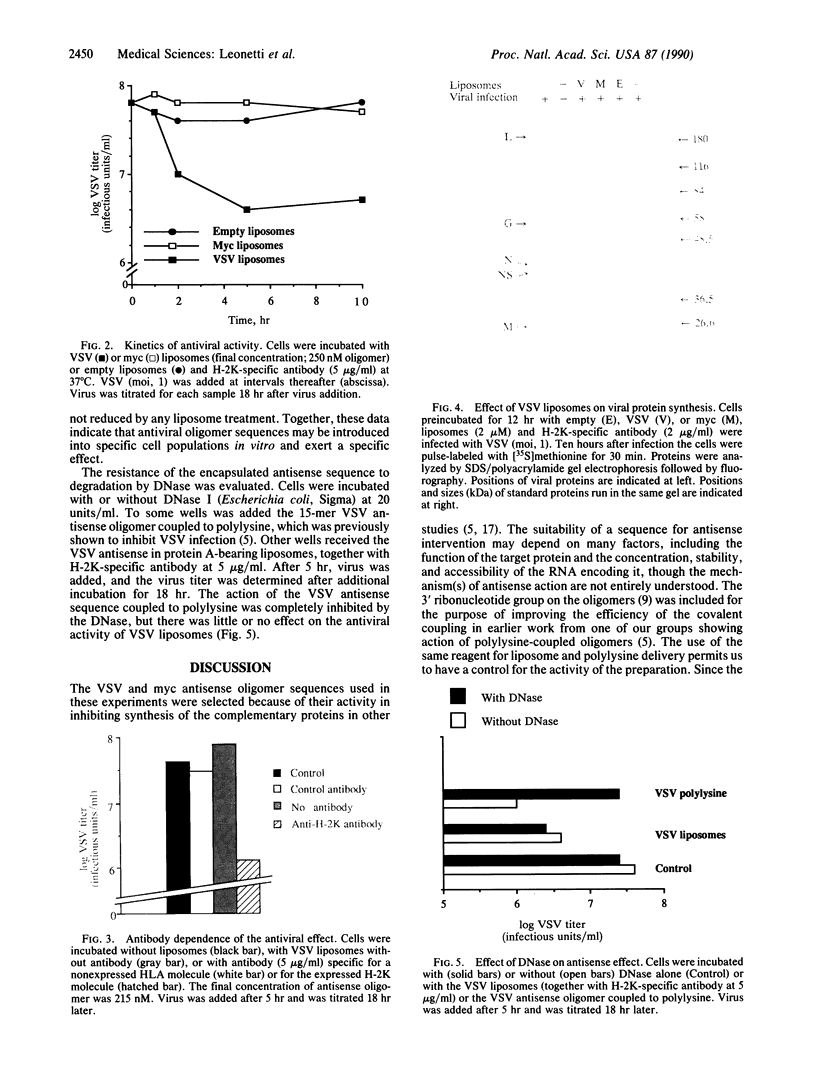
Abstract
Mouse L929 cells were incubated with antibody-targeted liposomes containing oligodeoxyribonucleotides (oligomers). When the oligomer was a 15-mer complementary to the 5'-end region of the mRNA encoding the N protein of vesicular stomatitis virus, the cells became less permissive for multiplication of that virus; greater than 95% reduction of viral multiplication was achieved. Protection was not seen for "empty" liposomes, liposomes containing a random oligomer sequence, or liposomes containing a sequence complementary to the 5' end of c-myc protooncogene mRNA targeted by the same antibody, nor was it seen when the liposomes containing the N-protein antisense oligomer were targeted by an antibody that does not bind to L929 cells. Antibody-bearing liposomes containing antisense oligomers thus have a double specificity: a particular cell selected by the targeting antibody on the liposome and a particular mRNA in the cell selected by sequence complementarity with the liposome-encapsulated oligomer. Nonencapsulated oligomers are sensitive to nucleases and usually must be administered to cells at high concentrations. Oligomers encapsulated in liposomes resist DNase and are active in amounts 1-2 orders of magnitude lower than for those reported for unencapsulated oligomer sequences.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris C. H., Blake K. R., Miller P. S., Reddy M. P., Ts'o P. O. Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence-specific oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Oct 7;25(20):6268–6275. doi: 10.1021/bi00368a065. [DOI] [PubMed] [Google Scholar]
- Bayard B., Leserman L. D., Bisbal C., Lebleu B. Antiviral activity in L1210 cells of liposome-encapsulated (2'-5')oligo(adenylate) analogues. Eur J Biochem. 1985 Sep 2;151(2):319–325. doi: 10.1111/j.1432-1033.1985.tb09103.x. [DOI] [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaitre M., Bayard B., Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci U S A. 1987 Feb;84(3):648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti J. P., Rayner B., Lemaitre M., Gagnor C., Milhaud P. G., Imbach J. L., Lebleu B. Antiviral activity of conjugates between poly(L-lysine) and synthetic oligodeoxyribonucleotides. Gene. 1988 Dec 10;72(1-2):323–332. doi: 10.1016/0378-1119(88)90159-x. [DOI] [PubMed] [Google Scholar]
- Leserman L. D., Barbet J., Kourilsky F., Weinstein J. N. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature. 1980 Dec 11;288(5791):602–604. doi: 10.1038/288602a0. [DOI] [PubMed] [Google Scholar]
- Machy P., Leserman L. D. Small liposomes are better than large liposomes for specific drug delivery in vitro. Biochim Biophys Acta. 1983 May 5;730(2):313–320. doi: 10.1016/0005-2736(83)90348-6. [DOI] [PubMed] [Google Scholar]
- Machy P., Pierres M., Barbet J., Leserman L. D. Drug transfer into lymphoblasts mediated by liposomes bound to distinct sites on H-2 encoded I-A, I-E, and K molecules. J Immunol. 1982 Nov;129(5):2098–2102. [PubMed] [Google Scholar]
- Machy P., Truneh A., Gennaro D., Hoffstein S. Endocytosis and de novo expression of major histocompatibility complex encoded class I molecules: kinetic and ultrastructural studies. Eur J Cell Biol. 1987 Dec;45(1):126–136. [PubMed] [Google Scholar]
- Machy P., Truneh A., Gennaro D., Hoffstein S. Major histocompatibility complex class I molecules internalized via coated pits in T lymphocytes. Nature. 1987 Aug 20;328(6132):724–726. doi: 10.1038/328724a0. [DOI] [PubMed] [Google Scholar]
- Milhaud P. G., Machy P., Lebleu B., Leserman L. Antibody targeted liposomes containing poly(rI).poly(rC) exert a specific antiviral and toxic effect on cells primed with interferons alpha/beta or gamma. Biochim Biophys Acta. 1989 Dec 11;987(1):15–20. doi: 10.1016/0005-2736(89)90449-5. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A., Mishal Z., Barbet J., Machy P., Leserman L. D. Endocytosis of liposomes bound to cell surface proteins measured by flow cytofluorometry. Biochem J. 1983 Jul 15;214(1):189–194. doi: 10.1042/bj2140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaia J. A., Rossi J. J., Murakawa G. J., Spallone P. A., Stephens D. A., Kaplan B. E., Eritja R., Wallace R. B., Cantin E. M. Inhibition of human immunodeficiency virus by using an oligonucleoside methylphosphonate targeted to the tat-3 gene. J Virol. 1988 Oct;62(10):3914–3917. doi: 10.1128/jvi.62.10.3914-3917.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]